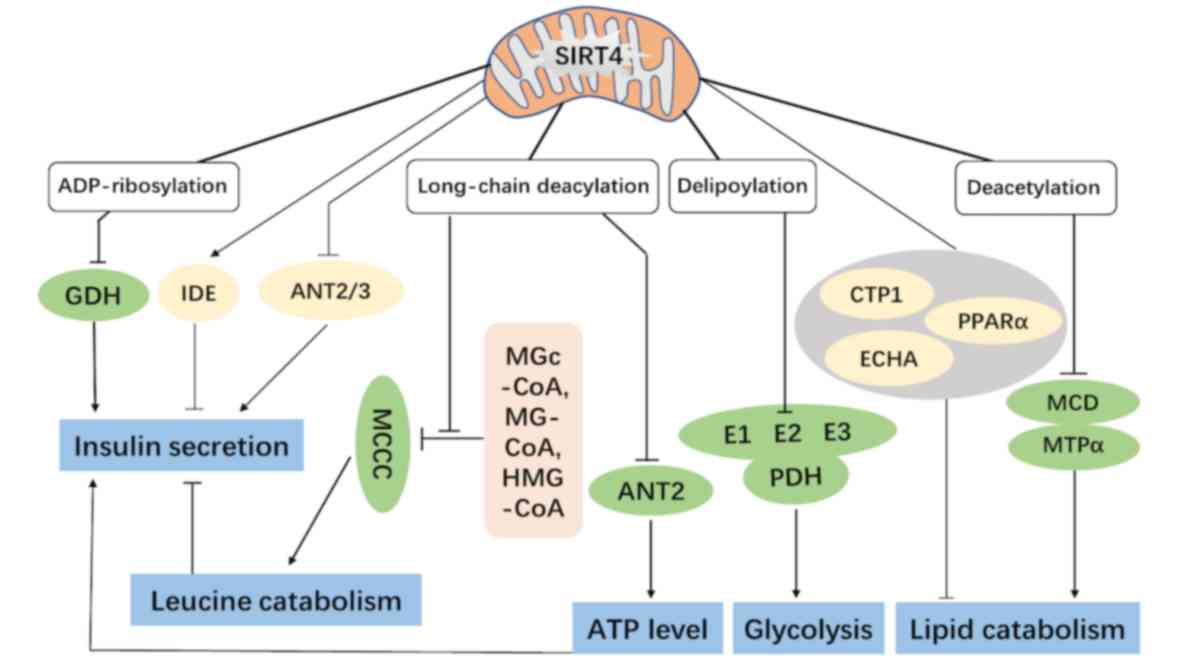
|
1
|
Wang Y, He J, Liao M, Hu M, Li W, Ouyang
H, Wang X, Ye T, Zhang Y and Ouyang L: An overview of Sirtuins as
potential therapeutic target: Structure, function and modulators.
Eur J Med Chem. 161:48–77. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jing H and Lin H: Sirtuins in epigenetic
regulation. Chem Rev. 115:2350–2375. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirschey MD: Old enzymes, new tricks:
Sirtuins are NAD(+)-dependent de-acylases. Cell Metab. 14:718–719.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klar AJ, Fogel S and Macleod K: MAR1-a
Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE.
Genetics. 93:37–50. 1979.PubMed/NCBI
|
|
5
|
Michan S and Sinclair D: Sirtuins in
mammals: Insights into their biological function. Biochem J.
404:1–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frye RA: Phylogenetic classification of
prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res
Commun. 273:793–798. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Zhou Y, Wang F, Chen X, Wang C, Wang
J, Liu T, Li Y and He B: SIRT4 is the last puzzle of mitochondrial
sirtuins. Bioorg Med Chem. 26:3861–3865. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanno M, Sakamoto J, Miura T, Shimamoto K
and Horio Y: Nucleocytoplasmic shuttling of the NAD+-dependent
histone deacetylase SIRT1. J Biol Chem. 282:6823–6832. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liszt G, Ford E, Kurtev M and Guarente L:
Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J
Biol Chem. 280:21313–21320. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar S and Lombard DB: Mitochondrial
sirtuins and their relationships with metabolic disease and cancer.
