Introduction
Osteosarcoma is the most common malignant tumor
among children, adolescents and young adults (1). Osteosarcoma originates from primary
bone-forming mesenchymal cells, accounting for 20% of all primary
osteosarcoma, and is the most common primary bone malignant tumor
(2). Osteosarcoma usually occurs in
the long bone of limbs near the metaphyseal plate. The most common
sites are the femur, tibia and humerus (3). Before 1970, the treatment for
osteosarcoma was mainly surgical resection. With the application of
multi-drug regimens, chemotherapy has markedly improved the 5-year
survival rate of patients with localized osteosarcoma from <20
to 65%; however, its prognosis is still very poor (4). Moreover, the mortality rate of patients
with recurrent and metastatic osteosarcoma is still very high.
Therefore, it of utmost importance to explore novel prognostic
factors for osteosarcoma patients, particularly those diagnosed
with metastatic disease.
Notch1 is a type 1 transmembrane receptor protein,
which is important for cell fate regulation, the differentiation of
various systems and neuronal development, such as neurogenesis and
the maintenance of neural stem cells (5). The increased expression of Notch1 is
related to the low survival rate of patients with various types of
cancer (6–8). The proliferation of cells from these
types of cancer can be inhibited by the pharmacological inhibition
of Notch1. Therefore, preventing the occurrence of Notch1 is a
potential strategy for the treatment of various types of cancers
(9). The transcription factor hairy
and enhancer of split-1 (HES1) is a member of the basic
helix-loop-helix (BHLH) of transcription inhibitor family, and is
the downstream target of Notch signal pathway (10). HES1 is overexpressed in a number of
tumor types, including colon cancer (11), breast cancer (12), non-small cell lung cancer (13), etc., suggesting that HES1 has
carcinogenic activity and is closely associated with cancer.
Therefore, the present study examined the changes in
the expression of Notch1 and HES1 in osteosarcoma patients
following surgery. The correlation between Notch1 expression and
HES1 expression, and its association with prognosis were also
investigated, so as to identify novel potential diagnostic and
treatment targets that may be used clinical practice.
Patients and methods
General patient information
In the present study, samples from 62 patients with
osteosarcoma treated at Shandong Cancer Hospital from April, 2011
to June, 2013 were collected as the research group, and those from
52 healthy individuals undergoing a physical examination were
collected as the control group. There were 33 males and 29 females
in the research group, with an average age of 18.6±10.1 years,
while the control group consisted of 28 males and 24 females with
an average age of 19.1±10.3 years. The present study was approved
by the Ethics Committee of Shandong Cancer Hospital. Signed written
informed consents were obtained from the patients and/or parents or
guardians.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Patients who
met the ESMO diagnostic criteria (14), or received treatment at Shandong
Cancer Hospital after diagnosis; patients who did not receive
radiotherapy or chemotherapy prior to surgery; patients who did not
receive any treatment within 30 days after surgery; patients aged
between 10 to 40 years; patients with complete case data; patients
who agreed to cooperate with the work arrangement of the medical
staff at Shandong Cancer Hospital; patients or their immediate
family members signed informed consents.
The exclusion criteria were as follows: Patients who
died during the course of treatment; patients with injury to
important organs; patients suffering from other cardiovascular and
cerebrovascular diseases, as well as any physical disability;
pregnant mothers; patients suffering from other autoimmune diseases
and chronic diseases; patients transferred to Shandong Cancer
Hospital; patients with contraindications to surgery, mental
diseases and language dysfunction.
Surgical treatment plan
The patients were subjected to limb preservation
surgery according to the strategies outlined in the study by Ando
et al (15) and references
listed in that study.
Blood sample processing
Before surgery and at 30 days after surgery, early
in the morning on an empty stomach, venous blood was drawn and
stored at 4°C for 30 min, and the serum samples were then
centrifuged for 10 min at 4°C (1,500 × g). The supernatant was then
extracted and stored in a refrigerator at −80°C.
Main reagents
Notch1 and HES1 kits were purchased from Wuhan Feien
Biotechnology Co., Ltd. (cat. nos. EH0926 and EH3223), and were
used strictly in accordance with the operating instructions
provided with the kits. The Eppendorf CryoCube F740hi ultra-low
temperature refrigerator was purchased from Eppendorf Co., Ltd.
