Introduction
Liver cancer was the fourth leading cause of
cancer-associated and the sixth most commonly diagnosed cancer
worldwide in 2018 (1). Surgical
resection, ablation and liver transplantation are the current
curative treatment modalities for patients with HCC. However, a
high rate of local recurrence or distant metastasis has impeded the
improvement of patient outcome following curative treatments
(2). Other treatments, such as
transcatheter arterial chemoembolization and multi-tyrosine kinase
inhibitor are used for advanced HCC (2). Recently, therapeutic monoclonal
antibodies targeting immune checkpoint inhibitors (ICIs), such as
the programmed cell death protein 1 (PD-1)/programmed death-ligand
1 (PD-L1) axis, have been approved in HCC treatment, and
unprecedented improvement in tumor control has been reported
(3). However, only a small subset of
patients with HCC exhibit a marked response to a single antibody
against ICIs, possibly due to the high complexity of the tumor
microenvironment (4).
Herpes virus entry mediator (HVEM), a member of the
tumor necrosis factor (TNF) receptor super family, interacts with
B- and T-lymphocyte attenuator (BTLA), which activates its
cytoplasmic domain that contains an inhibitory tyrosine-based
motif, attenuating proliferation signals in antigen-activated
lymphocytes (5). HVEM is widely
expressed on multiple cells, such as T, B, natural killer,
dendritic and myeloid cells (6).
Furthermore, the lung, liver and kidneys have been reported to
express HVEM (7). In addition to
BTLA, HVEM is also a ligand for CD160, and the TNF superfamily
members LIGHT and lymphotoxin-β (6).
HVEM interacts with CD160 or BTLA to mediate inhibitory signals in
T cell proliferation and cytokine secretion (8). Conversely, ligation of HVEM with LIGHT
mediates the activation of naïve T cells and clonal expansion
(9). HVEM exhibits a bidirectional
effect on T cell activation depending on the engaged ligands.
However, the overall function of HVEM is inhibitory, based on the
evidence that HVEM−/− T cells exhibit an enhanced
activation profile and increased susceptibility to the development
of Con A mitogen-induced, T cell-dependent autoimmune hepatitis and
experimental autoimmune encephalopathy in HVEM-deficient mice
(10). Ectopic HVEM expression has
been demonstrated to be associated with obesity, autoimmune disease
and inflammation (11,12). Furthermore, different types of tumor,
including esophageal squamous cell carcinoma (ESCC), HCC and
melanoma, exhibit higher HVEM expression in tumors compared with
adjacent normal tissues (13–15).
Downregulation of HVEM in ESCC cells induces cell cycle arrest and
inhibits tumor growth in vivo (13). An inverse association between HVEM
and tumor-infiltrating immune cells, including CD4+,
CD8+ and CD45RO+ lymphocytes has been
reported in both human ESCC and hepatitis C virus (HCV)-related HCC
(13,14). Notably, high HVEM expression in
cancer serves as an independent predictor of poor survival outcomes
in patients with ESCC or HCC, following radical resection (13,14).
However, the expression status and clinical significance of HVEM in
HCC with hepatitis B virus (HBV) remain largely unknown.
Thus, the present study aimed to investigate the
clinical significance of HVEM in HBV-related HCC, and determine the
association between HVEM and subsets of HCC-infiltrating immune
cells. Furthermore, BTLA expression in subsets of CD8+ T
cells was investigated.
Materials and methods
Patients and specimens
The clinicopathological characteristics of patients
with HCC included in the current study are presented in Table SI. For tissue microarray
construction, 221 patients with a median age of 52 years and age
range, 18–81 years, who underwent radical resection for HCC at the
Department of Liver Surgery and Transplantation, Zhongshan Hospital
(Shanghai, China) were enrolled between April 2002 and December
2007. The patients were comprised of 188 males and 33 females. The
inclusion and exclusion criterion of the cohort are described in
previous studies (16,17). The patient cohort was divided into
the HVEMhigh group (≥50% of HVEM+ tumor cells; n=139)
and HVEMlow group (<50% of HVEM+ tumor cells; n=82).
Follow-up was performed until mortality or May 2017. Patients were
followed up every 2 months during the first postoperative year and
then every 3 to 4 months for the remainder of the duration. The
median follow-up for all patients was 53 months, with a range of
2–180 months. Time to recurrence (TTR) and overall survival (OS)
time were calculated as the interval between primary surgical
resection and the first recurrence or mortality, respectively.
Briefly, the inclusion criteria were as follows: i) Underwent
radical resection for HCC with distinctive pathological diagnosis;
ii) no preoperative anticancer treatment or extrahepatic
metastasis; and iii) complete follow-up data. Paired peripheral
blood and fresh tissue samples were obtained from 20 patients with
HCC randomly from the total patient cohort for lymphocyte
isolation. Frozen HCC tissue samples and adjacent liver tissues (1
cm away from tumor tissues) from another 28 patients with HCC were
randomly selected from the tissue bank of Zhongshan Hospital for
PCR analysis. The present study was approved by the Zhongshan
Hospital Research Ethics Committee (approval no. Y2017-186) and
written informed consent was provided by all patients prior to the
study.
Reverse transcription-quantitative
(RT-q)PCR
Following radical resection of HCC tissue samples
and adjacent liver tissues, RNA extraction was immediately
performed, and both tissue samples and extracted RNA were preserved
at −80°C. Total RNA was extracted from frozen tissue samples using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse transcribed into complementary DNA at 45°C (cDNA; 0.5 µg)
using the cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.). qPCR was subsequently performed
using the TaqMan Universal PCR Master Mix (Thermo Fisher
Scientific, Inc.) with 1 µl cDNA in a 25 µl final reaction volume
and an ABI Prism 7300 system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following primer sequences were used for
qPCR: HVEM forward, 5′-CTTGAGGCTGGTGCTGTATC-3′ and reverse,
5′-GGTGGGCAATGTAGGTG-3′; and GAPDH forward,
5′-CACCCACTCCTCCACCTTTG-3′ and reverse, 5′-CCACCACCCTGTTGCTGTAG-3′.