Antioxid Redox Signal. 22:1060–1077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michishita E, Park JY, Burneskis JM,
Barrett JC and Horikawa I: Evolutionarily conserved and
nonconserved cellular localizations and functions of human SIRT
proteins. Mol Biol Cell. 16:4623–4635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mei Z, Zhang X, Yi J, Huang J, He J and
Tao Y: Sirtuins in metabolism, DNA repair and cancer. J Exp Clin
Cancer Res. 35:1822016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vaquero A, Scher MB, Lee DH, Sutton A,
Cheng HL, Alt FW, Serrano L, Sternglanz R and Reinberg D: SirT2 is
a histone deacetylase with preference for histone H4 Lys 16 during
mitosis. Genes Dev. 20:1256–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeong SM and Haigis MC: Sirtuins in
cancer: A balancing act between genome stability and metabolism.
Mol Cells. 38:750–758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
German NJ and Haigis MC: Sirtuins and the
metabolic hurdles in cancer. Curr Biol. 25:R569–R583. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grabowska W, Sikora E and Bielak-Zmijewska
A: Sirtuins, a promising target in slowing down the ageing process.
Biogerontology. 18:447–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kupis W, Palyga J, Tomal E and
Niewiadomska E: The role of sirtuins in cellular homeostasis. J
Physiol Biochem. 72:371–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frye RA: Characterization of five human
cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins
(sirtuins) metabolize NAD and may have protein
ADP-ribosyltransferase activity. Biochem Biophys Res Commun.
260:273–279. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
North BJ, Marshall BL, Borra MT, Denu JM
and Verdin E: The human Sir2 ortholog, SIRT2, is an NAD+-dependent
tubulin deacetylase. Mol Cell. 11:437–444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haigis MC, Mostoslavsky R, Haigis KM,
Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos
GD, Karow M, Blander G, et al: SIRT4 inhibits glutamate
dehydrogenase and opposes the effects of calorie restriction in
pancreatic beta cells. Cell. 126:941–954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar S and Lombard DB: For certain, SIRT4
activities! Trends Biochem Sci. 42:499–501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi JX, Wang QJ, Li H and Huang Q: SIRT4
overexpression protects against diabetic nephropathy by inhibiting
podocyte apoptosis. Exp Ther Med. 13:342–348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kane AE and Sinclair DA: Sirtuins and
NAD(+) in the development and treatment of metabolic and
cardiovascular diseases. Circ Res. 123:868–885. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McGuinness D, McGuinness DH, McCaul JA and
Shiels PG: Sirtuins, bioageing, and cancer. J Aging Res.
2011:2357542011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Onyango P, Celic I, McCaffery JM, Boeke JD
and Feinberg AP: SIRT3, a human SIR2 homologue, is an NAD-dependent
deacetylase localized to mitochondria. Proc Natl Acad Sci USA.
99:13653–13658. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schwer B, North BJ, Frye RA, Ott M and
Verdin E: The human silent information regulator (Sir)2 homologue
hSIRT3 is a mitochondrial nicotinamide adenine
dinucleotide-dependent deacetylase. J Cell Biol. 158:647–657. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Finnin MS, Donigian JR and Pavletich NP:
Structure of the histone deacetylase SIRT2. Nat Struct Biol.
8:621–625. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bellamacina CR: The nicotinamide
dinucleotide binding motif: A comparison of nucleotide binding
proteins. FASEB J. 10:1257–1269. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sanders BD, Jackson B and Marmorstein R:
Structural basis for sirtuin function: What we know and what we
don't. Biochim Biophys Acta. 1804:1604–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moniot S, Weyand M and Steegborn C:
Structures, substrates, and regulators of Mammalian
sirtuins-opportunities and challenges for drug development. Front
Pharmacol. 3:162012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan H and Marmorstein R: Structural basis
for sirtuin activity and inhibition. J Biol Chem. 287:42428–42435.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moniot S, Schutkowski M and Steegborn C:
Crystal structure analysis of human Sirt2 and its ADP-ribose
complex. J Struct Biol. 182:136–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Zhang H, He B, Du J, Lin H,
Cerione RA and Hao Q: The bicyclic intermediate structure provides
insights into the desuccinylation mechanism of human sirtuin 5
(SIRT5). J Biol Chem. 287:28307–28314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin L, Wei W, Jiang Y, Peng H, Cai J, Mao
C, Dai H, Choy W, Bemis JE, Jirousek, et al: Crystal structures of
human SIRT3 displaying substrate-induced conformational changes. J
Biol Chem. 284:24394–24405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahuja N, Schwer B, Carobbio S, Waltregny
D, North BJ, Castronovo V, Maechler P and Verdin E: Regulation of
insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase.
