Introduction
Lung cancer is the most common malignancy worldwide
and has the highest mortality rate (1). Numerous patients present with advanced
stages at diagnosis due to concealed symptoms. Additionally, it is
the most common type of cancer in China (2). In the United States, there was
estimated to be >230,000 new cases of lung cancer in 2018 and
lung cancer was suggested to lead to more deaths than breast,
prostate and colon cancer combined (3). Non-small cell lung cancer (NSCLC) is
the most typical form of lung cancer, accounting for ~85% of all
cases (4). The improvement of
diagnostic technologies and the emergence of effective new
treatment methods, such as targeted therapies and immunotherapy,
have improved the therapeutic management of lung cancer; however,
the overall 5-year survival rate of this type of cancer remains low
at 17.4% (5). Therefore, the early
diagnosis and the identification of effective biomarkers are
important to improve the prognosis of patients with lung
cancer.
Autophagy, also known as type II programmed cell
death, is a genetically regulated process that degrades cellular
proteins and organelles through lysosomes (6). There is a close and complex association
between autophagy and tumors. Furthermore, the role of autophagy in
different tumor types depends on the different stages of
tumorigenesis. As the first identified mammalian autophagy protein,
Beclin-1 has been used to investigate autophagy in cancer (7). However, to the best of our knowledge,
there is no research into the association between Beclin-1 and
cancer pathogenesis, and the nature of this association and its
underlying mechanism remain controversial.
Bcl-2 is a member of the Bcl-2 anti-apoptotic
protein family. In addition to being a key regulator of apoptosis,
Bcl-2 modulates other important cell functions, such as cell cycle
and mitochondrial signaling pathway (8). Additionally, Bcl-2 can bind to Beclin-1
to form the Beclin-1/Bcl-2 complex and then inhibit autophagy.
However, the binding of Bcl-2 to Beclin-1 is regulated by a variety
of proteins, which enhance or inhibit the Beclin-1/Bcl-2
interaction and further inhibiting or activating autophagy and
apoptosis, thus the Beclin-1/Bcl-2 complex act as a crosstalk
between autophagy and apoptosis (9).
Previous studies have demonstrated that the expression levels of
Beclin1 and Bcl-2 in tumor cells depend on the tumor and tissue
type (10–12); however, this requires further
investigation.
To the best of our knowledge, the association
between Beclin-1 and Bcl-2 in lung cancer has not been yet
elucidated. Therefore, the present study aimed to evaluate the
roles of Beclin-1 and Bcl-2 on the clinicopathological features and
survival of patients with NSCLC, and to estimate their value as
markers of the development and prognosis of this type of
cancer.
Materials and methods
Patients and tissue samples
A total of 120 patients with NSCLC who underwent
surgical resection between January 2014 and December 2014 were
selected from the archived materials of the Department of Pathology
of Ruijin Hospital, Shanghai Jiaotong University School of Medicine
(Shanghai, China). All patients were diagnosed by postoperative
paraffin pathology, and no neoadjuvant treatment was performed.
Patients younger than 18 or older than 75 years old, or patients
with distant metastases were excluded from the present study. Of
the 120 patients (median age, 61.5 years; age range, 45–75 years),
56 were male and 64 were female. The 2017 Union for International
Cancer Control 8th Edition TNM staging (13) as used to classify tumors into stages
I, II and III. All patients were followed-up until November 2019
(mean follow-up time, 60.1 months; range, 12–70 months). The
present study was approved by the Clinical Ethics Committee of
Ruijin Hospital, Shanghai Jiaotong University School of Medicine
(approval no. 2017138).
Immunohistochemistry (IHC)
IHC staining was performed on 10% formalin-fixed and
paraffin-embedded tumor and adjacent non-tumor tissues (≥3 cm from
the margins of tumors). Slides of 4-µm sections were deparaffinized
with xylene and antigen retrieval was accomplished by using
microwave oven. The sections were then incubated in 3% hydrogen
peroxide at room temperature for 15 min to block endogenous
peroxidase activity. Slides were then incubated with anti-Beclin-1
(1:200; cat. no. SC-11427; Santa Cruz Biotechnology, Inc.) and
anti-Bcl-2 (1:100; cat. no. ab32124; Abcam) primary antibodies at
4°C overnight. The slides were then washed three times in phosphate
buffer solution (PBS) for 5 min each and incubated in
biotin-labeled secondary antibodies (1:2,000; cat. no. ab205718;
Abcam) for 30 min at 37°C. Images were captured with a light
microscope. Immunohistochemical readings were performed by two
different pathologists. Using a double-blind reading scoring
system, five fields (magnification, ×400) were randomly selected
and 100 cells were counted in each field. Discordant results were
discussed and scored as follows. Score A: 1, ≤10% positive cells;
2, 11–50% positive cells; 3, 51–75% positive cells; and 4, >75%
positive cells. The staining intensity was observed under low
magnification (×100) and scored according to the staining intensity
score B: 0, not stained; 1, light yellow; 2, brownish yellow; and
3, brownish. The final score was calculated as score A × score B. A
final score ≤3 was considered low expression, while a score >3
was considered as high expression.