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 10 min; 40 cycles of denaturation at 95°C
for 15 sec, annealing at 55°C for 45 sec and extension at 60°C for
15 sec. Relative HVEM expression levels were calculated using the
2−ΔΔCq method (18) and
normalized to the internal reference gene GAPDH.
Immunohistochemistry (IHC) and
evaluation
Tissue microarray was constructed as previously
described (16,17). The tissues were fixed by immersion in
a 10% formalin solution for 4–8 h at room temperature. The
thickness of sections was 5–15 µm. Briefly, following
deparaffinization and rehydration in a graded series of ethanol
(100, 95, 80 and 50%), sections were incubated with 0.3%
H2O2 for 20 min at room temperature to
inhibit endogenous peroxidase activity. Antigen retrieval was
performed with Tris-ETDA (pH 9.0) using a microwave oven at
99–100°C for 20 min. Subsequently, sections were blocked with 5%
BSA (Sigma-Aldrich; Merck KGaA) at room temperature for 30 min,
prior to incubation with primary antibodies directed against human
HVEM/TNFRSF14 (ab47677; 1:20; Abcam), human CD8 (1:50; Dako;
Agilent Technologies, Inc.) and human forkhead box P3 (FOXP3; cat.
no. ab20034; 1:100; Abcam) overnight at 4°C. A 100 µl of diluted
biotinylated secondary antibody (1:1,000; cat. no. ab98624; Abcam)
was then added and the sections were incubated in a humidified
chamber at room temperature for 30 min. The components of the
Histostain®-Plus kit (Invitrogen; Thermo Fisher
Scientific, Inc.) were used for signal amplification and
visualization.
Immunohistochemical staining for HVEM was blindly
evaluated by two independent pathologists using confocal
laser-scanning microscope (magnification, ×100) (Olympus FluoView
FV1000; Olympus Corporation), as previously described (13,15).
Tumor cells (≥1,000) were counted and the percentage of tumor cells
with positive staining was calculated. Integrated absorbance and
the area in a photograph was measured using Image-Pro Plus software
(version 6.0; Media Cybernetic, Inc.), in order to compare the
expression levels of HVEM between HCC and peritumor tissue samples.
The density of interest (DOI) was calculated as the product of
integrated absorbance/total area. Tumor-infiltrating
CD8+ and FOXP3+ T cells were counted in five
randomly selected fields (magnification, ×400) per sample by two
independent investigators.
Cell lines
Human HCC cell lines, including HCCLM3, MHCC97H and
MHCC97L [human HCC cell lines with high, moderate and low
metastatic potential, respectively, which were derived from the
same parental cell line and established in Liver Cancer Institute,
Zhongshan Hospital, Fudan University (19,20)],
and PLC (Japanese Cancer Research Bank) were used in the present
study. All cell lines were maintained in DMEM (Thermo Fisher
Scientific, Inc.) containing 10% FBS (Thermo Fisher Scientific,
Inc.) supplemented with 100 IU/ml penicillin and 100 µg/ml
streptomycin and incubated at 37°C with 5% CO2.
Immunocytochemistry
Immunocytochemistry was performed according to
standardized protocols (21).
Briefly, seed adheren T cells were cultured in 6-well tissue plates
in a sterile tissue culture hood at room temperature overnight and
then fixed with 4% formaldehyde for 10 min at room temperature and
permeabilized using 0.2% Triton X-100 at room temperature for 15
min. Subsequently, cells were blocked with 1% BSA for 30 min at
room temperature, prior to incubation with anti-human HVEM/TNFRSF14
antibody (1:10; cat. no. ab47677; Abcam) overnight at 4°C. Next,
the cells were incubated with the secondary antibody (1:2,000; cat.
no. ab205718; Abcam) for 1 h at room temperature. The components of
the Alexa Fluor Plus 488 (Invitrogen; Thermo Fisher Scientific,
Inc.) were used for signal amplification, and DAPI at 300 ng/ml
incubated for 10 min away from light was used as a nuclear
counterstain. The slides were observed under a confocal
laser-scanning microscope (magnification, ×100) (Olympus FluoView
FV1000; Olympus Corporation).
Cell isolation and flow cytometric
analysis
Isolation of peripheral blood mononuclear cells
(PBMCs), peritumor-infiltrating lymphocytes (PILs) and
tumor-infiltrating lymphocytes (TILs) was performed as previously
described (16). Briefly, PBMCs were
isolated by Ficoll density gradient centrifugation, according to
the manufacturer's protocol (GE Healthcare). PILs and TILs were
isolated from clinical HCC specimens by Percoll gradient
centrifugation as previously described (22). Analysis of surface antigen expression
was performed, according to the manufacturer's protocol (BD
Biosciences). Briefly, BTLA expression on CD8+ T cells
was investigated in relation to the differentiation stage
discriminated by the expression of chemokine receptor CCR7 in
combination with the naïve cell marker CD45RA. CD8+ T
cells were gated as follows: i) Naïve (CCR7+
CD45RA+); ii) central memory (TCM, CCR7+
CD45RA−); iii) effector memory (CCR7−
CD45RA−); and iv) effector memory RA (CCR7−
CD45RA+). 1×106 PBMCs, PILs and TILs were washed in PBS
supplemented with 1% BSA and 0.1% sodium azide (Sigma-Aldrich;
Merck KGaA) three times, and incubated with anti-BTLA (1:10; cat.