J Biol Chem. 282:33583–33592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pannek M, Simic Z, Fuszard M, Meleshin M,
Rotili D, Mai A, Schutkowski M and Steegborn C: Crystal structures
of the mitochondrial deacylase Sirtuin 4 reveal isoform-specific
acyl recognition and regulation features. Nat Commun. 8:15132017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kato Y, Kihara H, Fukui K and Kojima M: A
ternary complex model of Sirtuin4-NAD+-Glutamate
dehydrogenase. Comput Biol Chem. 74:94–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Madsen AS, Andersen C, Daoud M, Anderson
KA, Laursen JS, Chakladar S, Huynh FK, Colaço AR, Backos DS,
Fristrup P, et al: Investigating the sensitivity of NAD+-dependent
sirtuin deacylation activities to NADH. J Biol Chem. 291:7128–7141.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feldman JL, Dittenhafer-Reed KE, Kudo N,
Thelen JN, Ito A, Yoshida M and Denu JM: Kinetic and structural
basis for Acyl-Group Selectivity and NAD(+) Dependence in
Sirtuin-Catalyzed Deacylation. Biochemistry. 54:3037–3050. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fernandez-Marcos PJ and Serrano M: Sirt4:
The glutamine gatekeeper. Cancer Cell. 23:427–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Altman BJ, Stine ZE and Dang CV: From
Krebs to clinic: Glutamine metabolism to cancer therapy. Nat Rev
Cancer. 16:7492016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Argmann C and Auwerx J: Insulin secretion:
SIRT4 gets in on the act. Cell. 126:837–839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lang J: Molecular mechanisms and
regulation of insulin exocytosis as a paradigm of endocrine
secretion. Eur J Biochem. 259:3–17. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ashcroft FM, Proks P, Smith PA, Ammala C,
Bokvist K and Rorsman P: Stimulus-secretion coupling in pancreatic
beta cells. J Cell Biochem. 55 (Suppl):S54–S65. 1994. View Article : Google Scholar
|
|
47
|
Glozak MA, Sengupta N, Zhang X and Seto E:
Acetylation and deacetylation of non-histone proteins. Gene.
363:15–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sauve AA, Wolberger C, Schramm VL and
Boeke JD: The biochemistry of sirtuins. Annu Rev Biochem.
75:435–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Feldman JL, Baeza J and Denu JM:
Activation of the protein deacetylase SIRT6 by long-chain fatty
acids and widespread deacylation by mammalian sirtuins. J Biol
Chem. 288:31350–31356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nasrin N, Wu X, Fortier E, Feng Y, Bare'
OC, Chen S, Ren X, Wu Z, Streeper RS and Bordone L: SIRT4 regulates
fatty acid oxidation and mitochondrial gene expression in liver and
muscle cells. J Biol Chem. 285:31995–32002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hardie DG: Sensing of energy and nutrients
by AMP-activated protein kinase. Am J Clin Nutr. 93:891S–896. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Laurent G, German NJ, Saha AK, de Boer VC,
Davies M, Koves TR, Dephoure N, Fischer F, Boanca G, Vaitheesvaran
B, et al: SIRT4 coordinates the balance between lipid synthesis and
catabolism by repressing malonyl CoA decarboxylase. Mol Cell.
