Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of non-Hodgkin's lymphoma worldwide with the morbidity
frequently in elderly people (1).
DLBCL is an aggressive malignancy of large transformed
B-lymphocytes that often originates from the lymph nodes, and that
exhibits a notable molecular heterogeneity in gene profiles and
clinical outcomes (2). DLBCL is
potentially curable. Patients with DLBCL at an early stage usually
undergo a short course of chemotherapy consisting of four drugs
(cyclophosphamide, doxorubicin, vincristine and prednisone) known
as CHOP, or chemo-immunotherapy, which is a combination of
chemotherapy and the monoclonal antibody rituximab
(Rituxan®) (3). For
patients with late-stage DLBCL with a higher risk of recurrence
after treatment, high-dose chemotherapy followed by a stem cell
transplant is provided as an option (2). Allogeneic transplantation from a
sibling or matched unrelated donor may be considered for patients
with refractory disease, early relapse or relapse after autologous
stem cell transplantation (4). All
these therapeutic strategies have greatly improved the survival
time of patients with DLBCL (5).
Although DLBCL can now be successfully treated in ~50% of patients,
certain individuals, especially those with relapsed or refractory
DLBCL, fail to respond to these conventional treatments or to
achieve long-term outcomes (2).
A number of novel therapies or procedures are being
tested in various clinical trials for DLBCL, including
immunomodulators, tyrosine kinase inhibitors, BCL2 inhibitors and
immune checkpoint inhibitors (6).
Chimeric antigen receptor (CAR) T-cell therapy is one of the most
promising immunotherapies for patients with DLBCL (7). As of December 10, 2019, clinicaltrials.gov has registered a total of 896 CAR
T-cell-associated clinical trials worldwide, including 43 for
DLBCL. There are currently 15 clinical trials being performed in
China for DLBC, including one using CD19- and CD22-targeted
sequential treatment (8), nine
against CD19 (9), two against CD22,
two against CD20 and one against CD19/22 (10). The principle of CAR T-cell therapy is
to genetically modify autologous T cells with a recombinant
receptor construct composed of an antibody-derived extracellular
single-chain variable fragment (scFv) linked to intracellular
T-cell signaling domains of the T-cell receptor. The T cell-antigen
interaction is independent from molecules of the major
histocompatibility complex, and is therefore not regulated by the
immune escape promoted by tumor cells (11). Choosing the right tumor antigen as a
target is the key to designing safe and effective CAR T-cell
therapies. B-cell malignancies commonly express the surface
antigens CD19 and CD22, which are not expressed on other non-B
cells (such as hematopoietic stem cells) (12). At present, CD19 CAR T-cell therapy is
widely used in clinical trials of malignant B-cell tumors,
including B-cell acute lymphoblastic leukemia (ALL), chronic
lymphocytic leukemia, mantle cell lymphoma, multiple myeloma and
B-cell non-Hodgkin's lymphoma, particularly for aggressive B-cell
lymphomas (13). Single- and
multi-center clinical trials using anti-CD19 CAR T-cell therapy
have demonstrated the effectiveness of this cell therapy, it has
great efficacy and long-term remissions in patients with poor-risk
DLBCL, when no other effective treatment options are available
(14). With no other effective
treatment options available, the single and multi-center clinical
trials have demonstrated that the anti-CD19 CAR T-cell therapy can
provide long-term remission in patients with poor-risk DLBCL
(15,16). As a synergistic targeting strategy,
compared with targeting a single antigen, dual specific CD19- and
CD22-targeted CAR T-cell therapy may represent a potential approach
to improve the outcomes in patients with DLBCL with heterogeneous
expression of CD19 and CD22 on leukemic blasts (16).
Cytokine release syndrome (CRS) is a systemic
inflammatory response that can be triggered after infusion of
antibody-based therapies, such as CAR T-cell therapy. According to
the ZUMA-1 (Yescarta®) trial data published in January
2019, 83% of the 101 patients with assessable efficacy achieved an
objective response and 58% achieved a complete response (14). Among the 108 patients whose safety
could be assessed, 48% developed grade ≥3 serious adverse events
and 11% of patients exhibited grade ≥3 CRS (17). CRS represents one of the most
frequent serious adverse effects and is one of the challenges of
using bispecific antibody (such as CD19/CD22) CAR T-cell therapies
(18–20). To the best of our knowledge, the
present case report describes the first clinical case of a patient
with refractory DLBCL who underwent both single CD19- and dual
CD19/CD22-targeted CAR T-cell therapies after multi-line
chemotherapy regimens, and who achieved complete remission (CR)
with minor CRS-associated adverse events.
Case report
A 31-year-old man with no prior medical history
presented with persistent epigastric pain for 1 week was admitted
to the Fourth Hospital of Hebei Medical University on April 3rd,
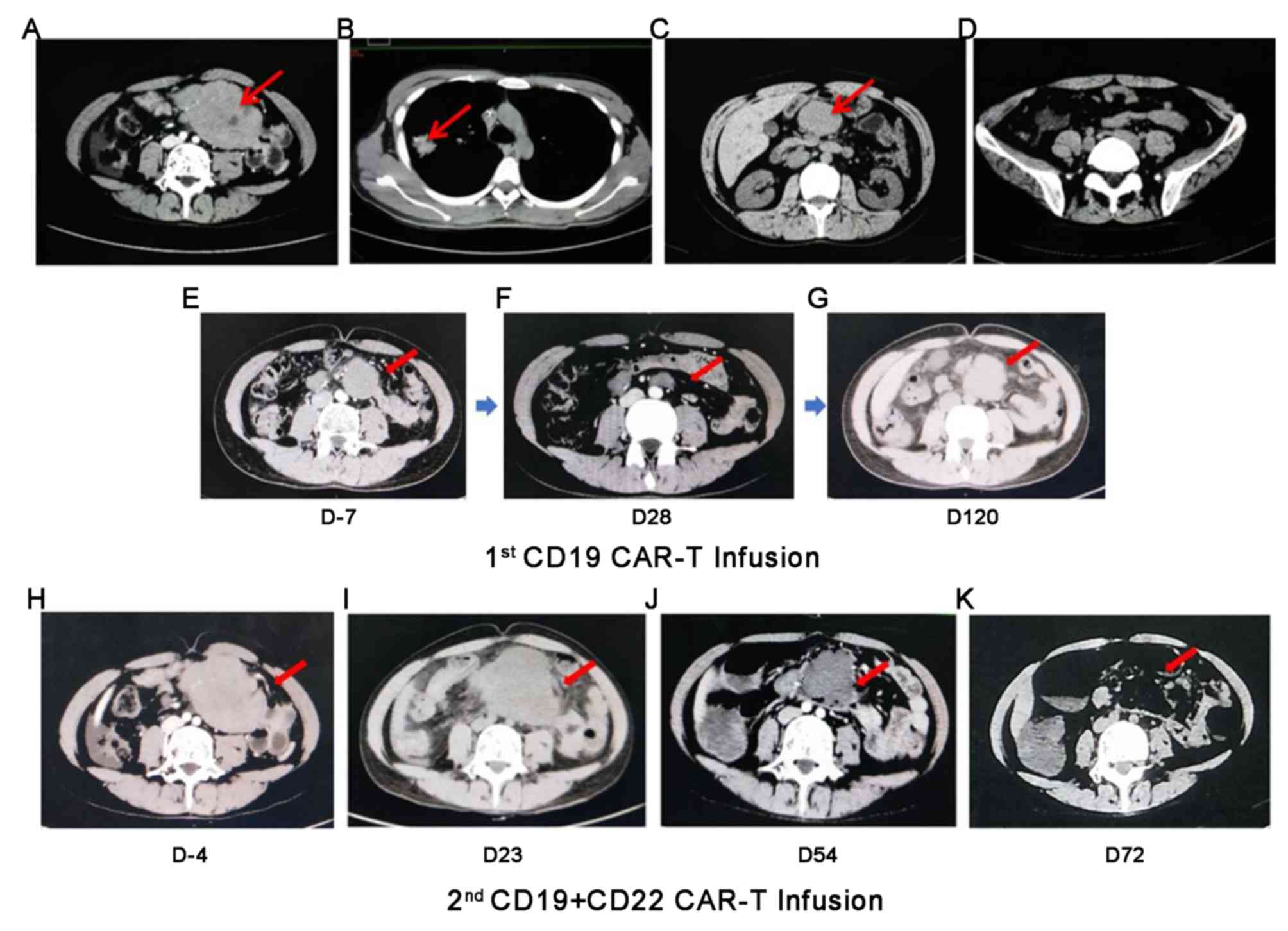
2015. Imaging scans demonstrated a large mass-like conglomerate in
the abdomen, with the maximum clast length measuring up to 13 cm
(Fig. 1A). After that,
immunohistochemistry examination of the biopsy specimen was
performed as described below. Formalin-fixed (at 4°C for 24 h) and
paraffin-embedded tissues were cut into 5 µm thick sections. After
drying at 65°C for 2 h, tissues were deparaffinized and hydrated in
graded alcohol and PBS. The sections were blocked at room
temperature with 0.3% hydrogen peroxide to inhibit endogenous
peroxidase activity for 5 min. EDTA pre-incubated with 5% normal
bovine serum (Wuhan Boster Biological Technology Ltd.) was applied
for antigen retrieval at room temperature for 20 min. Sections were
subsequently incubated with antibodies against CD20 (cat. no.