no. 746759, BD Pharmingen) for 30 min at 4°C. Following washing
three times using ice cold PBS, 10% FCS, 1% sodium azide and
centrifugation at 400 × g at 4°C for 5 min, samples were fixed in
PBS/1% paraformaldehyde (Sigma-Aldrich; Merck KGaA) overnight at
4°C. Lymphocytes were gated based on forward and side scatters, and
at least 1×105 gated events were acquired for each sample and
analyzed using FlowJo v.10.5.3 software (FlowJo LLC).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 16.0; SPSS, Inc.). Paired Student's t-tests were
used for comparing two groups. A χ2 test was used to determine the
association between HVEM expression and clinicopathological
characteristics of patients with HCC. One-way ANOVA followed by
Bonferroni's post-hoc test was used to compare differences between
multiple groups. Univariate survival analysis was performed using
the Kaplan-Meier method and the log-rank test, while Cox
multivariate analysis was performed to adjust for potential
confounding variables in a stepwise manner (forward, likelihood
ratio) and determine the independent prognostic factors. Data are
shown as the mean ± standard deviation of three independent
experiments, and t-tests were used to compare group averages.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HVEM expression in HBV-related HCC and
HCC cell lines
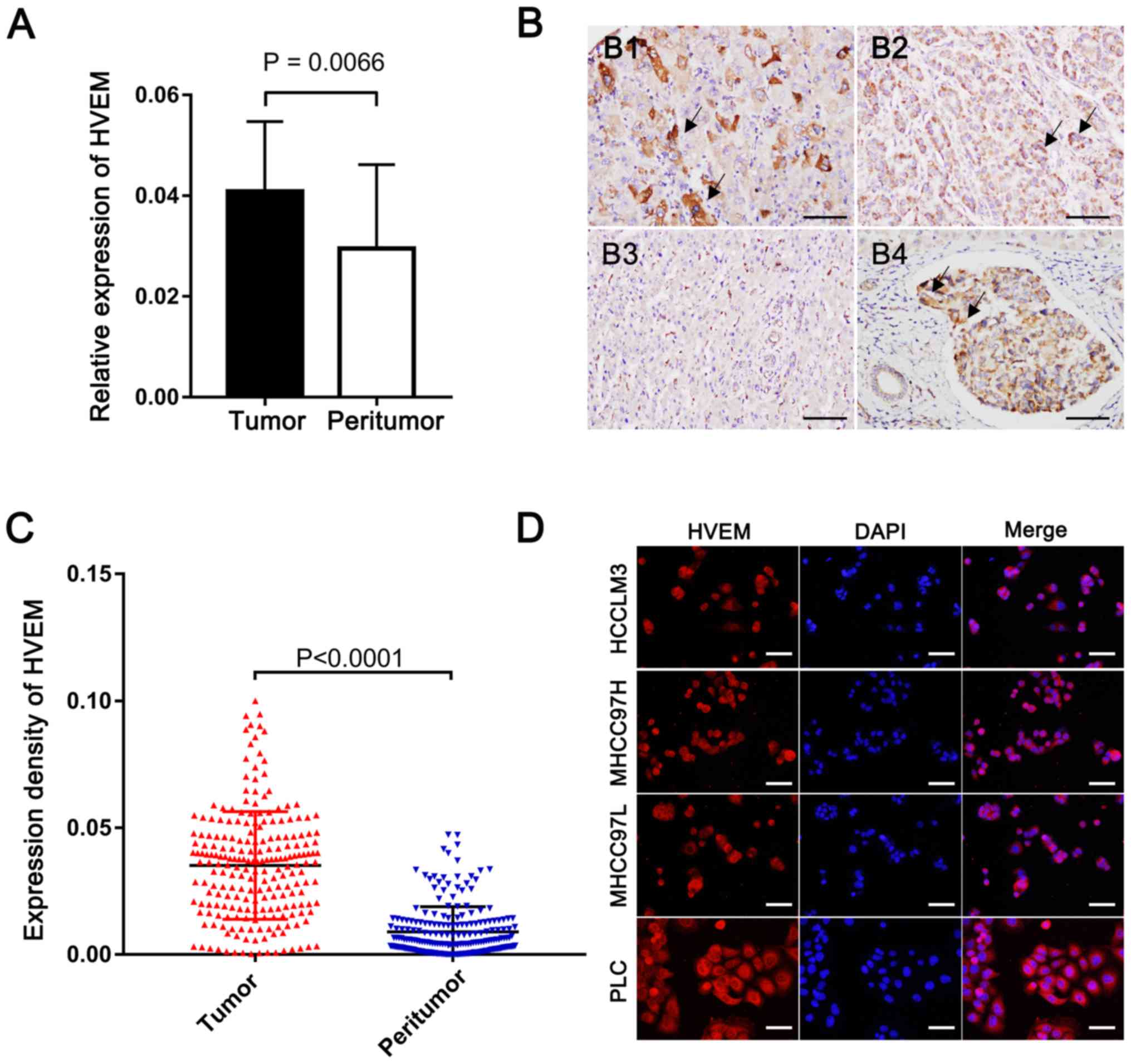
RT-qPCR was performed to determine HVEM expression
in patients with HCC with a background of HBV. The results
demonstrated that HVEM expression was significantly higher in HCC
tissues compared with paired peritumor tissues (P=0.0066; Fig. 1A). Furthermore, HVEM expression was
assessed in HCC via IHC analysis. Positive staining of HVEM was
identified predominantly on the membrane and in the cytoplasm of
tumor cells. Scatter positive staining of the stromal cells was
also observed in peritumor tissue, and accidentally observed
embolus also demonstrated higher HVEM expression compared with
surrounding liver tissue (Fig. 1B).
HCC tissue was demonstrated to have a significantly stronger HVEM
expression intensity when evaluated by DOI (0.035±0.021 vs.
0008±0.009; P<0.0001; Fig. 1C).
Furthermore, high HVEM expression was demonstrated in HCC cell
lines via immunocytochemistry analysis (Fig. 1D).
Association between HVEM expression
and clinicopathological characteristics of HCC
The patient cohort was divided into the HVEMhigh
group (≥50% of HVEM+ tumor cells, n=139) and HVEMlow
group (<50% of HVEM+ tumor cells, n=82), as
previously described (10,11). Vascular invasion was significantly
higher and tumor encapsulation was significantly lower in the
HVEMhigh group compared with the HVEMlow group (P=0.048 and
P=0.036, respectively; Table I).
Furthermore, tumors with high HVEM expression had a relatively
higher rate of advanced BCLC stage; however, no significant
difference was observed between the groups (P=0.087; Table I). AFP level and TNM stage failed to
demonstrate a prognostic value for patients with HCC (P=0.081 and
P=0.211, respectively; Table I).