(cat. no. ep000000).
Follow-up of patients
The patients were followed-up for 5 years, and their
survival rates were recorded via telephone communications and
outpatient medical records. The follow-up time points were the 3rd,
6, 9 and 12th month of each year.
Observation indicators
The main observation indicators were as follows: The
expression levels of Notch1 and HES1 in osteosarcoma patients
before and after surgery were observed, and the diagnostic value of
Notch1 and HES1 in osteosarcoma was determined.
The secondary observation indicators were the
following: Pearson's correlation analysis was used to analyze the
correlation between Notch1 expression and HES1 expression in
osteosarcoma patients. According to the expression levels of Notch1
and HES1 (obtained by ELISA), the patients were divided into the
high and low expression groups, and the 5-year survival rate of the
patients was observed.
Statistical analysis
In the present study, the SPSS20.0 software package
was used to perform the statistical analysis on the collected data.
The GraphPad 7 software package was used to obtain the required
graphs, and the Kolmogorov-Smirnov test was used to analyze the
distribution of these data, in which normally distribution data
were expressed as the mean ± standard deviation (means ± SD).
Inter-group comparisons were conducted using an independent-samples
t-test, and intra-group comparisons were conducted using a paired
t-test. Count data are expressed as a percentage (%) and analyzed
using the Chi-squared (χ2) test. A receiver operating
characteristic (ROC) curve was created to plot the diagnostic value
of Notch1 and HES1 in osteosarcoma, which was represented by the
χ2 value. Cut-off values were calculated using Youden's
index (YI) calculation formula as follows: YI=[a/(a + c) + d/(b +
d)]-1. Pearson's correlation analysis was used to analyze the
correlation between Notch1 expression and HES1 expression in the
osteosarcoma patients. The 5-year survival of the patients was
plotted by the Kaplan-Meier survival curve and data were analyzed
using the log-rank test. In addition, univariate and multivariate
logistic regression were performed to analyze the independent risk
factors affecting the prognosis of the patients. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical data
No significant differences were observed in the
clinical data of the research group and the control group,
including age, sex, body mass index (BMI), marital status,
nationality, place of residence, smoking, alcohol consumption and
exercise, which proved comparability (P>0.05), as shown in
Table I.
 | Table I.Clinical basic data of the
patients. |
Table I.
Clinical basic data of the
patients.
| Characteristic | Research group
(n=62) | Control group
(n=52) | χ2 or
t-test value | P-value |
|---|
| Age (years) | 18.6±10.1 | 19.1±10.3 | 0.261 | 0.795 |
| Sex, no. (%) |
|
| 0.004 | 0.947 |
| Male | 33 (53.23) | 28 (53.85) |
|
|
|
Female | 29 (46.77) | 24 (46.15) |
|
|
| BMI
(kg/m2) | 22.26±0.37 | 22.21±0.25 | 0.828 | 0.409 |
| Marital status, no.
(%) |
|
| 0.001 | 0.981 |
|
Married | 13 (20.97) | 11 (21.15) |
|
|
|
Unmarried | 49 (79.03) | 41 (78.85) |
|
|
| Nationality, no.