IS60430-2; 1:200; Dako; Agilent Technologies, Inc.), CD19 (cat. no.
551520; 1:100; Ventana Medical Systems, Inc.), CD22 (cat. no.
563941; 1:100; Ventana Medical Systems, Inc.), CD10 (cat. no.
561002; 1:1; Ventana Medical Systems, Inc.), BCL2 (cat. no.
IS61430-2; 1:10; Agilent Technologies, Inc) and BCL6 (cat. no.
1306055; 1:75; Santa Cruz Biotechnology, Inc.), overnight at 4°C.
The sections were subsequently incubated with secondary antibody
(cat. no. KIT-5220; 1:200; Maxim Biotech, Inc.) for 20 min at room
temperature. The reaction products were treated with
diaminobenzidine and counterstained with hematoxylin at room
temperature for 5–10 min. Tissue sections were observed under a
light microscope (magnification, ×20). The results of
immunohistochemistry stains revealed the infiltration of large
atypical pleomorphic lymphoid cells, which expressed CD20, CD19,
CD22 and BCL2, but not BCL6 and CD10. Furthermore, >70 and
>50% of cells were positive for Ki-67 and c-Myc staining,
respectively. Chest computed tomography (CT) scan revealed a shadow
in the right upper lobe of the lung (Fig. 1B), while pathology tests of CT-guided
percutaneous lung biopsy revealed epithelioid granulomas. The
purified protein derivative skin test was negative, while a more
accurate T cell-based test of tuberculosis infection was positive,
indicating a prior mycobacteria infection (21). According to Ann Arbor staging system
(22), the patient who diagnosed
with DLBCL was classified as stage I after the biopsy procedure and
immunohistochemical analysis. This type of DLBCL was also
characterized as a non-germinal center B-cell-like (non-GCB)
subtype (23). Due to the age of the
patient, the international prognostic index was evaluated as 2
(2), and due to the persistent
residual mass in the abdomen, the patient was considered to be at
high-intermediate risk. The flow diagram of the treatments used is
presented in Fig. 2.
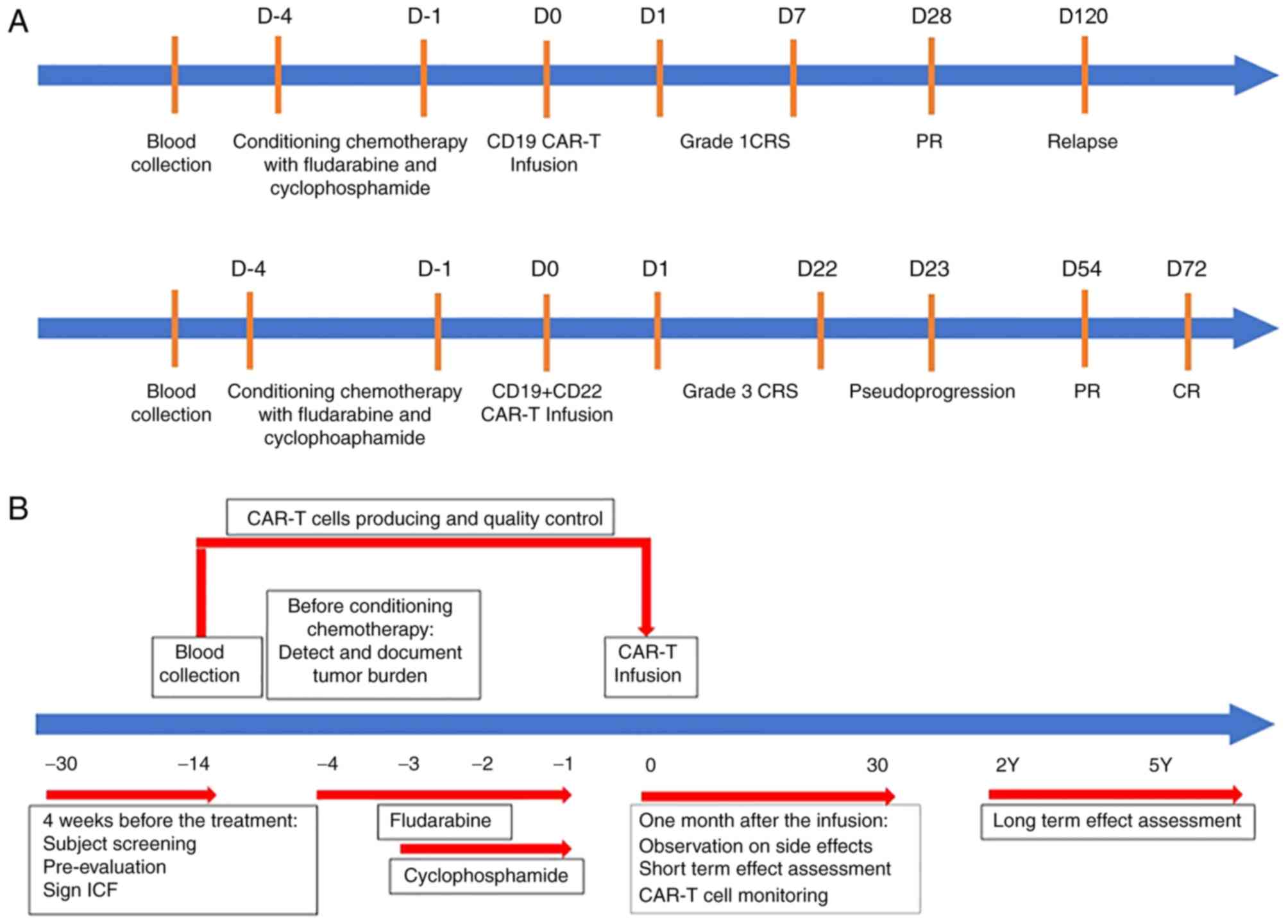 | Figure 2.Schematic representation of the
patient's progress and clinical protocol design. (A) Record of
patient's progress after single CD19 CAR-T infusion or CD19 + CD22
CAR-T infusion treatment. Blood was collected from the patient, and
conditioning chemotherapy with fludarabine and cyclophoamide was
conducted, following by giving either CD19 CAR-T infusion or
CD19+CD22 CAR-T infusion treatment; the grade of CRS, PR or
pseudoprogression and CR or relapse were recorded. (B) Clinical
protocol design with time frame ranged from −30 days to 5 years. 4
weeks before the treatment, patient had screening, pre-evaluation
and signed ICF, followed by detection and documentation the size of
tumor burden before conditioning chemotherapy. One month after the
infusion, any side effects and short-term effects were observed and
assessed. At the conclusion of the CAR-T cell monitoring, the
patient was asked for long-term follow up (2–5 years). CAR,
chimeric antigen receptor; PR, partial remission; CR, complete
remission; CRS, cytokine release; ICF, informed consent form; D,
day; M, month; Y, years. |
The patient only achieved partial remission after
two cycles of standard therapy, including 750 mg/m2 of
cyclophosphamide, 1.4 mg/m2 of vincristine (max dose of
2 mg), 50 mg/m2 of doxorubicin, 100 mg of prednisone,
and 375 mg/m2 of rituximab (R-CHOP). After that, an
intensified immunochemotherapy regimen therapy was applied, as
shown by cyclophosphamide 1200 mg/m2, vincristine 2
mg/m2 (max dose of 2 mg), doxorubicin 75
mg/m2, prednisone 60 mg, and rituximab 375
mg/m2 (R-ACVBP). The patient finally achieved CR after
two courses of the R-ACVBP regimen, followed by another two courses
of chemotherapy for consolidation. At 2 months post-CR, imaging
scans revealed that the abdominal mass was ~7.6×5.1 cm in size
(Fig. 1C), which was considered as a
recurrence. The patient received sequential salvage chemotherapies,
including two cycles of rituximab, ifosfamide, carboplatin and