Assessment of clinical outcome
Overall survival (OS) was defined as the survival
from the date of surgery to the date of death from any cause.
Statistical analysis
SPSS software (v18.0, SPSS, Inc.) was used to
analyze the data. Pearson's χ2 test was used to evaluate
the association between Beclin-1 and Bcl-2 expression with several
clinicopathological variables. The Kaplan-Meier method was used to
determine the probability of survival, and the data were analyzed
using the log-rank test. The Cox proportional hazards model was
used for univariate and multivariate analyses of prognostic
factors. P<0.05 was considered to indicate a statistically
significant difference.
Results
Beclin-1 and Bcl-2 expression in NSCLC
lesions and adjacent tissues
High Beclin-1 expression was observed in 38 (31.67%)
NSCLC samples, while 82 (68.33%) NSCLC samples exhibited low
Beclin-1 expression. The adjacent tissues presented with
significantly higher Beclin-1 expression compared with the NSCLC
tissues (P<0.01). By contrast, high Bcl-2 expression was
identified in 55 (45.83%) NSCLC samples, while only 26 (21.67%)
samples exhibited high Bcl-2 expression in adjacent tissues. NSCLC
samples exhibited significantly higher Bcl-2 expression compared
with their adjacent tissues (P<0.01; Table I). The expression levels of Beclin-1
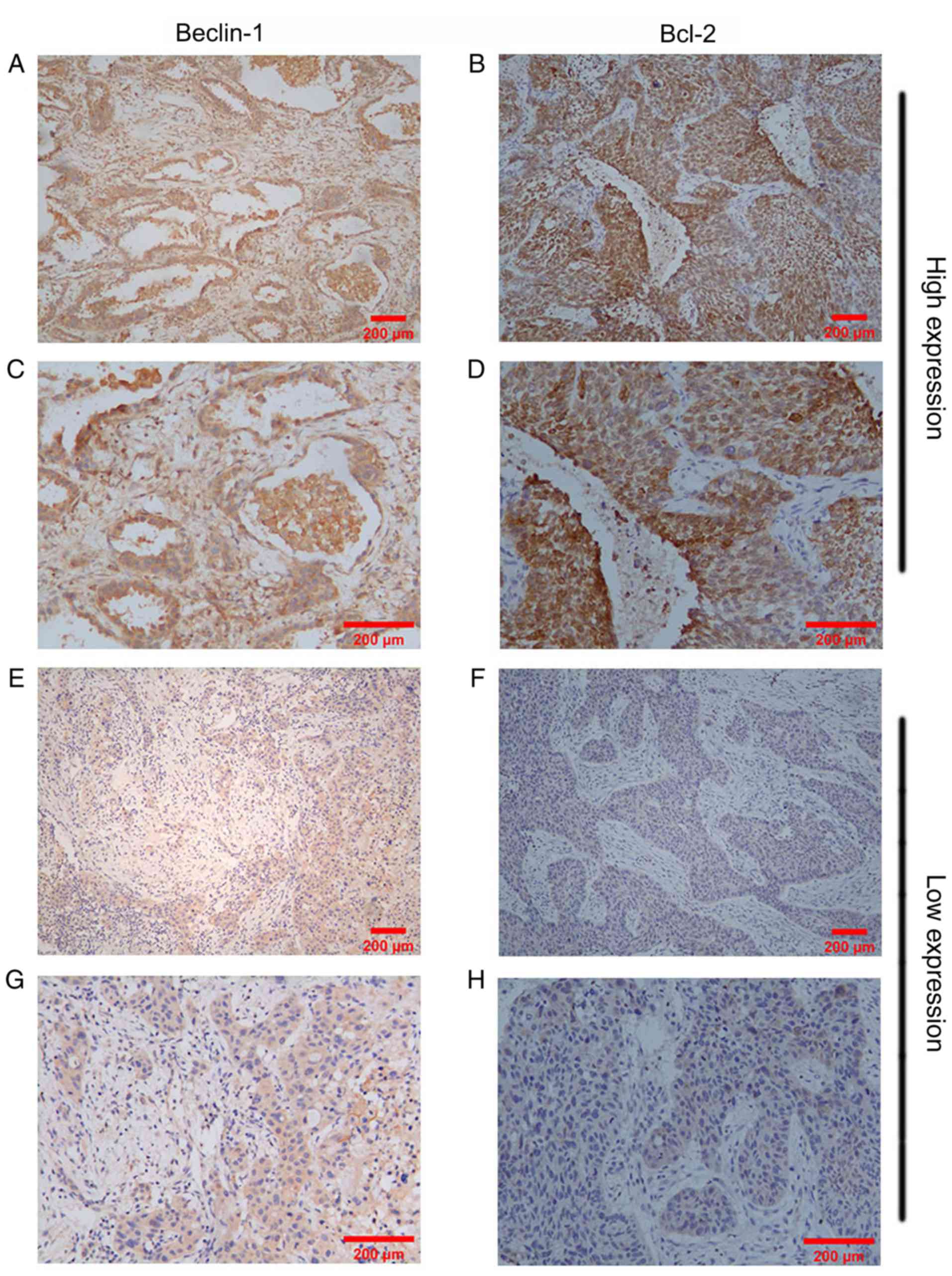
and Bcl-2 in NSCLC tissues, as assessed via IHC, are shown in
Fig. 1. High or low Beclin-1 and
Bcl-2 expression was detected in NSCLC tissues.