(%) |
|
| 1.077 | 0.299 |
| Han | 55 (88.71) | 49 (94.23) |
|
|
|
Minority | 7 (11.29) | 3 (5.77) |
|
|
| Place of residence,
no. (%) |
|
| 0.129 | 0.719 |
| Cities
and towns | 32 (51.61) | 30 (48.39) |
|
|
|
Countryside | 30 (48.39) | 32 (51.56) |
|
|
| Smoking history,
no. (%) |
|
| 0.500 | 0.480 |
|
Yes | 11 (17.74) | 12 (23.08) |
|
|
| No | 51 (82.26) | 40 (76.92) |
|
|
| Alcohol consumption
history, no. (%) |
|
| 1.273 | 0.259 |
|
Yes | 28 (45.16) | 29 (55.77) |
|
|
| No | 34 (54.84) | 23 (44.23) |
|
|
| Exercise habits,
no. (%) |
|
| 1.433 | 0.231 |
|
Yes | 30 (48.39) | 31 (59.62) |
|
|
| No | 32 (51.61) | 21 (40.38) |
|
|
Expression levels of Notch1 and HES1
in osteosarcoma patients before and after surgery
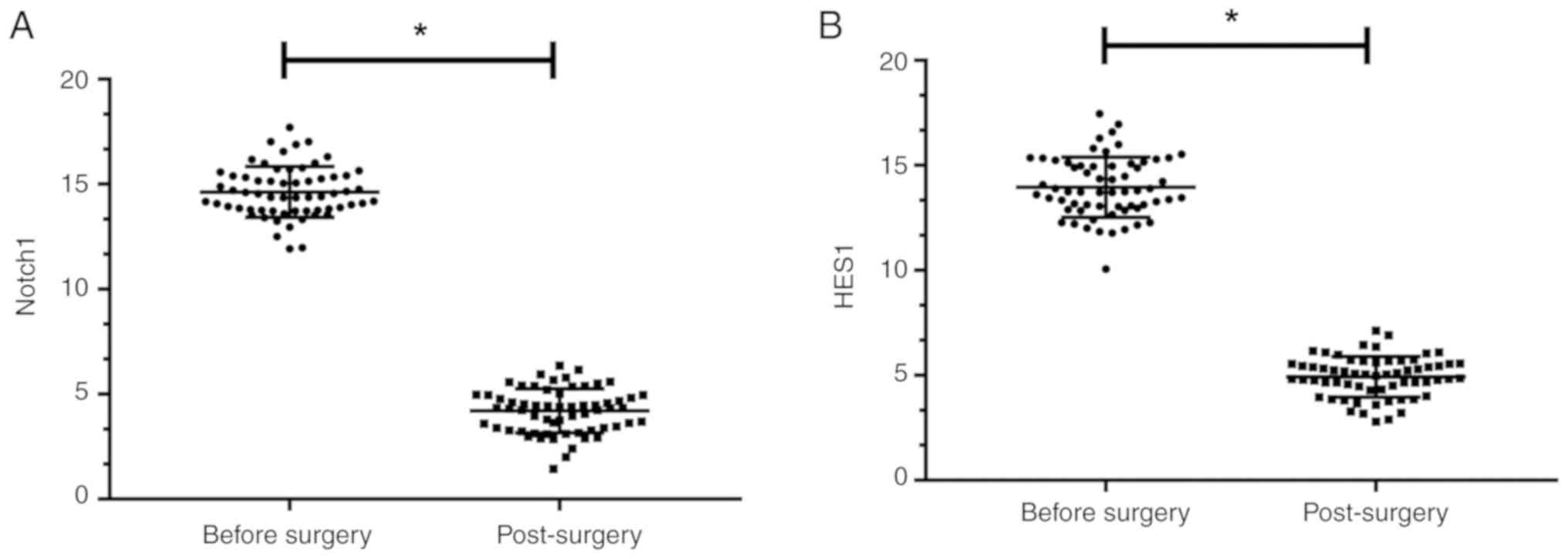
The expression levels of Notch1 and HES1 in the
osteosarcoma patients before surgery were 15.03±1.35 and
13.86±1.53, while the expression levels of Notch1 and HES1 in the
osteosarcoma patients after surgery were 4.12±1.01 and 5.02±0.99,
respectively. Significant differences were observed in the
comparisons of Notch1 and HES1 expression levels before and after
surgery (P<0.05), as shown in Fig.
1.