etoposide, two cycles of rituximab and lenalidomide, one cycle of
gemcitabine, dexamethasone and cisplatin, and one cycle of
etoposide, methyl prednisolone, cisplatin and cytarabine. Despite
many attempts at treatment, the patient with refractory DLBCL
exhibited no significant response to the salvage therapies.
Instead of stem cell transplantation, the patient
received radiotherapy at a dose of 45 Gy in 25 fractions in order
to treat the retroperitoneal soft-tissue masses. Although the tumor
burden decreased (Fig. 1D), the
course of radiotherapy was interrupted due to the development of
severe bone marrow suppression and gastrointestinal intolerance. As
one of the most common acute side effects of radiation therapy, a
reduction of T cells was observed in the peripheral blood of the
patient, and the biopsy of the abdominal mass resulted positive for
CD19 expression in the non-GCB subtype DLBCL. Additionally,
full-body CT scans revealed enlarged mesenteric lymph nodes located
in the abdomen, as compared with prior CT scans (Fig. 1E).
The CAR construct used in the present study was
composed of a CD19-scFv (FMC63), the costimulatory domains of 4-1BB
and the endodomain of CD3-θ (24,25).
After careful physical examination, the patient was recruited for a
CD19 CAR T-cell therapy clinical trial (NCT03121625). Peripheral
blood mononuclear cells (100 ml) were collected to prepare
CD19-directed CAR T cells. A lymphodepleting pretreatment (25
mg/m2 fludarabine on days-4 to −2, and 900
mg/m2 cyclophosphamide on days-2 to −1) was administered
prior to a 2×106 cells/kg CAR T-cell infusion on day 0.
Within 12 h after infusion, the patient developed grade 1 CRS with
fever (26). On day 28
post-infusion, the patient exhibited partial remission. An imaging
test revealed that the size of the enlarged lymph nodes in the
abdomen was decreased (Fig. 1F).
At 4 months post-infusion, the patient experienced
disease progression, assessed via imaging examination revealing
enlarged lymph nodes (maximum diameter, 4.89 cm; Fig. 1G). Immunohistochemistry results from
the biopsy demonstrated that the infiltrates around the abdominal
mass were CD19 and CD22 double-positive cells. Therefore, a dual
CD19/CD22-targeted CAR T-cell therapy with the same dose of the FC
regimen (fludarabine, 25 mg/m2; cyclophosphamide, 250
mg/m2) was administered to the patient 150 days after
the first CD19 infusion. Imaging before the second CART treatment
showed that the abdominal mass was significantly larger than before
(Fig. 1H). The patient received
2×106/kg of both CAR T cells on day 0. The patient
developed grade 3 CRS with shivering, hypotension and hyperpyrexia,
and therefore received anti-infection and rehydration treatments.
Full body CT scans on day 23 revealed that the abdominal mass had
increased in size (maximum diameter, 9 cm), suggesting a poorer
prognosis (Fig. 1I). Accordingly,
the patient had persistent fever for >1 week, with pancytopenia,
a decreased fibrinogen level (<1.5 g/l; normal range, 2–4 g/l)
and an elevated serum ferritin level (23,410.00 ng/ml; normal
range, 15–200 ng/ml for adult male). The proliferation of
peripheral blood CAR T cells was analyzed via quantitative (q)PCR.
Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions, and reversed transcribed into cDNA using the reverse
transcription kit (cat. no. 18091050; Thermo Fisher Scientific,
Inc.). cDNA was amplified using EmeraldAmp® PCR Master
Mix (Thermo Fisher Scientific, Inc.). The algorithm
2−ΔΔCq method (27) was
used to normalize the relative expression of genes to GAPDH. The
following primer sequences were used for qPCR: CAR forward,
5′-CATCCTCCCTGTCTGCCTCT-3;′ and reverse,
5′-GCCTCCGCCATCTTATCTTT-3′; GAPDH forward, 5′-TGCATTCGCCCTCTTAA-3′
and reverse, 5′-CATCACGCCACAGTTTCC−3′; and CAR FQ-PCR forward,
5′-GGATTCGCCAGCCTCCAC−3′ and reverse, 5′-AAACTTGGCTCTTGGAGTTGT−3′.
CAR FQ-PCR-Probe: 5′-(FAM)-TCCCAGCCACTCCAGACCCTT-(MGB)-3′.