50:686–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Saggerson D: Malonyl-CoA, a key signaling
molecule in mammalian cells. Annu Rev Nutr. 28:253–272. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kersten S, Seydoux J, Peters JM, Gonzalez
FJ, Desvergne B and Wahli W: Peroxisome proliferator-activated
receptor alpha mediates the adaptive response to fasting. J Clin
Invest. 103:1489–1498. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Laurent G, de Boer VC, Finley LW, Sweeney
M, Lu H, Schug TT, Cen Y, Jeong SM, Li X, Sauve AA and Haigis MC:
SIRT4 represses peroxisome proliferator-activated receptor α
activity to suppress hepatic fat oxidation. Mol Cell Biol.
33:4552–4561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ibdah JA, Paul H, Zhao Y, Binford S,
Salleng K, Cline M, Matern D, Bennett MJ, Rinaldo P and Strauss AW:
Lack of mitochondrial trifunctional protein in mice causes neonatal
hypoglycemia and sudden death. J Clin Invest. 107:1403–1409. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guo L, Zhou SR, Wei XB, Liu Y, Chang XX,
Liu Y, Ge X, Dou X, Huang HY, Qian SW, et al: Acetylation of
mitochondrial trifunctional protein α-subunit enhances its
stability to promote fatty acid oxidation and is decreased in
nonalcoholic fatty liver disease. Mol Cell Biol. 36:2553–2567.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou ZH, McCarthy DB, O'Connor CM, Reed LJ
and Stoops JK: The remarkable structural and functional
organization of the eukaryotic pyruvate dehydrogenase complexes.
Proc Natl Acad Sci USA. 98:14802–14807. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mathias RA, Greco TM, Oberstein A,
Budayeva HG, Chakrabarti R, Rowland EA, Kang Y, Shenk T and Cristea
IM: Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase
complex activity. Cell. 159:1615–1625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
MacDonald MJ, Fahien LA, Brown LJ, Hasan
NM, Buss JD and Kendrick MA: Perspective: Emerging evidence for
signaling roles of mitochondrial anaplerotic products in insulin
secretion. Am J Physiol Endocrinol Metab. 288:E1–E15. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sener A and Malaisse WJ: L-leucine and a
nonmetabolized analogue activate pancreatic islet glutamate
dehydrogenase. Nature. 288:187–189. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Anderson KA, Huynh FK, Fisher-Wellman K,
Stuart JD, Peterson BS, Douros JD, Wagner GR, Thompson JW, Madsen
AS, Green MF, et al: SIRT4 is a lysine deacylase that controls
leucine metabolism and insulin secretion. Cell Metab. 25:838–855
e15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Huynh FK, Hu X, Lin Z, Johnson JD and
Hirschey MD: Loss of sirtuin 4 leads to elevated glucose- and
leucine-stimulated insulin levels and accelerated age-induced
insulin resistance in multiple murine genetic backgrounds. J
Inherit Metab Dis. 41:59–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zaganjor E, Vyas S and Haigis MC: SIRT4 is
a regulator of insulin secretion. Cell Chem Biol. 24:656–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Klingenberg M: The ADP and ATP transport
in mitochondria and its carrier. Biochim Biophys Acta.
1778:1978–2021. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ho L, Titus AS, Banerjee KK, George S, Lin
W, Deota S, Saha AK, Nakamura K, Gut P, Verdin E and
Kolthur-Seetharam U: SIRT4 regulates ATP homeostasis and mediates a
retrograde signaling via AMPK. Aging (Albany NY). 5:835–849. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vander Heiden MG, Lunt SY, Dayton TL,
Fiske BP, Israelsen WJ, Mattaini KR, Vokes NI, Stephanopoulos G,
Cantley LC, Metallo CM and Locasale JW: Metabolic pathway
alterations that support cell proliferation. Cold Spring Harb Symp
Quant Biol. 76:325–334. 2011. View Article : Google Scholar
|
|
69
|
Fuchs BC and Bode BP: Stressing out over
survival: Glutamine as an apoptotic modulator. J Surg Res.
131:26–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wise DR, DeBerardinis RJ, Mancuso A, Sayed
N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon
SB and Thompson CB: Myc regulates a transcriptional program that
stimulates mitochondrial glutaminolysis and leads to glutamine
addiction. Proc Natl Acad Sci USA. 105:18782–18787. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
DeBerardinis RJ, Mancuso A, Daikhin E,
Nissim I, Yudkoff M, Wehrli S and Thompson CB: Beyond aerobic
glycolysis: Transformed cells can engage in glutamine metabolism
that exceeds the requirement for protein and nucleotide synthesis.