 | Table I.Beclin-1 and Bcl-2 expression in
non-small cell lung cancer tissues and adjacent tissues
(n=120). |
Table I.
Beclin-1 and Bcl-2 expression in
non-small cell lung cancer tissues and adjacent tissues
(n=120).
|
| Beclin-1
expression |
|
| Bcl-2 expression |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Tissue type | + | − | χ2 | P-value | + | − | χ2 | P-value |
|---|
| Lung cancer
tissues | 38 | 82 | 7.63 | <0.01a | 55 | 65 | 15.67 | <0.01a |
| Adjacent tissues | 59 | 61 |
|
| 26 | 94 |
|
|
Association between Beclin-1 and Bcl-2
expression
The association between Beclin-1 and Bcl-2
expression in NSCLC tissues was examined using χ2 tests,
which revealed a strong association between the two proteins in
NSCLC tissues (χ2=4.84; P<0.05; Table II).
 | Table II.Association between Beclin-1 and Bcl-2
expression in non-small cell lung cancer tissues (n=120). |
Table II.
Association between Beclin-1 and Bcl-2
expression in non-small cell lung cancer tissues (n=120).
|
| Bcl-2 expression |
|
|
|---|
|
|
|
|
|
|---|
| Beclin-1
expression | + | − | χ2 | P-value |
|---|
| + | 23 | 15 | 4.84 | 0.05a |
| − | 32 | 50 |
|
|
Association between Beclin-1 and Bcl-2
expression with the clinicopathological characteristics of patients
with NSCLC
In the present study, the characteristics of the
patients, such as age, sex, smoking history, pathological staging,
lymph node metastasis, pathological type, degree of tumor
differentiation and preoperative serum carcinoembryonic antigen
(CEA) levels, obtained from the patients' medical records, were
analyzed. All patients were divided into high and low expression
groups regarding Beclin-1 or Bcl-2 expression. As shown in Table III, Beclin-1 expression in NSCLC
was not associated with age, sex, smoking history, pathological
type and preoperative serum CEA levels (P>0.05). However,
Beclin-1 expression was associated with lymph node metastasis,
pathological staging and degree of tumor differentiation
(P<0.05). Furthermore, Bcl-2 expression in NSCLC was not
associated with age, sex, smoking history, pathological staging,
pathological type and preoperative serum CEA levels (P>0.05).
However, Beclin-1 expression was associated with lymph node
metastasis and the degree of tumor differentiation (P<0.05;
Table IV).
 | Table III.Association between Beclin-1
expression and clinicopathological characteristics in patients with
non-small cell lung cancer (n=120). |
Table III.
Association between Beclin-1
expression and clinicopathological characteristics in patients with
non-small cell lung cancer (n=120).
|
|
| Beclin-1
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Pathologic
parameter | Cases, n | High | Low | χ2 | P-value |
|---|
| Sex |
|
|
| 0.46 | >0.05 |
|
Male | 56 | 16 | 40 |
|
|
|
Female | 64 | 22 | 42 |
|
|
| Smoking
history |
|
|
| 2.62 | >0.05 |
|
Yes | 54 | 13 | 41 |
|
|
| No | 66 | 25 | 41 |
|
|
| Age, years |
|
|
| 1.56 | >0.05 |
|
≤60 | 41 | 16 | 25 |
|
|
|
>60 | 79 | 22 | 57 |
|
|
| Pathological
stage |
|
|
| 6.54 |
<0.05a |
| I | 59 | 25 | 34 |
|
|
| II | 42 | 10 | 32 |
|
|
|
III | 19 | 3 | 16 |
|
|
| Lymph node
metastasis |
|
|
| 4.53 |
<0.05a |
|
Positive | 45 | 9 | 36 |
|
|
|
Negative | 75 | 29 | 46 |
|
|
| Pathological
type |
|
|
| 0.89 | >0.05 |
|
Adenocarcinoma | 83 | 28 | 55 |
|
|
|
Squamous | 31 | 9 | 22 |
|
|
|
Others | 6 | 1 | 5 |
|
|
| Degree of
differentiation |
|
|
| 6.90 |
<0.05a |
|
Well | 26 | 12 | 14 |
|
|
|
Moderate | 61 | 21 | 40 |
|
|
|
Poor | 33 | 5 | 28 |
|
|
| Carcinoembryonic
antigen, ng/ml |
|
|
| 1.99 | >0.05 |
| ≤5 | 55 | 21 | 34 |
|
|
|
>5 | 65 | 17 | 48 |
|
|
 | Table IV.Association between Bcl-2 expression
and clinicopathological characteristics in patients with non-small
cell lung cancer (n=120). |
Table IV.