Additionally, qPCR was carried out with an ABI 7500 machine
(Applied Biosystems, Carlsbad, CA). The following thermocycling
conditions were applied: 1 cycle at 50°C for 2 min, 1 cycle at 95°C
for 10 min and 40 cycles at 95°C for 15 s, while 60°C for 60 s
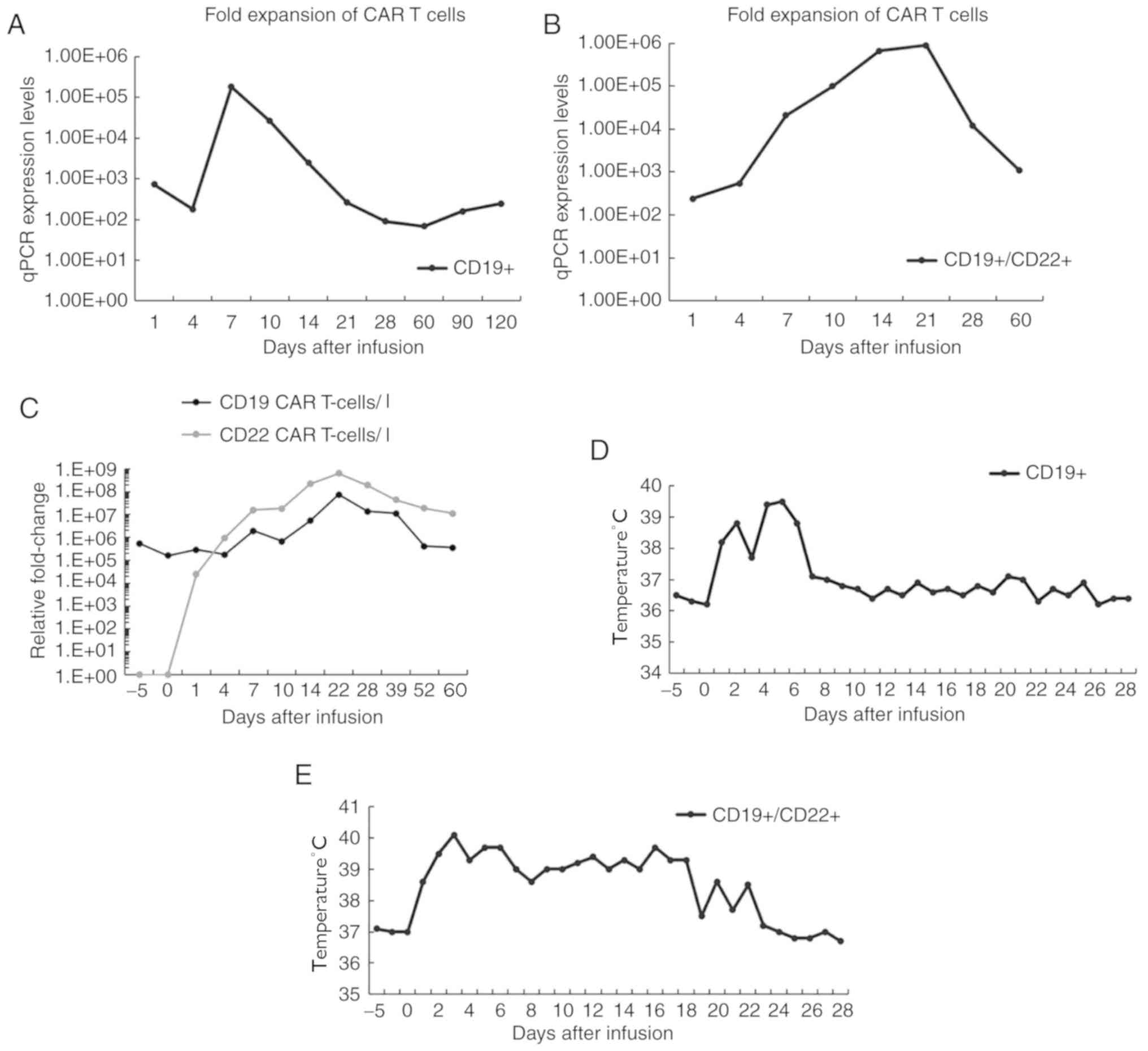
(27). The data demonstrated that
both CD19 and CD19/CD22 CAR T cells began to expand after 4 days,
peaked on days 7 and 18, and then gradually decreased (Fig. 3A-C). Notably, dual-CAR T cells were
mixed after individual transduction, and their expansion was ~5
times higher than single CD19 CAR T cells; additionally, both
CD19+ and CD22+ CAR T cells remained
sustained at higher levels for 2 further months. After infusion of
both CAR T cells, the patient exhibited an intermittent high fever
during the first few days but, subsequently, the body temperature
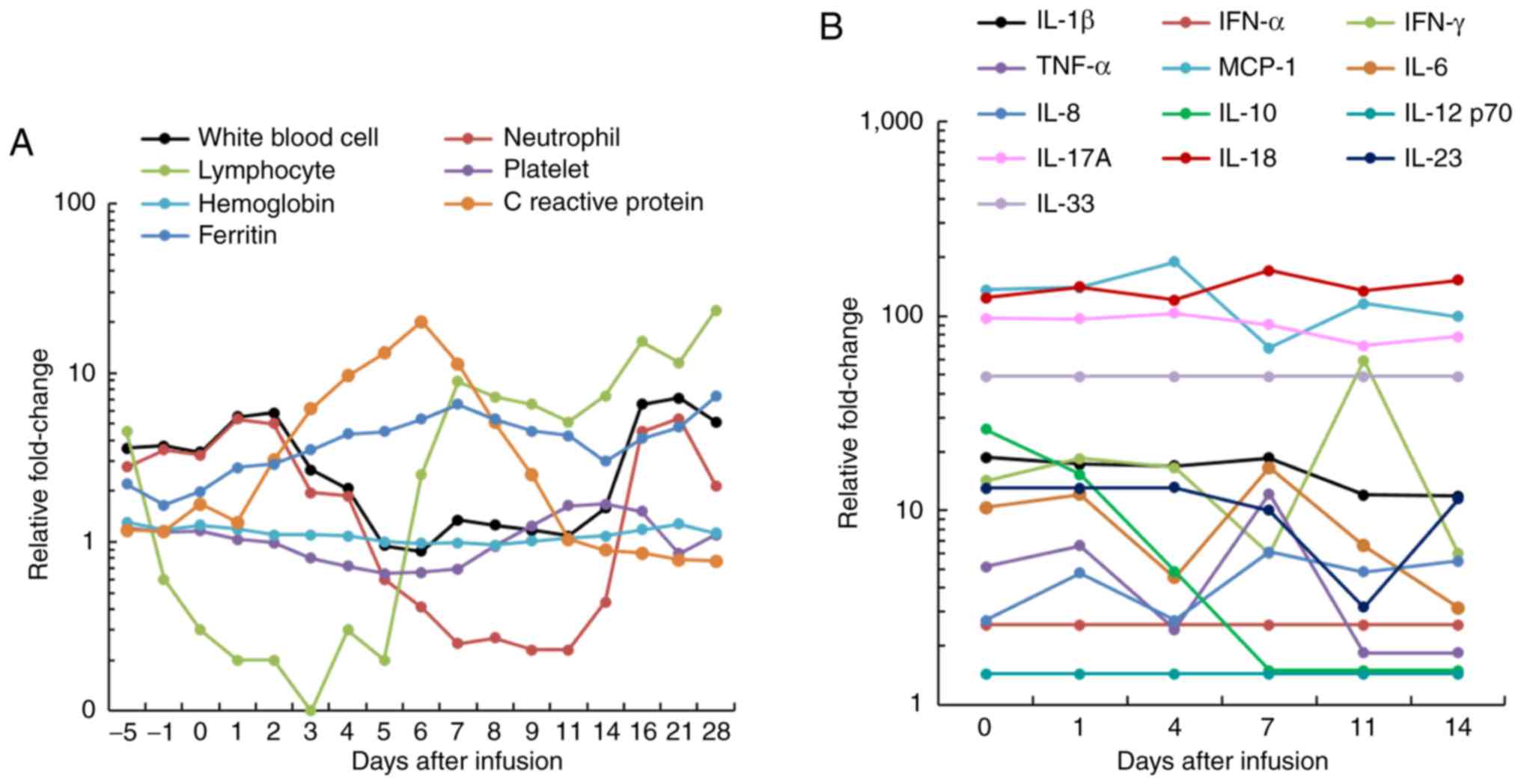
gradually returned to normal and remained stable (Fig. 3D and E). The levels of lymphocytes,
ferritin and C-reactive protein gradually decreased after day 7 for
CD19 single CAR T-cell infusion (Fig.
