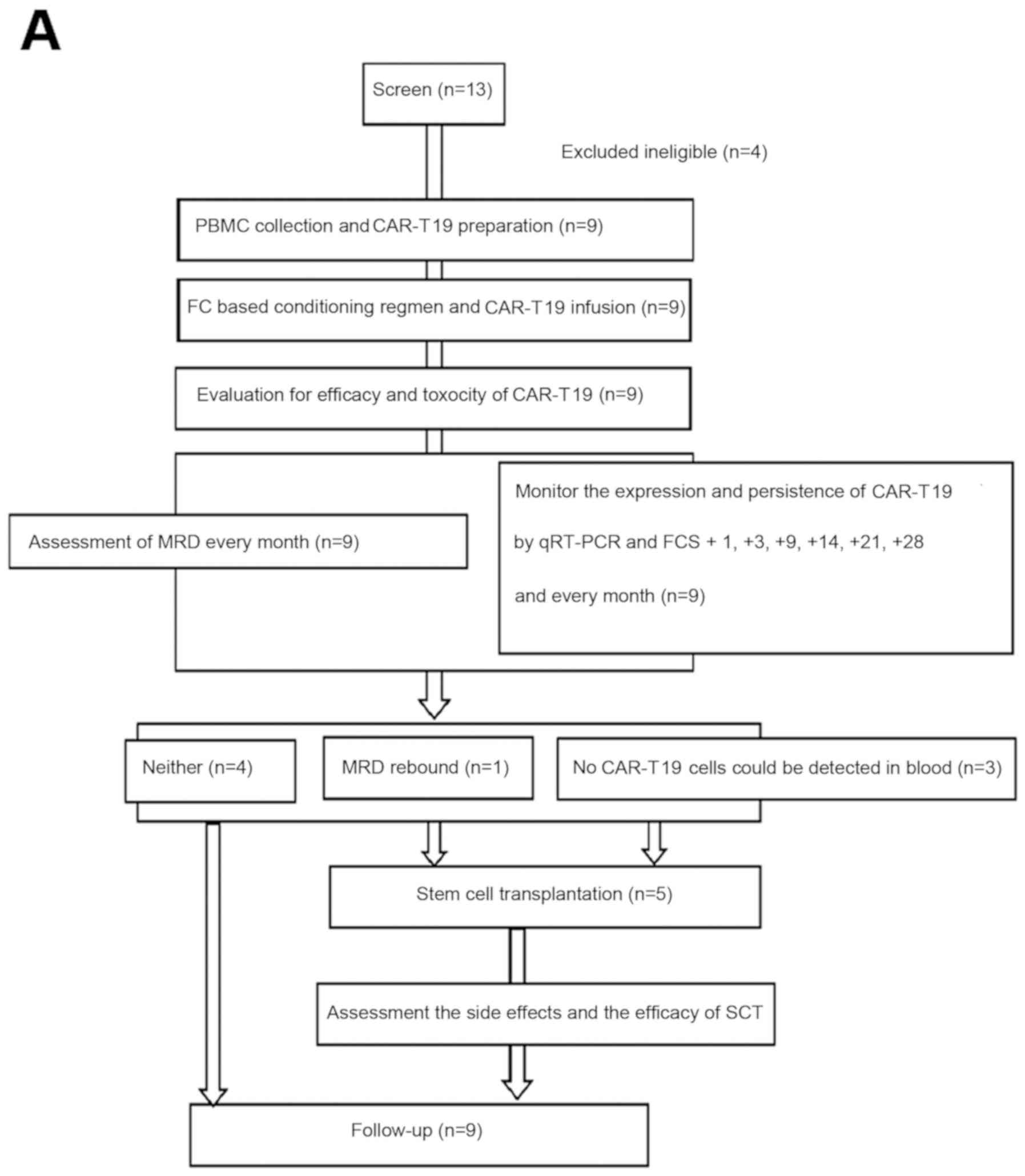
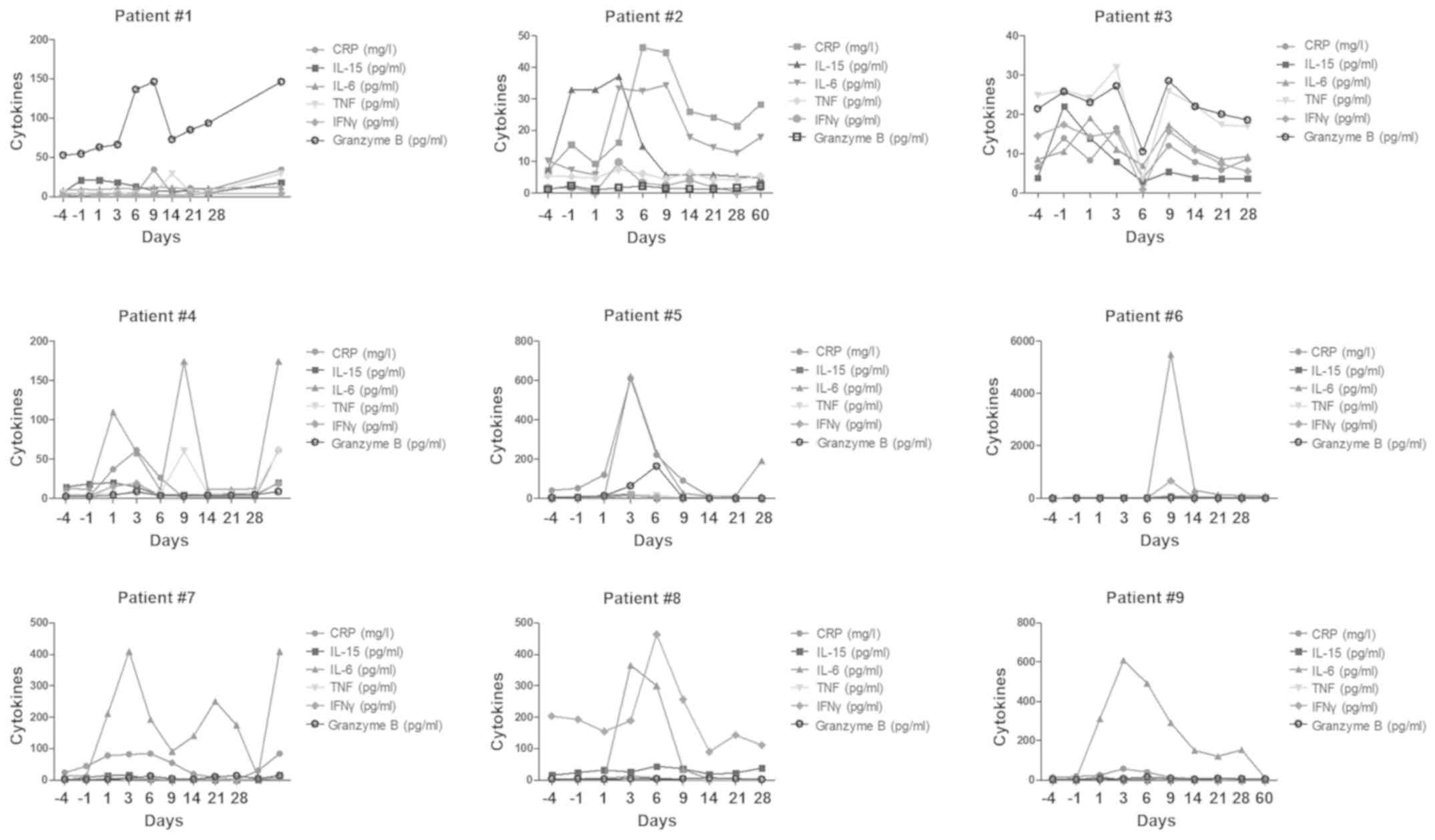
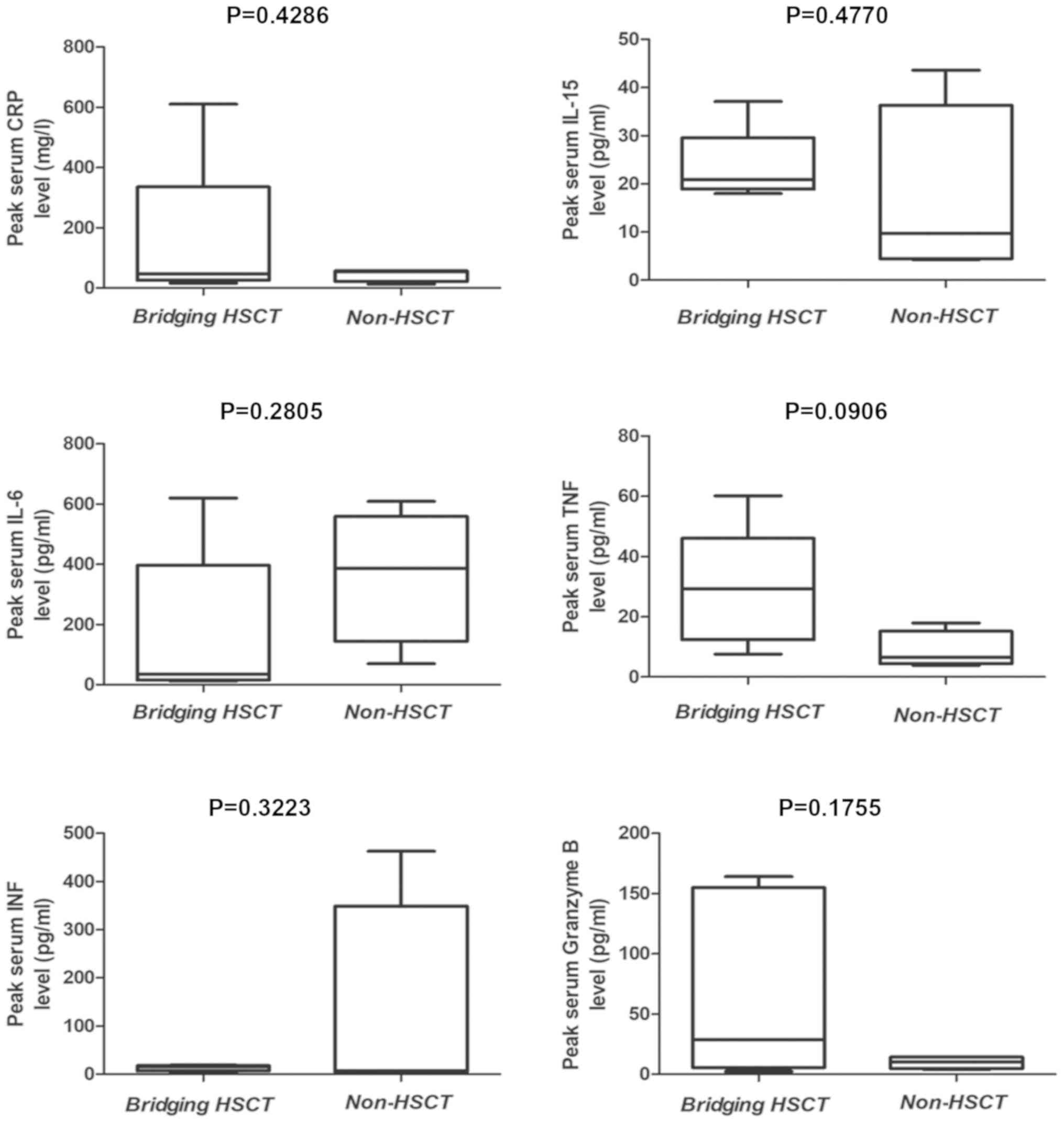