Proc Natl Acad Sci USA. 104:19345–19350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Daye D and Wellen KE: Metabolic
reprogramming in cancer: Unraveling the role of glutamine in
tumorigenesis. Semin Cell Dev Biol. 23:362–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lukey MJ, Wilson KF and Cerione RA:
Therapeutic strategies impacting cancer cell glutamine metabolism.
Future Med Chem. 5:1685–1700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yuan H, Su L and Chen WY: The emerging and
diverse roles of sirtuins in cancer: A clinical perspective. Onco
Targets Ther. 6:1399–1416. 2013.PubMed/NCBI
|
|
75
|
Garber ME, Troyanskaya OG, Schluens K,
Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen
GD, Perou CM, Whyte RI, et al: Diversity of gene expression in
adenocarcinoma of the lung. Proc Natl Acad Sci USA. 98:13784–13789.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Blaveri E, Simko JP, Korkola JE, Brewer
JL, Baehner F, Mehta K, Devries S, Koppie T, Pejavar S, Carroll P
and Waldman FM: Bladder cancer outcome and subtype classification
by gene expression. Clin Cancer Res. 11:4044–4055. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Choi YL, Tsukasaki K, O'Neill MC, Yamada
Y, Onimaru Y, Matsumoto K, Ohashi J, Yamashita Y, Tsutsumi S,
Kaneda R, et al: A genomic analysis of adult T-cell leukemia.
Oncogene. 26:1245–1255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jeong SM, Xiao C, Finley LW, Lahusen T,
Souza AL, Pierce K, Li YH, Wang X, Laurent G, German NJ, et al:
SIRT4 has tumor-suppressive activity and regulates the cellular
metabolic response to DNA damage by inhibiting mitochondrial
glutamine metabolism. Cancer Cell. 23:450–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mahjabeen I and Kayani MA: Loss of
mitochondrial tumor suppressor genes expression is associated with
unfavorable clinical outcome in head and neck squamous cell
carcinoma: Data from retrospective study. PLoS One.
11:e01469482016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang YS, Du L, Liang X, Meng P, Bi L, Wang
YL, Wang C and Tang B: Sirtuin 4 depletion promotes hepatocellular
carcinoma tumorigenesis through regulating
adenosine-monophosphate-activated protein kinase alpha/mammalian
target of rapamycin axis in mice. Hepatology. 69:1614–1631. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fu L, Dong Q, He J, Wang X, Xing J, Wang
E, Qiu X and Li Q: SIRT4 inhibits malignancy progression of NSCLCs,
through mitochondrial dynamics mediated by the ERK-Drp1 pathway.
Oncogene. 36:2724–2736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen X, Lai X, Wu C, Tian Q, Lei T, Pan J
and Huang G: Decreased SIRT4 protein levels in endometrioid
adenocarcinoma tissues are associated with advanced AJCC stage.
Cancer Biomark. 19:419–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang Y, Guo Y, Gao J and Yuan X:
Tumor-suppressive function of SIRT4 in neuroblastoma through
mitochondrial damage. Cancer Manag Res. 10:5591–5603. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sun H, Huang D, Liu G, Jian F, Zhu J and
Zhang L: SIRT4 acts as a tumor suppressor in gastric cancer by
inhibiting cell proliferation, migration, and invasion. Onco
Targets Ther. 11:3959–3968. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Huang G, Cui F, Yu F, Lu H, Zhang M, Tang
H and Peng Z: Sirtuin-4 (SIRT4) is downregulated and associated
with some clinicopathological features in gastric adenocarcinoma.
Biomed Pharmacother. 72:135–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Shen X, Li P, Xu Y, Chen X, Sun H, Zhao Y,
Liu M and Zhang W: Association of sirtuins with clinicopathological
parameters and overall survival in gastric cancer. Oncotarget.