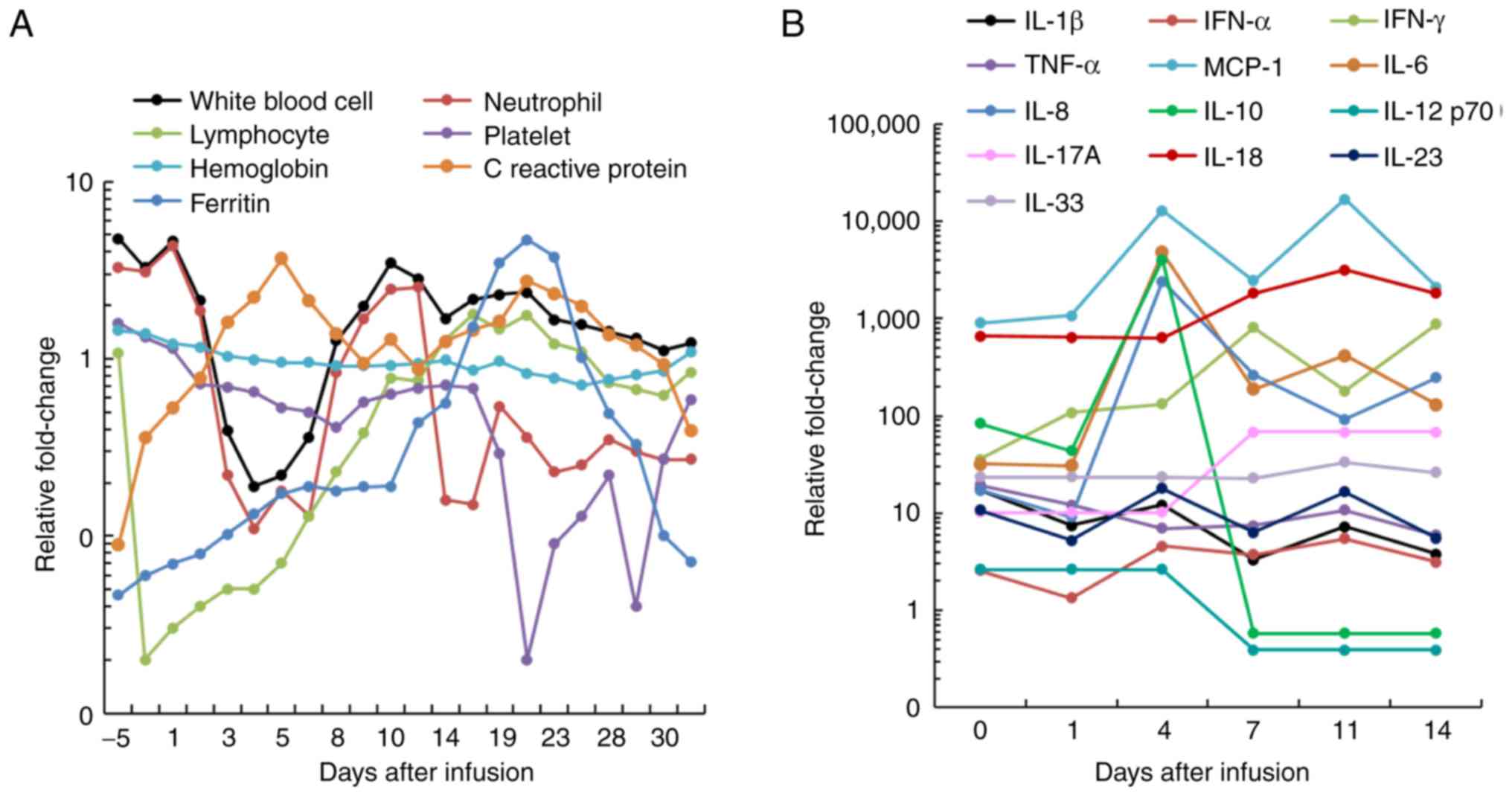
4A), and for dual CAR T-cell infusion, the level of platelets
drastically decreased after day 18, while the levels of ferritin
and C reactive protein decreased after day 20 (Fig. 5A). Although no immediate
infusion-associated toxic effect was observed, a febrile syndrome
with elevated cytokine levels was subsequently observed after CAR
T-cell infusions (Figs. 4B and
5B). Overall, the present results
indicated that the patient developed grade 1 CRS after single CD19
CAR T-cell infusion, and grade 3 CRS after dual CAR T-cell
infusion.
Additionally, hemophagocytosis was observed in the
patient's bone marrow, supporting a diagnosis of hemophagocytic
syndrome. The patient was treated with dexamethasone (20 mg/day),
which was later replaced by methylprednisolone (80 mg/day) on day
33. The body temperature of the patient was well controlled from
day 48. Additionally, the patient developed gastrointestinal
bleeding and was therefore administered proton pump inhibitors,
intravenous fluids and electrolytes, while the oral intake of
liquids or solids was prohibited. There was a marked reduction in
the size of the abdominal mass after day 54 (Fig. 1J). The hemophagocytosis symptom was
not observed after day 57, while at the same time several blood
tests, including ferritin levels and coagulation function, returned
to normal. A full-body CT scan on day 72 demonstrated CR (Fig. 1K) that continued until day 100. Flow
cytometric analysis (28) revealed
that most of the lymphocytes before infusion were CD3+
and CD3+/CD4+ T cells, and these cells were
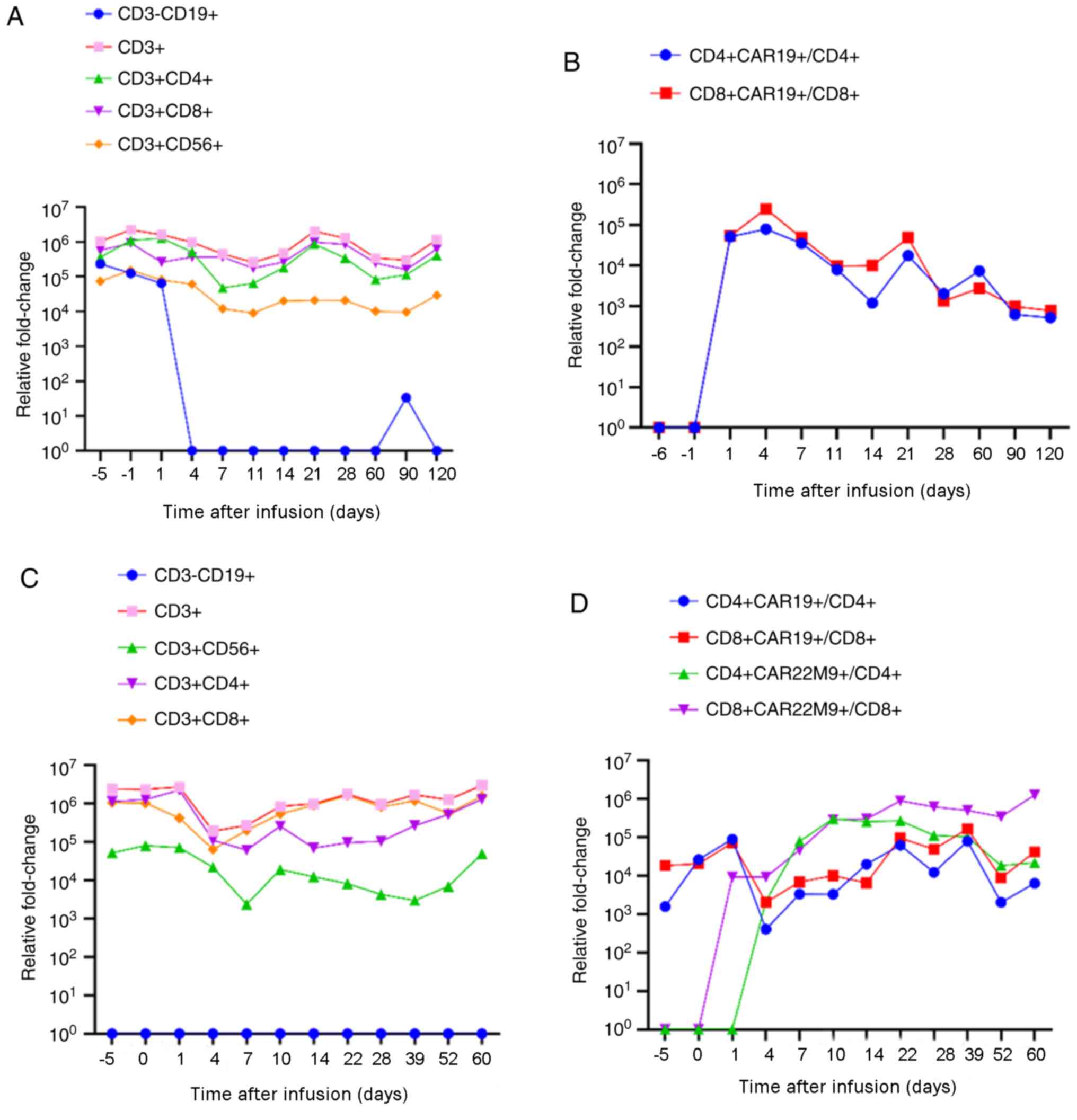
replaced by CAR T cells rapidly after infusion (Fig. 6). The proportion of CAR T cells
increased and then decreased within 1-month post-infusion for
single CAR T-cell infusion but remained high in dual CAR T-cell
infusion for at least 2 months. Overall, the present data suggest a
synergistic efficacy of CD19- and CD22-targeted CAR T cells in the
present patient. After monitoring (up to D60) and infecting with
H1N1 (at D85), the patient was transferred to the Hebei Provincial
Chest Hospital because of gastrointestinal hemorrhage.
Discussion
Cancer immunotherapy is an emerging therapeutic
strategy that has demonstrated significant efficiency compared with
conventional treatments such as radiotherapy, chemotherapy and
surgery (29). At present, >800
CAR T-cell studies are registered on clinicaltrials.gov, with one-third being CD19-targeted
CAR T-cell trials. Tumor cells of B-cell malignancies typically
express both CD19 and CD22 surface antigens (30), making dual targeted CAR T cells a
more broadly active therapy (31).
To the best of our knowledge, there are no reports regarding the
efficacy of dual CD19/CD22-targeted CAR T-cell therapy in DLBCL. In
addition, the toxicity and safety have not yet been investigated.
The present case report describes the first clinical experience in
a patient with DLBCL treated with bispecific CD19/CD22-targeted CAR
T cells.
Single-agent MOR208 therapy has previously
demonstrated a good clinical performance in patients with
relapse/refractory (r/r) DLBCL and r/r follicular lymphoma,
including in patients refractory to rituximab (32). A number of published reports have
identified CD19 as a promising target for CAR T-cell therapy for
most B-cell malignancies, including ALL (33–35). The