Diagnostic value of Notch1 and HES1
expression in osteosarcoma
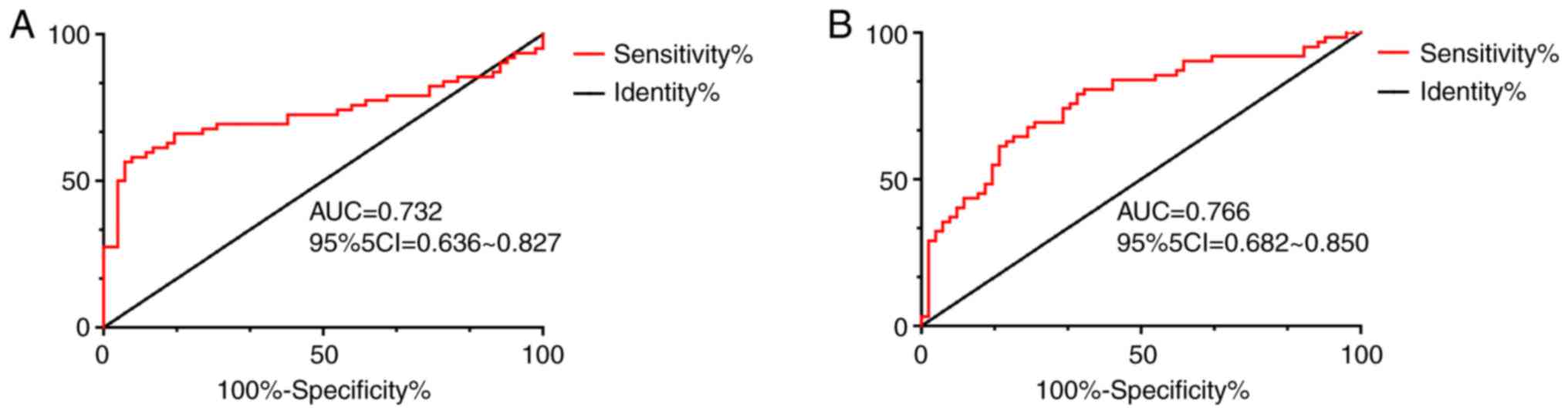
ROC curve analysis demonstrated that when the
cut-off value was 13.230, the sensitivity, specificity and AUC of
Notch1 in the diagnosis of osteosarcoma were 93.55%, 58.06% and
0.732, respectively (P<0.001); when the cut-off value was
12.810, the sensitivity, specificity and AUC of HES1 in the
diagnosis of osteosarcoma were 82.26%, 61.29% and 0.766,
respectively (P<0.001), as shown in Table II and Fig. 2.
 | Table II.ROC curve diagnosis. |
Table II.
ROC curve diagnosis.
| Item | Notch1 | HES1 |
|---|
| AUC | 0.732 | 0.766 |
| Std.Error | 0.049 | 0.043 |
| 95% CI | 0.636-0.827 | 0.682-0.850 |
| P-value | 0.001 | 0.001 |
| Cut-off | 13.230 | 12.810 |
| Sensitivity
(%) | 93.55 | 82.26 |
| Specificity
(%) | 58.06 | 61.29 |
Correlation between Notch1 and HES1
expression in patients with osteosarcoma
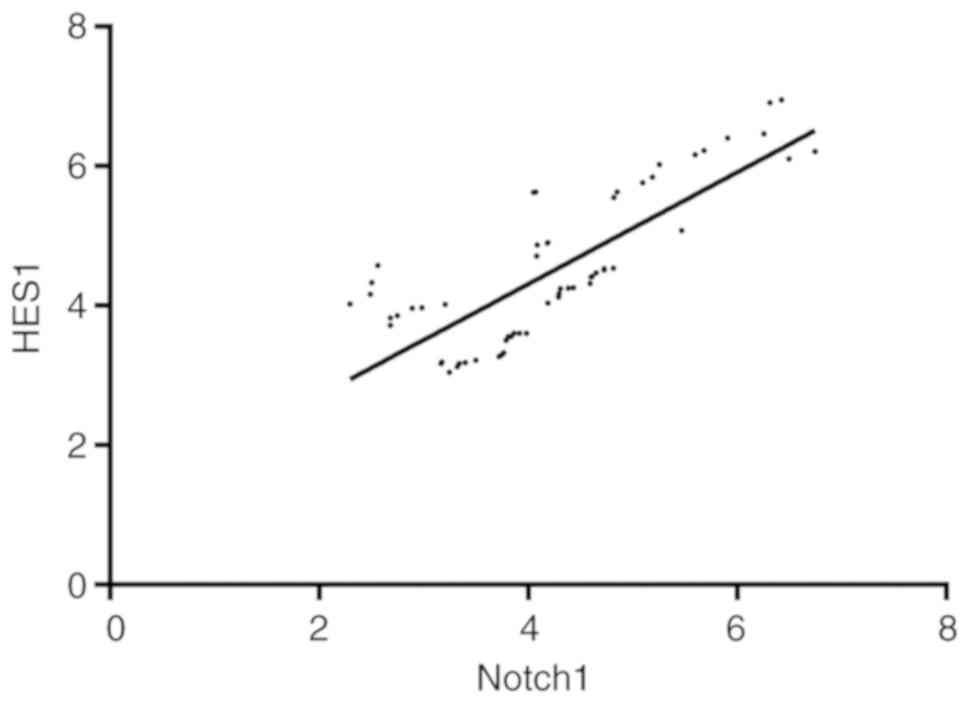
Pearson's correlation analysis identified that the
expression level of Notch1 positively correlated with that of HES1
in the osteosarcoma patients (r=0.795, P<0.001), 95% CI:
0.681-0.872, as shown in Fig. 3.