Association between Bcl-2 expression
and clinicopathological characteristics in patients with non-small
cell lung cancer (n=120).
|
|
| Bcl-2
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Pathologic
parameter | Cases, n | High | Low | χ2 | P-value |
|---|
| Sex |
|
|
| 0.37 | >0.05 |
|
Male | 56 | 24 | 32 |
|
|
|
Female | 64 | 31 | 33 |
|
|
| Smoking
history |
|
|
| 0.42 | >0.05 |
|
Yes | 54 | 23 | 31 |
|
|
| No | 66 | 32 | 34 |
|
|
| Age, years |
|
|
| 1.16 | >0.05 |
|
≤60 | 41 | 16 | 25 |
|
|
|
>60 | 79 | 39 | 40 |
|
|
| Pathological
stage |
|
|
| 0.02 | >0.05 |
| I | 59 | 27 | 32 |
|
|
| II | 42 | 19 | 23 |
|
|
|
III | 19 | 9 | 10 |
|
|
| Lymph node
metastasis |
|
|
| 4.14 |
<0.05a |
|
Positive | 45 | 26 | 19 |
|
|
|
Negative | 75 | 29 | 46 |
|
|
| Pathological
type |
|
|
| 2.70 | >0.05 |
|
Adenocarcinoma | 83 | 34 | 49 |
|
|
|
Squamous | 31 | 18 | 13 |
|
|
|
Others | 6 | 3 | 3 |
|
|
| Degree of
differentiation |
|
|
| 9.02 |
<0.05a |
|
Well | 26 | 18 | 8 |
|
|
|
Moderate | 61 | 21 | 40 |
|
|
|
Poor | 33 | 16 | 17 |
|
|
| Carcinoembryonic
antigen, ng/ml |
|
|
| 0.43 | >0.05 |
| ≤5 | 55 | 27 | 28 |
|
|
|
>5 | 65 | 28 | 37 |
|
|
Univariate and multivariate analyses
of OS in patients with NSCLC
In the present study, the OS time of patients with
NSCLC was available for 120 cases; the mean OS time was 60.10
months (range, 12–70 months). Further univariate and multivariate
analyses were performed for the main factors associated with OS in
patients with NSCLC. The results demonstrated that a lower OS was