peak of CAR T-cell expansion was positively correlated with
post-treatment efficiency and survival time, in accordance with
previous studies (36,37). Due to the short duration and small
number of CD19 CAR T-cell expansions, the patient in the present
case report only reached partial remission for 3 months.
Although most B-cell ALL cases can be targeted by
CD19 CAR T-cell therapy, 5–10% of relapses occur in patients with
absent or low cell-surface expression of CD19 (38,39). In
the PLAT-02 clinical trial, 93% of patients with r/r ALL exhibited
a good response after anti-CD19 CAR T-cell therapy; however, 50% of
patients relapsed at the end of the trial (40). Instead of CD19 expression, CD22
expression was identified in patients with relapsed leukemia. The
Stanford University School of Medicine and the National Cancer
Institute have launched a phase I clinical trial of anti-CD22 CAR
T-cell therapy in patients with relapsed B-cell ALL and obtained
significant progress. This includes CD-22 CAR that can mediate
similar potent antineoplastic effects as CD19, while the dual
CD19/CD22 targeted immunotherapeutic plays an important role to
overcome the resistance to immunotherapy via antigen loss (41). In November 2019, Tongji Hospital
Affiliated to Tongji Medical College of Huazhong University of
Science and Technology (Wuhan, China) published a single case
report of HBV reactivation after sequential treatment with CD19 and
CD22 in a patient with DLBCL; after 2.5 months of CAR T-cell
treatment, the tumor condition remained stable and superficial
lymph nodes could not be detected (42). In the present report, the proportion
of CAR T cells remained high throughout the two CAR T-cell
infusions, and gradually restored the body temperature of the
patient to normal, thereafter remaining stable. Therefore, the
synergistic efficacy of CD19 and CD22 CAR T-cell infusion was
observed in the present patient with refractory DLBCL.
The dose of CAR T-cell infusion is dependent on body
weight. Due to the different weight of patients, the total number
of CAR T cells returned to each individual is not the same, making
it difficult to analyze all patients by a specific number of CAR T
cells and therefore having to rely on CAR T-cell expansion trends
and patient symptoms as a marker of treatment efficacy. In the
present report, the CAR T cells began to expand on days 4–7, peaked
on days 7–10 and began to decline on day 14. CRS is one of the most
notable adverse reactions in the clinical application of CAR T-cell
technology (43,44). If patients experience severe CRS
reactions, such as high fever, >20% blood pressure reduction,
dyspnea and grade 4 organ damage, the test should be automatically
suspended, and restorative treatment should be initiated
immediately. In the present report, due to the large release of
cytokines caused by T-cell expansion, the patient developed
manageable CRS symptoms, such as fever, hypotension, myalgia and
respiratory failure. Currently, although there are drugs that can
control CRS, complications remain a barrier to standard treatment.
It has been demonstrated that the degree of CRS severity is
associated with disease burden at the time of infusion, as a higher
tumor burden results in more serious CRS (45), suggesting that in the case of low
tumor burden, such as early disease, the risk and severity of CRS
in patients undergoing CAR T-cell therapy may be markedly reduced.