High and low expression levels of
Notch1 and HES1, and the 5-year survival rate of patients with
osteosarcoma
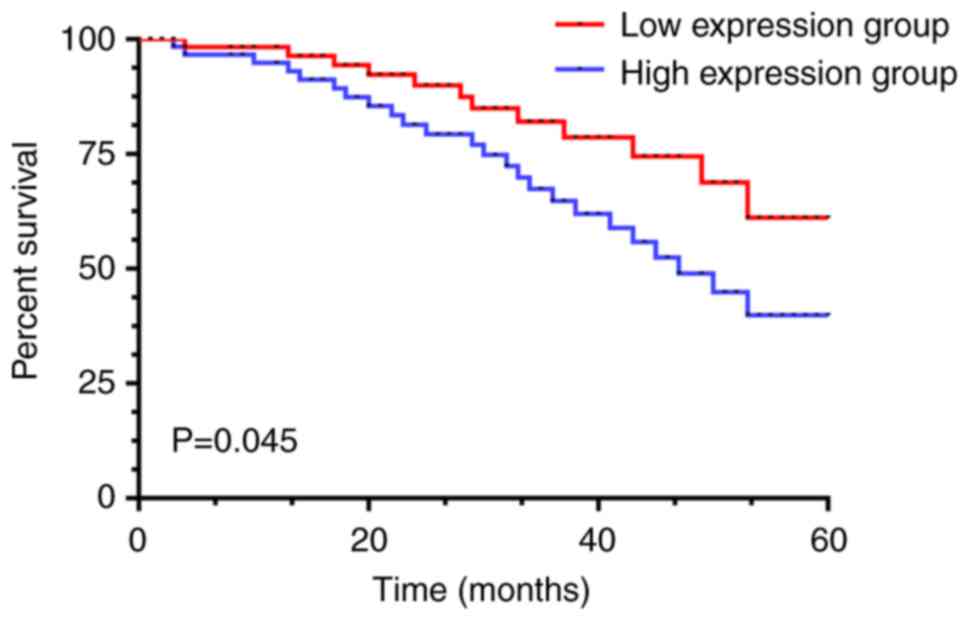
The patients in the present study were then divided
into the Notch1 high expression group (≥16.57), the HES1 high
expression group (≥15.18) (31 cases), the Notch1 low expression
group (<16.57) and the HES1 low expression group (<15.18) (31
cases) according to the median value of the expression levels of
Notch1 and HES1. All the patients were interviewed at follow-up. In
the Notch1 and HES1 low expression groups, 10 patients died, with a
5-year survival rate of 67.74%; there were 16 patients that died in
the high expression group, with a 5-year survival rate of 48.39%.
The survival rate of the patients in the low expression group was
significantly higher than that of the patients in the high
expression group (P=0.045), as shown in Fig. 4.
Univariate logistic regression
analysis
The patients were divided into the survival group
(36 cases) and the mortality group (26 cases) according to their
survival conditions. Univariate analysis based on the clinical data
of the survival group and mortality group illustrated that there
were no significant differences in age, sex and TNM staging between
the groups (P>0.05). Significant differences were observed for
in tumor location, chemotherapy response, tumor size, and Notch1
and HES1 expression (P<0.05), as shown in Table III.
 | Table III.Univariate analysis. |
Table III.