significantly associated with a more advanced stage (HR, 10.844;
95% CI, 3.885–30.256; P<0.01), poor differentiation (HR, 2.819;
95% CI, 1.425–5.575; P<0.01), high CEA levels (HR, 5.678; 95%
CI, 1.035–31.156; P<0.05) and low Beclin-1 expression (HR,
5.319; 95% CI, 1.844–15.348; P<0.01) (Table V). The mean OS time of patients with
NSCLC with high Beclin-1 expression was 63.17 months, while it was
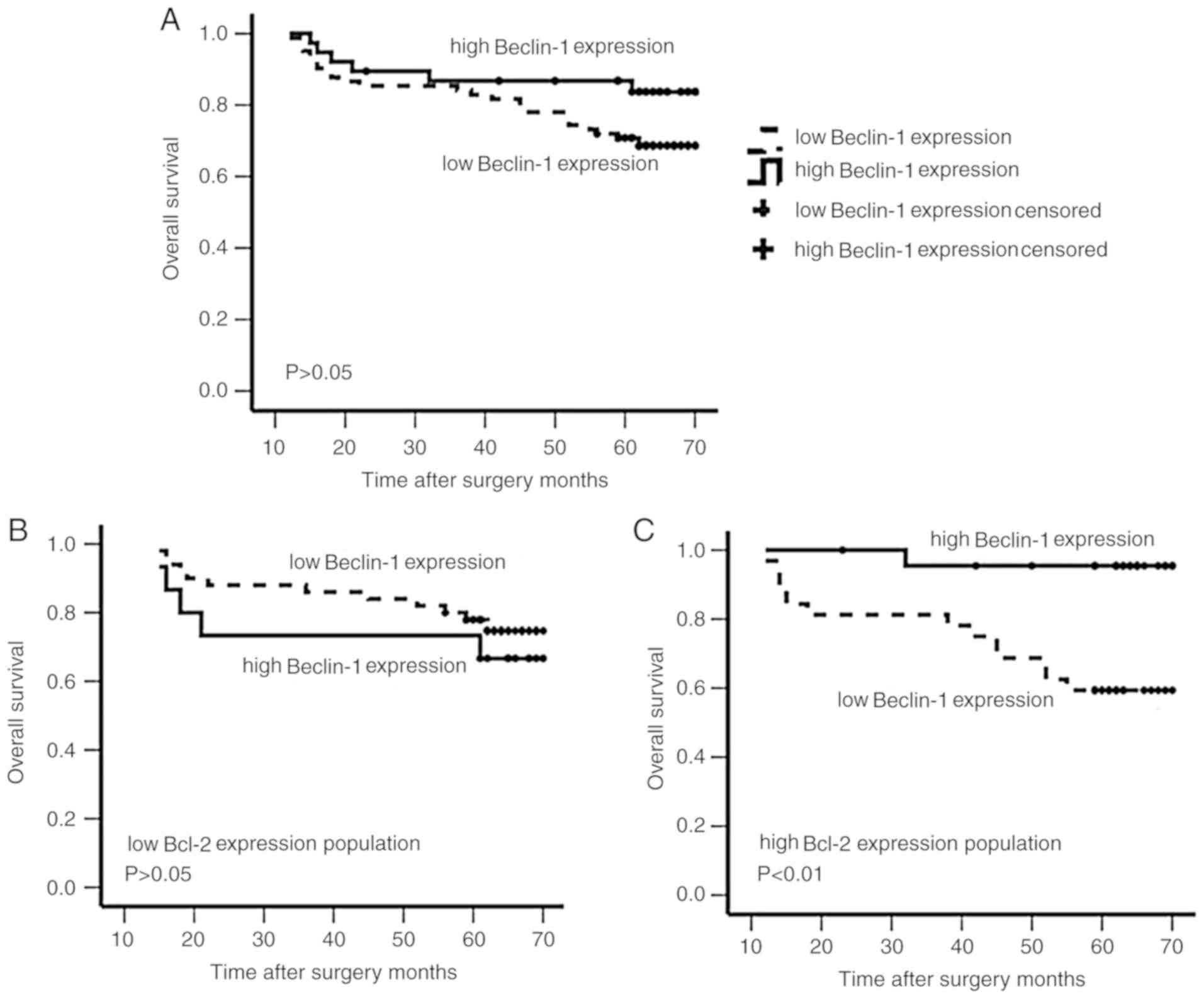
58.72 months for patients with low Beclin-1 expression. However,
this difference was not significant (63.17±2.75 vs. 58.72±2.15
months; P>0.05; Fig. 2A).
Subsequently, the association between the combination of Beclin-1
and Bcl-2 expression status with OS was examined. Patients with
NSCLC with high Beclin-1 expression and high Bcl-2 expression had a
significantly longer mean OS time than those with high Bcl-2 but
low Beclin-1 expression (68.27±1.69 vs. 54.53±3.81 months;
P<0.05; Fig. 2C). In patients
with low Bcl-2 expression, there was no significant difference in
mean OS time according to Beclin-1 expression status (55.40±5.94
vs. 61.42±2.48 months for high and low Beclin-1 expression,
respectively; P>0.05; Fig. 2B).
However, patients with low Bcl-2 and low Beclin-1 expression tended
to have an improved OS compared with patients with low Bcl-2 and
high Beclin-1 expression.
 | Table V.Univariate and multivariate analyses
of prognostic factors in non-small cell lung cancer. |
Table V.
Univariate and multivariate analyses
of prognostic factors in non-small cell lung cancer.