Therefore, in the present study, radiotherapy was used prior to CAR
T-cell therapy, successfully decreasing the tumor burden of the
patient. It has been suggested that CRS is over-activated by immune
effector cells, resulting in excessive release of inflammatory
cytokines, such as interleukin (IL)-1, IL-2, IL-6, IL-10, IL-15,
interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) (46). Therefore, the associations between
the number of CAR T-cell expansions and IL-6, TNF-α and IFN-γ
levels were analyzed in the present report. The analysis revealed
that when the patient was infused with a single CD19 CAR T-cell
infusion for the first time, the qPCR amplification curve and the
fold-change curve of IL-6 seemed to have the same trend, suggesting
that there was no association between qPCR and the changes in the
three factors (IL-6, IFN-γ and TNF-α). However, upon further
analysis of the three cytokines and qPCR via one-way ANOVA, no
statistical differences were observed (P>0.2), indicating that
it may be due to insufficient sample size. IL-6 is a cytokine known
to cause side effects, such as fever, hypotension, myalgia and
respiratory failure (46). In
addition to T cells, macrophages are a typical cell subset that
produce IL-6 (47). In a mouse
model, the severity of CRS was reduced when monocytes depleted,
which provided the major source of IL-1 and IL-6 or can block IL-6
receptor with tociizumab, suggesting that IL-6 inhibitors or
anti-IL-6 receptor antibodies may reverse the syndrome (48). Recently, two independent trials from
two research teams at the San Rafael Institute of Science and the
Memorial Sloan Kettering Cancer Center (MSKCC) demonstrated that
CRS is triggered by the inflammatory molecule IL-1 (46,47).
Anakinra is an IL-1 inhibitor that can be combined with CAR T-cell
therapy and is effective in managing CRS and neurotoxicity
(49). In addition, researchers from
the MSKCC have designed CAR T cells that secrete an IL-1 inhibitor
to prevent CRS.
The present case report demonstrates the efficacy
and safety of dual CD19/CD22-targeted CAR T-cell therapy in the
treatment of DLBCL. The present results provide evidence that dual
CAR T-cell therapy may be a promising option for the treatment of
relapsed or refractory DLBCL in patients who do not benefit from
single CD19-targeted CAR T-cell therapy. However, CRS is the major
adverse effect of dual CD19/CD22-targeted CAR T-cell therapy and
caution should be taken for patients receiving this treatment.
However, this was only one case report on a single patient;
therefore, the optimal dose of CAR T cells and the follow-up
treatment remain to be clarified in well-designed studies with
larger sample sizes.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CH designed the experiments and wrote the article.
HZ performed the histopathological and serum marker analyses; JH,
RL and LW performed the experiments. NK and MZ were responsible for
data collection and analysis, and checked the references. LL and JL
assisted with the study design and made the figures of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present report was approved by the Ethics
Committee of Drug Clinical Trials of The Fourth Hospital of Hebei
Medical University (Shijiazhuang, China; approval no. 2016040).
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the present report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sieniawski MK: Epidemiology, prognosis and
treatment of aggressive non-Hodgkin lymphomas. Newcastle
University. 2017.
|
|
2
|
Vitolo U, Seymour JF, Martelli M,
Illerhaus G, Illidge T, Zucca E, Campo E and Ladetto M; ESMO
Guidelines Committee, : Extranodal diffuse large B-cell lymphoma
(DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 27 (Suppl 5):v91–v102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tilly H, Gomes da Silva M, Vitolo U, Jack
A, Meignan M, Lopez-Guillermo A, Walewski J, André M, Johnson PW,
Pfreundschuh M, et al: Diffuse large B-cell lymphoma (DLBCL): ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 26 (Suppl 5):v116–v125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Avivi I, Canals C, Vernant JP, Wulf G,
Nagler A, Hermine O, Petersen E, Yakoub-Agha I, Craddock C,
Schattenberg A, Niederwieser D, et al: Matched unrelated donor
allogeneic transplantation provides comparable long-term outcome to
HLA-identical sibling transplantation in relapsed diffuse large
B-cell lymphoma. Bone Marrow Transplant. 49:671–678. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aviles A, Calva A, Neri N, Cleto S and
Silva L: Role of radiotherapy in diffuse large B-cell lymphoma in
advanced stages on complete response after administration of
cyclophosphamide, doxorubicin, vincristine, prednisone, and
rituximab. Precision Radiation Oncol. 3:100–104. 2019. View Article : Google Scholar
|
|
6
|
Maziarz RT, Hao Y, Guerin A, Gauthier G,
Gauthierloiselle M, Thomas SK and Eldjerou L: Economic burden
following allogeneic hematopoietic stem cell transplant in patients
with diffuse large B-cell lymphoma. Leuk Lymphoma. 59:1133–1142.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brudno JN and Kochenderfer JN: Chimeric
antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol.
15:31–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang F, Zhang J, Zhang X, Tian M, Wang J,
Kang L, Qiu H and Wu D: Delayed remission following sequential
infusion of humanized CD19- and CD22-modified CAR-T cells in a
patient with relapsed/refractory acute lymphoblastic leukemia and
prior exposure to murine-derived CD19-directed CAR-T cells. Onco
Targets Ther. 12:2187–2191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dai H, Zhang W, Li X, Han Q, Guo Y, Zhang
Y, Wang Y, Wang C, Shi F, Zhang Y, et al: Tolerance and efficacy of
autologous or donor-derived T cells expressing CD19 chimeric
antigen receptors in adult B-ALL with extramedullary leukemia.
Oncoimmunology. 4:e10274692015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang N, Hu X, Cao W, Li C, Xiao Y, Cao Y,
Gu C, Zhang S, Chen L, Cheng J, et al: Efficacy and Safety of
CAR19/22 T-cell Cocktail therapy in patients with
Refractory/Relapsed B-cell Malignancies. Blood. 135:17–27. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
John M: Clinical immunotherapy of B-cell
malignancy using CD19-targeted CAR T-cells. Curr Gene Ther.
14:35–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Banihashemi SR, Hosseini AZ, Rahbarizadeh
F and Ahmadvand D: Development of Specific Nanobodies (VHH) for
CD19 Immuno-targeting of Human B-Lymphocytes. Iran J Basic Med Sci.
21:455–464. 2018.PubMed/NCBI
|
|
13
|
Locke FL, Neelapu SS, Bartlett NL, Siddiqi
T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, et
al: Phase 1 results of ZUMA-1: A multicenter study of KTE-C19
Anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol
Ther. 25:285–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chavez JC, Bachmeier C and Kharfan-Dabaja
MA: CAR T-cell therapy for B-cell lymphomas: Clinical trial results
of available products. Ther Adv Hematol. 10:20406207198415812019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kochenderfer JN, Somerville RPT, Lu T,
Yang JC, Sherry RM, Feldman SA, McIntyre L, Bot A, Rossi J, Lam N
and Rosenberg SA: Long-duration complete remissions of diffuse
large B-cell Lymphoma after Anti-CD19 chimeric antigen receptor
therapy. Mol Ther. 25:2245–2253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leonard JP, Coleman M, Ketas JC, Chadburn
A, Furman R, Schuster MW, Feldman EJ, Ashe M, Schuster SJ, Wegener
WA, et al: Epratuzumab, a Humanized Anti-CD22 antibody, in
aggressive Non-Hodgkin's lymphoma: Phase I/II clinical trial
results. Clin Cancer Res. 10:5327–5334. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Locke FL, Ghobadi A, Jacobson CA, Miklos
DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT,
Timmerman JM, et al: Long-term safety and activity of axicabtagene
ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A
single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 20:31–42.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kochenderfer JN, Dudley ME, Kassim SH,
Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ,
Hughes MS, Sherry RM, et al: Chemotherapy-refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies Can Be effectively
treated with autologous T cells expressing an Anti-CD19 chimeric
antigen receptor. J Clin Oncol. 33:540–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maude SL, Teachey DT, Porter DL and Grupp
SA: CD19-targeted chimeric antigen receptor T-cell therapy for
acute lymphoblastic leukemia. Blood. 125:4017–4023. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maude SL, Barrett D, Teachey DT and Grupp
SA: Managing cytokine release syndrome associated with novel T
cell-engaging therapies. Cancer J. 20:119–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meier JD and Grimmer JF: Evaluation and
Management of Neck Masses in Children. Am Fam Physician.