8:74359–74370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hu Y, Lin J, Lin Y, Chen X, Zhu G and
Huang G: Overexpression of SIRT4 inhibits the proliferation of
gastric cancer cells through cell cycle arrest. Oncol Lett.
17:2171–2176. 2019.PubMed/NCBI
|
|
88
|
Miyo M, Yamamoto H, Konno M, Colvin H,
Nishida N, Koseki J, Kawamoto K, Ogawa H, Hamabe A, Uemura M, et
al: Tumour-suppressive function of SIRT4 in human colorectal
cancer. Br J Cancer. 113:492–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Huang G, Cheng J, Yu F, Liu X, Yuan C, Liu
C, Chen X and Peng Z: Clinical and therapeutic significance of
sirtuin-4 expression in colorectal cancer. Oncol Rep. 35:2801–2810.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhu Y, Wang G, Li X, Wang T, Weng M and
Zhang Y: Knockout of SIRT4 decreases chemosensitivity to 5-FU in
colorectal cancer cells. Oncol Lett. 16:1675–1681. 2018.PubMed/NCBI
|
|
91
|
Shi Q, Liu T, Zhang X, Geng J, He X, Nu M
and Pang D: Decreased sirtuin 4 expression is associated with poor
prognosis in patients with invasive breast cancer. Oncol Lett.
12:2606–2612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Nakahara Y, Yamasaki M, Sawada G, Miyazaki
Y, Makino T, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S,
Mimori K, et al: Downregulation of SIRT4 expression is associated
with poor prognosis in esophageal squamous cell carcinoma.
Oncology. 90:347–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jeong SM, Lee A, Lee J and Haigis MC:
SIRT4 protein suppresses tumor formation in genetic models of
Myc-induced B cell lymphoma. J Biol Chem. 289:4135–4144. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chen Z, Lin J, Feng S, Chen X, Huang H,
Wang C, Yu Y, He Y, Han S, Zheng L and Huang G: SIRT4 inhibits the
proliferation, migration, and invasion abilities of thyroid cancer
cells by inhibiting glutamine metabolism. Onco Targets Ther.
12:2397–2408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hu Q, Qin Y, Ji S, Xu W, Liu W, Sun Q,
Zhang Z, Liu M, Ni Q, Yu X and Xu X: UHRF1 promotes aerobic
glycolysis and proliferation via suppression of SIRT4 in pancreatic
cancer. Cancer Lett. 452:226–236. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Negrini S, Gorgoulis VG and Halazonetis
TD: Genomic instability-an evolving hallmark of cancer. Nat Rev Mol
Cell Biol. 11:220–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Colombo SL, Palacios-Callender M, Frakich
N, Carcamo S, Kovacs I, Tudzarova S and Moncada S: Molecular basis
for the differential use of glucose and glutamine in cell
proliferation as revealed by synchronized HeLa cells. Proc Natl
Acad Sci USA. 108:21069–21074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang L, Zhou H, Wang Y, Cui G and Di LJ:
CtBP maintains cancer cell growth and metabolic homeostasis via
regulating SIRT4. Cell Death Dis. 6:e16202015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wang L, Li JJ, Guo LY, Li P, Zhao Z, Zhou
H and Di LJ: Molecular link between glucose and glutamine
consumption in cancer cells mediated by CtBP and SIRT4.
Oncogenesis. 7:262018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Csibi A, Fendt SM, Li C, Poulogiannis G,
Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T,
et al: The mTORC1 pathway stimulates glutamine metabolism and cell
proliferation by repressing SIRT4. Cell. 153:840–854. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
van de Ven RAH, Santos D and Haigis MC:
Mitochondrial Sirtuins and molecular mechanisms of aging. Trends
Mol Med. 23:320–331. 2017. View Article : Google Scholar
|
|
102
|
Xing J, Li J, Fu L, Gai J, Guan J and Li
Q: SIRT4 enhances the sensitivity of ER-positive breast cancer to
tamoxifen by inhibiting the IL-6/STAT3 signal pathway. Cancer Med.