| Variable | Univariate
analysis, HR (95% CI) | P-value | Multivariate
analysis, HR (95% CI) | P-value |
|---|
| Sex | 2.977
(0.612–14.492) | 0.177 |
|
|
|
Male |
|
Female |
| Age, years | 1.132
(0.534–3.221) | 0.554 |
|
|
|
≤60 |
|
>60 |
| Smoking
history | 0.421
(0.093–1.910) | 0.262 |
|
|
|
Yes |
| No |
| Degree of
differentiation | 2.819
(1.425–5.575) | 0.003a | 2.114
(1.185–3.770) | 0.011a |
|
Well |
|
Moderate and poor |
| Pathological
stage | 10.844
(3.885–30.265) |
5.328×10−6a | 13.707
(5.553–33.863) |
1.359×10−8a |
| I |
| II and
III |
| Lymph node
metastasis | 0.964
(0.295–3.156) | 0.952 |
|
|
|
Positive |
|
Negative |
| Pathological
type | 1.221
(0.627–2.380) | 0.557 |
|
|
|
Adenocarcinoma |
|
Squamous and others |
| Beclin-1
expression | 5.319
(1.844–15.348) | 0.002a | 4.508
(1.753–11.591) | 0.002a |
|
High |
|
Low |
| Bcl-2
expression | 0.436
(0.166–1.144) | 0.092 |
|
|
|
High |
|
Low |
| Carcinoembryonic
antigen, ng/ml | 5.678
(1.035–31.156) | 0.046a | 4.373
(0.958–19.954) | 0.057 |
| ≤5 |
|
>5 |
Beclin-1 expression was an independent risk factor
for OS in patients with NSCLC [hazard ratio (HR), 4.508; 95% CI,
1.753–11.591; P<0.01], whereas Bcl-2 expression was not an
independent biomarker of OS (HR, 0.436; 95% CI, 0.166–1.144;
P>0.05; Table V). Furthermore,
the present study indicated that earlier pathologic stage (HR,
13.707; 95% CI, 5.553–33.863; P<0.01) and improved
differentiation (HR, 2.114; 95% CI, 1.185–3.770; P<0.05) were
associated with improved OS in patients with NSCLC (Table V).
Discussion
Autophagy and apoptosis, as type I and II programmed
cell death, respectively, are closely associated with tumor
progression. Studies of Beclin-1 and Bcl-2, which are key molecules
that regulate these two types of programmed cell death, can help
shed light on autophagy and apoptosis, as well as the role of cell
death in NSCLC (14). The present
study focused on the expression levels of Beclin-1 and Bcl-2 in
NSCLC, as well as on the analysis of the association between the
two proteins, to further explore the roles of autophagy and
apoptosis on the biological and clinical behaviors of NSCLC. As a
specific marker of autophagy, Beclin-1 has been the focus of
previous research. Recent studies have reported that Beclin-1 is
downregulated in glioblastoma, liver cancer, bladder cancer and
breast cancer (15–18), while it is upregulated in colon
cancer (19). The present study
revealed that Beclin-1 expression was downregulated in NSCLC
tissues, consistent with a study by Zheng et al (20), which demonstrated that the regulation
of Beclin-1 may serve a role in the development of this type of
cancer.
The association between Beclin-1 expression and the
clinicopathological characteristics of patients has different
manifestations in different types of tumor. In primary
hepatocellular carcinoma, low Beclin-1 protein expression is
associated with the degree of tumor cell differentiation and
postoperative pathological stage, indicating a poor OS (21). In colon cancer, a meta-analysis of
six studies has revealed that high Beclin-1 protein expression is
associated with tumor metastasis and predicts a poor OS (22). The present study revealed that
Beclin-1 was upregulated in the tumor tissues of 31.7% (38/120) of
patients with NSCLC, whereas Beclin-1 was downregulated in 68.3%
(82/120) of these patients. Compared with the normal tissues
adjacent to the tumors, the positive expression rate of Beclin-1
was significantly lower in NSCLC than in adjacent tissues
(χ2=7.63; P<0.01). A subsequent clinicopathological
analysis revealed that low Beclin-1 expression was associated with
the degree of tumor cell differentiation, postoperative
pathological stage and lymphatic metastasis status in patients with
NSCLC (P<0.05). Furthermore, a Cox regression analysis
demonstrated that low Beclin-1 expression may be used as an
independent risk factor for poor prognosis and as an independent
predictor of prognosis in patients with NSCLC.
The Bcl-2 protein, an anti-apoptotic protein that
helps inhibit apoptosis, has been identified as an oncogenic
protein (23); the tumorigenic
effect of Bcl-2 has been confirmed in animal model experiments
(24). However, in some solid
tumors, Bcl-2 appears to have an inhibitory effect, and its
expression is associated with good prognostic characteristics, such
as in gastric cancer (25) and
colorectal cancer (26). A
meta-analysis of ~5,892 patients with breast cancer from 17 studies
examined the effect of Bcl-2 expression on breast cancer prognosis;
its results revealed that Bcl-2 is associated with disease-free
survival (DFS) and OS times (27).
However, the mechanism via which Bcl-2 exerts its protective effect
is unclear. The present study demonstrated that Bcl-2 expression
was significantly higher in lung cancer tissues than in adjacent
tissues (P<0.01). High Bcl-2 expression was associated with the
degree of tumor cell differentiation and lymphatic metastasis in
patients with NSCLC (P<0.05). However, a subsequent Cox
regression analysis did not reveal its role as an independent risk
factor for poor prognosis in patients with NSCLC. Therefore, the
present results suggested that Bcl-2 may not be used as an
independent predictor of prognosis in patients with NSCLC. However,
this observation needs to be confirmed using a larger sample size
in future studies.
Beclin-1 and Bcl-2 are the main factors underlying
two programmed cell death mechanisms. The association between
autophagy and apoptosis is complex and varies according to cell
type and stress stage (28).