Taken together, these results suggest that HVEM may be involved in
disease progression of patients with HCC.
 | Table I.Association between HVEM expression
and clinicopathological characteristics of patients with
hepatocellular carcinoma (n=221). |
Table I.
Association between HVEM expression
and clinicopathological characteristics of patients with
hepatocellular carcinoma (n=221).
|
|
| HVEM
expression |
|---|
|
|
|
|
|---|
| Characteristic | Number of patients,
n | Low, n | High, n | P-value |
|---|
| Age, years |
|
|
| >0.999 |
|
≤52 | 110 | 69 | 41 |
|
|
>52 | 111 | 70 | 41 |
|
| Sex |
|
|
| 0.050 |
|
Male | 188 | 113 | 75 |
|
|
Female | 33 | 26 | 7 |
|
| HBV history |
|
|
| 0.606 |
|
Yes | 204 | 127 | 77 |
|
| No | 17 | 12 | 5 |
|
| Liver
cirrhosis |
|
|
| 0.534 |
|
Yes | 193 | 123 | 70 |
|
| No | 28 | 16 | 12 |
|
| AFP, ng/ml |
|
|
| 0.081 |
|
≤20 | 79 | 56 | 23 |
|
|
>20 | 142 | 83 | 59 |
|
| γ-GT, U/l |
|
|
| 0.576 |
|
≤56 | 98 | 64 | 34 |
|
|
>56 | 123 | 75 | 48 |
|
| Tumor size, cm |
|
|
| 0.051 |
| ≤5 | 114 | 79 | 35 |
|
|
>5 | 107 | 60 | 47 |
|
| Tumor number |
|
|
| >0.999 |
|
Single | 172 | 108 | 64 |
|
|
Multiple | 49 | 31 | 18 |
|
| Tumor
encapsulation |
|
|
| 0.036 |
|
None | 113 | 79 | 34 |
|
|
Complete | 108 | 60 | 48 |
|
| Tumor
differentiation |
|
|
| 0.205 |
|
I–II | 131 | 87 | 44 |
|
|
III–IV | 90 | 52 | 38 |
|
| Vascular
invasion |
|
|
|
0.048a |
|
Yes | 130 | 89 | 41 |
|
| No | 91 | 50 | 41 |
|
| TNM stage |
|
|
| 0.211 |
| I | 107 | 72 | 35 |
|
|
II–III | 114 | 67 | 47 |
|
| BCLC stage |
|
|
| 0.087 |
| A | 80 | 57 | 23 |
|
| B | 50 | 32 | 18 |
|
| C | 91 | 50 | 41 |
|
| FOXP3 |
|
|
| 0.005 |
|
Low | 117 | 84 | 33 |
|
|
High | 104 | 55 | 49 |
|
| CD8 |
|
|
| 0.676 |
|
Low | 108 | 66 | 42 |
|
|
High | 113 | 73 | 40 |
|
Association between HVEM and
FOXP3+ T cells in patients with HCC
HVEM may be involved in tumor progression by
inducing the immune escape of tumor cells (23). The present study evaluated the
associations between HVEM with CD8+ T cells and
FOXP3+ regulatory T cells (Tregs). High FOXP3 expression
was significantly higher in the HVEMhigh group compared with the
HVEMlow group (P=0.005); however, no significant association was
observed between HVEM expression and CD8+ T cells
(Table I).
Prognostic significance of HVEM in
HBV-related HCC
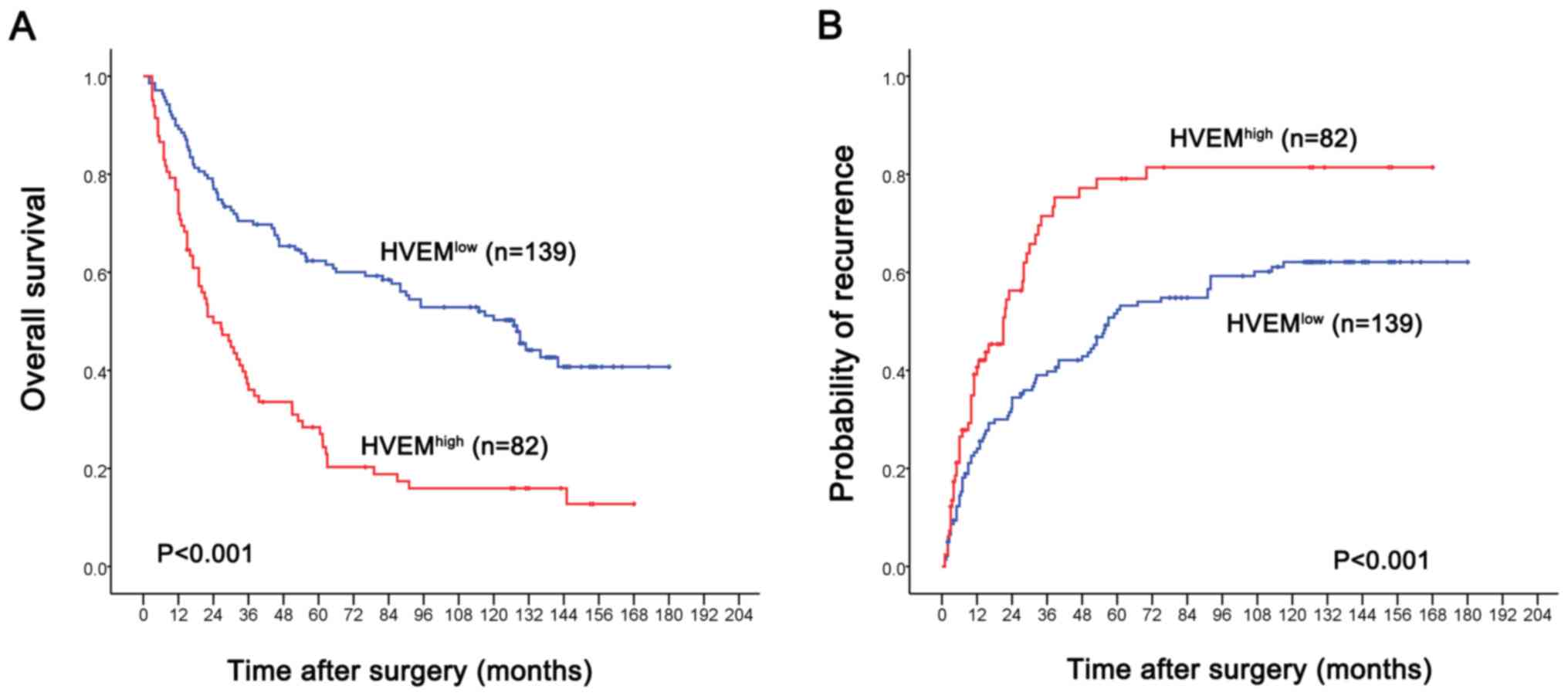
Kaplan-Meier analysis demonstrated that high HVEM
expression in HCC tissue was associated with a shorter TTR time and
shorter OS time compared with the HVEMlow group (P<0.001;
Fig. 2). Factors that demonstrated
significance by univariate analysis presented in Table II were enrolled in a multivariate
Cox proportional hazards model. We found that α-fetoprotein,
γ-glutamyl transferase, tumor size, vascular invasion, FOXP3 and
CD8 are independent prognostic factor for predicting OS.