8:7086–7097. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jeong SM, Hwang S and Seong RH: SIRT4
regulates cancer cell survival and growth after stress. Biochem
Biophys Res Commun. 470:251–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Liu M, Wang Z, Ren M, Yang X, Liu B, Qi H,
Yu M, Song S, Chen S, Liu L, et al: SIRT4 regulates PTEN stability
through IDE in response to cellular stresses. FASEB J.
33:5535–5547. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lee JO, Yang H, Georgescu MM, Di
Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P and Pavletich
NP: Crystal structure of the PTEN tumor suppressor: Implications
for its phosphoinositide phosphatase activity and membrane
association. Cell. 99:323–334. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Georgescu MM, Kirsch KH, Kaloudis P, Yang
H, Pavletich NP and Hanafusa H: Stabilization and productive
positioning roles of the C2 domain of PTEN tumor suppressor. Cancer
Res. 60:7033–7038. 2000.PubMed/NCBI
|
|
107
|
Mizushima N and Klionsky DJ: Protein
turnover via autophagy: Implications for metabolism. Annu Rev Nutr.
27:19–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Luo YX, Tang X, An XZ, Xie XM, Chen XF,
Zhao X, Hao DL, Chen HZ and Liu DP: SIRT4 accelerates Ang
II-induced pathological cardiac hypertrophy by inhibiting manganese
superoxide dismutase activity. Eur Heart J. 38:1389–1398.
2017.PubMed/NCBI
|
|
109
|
Xiao Y, Zhang X, Fan S, Cui G and Shen Z:
MicroRNA-497 inhibits cardiac hypertrophy by targeting Sirt4. PLoS
One. 11:e01680782016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zeng G, Liu H and Wang H: Amelioration of
myocardial ischemia-reperfusion injury by SIRT4 involves
mitochondrial protection and reduced apoptosis. Biochem Biophys Res
Commun. 502:15–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Shih J, Liu L, Mason A, Higashimori H and
Donmez G: Loss of SIRT4 decreases GLT-1-dependent glutamate uptake
and increases sensitivity to kainic acid. J Neurochem. 131:573–581.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Komlos D, Mann KD, Zhuo Y, Ricupero CL,
Hart RP, Liu AY and Firestein BL: Glutamate dehydrogenase 1 and
SIRT4 regulate glial development. Glia. 61:394–408. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ramatchandirin B, Sadasivam M, Kannan A
and Prahalathan C: Sirtuin 4 regulates lipopolysaccharide mediated
leydig cell dysfunction. J Cell Biochem. 117:904–916. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zeng J, Jiang M, Wu X, Diao F, Qiu D, Hou
X, Wang H, Li L, Li C, Ge J, et al: SIRT4 is essential for
metabolic control and meiotic structure during mouse oocyte
maturation. Aging Cell. 17:e127892018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Nunnari J and Suomalainen A: Mitochondria:
In sickness and in health. Cell. 148:1145–1159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Han Y, Zhou S, Coetzee S and Chen A: SIRT4
and its roles in energy and redox metabolism in health, disease and
during exercise. Front Physiol. 10:10062019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Min Z, Gao J and Yu Y: The roles of
mitochondrial SIRT4 in cellular metabolism. Front Endocrinol
(Lausanne). 9:7832019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li S and Zheng W: Mammalian Sirtuins SIRT4
and SIRT7. Prog Mol Biol Transl Sci. 154:147–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Song R, Xu W, Chen Y, Li Z, Zeng Y and Fu
Y: The expression of Sirtuins 1 and 4 in peripheral blood
leukocytes from patients with type 2 diabetes. Eur J Histochem.
55:e102011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Kim EA, Yang SJ, Choi SY, Lee WJ and Cho
SW: Inhibition of glutamate dehydrogenase and insulin secretion by
KHG26377 does not involve ADP-ribosylation by SIRT4 or
deacetylation by SIRT3. BMB Rep. 45:458–463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lappas M: Anti-inflammatory properties of
sirtuin 6 in human umbilical vein endothelial cells. Mediators
Inflamm. 2012:5975142012. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sebastian C, Satterstrom FK, Haigis MC and
Mostoslavsky R: From sirtuin biology to human diseases: An update.
J Biol Chem. 287:42444–42452. 2012. View Article : Google Scholar : PubMed/NCBI
|