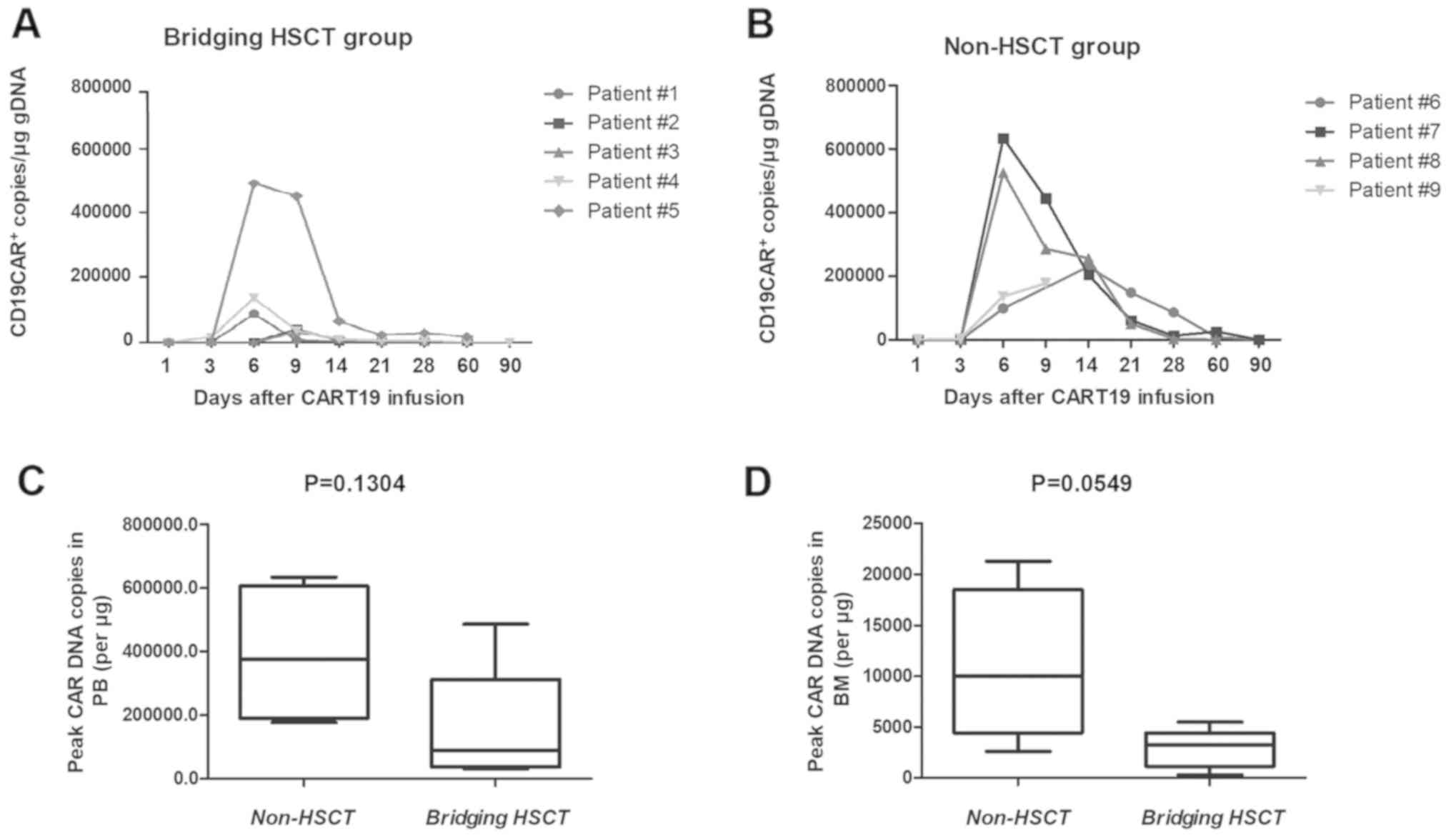
|
1
|
Iacobucci I and Mullighan CG: Genetic
basis of acute lymphoblastic leukemia. J Clin Oncol. 35:975–983.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Terwilliger T and Abdul-Hay M: Acute
lymphoblastic leukemia: A comprehensive review and 2017 update.