Importantly, HVEM was revealed to be an independent prognostic
factor for predicting recurrence and OS (Table II).
 | Table II.Univariate and multivariate analyses
of prognostic factors in patients with hepatocellular carcinoma
(n=221). |
Table II.
Univariate and multivariate analyses
of prognostic factors in patients with hepatocellular carcinoma
(n=221).
|
| Overall survival
time | Time to
recurrence |
|---|
|
|
|
|
|---|
|
|
| Multivariate
analysis |
| Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Factor | Univariate
P-value | HR (95% CI) | P-value | Univariate
P-value | HR (95% CI) | P-value |
|---|
| α-fetoprotein,
ng/ml (≤20 vs. >20) | 0.001 | 1.553
(1.054-2.288) | 0.026 | 0.066 | NA | NA |
| γ-glutamyl
transferase, units/l (≤56 vs. >56) | <0.001 | 1.845
(1.297-2.625) | 0.001 | 0.089 | NA | NA |
| Liver cirrhosis (No
vs. Yes) | 0.033 | NS | NS | 0.076 | NA | NA |
| Tumor size, cm (≤5
vs. >5) | <0.001 | 1.436
(1.006-2.050) | 0.046 | 0.002 | NS | NS |
| Tumor multiplicity
(Single vs. Multiple) | 0.001 | NS | NS | 0.001 | 1.785
(1.217-2.617) | 0.003 |
| Tumor encapsulation
(Complete vs. None) | 0.009 | NS | NS | 0.003 | NS | NS |
| Tumor
differentiation (I/II vs. III/IV) | 0.082 | NA | NA | 0.248 | NA | NA |
| Vascular
invasion(No vs. Yes) | <0.001 | 2.974
(2.057-4.298) | <0.001 | <0.001 | 2.895
(2.037-4.117) | <0.001 |
| HVEM (Positive vs.
Negative) | <0.001 | 2.162
(1.528-3.059) | <0.001 | 0.003 | 1.752
(1.222-2.513) | 0.002 |
| FOXP3 (High vs.
Low) | <0.001 | 2.314
(1.630-3.284) | <0.001 | 0.013 | NS | NS |
| CD8 (High vs.
Low) | 0.001 | 0.523
(0.367-0.746) | <0.001 | 0.084 | NA | NA |
BTLA expression in CD8+ T
cells
CD8+ T cells act as the main effector
cells in the tumor microenvironment (24). As expected, the infiltration of
CD8+ T cells decreased in HCC compared with that in
paired peritumor tissue and peripheral blood (Fig. 3A). The ligation of BTLA by HVEM
expressed by HCC cells may result in decreased T cell proliferation
and cytokine secretion (25). Thus,
BTLA expression on CD8+ T cells was investigated in
relation to the differentiation stage discriminated by the
expression of chemokine receptor CCR7 in combination with the naïve
cell marker CD45RA. CD8+ T cells were gated as follows:
i) Naïve (CCR7+ CD45RA+); ii) central memory
(TCM, CCR7+ CD45RA−); iii) effector memory
(CCR7− CD45RA−) and iv) effector memory RA
(CCR7− CD45RA+). The results demonstrated
that the surface expression of BTLA in these cell subtypes
gradually decreased with differentiation stage (Fig. 3A). However, HCC-infiltrating
CD8+ T cells still exhibited persistently high levels of
BTLA. Specifically, both effector memory and effector memory RA
CD8+ T cells exhibited higher levels of BTLA in HCC
tissues compared with peritumor tissues (Fig. 3B).