Autophagy may initiate or inhibit apoptosis according to the
environment and stimulation of the cell, and inhibition of
autophagy may increase the sensitivity of the cell to apoptotic
signals (29). Furthermore, the
coordination between autophagy and apoptosis may serve an important
role in tumorigenesis and tumor development. A previous study has
indicated that in breast cancer Beclin-1 may serve a role in the
inhibition of the development of breast cancer, which may be due to
an interaction with the Bcl-2 protein (30). In pancreatic cancer, a study by
Shanshan et al (10)
demonstrated that high Bcl-2 and low Beclin-1 expression was
associated with an improved DFS and OS. The present study further
evaluated the expression levels of Beclin-1 in NSCLC tissue
specimens with different expression levels of Bcl-2 via
immunohistochemical staining.
It was revealed that in the high Bcl-2 expression
group, low Beclin-1 expression in NSCLC tissues indicated a poor
prognosis, while high Beclin-1 expression indicated an improved
prognosis. In turn, in the low Bcl-2 expression group, Beclin-1
expression was not associated with the prognosis in patients with
NSCLC (P>0.05). Therefore, the prognosis of NSCLC was closely
associated with Beclin-1 expression only in the presence of high
expression levels of Bcl-2. It was hypothesized that, regardless of
the functional status of autophagy, tumor cells can be destroyed by
apoptosis and that programmed cell death via autophagy may occur
when apoptosis is inhibited; by contrast, when apoptosis is
activated, autophagy may mainly serve a role in protecting tumor
cells from apoptotic death. Nevertheless, the regulatory mechanisms
of action behind the tumorigenesis and development of NSCLC are
complicated and alternative pathways that are independent of
apoptosis or autophagy, or independent of Bcl-2 and Beclin-1, may
be involved. Therefore, further studies are required to confirm
these observations. It should be noted that there is a limitation
in the present study. The results about autophagy activity were
only based on Beclin-1 expression, which was not used in
conjunction with other autophagy markers, such as LC3-II, to assess
autophagy.
In conclusion, the present study revealed that
autophagy activity was decreased in NSCLC tumor tissues, and that
Beclin-1 was downregulated and Bcl-2 was upregulated in the tumor
tissues of these patients. Beclin-1 may be a promising prognostic
marker for patients with NSCLC with high Bcl-2 expression. The
current findings provide a more accurate prognostic assessment for
patients with NSCLC. Additionally, they may be used to actively
follow-up and promptly treat patients with a poor prognosis, which
may benefit a large number of patients with NSCLC.
Acknowledgements
The authors would like to acknowledge Dr Ruyuan
Zhang from Shanghai Ruijin Hospital (Shanghai, China) for his
assistance on this manuscript.
Funding
The present study was partly supported by a grant
from a project of the Shanghai Jiading District Health Committee
(grant no. 2017KY02)
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QC made contributions towards the conception and
design. HD and LC are responsible for the collection of clinical
pathological data and patient follow-up. FL carried out the
immunohistochemistry experiments. XC and YL performed the
statistical analysis. All authors were involved in the writing of
the manuscript, and all authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present retrospective biomarker study was
approved by the Clinical Ethics Committee of Ruijin Hospital,
Shanghai Jiaotong University School of Medicine (approval no.
2017138; Shanghai, China). Written informed consent was provided by
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nasim F, Sabath BF and Eapen GA: Lung
cancer. Med Clin North Am. 103:463–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao M and Chen W: Epidemiology of lung
cancer in China. Thorac Cancer. 10:3–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoy H, Lynch T and Beck M: Surgical
treatment of lung cancer. Crit Care Nurs Clin North Am. 31:303–313.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao Y, Yang D, He J and Krasna MJ:
Epidemiology of lung cancer. Surg Oncol Clin N Am. 25:439–445.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cotzomi-Ortega I, Aguilar-Alonso P,
Reyes-Leyva J and Maycotte P: Autophagy and its role in protein
secretion: Implications for cancer therapy. Mediators Inflamm.
2018:42315912018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levy JMM, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schenk RL, Strasser A and Dewson G: BCL-2:
Long and winding path from discovery to therapeutic target. Biochem
Biophys Res Commun. 482:459–469. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rahman MA, Bishayee K, Habib K, Sadra A
and Huh SO: 18α-Glycyrrhetinic acid lethality for neuroblastoma
cells via de-regulating the Beclin-1/Bcl-2 complex and inducing
apoptosis. Biochem Pharmacol. 117:97–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song S, Wang B, Gu S, Li X and Sun S:
Expression of Beclin 1 and Bcl-2 in pancreatic neoplasms and its
effect on pancreatic ductal adenocarcinoma prognosis. Oncol Lett.