Blood Cancer J. 7:e5772017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Styczynski J, Tallamy B, Waxman I, van de
Ven C, Milone MC, Shaw LM, Harrison L, Morris E, Satwani P, Bhatia
M, et al: A pilot study of reduced toxicity conditioning with BU,
fludarabine and alemtuzumab before the allogeneic hematopoietic SCT
in children and adolescents. Bone Marrow Transplant. 46:790–799.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu LP, Zhang XH, Wang FR, Mo XD, Han TT,
Han W, Chen YH, Zhang YY, Wang JZ, Yan CH, et al: Haploidentical
transplantation for pediatric patients with acquired severe
aplastic anemia. Bone Marrow Transplant. 52:381–387. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang J, Graves SS, Butts-Miwongtum T,
Sale GE, Storb R and Mathes DW: Long-term tolerance toward
Haploidentical Vascularized Composite Allograft Transplantation in
a Canine Model Using Bone Marrow or Mobilized Stem Cells.
Transplantation. 100:e120–e127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luznik L, O'Donnell PV and Fuchs EJ:
Post-transplantation cyclophosphamide for tolerance induction in
HLA-haploidentical bone marrow transplantation. Semin Oncol.
39:683–693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Logan AC, Zhang B, Narasimhan B, Carlton
V, Zheng J, Moorhead M, Krampf MR, Jones CD, Waqar AN, Faham M, et
al: Minimal residual disease quantification using consensus primers
and high-throughput IGH sequencing predicts post-transplant relapse
in chronic lymphocytic leukemia. Leukemia. 27:1659–1665. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bassan R, Spinelli O, Oldani E,
Intermesoli T, Tosi M, Peruta B, Rossi G, Borlenghi E, Pogliani EM,
Terruzzi E, et al: Improved risk classification for risk-specific
therapy based on the molecular study of minimal residual disease
(MRD) in adult acute lymphoblastic leukemia (ALL). Blood.
113:4153–4162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maude S and Barrett DM: Current status of
chimeric antigen receptor therapy for haematological malignancies.
Br J Haematol. 172:11–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X,
Lin YH, Wu YN, Deng ZL, Zhang YL, Liu SH, et al: High efficacy and
safety of low-dose CD19-directed CAR-T cell therapy in 51
refractory or relapsed B acute lymphoblastic leukemia patients.
Leukemia. 31:2587–2593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene Ciloleucel CAR T-Cell Therapy in
Refractory Large B-Cell Lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tiberghien P, Deconinck E and Adotevi O:
More on Anti-CD19 CAR T Cells in CNS Diffuse Large-B-Cell Lymphoma.
N Engl J Med. 377:2101–2102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brentjens RJ, Davila ML, Riviere I, Park
J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska
M, et al: CD19-targeted T cells rapidly induce molecular remissions
in adults with chemotherapy-refractory acute lymphoblastic
leukemia. Sci Transl Med. 5:177ra382013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19-28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6:224ra252014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maude SL, Teachey DT, Porter DL and Grupp
SA: CD19-targeted chimeric antigen receptor T-cell therapy for
acute lymphoblastic leukemia. Blood. 125:4017–4023. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JH, Rivière I, Gonen M, Wang X,
Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, et
al: Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic
Leukemia. N Engl J Med. 378:449–459. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y, Wu Z, Luo Y, Shi J, Yu J, Pu C,
Liang Z, Wei G, Cui Q, Sun J, et al: Potent Anti-leukemia
Activities of Chimeric Antigen Receptor-Modified T Cells against
CD19 in Chinese Patients with Relapsed/Refractory Acute Lymphocytic
Leukemia. Clin Cancer Res. 23:3297–3306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park J, Riviere I, Wang XY, Bernal Y,
Purdon T, Halton E, Wang Y, Curran KJ, Sauter CS, Sadelain M, et
al: Implications of Minimal Residual Disease Negative Complete
Remission (MRD-CR) and Allogeneic Stem Cell Transplant on Safety
and Clinical Outcome of CD19-Targeted 19-28z CAR Modified T Cells
in Adult Patients with relapsed, Refractory B-Cell ALL. Blood.