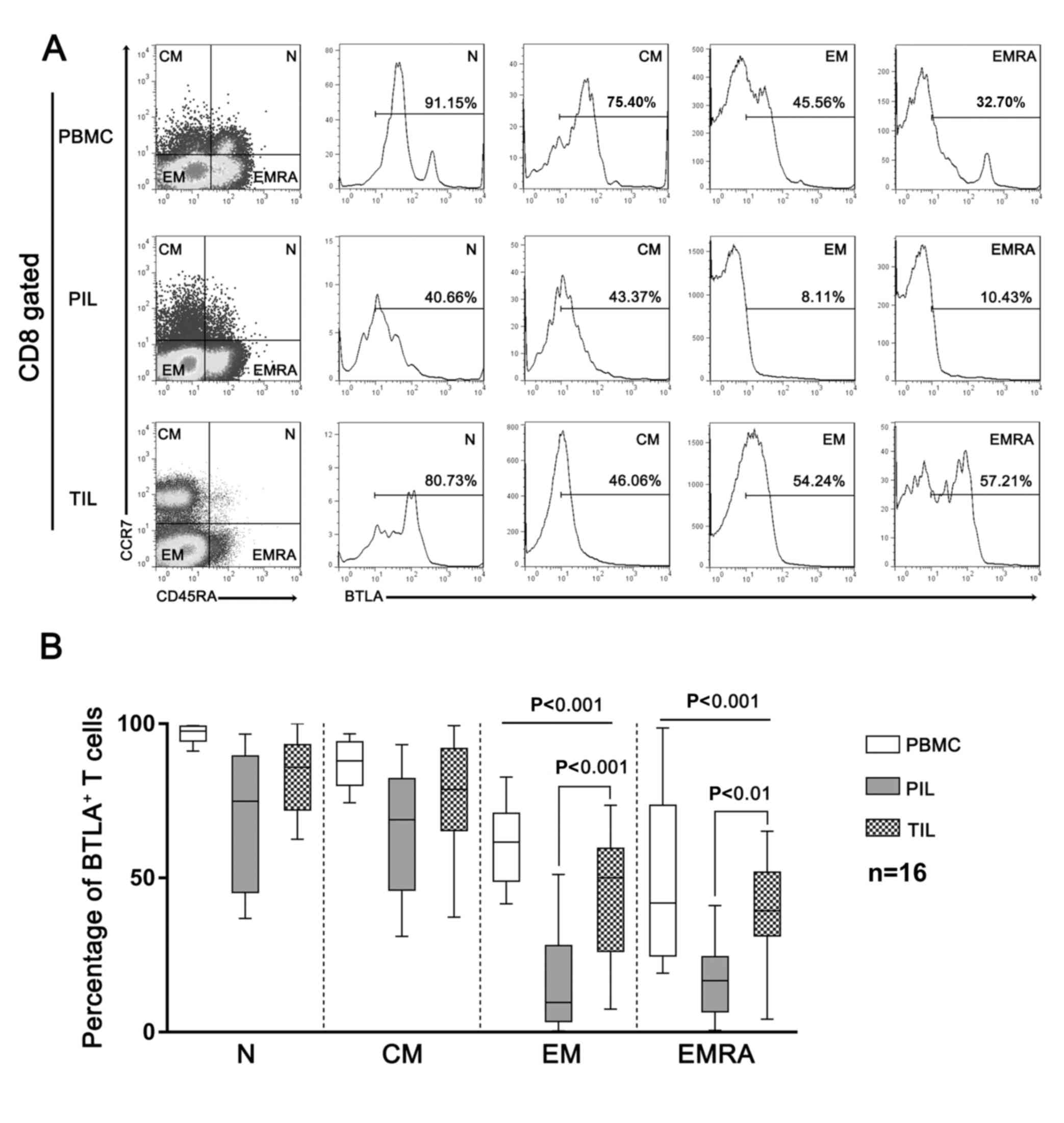 | Figure 3.BTLA expression in HCC-infiltrating
CD8+ T cells. (A) Representative figures of BTLA
expression in CD8+ T cells from peripheral blood,
peritumor tissue and tumor tissue of patients with HCC.
CD8+ T cells were divided into four subsets according to
CD45RA and CCR7 expression (N, CCR7+ CD45RA+;
CM, CCR7+ CD45RA−; EM, CCR7−
CD45RA− and; EMRA, CCR7− CD45RA+).
BTLA+ T cells were gated from BTLA− T cells
using an established threshold, according to the autologous naïve T
cell subsets of PBMC, which is always BTLA positive. (B)
Statistical analysis of BTLA+CD8+ T cell
subsets in PBMCs, PILs and TILs derived from patients with HCC.
HCC, hepatocellular carcinoma; CD8, cluster of differentiation 8;
BTLA, B- and T-lymphocyte attenuator; PBMCs, peripheral blood
mononuclear cells; PILs, peritumor-infiltrating lymphocytes; TILs,
tumor-infiltrating lymphocytes; N, naïve; CM, central memory, EM,
effector memory; EMRA, effector memory RA+. |
Discussion
It has been demonstrated that HVEM plays dynamic
immune regulatory functions in various physiological and
pathological conditions (6,12). In the tumor microenvironment, HVEM is
involved in tumor immune evasion through ligation with BTLA, a
coinhibitory receptor with functional similarities to PD-1 and
CTLA-4 (3). The present study
demonstrated a significantly higher expression of HVEM in HCC
compared with paired peritumor tissue. Furthermore, high HVEM
expression was associated with poor clinical outcome and invasive
characteristics, such as high rate of vascular invasion and
infiltration of suppressive Tregs. It has been reported that
HCC-infiltrating CD8+ T cells differentiate from naïve
to effector cells, and these cells were important in HCC and were
demonstrated to persistently express high levels of BTLA (26), which underlines the importance of the
HVEM/BTLA signaling pathway in HCC.
Immunotherapy based on ICIs has reported promising
therapeutic outcomes in patients with cancer (27). PD-1/PD-L1 and CTLA-4 inhibitors have
been approved for certain cancer treatments, and some are currently
under clinical trials (4). However,
the low response rate is one of the major difficulties for
ICI-based treatments in some patients with cancer (28). In HCC, it has been reported that only
10–30% of treated patients respond to anti-PD-1/PD-L1 therapy
(29). Conversely, several other
ICIs, including HVEM/BTLA, CD73 and mucin domain 3, also regulate
immune responses in tumor niches and may be alternative targets for
novel immune therapy (3). The
HVEM/BTLA signaling pathway is considered a novel target for
checkpoint blockade, based on the fact that HVEM/BTLA inhibition
enhances human T cell responses when used alone or in combination
with anti-PD-1 treatment (30–33). It
is reasonable to expect the combination of HVEM blockade and other
anticancer treatments, such as resection, ablation, chemotherapy
and anti-PD-1/PD-L1 treatment, may induce a synergistic anticancer
effect. However, clinical trials are required to effectively
evaluate the combined modality.
HVEM is involved in cancer progression through
mediating immune evasion; a higher expression of HVEM in cancer
tissue is associated with relatively poorer survival outcomes, as
reported in patients with ESCC, HCV-related hepatocellular
carcinoma and colorectal cancer (CRC) (13,14,34).
However, to the best of our knowledge, the significance of HVEM in
HBV-related HCC was previously undetermined. The present study
verified the prognostic role of HVEM in HBV-related HCC.