14:7849–7861. 2017.PubMed/NCBI
|
|
11
|
Jiang LC, Huang SY, Zhang DS, Zhang SH, Li
WG, Zheng PH and Chen ZW: Expression of beclin 1 in primary
salivary adenoid cystic carcinoma and its relation to Bcl-2 and p53
and prognosis. Braz J Med Biol Res. 47:252–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baspinar S, Bircan S, Orhan H, Kapucuoglu
N and Bozkurt KK: The relation of Beclin 1 and Bcl-2 expressions in
high grade prostatic intraepithelial neoplasia and prostate
adenocarcinoma: A tissue microarray study. Pathol Res Pract.
210:412–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu HD and Qin ZH: Beclin 1, Bcl-2 and
autophagy. Adv Exp Med Biol. 1206:109–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guadagno E, Borrelli G, Pignatiello S,
Donato A, Presta I, Arcidiacono B, Malara N, Solari D, Somma T,
Cappabianca P, et al: Anti-apoptotic and anti-oxidant proteins in
glioblastomas: Immunohistochemical expression of Beclin and DJ-1
and its correlation with prognosis. Int J Mol Sci. 20:40662019.
View Article : Google Scholar
|
|
16
|
Sun H, Yu J, Wen Z, Wang M and Chen W:
Decreased expression of Beclin-1 in patients with hepatocellular
carcinoma. J BUON. 24:634–641. 2019.PubMed/NCBI
|
|
17
|
Chen L, Liu Y, Zhang Q, Zhang M, Han X, Li
Q, Xie T, Wu Q and Sui X: p53/PCDH17/Beclin-1 proteins as
prognostic predictors for urinary bladder cancer. J Cancer.
10:6207–6216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Wang X, Wang G, Li Z, Wang J,
Huang L, Qin Z, Yuan X, Cheng Z, Zhang S, et al: Integrating
multiple omics data for the discovery of potential Beclin-1
interactions in breast cancer. Mol Biosyst. 13:991–999. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Ghoorun RA, Fan X, Wu P, Bai Y, Li
J, Chen H, Wang L and Wang J: High expression of Beclin-1 predicts
favorable prognosis for patients with colorectal cancer. Clin Res
Hepatol Gastroenterol. 39:98–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng T, Li D, He Z, Feng S and Zhao S:
Prognostic and clinicopathological significance of Beclin-1 in
non-small-cell lung cancer: A meta-analysis. Onco Targets Ther.
11:4167–4175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu DM, Wang GL, Chen L, Xu YY, He S, Cao
XL, Qin J, Zhou JM, Zhang YX and E Q: The expression of Beclin-1,
an autophagic gene, in hepatocellular carcinoma associated with
clinical pathological and prognostic significance. BMC Cancer.
14:3272014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han Y, Xue XF, Shen HG, Guo XB, Wang X,
Yuan B, Guo XP, Kuang YT, Zhi QM and Zhao H: Prognostic
significance of Beclin-1 expression in colorectal cancer: A
meta-analysis. Asian Pac J Cancer Prev. 15:4583–4587. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu XG, Chen YD, Yuan J, Zhang N, Lei T,
Liu J and Yang M: Functional BCL-2 rs2279115 promoter noncoding
variant contributes to glioma predisposition, especially in males.
DNA Cell Biol. 38:85–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chi XX, Zhang T, Chu XL, Zhen JL and Zhang
DJ: The regulatory effect of Genistein on granulosa cell in ovary
of rat with PCOS through Bcl-2 and Bax signaling pathways. J Vet
Med Sci. 80:1348–1355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Cai H, Huang H, Long Z, Shi Y and
Wang Y: The prognostic significance of apoptosis-related biological
markers in Chinese gastric cancer patients. PLoS One. 6:e296702011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Q, Li S, Cheng P, Deng M, He X, Wang
Z, Yang CH, Zhao XY and Huang J: High expression of anti-apoptotic
protein Bcl-2 is a good prognostic factor in colorectal cancer:
Result of a meta-analysis. World J Gastroenterol. 23:5018–5033.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Callagy GM, Webbe MJ, Pharoah PD and
Caldas C: Meta-analysis confirms BCL2 is an independent prognostic
marker in breast cancer. BMC Cancer. 8:1532008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu Y, Li W, Ren L, Yang C, Li D, Han X,
Sun Y, Lv C and Han F: Inhibition of autophagy enhanced cobalt
chloride-induced apoptosis in rat alveolar type II epithelial
cells. Mol Med Rep. 18:2124–2132. 2018.PubMed/NCBI
|
|
30
|
Won KY, Kim GY, Kim YW, Song JY and Lim
SJ: Clinicopathologic correlation of Beclin-1 and Bcl-2 expression
in human breast cancer. Hum Pathol. 41:107–112. 2010. View Article : Google Scholar : PubMed/NCBI
|