126:6822015. View Article : Google Scholar
|
|
20
|
Summers C, Annesley C, Bleakley M,
Dahlberg A, Jensen MC and Gardner R: Long Term Follow-up after
SCRI-CAR19v1 Reveals Late Recurrences As Well As a Survival
Advantage to Consolidation with HCT after CAR T Cell Induced
Remission. Blood. 132 (Supp. 1):9672018. View Article : Google Scholar
|
|
21
|
Cheng Z, Wei R, Ma Q, Shi L, He F, Shi Z,
Jin T, Xie R, Wei B, Chen J, et al: In vivo expansion and antitumor
activity of coinfused CD28- and 4-1BB-engineered CAR-T cells in
patients with B cell leukemia. Mol Ther. 26:976–985. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kochenderfer JN, Dudley ME, Feldman SA,
Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes
MS, Sherry RM, et al: B-cell depletion and remissions of malignancy
along with cytokine-associated toxicity in a clinical trial of
anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood.
119:2709–2720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harb JG, Chyla BI and Huettner CS: Loss of
Bcl-x in Ph+ B-ALL increases cellular proliferation and
does not inhibit leukemogenesis. Blood. 111:3760–3769. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maude SL, Frey N, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Porter DL, Hwang WT, Frey NV, Lacey SF,
Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, et al:
Chimeric antigen receptor T cells persist and induce sustained
remissions in relapsed refractory chronic lymphocytic leukemia. Sci
Transl Med. 7:303ra1392015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grupp SA, Kalos M, Barrett D, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bozic I, Antal T, Ohtsuki H, Carter H, Kim
D, Chen S, Karchin R, Kinzler KW, Vogelstein B and Nowak MA:
Accumulation of driver and passenger mutations during tumor
progression. Proc Natl Acad Sci USA. 107:18545–18550. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brentjens RJ, Rivière I, Park JH, Davila
ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda
O, et al: Safety and persistence of adoptively transferred
autologous CD19-targeted T cells in patients with relapsed or
chemotherapy refractory B-cell leukemias. Blood. 118:4817–4828.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bachanova V, Sandhu K, Yohe S, Cao Q,
Burke MJ, Verneris MR and Weisdorf D: Allogeneic hematopoietic stem
cell transplantation overcomes the adverse prognostic impact of
CD20 expression in acute lymphoblastic leukemia. Blood.
117:5261–5263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang XJ, Zhu HH, Chang YJ, Xu LP, Liu DH,
Zhang XH, Jiang B, Jiang Q, Jiang H, Chen YH, et al: The
superiority of haploidentical related stem cell transplantation
over chemotherapy alone as postremission treatment for patients
with intermediate- or high-risk acute myeloid leukemia in first
complete remission. Blood. 119:5584–5590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Villalobos IB, Takahashi Y, Akatsuka Y,
Muramatsu H, Nishio N, Hama A, Yagasaki H, Saji H, Kato M, Ogawa S,
et al: Relapse of leukemia with loss of mismatched HLA resulting
from uniparental disomy after haploidentical hematopoietic stem
cell transplantation. Blood. 115:3158–3161. 2010. View Article : Google Scholar : PubMed/NCBI
|