Furthermore, a significant association between HVEM and aggressive
biological behavior of HCC, including vascular invasion and
incomplete tumor capsule, was identified. Similarly, overexpression
of HVEM in patients with non-small cell lung carcinoma of N2 lymph
node metastasis or late-stage has been observed (24). In CRC and gastric cancer, HVEM status
is significantly associated with tumor status and pathological
stage (34,35). Conversely, the present study
determined that HVEM expression levels are associated with
tumor-infiltrating Tregs, a robust immune inhibitor, rather than
CD8+ T cells (36).
Notably, Tao et al (36)
reported that Tregs exert their suppressive effect via the
upregulation of HVEM, which, upon ligation with BTLA expressed on
effector cells, helps control immune response. HVEM−/−
Tregs have been demonstrated to decrease suppressive activity
compared with wild-type Tregs (36).
These findings suggest that immunotherapy targeting HVEM may lead
to activation of effector T cells and dampening of Tregs.
Studies have reported that HVEM can activate BTLA,
thus inhibiting CD8+ T cell differentiation and cytokine
secretion (37,38). The present study identified aberrant
persistent high expression of BTLA by differentiated effector T
cells derived from HCC tissue, suggesting the HVEM/BTLA signaling
pathway may play a role in the inhibition of efficient immune
responses against cancer.
Overall, the results of the present study suggest a
prognostic value of HVEM in patients with HCC following radical
resection. Thus, the HVEM/BTLA signaling pathway may be a target in
cancer immunotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Key
Research and Development Program of China (grant nos.
2017YFC0908101 and 2017YFC0908102), the National Natural Science
Foundation of China (grant no. 81772510), Research Programs of
Science and Technology Commission Foundation of Shanghai (grant
nos. 16DZ0500300 and 18XD1401100), the Developing Foundation of
Zhongshan (grant no. 2019ZSFZ24) and Shanghai Municipal Key
Clinical Specialty.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YY and SJQ designed the present study and
contributed to the development of methodology. YY and JLH
contributed to the acquisition of data. XCN and GL performed the
experiments. JLH, WG, PYZ, RYG, CZ, YRY and BYS analyzed the data
and performed the studies. YY drafted the manuscript and reviewed
the manuscript for important intellectual content and SJQ acquired
the funding. YY contributed to the administrative, technical, or
material support. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Zhongshan
Hospital Research Ethics Committee (approval no. Y2017-186), and
written informed consent was provided by all patients prior to the
study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BTLA
|
B- and T-lymphocyte attenuator
|
|
CRC
|
colorectal cancer
|
|
DOI
|
density of interest
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
HBV
|
hepatitis B virus
|
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
HVEM
|
herpes virus entry mediator
|
|
OS
|
overall survival
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
PILs
|
peritumor-infiltrating lymphocytes
|
|
TILs
|
tumor-infiltrating lymphocytes
|
|
TNF
|
tumor necrosis factor
|
|
Tregs
|
regulatory T cells
|
|
TTR
|
time to recurrence
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greten TF and Sangro B: Targets for
immunotherapy of liver cancer. J Hepatol. Sep 18–2017.(Epub ahead
of print).
|
|
4
|
Okusaka T and Ikeda M: Immunotherapy for
hepatocellular carcinoma: Current status and future perspectives.
ESMO Open. 3 (Suppl 1):e0004552018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheung TC, Humphreys IR, Potter KG, Norris
PS, Shumway HM, Tran BR, Patterson G, Jean-Jacques R, Yoon M, Spear
PG, et al: Evolutionarily divergent herpesviruses modulate T cell
activation by targeting the herpesvirus entry mediator cosignaling
pathway. Proc Natl Acad Sci USA. 102:13218–13223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai G and Freeman GJ: The CD160, BTLA,
LIGHT/HVEM pathway: A bidirectional switch regulating T-cell
activation. Immunol Rev. 229:244–258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu X, Zheng Y, Mao R, Su Z and Zhang J:
BTLA/HVEM signaling: Milestones in research and role in chronic
hepatitis B virus infection. Front Immunol. 10:6172019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodriguez-Barbosa JI, Schneider P, Weigert
A, Lee KM, Kim TJ, Perez-Simon JA and Del Rio ML: HVEM, a
cosignaling molecular switch, and its interactions with BTLA, CD160
and LIGHT. Cell Mol Immunol. 16:679–682. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
del Rio ML, Lucas CL, Buhler L, Rayat G
and Rodriguez-Barbosa JI: HVEM/LIGHT/BTLA/CD160 cosignaling
pathways as targets for immune regulation. J Leukoc Biol.
87:223–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Subudhi SK, Anders RA, Lo J, Sun
Y, Blink S, Wang Y, Wang J, Liu X, Mink K, et al: The role of
herpesvirus entry mediator as a negative regulator of T
cell-mediated responses. J Clin Invest. 115:711–717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Croft M, Duan W, Choi H, Eun SY, Madireddi
S and Mehta A: TNF superfamily in inflammatory disease: Translating
basic insights. Trends Immunol. 33:144–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shui JW, Steinberg MW and Kronenberg M:
Regulation of inflammation, autoimmunity, and infection immunity by
HVEM-BTLA signaling. J Leukoc Biol. 89:517–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Migita K, Sho M, Shimada K, Yasuda S,
Yamato I, Takayama T, Matsumoto S, Wakatsuki K, Hotta K, Tanaka T,
et al: Significant involvement of herpesvirus entry mediator in
human esophageal squamous cell carcinoma. Cancer. 120:808–817.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hokuto D, Sho M, Yamato I, Yasuda S, Obara
S, Nomi T and Nakajima Y: Clinical impact of herpesvirus entry
mediator expression in human hepatocellular carcinoma. Eur J
Cancer. 51:157–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han L, Wang W, Lu J, Kong F, Ma G, Zhu Y,
Zhao D, Zhu J, Shuai W, Zhou Q, et al: AAV-sBTLA facilitates HSP70
vaccine-triggered prophylactic antitumor immunity against a murine
melanoma pulmonary metastasis model in vivo. Cancer Lett.