Univariate analysis.
| Clinicopathological
features | Survival group
(n=36) | Mortality group
(n=26) | χ2 or
t-test value | P-value |
|---|
| Age (years) |
|
| 0.261 | 0.610 |
|
<20 | 13 (41.94) | 15 (48.39) |
|
|
|
≥20 | 18 (58.06) | 16 (51.61) |
|
|
| Sex, n (%) |
|
| 0.272 | 0.602 |
|
Male | 20 (64.52) | 18 (58.06) |
|
|
|
Female | 11 (35.48) | 13 (41.94) |
|
|
| Tumor location, n
(%) |
|
| 5.248 | 0.022 |
|
Limbs | 10 (32.26) | 19 (61.29) |
|
|
| Not
limbs | 21 (67.74) | 12 (38.71) |
|
|
| Chemotherapy
response, n (%) |
|
| 4.239 | 0.040 |
| Adverse
reaction | 14 (45.15) | 22 (70.97) |
|
|
| Good
reaction | 17 (54.84) | 9 (29.03) |
|
|
| TNM staging, n
(%) |
|
| 0.369 | 0.544 |
| Stages
I–II | 23 (74.19) | 25 (80.65) |
|
|
| Stages
III–IV | 8 (25.81) | 6 (19.35) |
|
|
| Tumor size, n
(%) |
|
| 4.133 | 0.042 |
| ≥3
cm | 12 (38.71) | 20 (64.52) |
|
|
| <3
cm | 19 (61.29) | 11 (35.48) |
|
|
| Notch1
expression | 19.03±2.35 | 16.07±1.55 | 5.597 | 0.001 |
| HES1
expression | 16.86±1.53 | 14.02±1.32 | 7.630 | 0.001 |
Multivariate logistic regression
analysis
Multivariate difference indicators (tumor location,
chemotherapy response and tumor size) were assigned, as shown in
Table IV. Subsequently,
multivariate logistic regression analysis was performed to confirm
tumor location (OR, 3.521; 95% CI, 1.061-3.183), chemotherapy
response (OR, 5.020; 95% CI, 0.218-0.675), tumor size (OR, 3.227;
95% CI, 1.072-2.901), Notch1 expression (OR, 4.019; 95% CI,
1.467-4.218) and HES1 expression (OR, 4.629; 95% CI, 1.353-5.727).
Tumor location, chemotherapy response and tumor size, and Notch1
and HES1 expression were independent risk factors for the prognosis
of patients, as shown in Table
V.
 | Table IV.Assignment table. |
Table IV.
Assignment table.
| Factor | Assignment |
|---|
| Tumor location | Limbs,1; not limbs,
0 |
| Chemotherapy
response | Good, 1; poor,
0 |
| Tumor size | ≥3 cm, 1; <3 cm,
0 |
| Notch1
expression | Data were
continuous variables and were analyzed as original data. |
| HES1
expression | Data were
continuous variables and were analyzed as original data. |
 | Table V.Multivariate logistic regression
analysis. |
Table V.
Multivariate logistic regression
analysis.
|
|
|
|
|
|
| 95% CI of Exp
(B) |
|---|
|
|
|
|
|
|
|
|
|---|
| Factor | B | SE | Wals | Sig. | Exp (B) | Lower limit | Upper limit |
|---|
| Tumor location | 0.608 | 0.280 | 4.709 | 0.030 | 3.521 | 1.061 | 3.183 |
| Chemotherapy
response | −0.958 | 0.288 | 11.056 | 0.001 | 5.020 | 0.218 | 0.675 |
| Tumor size | 0.568 | 0.742 | 4.996 | 0.026 | 3.227 | 1.072 | 2.901 |
| Notch1
expression | 0.721 | 0.239 | 5.705 | 0.002 | 4.019 | 1.467 | 4.218 |
| HES1
expression | 0.856 | 0.423 | 5.512 | 0.031 | 4.629 | 1.353 | 5.727 |
Discussion
Osteosarcoma is one of the most common primary
malignant bone diseases, which severely threatens the health of
children and adolescents (16). It
has a high tendency of local invasion and early systemic
metastasis, such as lung metastasis (17,18). Its
morbidity rate is high, mainly among children and adolescents aged
between 10 and 25 years, whose skeleton is growing rapidly,
accounting for 70% of all osteosarcoma cases (19). Osteosarcoma has a high malignancy and
a poor prognosis. According to statistics, approximately 85% of
osteosarcoma patients exhibit metastasis (20). Chen et al (21) and Shin et al (22) demonstrated that the 5-year survival
rate of non-metastatic patients increased to 55–70% with the
application of high-dose combination chemotherapy. However, the
5-year survival rate of metastatic patients was only 5–20%.