89:353–358. 2014.PubMed/NCBI
|
|
22
|
McCarten KM, Nadel HR, Shulkin BL and Cho
SY: Imaging for diagnosis, staging and response assessment of
Hodgkin lymphoma and non-Hodgkin lymphoma. Pediat Radiol.
49:1545–1564. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee KC, Lee SH, Sung K, Ahn SH, Choi J,
Lee SH, Lee JH, Hong J and Park SH: A case of primary breast
diffuse large B-cell lymphoma treated with chemotherapy followed by
elective field radiation therapy: A brief treatment pattern review
from a radiation Oncologist's point of view. Case Rep Oncol Med.
2015:9079782015.PubMed/NCBI
|
|
24
|
Kahlon KS, Christine B, Cooper LJN, Andrew
R, Forman SJ and Jensen MC: Specific recognition and killing of
glioblastoma multiforme by interleukin 13-zetakine redirected
cytolytic T cells. Cancer Res. 64:9160–9166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Debinski W and Thompson JP: Retargeting
interleukin 13 for radioimmunodetection and radioimmunotherapy of
human high-grade gliomas. Clin Cancer Res. 5 (10
Suppl):3143S–3147S. 1999.PubMed/NCBI
|
|
26
|
Porter D, Frey N, Wood PA, Weng Y and
Grupp SA: Grading of cytokine release syndrome associated with the
CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 11:352018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kalos M, Levine BL, Porter DL, Katz S,
Grupp SA, Bagg A and June CH: T cells with chimeric antigen
receptors have potent antitumor effects and can establish memory in
patients with advanced leukemia. Sci Transl Med. 3:95ra732011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Olson B, Li Y, Lin Y, Liu ET and Patnaik
A: Mouse models for cancer immunotherapy research. Cancer Discov.
8:1358–1365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hill JA, Li D, Hay KA, Green ML, Cherian
S, Chen X, Riddell SR, Maloney DG, Boeckh M and Turtle CJ:
Infectious complications of CD19-targeted chimeric antigen
receptor-modified T cell immunotherapy. Blood. 131:121–130. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chaouchi N, Vazquez A, Galanaud P and
Leprince C: B cell antigen receptor-mediated apoptosis. Importance
of accessory molecules CD19 and CD22 and of surface IgM
cross-linking. J Immunol. 154:3096–3104. 1995.PubMed/NCBI
|
|
32
|
Jurczak W, Zinzani PL, Gaidano G, Goy A,
Provencio M, Nagy Z, Robak T, Maddocks K, Buske C, Ambarkhane S, et
al: Phase IIa study of the CD19 antibody MOR208 in patients with
relapsed or refractory B-cell non-Hodgkin's lymphoma. Ann Onco.
29:1266–1272. 2018. View Article : Google Scholar
|
|
33
|
Park JH, Rivière I, Gonen M, Wang X,
Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, et
al: Long-term Follow-up of CD19 CAR therapy in acute lymphoblastic
leukemia. N Engl J Med. 378:449–459. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schuster SJ, Svoboda J, Chong EA, Nasta
SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V,
Landsburg D, et al: Chimeric antigen receptor T cells in refractory
B-cell lymphomas. N Engl J Med. 377:2545–2554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Turtle CJ, Hanafi LA, Berger C, Hudecek M,
Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, et
al: Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of
CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T
cells. Sci Transl Med. 8:355ra1162016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kochenderfer JN, Somerville RPT, Lu T, Shi
V, Bot A, Rossi J, Xue A, Goff SL, Yang JC, Sherry RM, et al:
Lymphoma remissions caused by Anti-CD19 chimeric antigen receptor T
cells are associated with high serum Interleukin-15 levels. J Clin
Oncol. 35:1803–1813. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee DW, Kochenderfer JN, Stetlerstevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sotillo E, Barrett DM, Black KL, Bagashev
A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, et
al: Convergence of acquired mutations and alternative splicing of
CD19 enables resistance to CART-19 immunotherapy. Cancer Discov.
5:1282–1295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park JH, Riviere I, Wang X, Bernal Y,
Purdon T, Halton E, Wang Y, Curran KJ, Sauter CS, Sadelain M and
Brentjens RJ: Implications of minimal residual disease negative
complete remission (MRD-CR) and allogeneic stem cell transplant on
safety and clinical outcome of CD19-targeted 19-28z CAR modified T
cells in adult patients with relapsed, refractory B-cell ALL.
Blood. 126:6822015. View Article : Google Scholar
|
|
40
|
Fry TJ, Shah NN, Orentas RJ,
Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S,
Delbrook C, Yates B, et al: CD22-targeted CAR T cells induce
remission in B-ALL that is naive or resistant to CD19-targeted CAR
immunotherapy. Nat Med. 24:20–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wei J, Zhu X, Mao X, Huang L, Meng F and
Zhou J: Severe early hepatitis B reactivation in a patient
receiving anti-CD19 and anti-CD22 CAR T cells for the treatment of
diffuse large B-cell lymphoma. J Immunother Cancer. 7:3152019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maude SL, Frey N, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang H, Liu L, Guo T, Wu Y, Ai L, Deng J,
Dong J, Mei H and Hu Y: Improving the safety of CAR-T cell therapy
by controlling CRS-related coagulopathy. Ann Hematol. 98:1721–1732.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Y, Chen X, Wang D, Li H, Huang J,
Zhang Z, Qiao Y, Zhang H, Zeng Y, Tang C, et al: Hemofiltration
successfully eliminates severe cytokine release syndrome following
CD19 CAR-T-Cell Therapy. J Immunother. 41:406–410. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luo H, Wang N, Huang L, Zhou X, Jin J, Li
C, Wang D, Xu B, Xu J, Jiang L, et al: Inflammatory signatures for
quick diagnosis of life-threatening infection during the CAR T-cell
therapy. J Immunother Cancer. 7:2712019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chavez JC and Locke FL: CAR T cell therapy
for B-cell lymphomas. Best Pract Res Clin Haematol. 31:135–146.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Konstantinos AP and Maria TA: Systemic
consequences of intestinal inflammation. Bedside. 235–250.
2005.doi: 10.1007/0-387-25808-6_12.
|
|
48
|
Norelli M, Camisa B, Barbiera G, Falcone
L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C,
Cristofori P, et al: Monocyte-derived IL-1 and IL-6 are
differentially required for cytokine-release syndrome and
neurotoxicity due to CAR T cells. Nat Med. 24:739–748. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Giavridis T, Stegen SJCVD, Eyquem J,
Hamieh M, Piersigilli A and Sadelain M: CAR T cell-induced cytokine
release syndrome is mediated by macrophages and abated by IL-1
blockade. Nat Med. 24:731–738. 2018. View Article : Google Scholar : PubMed/NCBI
|