354:398–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yi Y, He HW, Wang JX, Cai XY, Li YW, Zhou
J, Cheng YF, Jin JJ, Fan J and Qiu SJ: The functional impairment of
HCC-infiltrating γδ T cells, partially mediated by regulatory T
cells in a TGFβ- and IL-10-dependent manner. J Hepatol. 58:977–983.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi Y, Wu H, Gao Q, He HW, Li YW, Cai XY,
Wang JX, Zhou J, Cheng YF, Jin JJ, et al: Interferon regulatory
factor (IRF)-1 and IRF-2 are associated with prognosis and tumor
invasion in HCC. Ann Surg Oncol. 20:267–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Tang Y, Ye L, Liu B, Liu K, Chen J
and Xue Q: Establishment of a hepatocellular carcinoma cell line
with unique metastatic characteristics through in vivo selection
and screening for metastasis-related genes through cDNA microarray.
J Cancer Res Clin Oncol. 129:43–51. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Tian B, Yang J, Zhao L, Wu X, Ye SL,
Liu YK and Tang ZY: Stepwise metastatic human hepatocellular
carcinoma cell model system with multiple metastatic potentials
established through consecutive in vivo selection and studies on
metastatic characteristics. J Cancer Res Clin Oncol. 130:460–468.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song J, Ge Z, Yang X, Luo Q, Wang C, You
H, Ge T, Deng Y, Lin H, Cui Y, et al: Hepatic stellate cells
activated by acidic tumor microenvironment promote the metastasis
of hepatocellular carcinoma via osteopontin. Cancer Lett.
356:713–720. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beldi G, Wu Y, Banz Y, Nowak M, Miller L,
Enjyoji K, Haschemi A, Yegutkin GG, Candinas D, Exley M and Robson
MC: Natural killer T cell dysfunction in CD39-null mice protects
against concanavalin A-induced hepatitis. Hepatology. 48:841–852.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren S, Tian Q, Amar N, Yu H, Rivard CJ,
Caldwell C, Ng TL, Tu M, Liu Y, Gao D, et al: The immune
checkpoint, HVEM may contribute to immune escape in non-small cell
lung cancer lacking PD-L1 expression. Lung Cancer. 125:115–120.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sine H, Marco D and Straten PT: Effector
CD4 and CD8 T cells and their role in the tumor microenvironment.
Cancer Microenviron. 6:123–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marin-Acevedo JA, Dholaria B, Soyano AE,
Knutson KL, Chumsri S and Lou Y: Next generation of immune
checkpoint therapy in cancer: New developments and challenges. J
Hematol Oncol. 11:392018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Q, Huang ZL, He M, Gao Z and Kuang
DM: BTLA identifies dysfunctional PD-1-expressing CD4+ T
cells in human hepatocellular carcinoma. Oncoimmunology.
5:e12548552016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng AL, Hsu C, Chan SL, Choo SP and Kudo
M: Challenges of combination therapy with immune checkpoint
inhibitors for hepatocellular carcinoma. J Hepatol. 72:307–319.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Darvin P, Toor SM, Sasidharan Nair V and
Elkord E: Immune checkpoint inhibitors: Recent progress and
potential biomarkers. Exp Mol Med. 50:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fourcade J, Sun Z, Pagliano O, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V and
Zarour HM: CD8(+) T cells specific for tumor antigens can be
rendered dysfunctional by the tumor microenvironment through
upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res.
72:887–896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grabmeier-Pfistershammer K, Stecher C,
Zettl M, Rosskopf S, Rieger A, Zlabinger GJ and Steinberger P:
Antibodies targeting BTLA or TIM-3 enhance HIV-1 specific T cell
responses in combination with PD-1 blockade. Clin Immunol.
183:167–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stecher C, Battin C, Leitner J, Zettl M,
Grabmeier-Pfistershammer K, Höller C, Zlabinger GJ and Steinberger
P: PD-1 blockade promotes emerging checkpoint inhibitors in
enhancing T cell responses to allogeneic dendritic cells. Front
Immunol. 8:5722017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu J, Li J, He M, Zhang GL and Zhao Q:
Distinct changes of BTLA and HVEM expressions in circulating
CD4+ and CD8+ T cells in hepatocellular
carcinoma patients. J Immunol Res. 2018:45615712018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Inoue T, Sho M, Yasuda S, Nishiwada S,
Nakamura S, Ueda T, Nishigori N, Kawasaki K, Obara S, Nakamoto T,
et al: HVEM expression contributes to tumor progression and
prognosis in human colorectal cancer. Anticancer Res. 35:1361–1367.
2015.PubMed/NCBI
|
|
35
|
Lan X, Li S, Gao H, Nanding A, Quan L,
Yang C, Ding S and Xue Y: Increased BTLA and HVEM in gastric cancer
are associated with progression and poor prognosis. Onco Targets
Ther. 10:919–926. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tao R, Wang L, Murphy KM, Fraser CC and
Hancock WW: Regulatory T cell expression of herpesvirus entry
mediator suppresses the function of B and T lymphocyte
attenuator-positive effector T cells. J Immunol. 180:6649–6655.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Derré L, Rivals JP, Jandus C, Pastor S,
Rimoldi D, Romero P, Michielin O, Olive D and Speiser DE: BTLA
mediates inhibition of human tumor-specific CD8+ T cells that can
be partially reversed by vaccination. J Clin Invest. 120:157–167.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haymaker CL, Wu RC, Ritthipichai K,
Bernatchez C, Forget MA, Chen JQ, Liu H, Wang E, Marincola F, Hwu P
and Radvanyi LG: BTLA marks a less-differentiated
tumor-infiltrating lymphocyte subset in melanoma with enhanced
survival properties. Oncoimmunology. 4:e10142462015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martins-Filho SN, Paiva C, Azevedo RS and
Alves VAF: Histological grading of hepatocellular carcinoma-a
systematic review of literature. Front Med (Lausanne). 4:1932017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y
and Makuuchi M: Staging of hepatocellular carcinoma: Assessment of
the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772
patients in Japan. Ann Surg. 245:909–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Forner A, Reig ME, de Lope CR and Bruix J:
Current strategy for staging and treatment: The BCLC update and
future prospects. Semin Liver Dis. 30:61–74. 2010. View Article : Google Scholar : PubMed/NCBI
|