Although the survival rate of osteosarcoma patients has improved,
there certain serious issues still exist, including severe
side-effects and recurrent or metastatic disease (23). Therefore, it is of utmost importance
to identify effective indicators for the diagnosis and prognosis of
patients with osteosarcoma.
Notch1 is an evolutionarily conserved
ligand-receptor signaling system that regulates cell proliferation,
survival, apoptosis and differentiation (24,25). The
dysfunction of the Notch1 signaling pathway may lead to abnormal
differentiation or undifferentiation, and may eventually lead to
the malignant transformation of these cells. Of note, it has been
revealed that changes in Notch1 signaling are associated with a
number of human cancers (26–28);
however, the role of Notch1 in osteosarcoma has yet not been
elucidated. HES1 is a highly conserved basic helix-loop-helix
transcription inhibitor, which mediates its biological effects by
binding to N-cassettes (CACNAG) in the entire genome and recruiting
chromatin modification factors to these sites (29,30).
HES1 is necessary for organogenesis and development of several
species as a component of Notch1 (31,32).
However, the molecular function of HES1 in adult tissues remains
unclear. Therefore, by investigating the clinical diagnostic values
of Notch1 and HES1 in osteosarcoma patients and their influence on
prognosis, this may provide the basis for the future clinical
diagnosis and treatment of osteosarcoma.
In the present study the expression levels of Notch1
and HES1 in osteosarcoma patients before and after surgery, we
first observed. It was found that Notch1 and HES1 in osteosarcoma
patients after surgery exhibited a low expression, which differed
significantly from that before surgery. This indicated that Notch1
and HES1 may become potential diagnostic and therapeutic targets
for osteosarcoma. Therefore, a ROC curve was then drawn and it was
found that the areas under the Notch1 and HES1 curves were 0.732
and 0.766, respectively, which were not associated with a high
specificity, but with a high sensitivity and were clinical
diagnostic indicators of osteosarcoma. Zhang et al (33) found a new regulatory pathway of
invasion and metastasis in osteosarcoma, as well as a novel
function of the Notch pathway: The regulation of metastasis. As the
Notch pathway can be pharmacologically inhibited, these findings
suggest possible novel therapeutic strategies with which to reduce
the invasion and metastasis of osteosarcoma. Subsequently,
Pearson's correlation analysis demonstrated that the expression
level of Notch1 positively correlated with the expression level of
HES1 in osteosarcoma patients (r=0.795, P<0.001). The patients
were further divided into the high and low expression groups
according to the median value of the expression levels of Notch1
and HES1 in osteosarcoma. Observing the 5-year survival rate of the
patients, it was found that the 5-year survival rate of the
patients in the Notch1 and HES1 high expression groups was 48.39%,
and that of the patients in the Notch1 and HES1 low expression
groups was 67.74%. The higher the expression levels of Notch1 and
HES1, the lower the survival rate of the osteosarcoma patients,
suggesting that Notch1 and HES1 may be used as prognostic survival
indicators of osteosarcoma patients. Finally, it was found that
tumor location, chemotherapy response, tumor size, and Notch1 and
HES1 expression were independent prognostic factors of patients
through logistic multivariate analysis, which indicated that tumor
location, chemotherapy response, tumor size, Notch1, HES1 and may
be used as prognostic indicators for patients with
osteosarcoma.
The present study preliminarily proved the clinical
value of Notch1 and HES1 through the above-mentioned findings.
However, there are still certain limitations to this research.
First, tissue samples were not collected and basic cell experiments
were not performed. Second, no animal experiments were conducted.
Thus, the authors aim to conduct further in-depth experimental
analyses as soon to confirm and further broaden the findings of the
present study.
In conclusion, the present study demonstrated that
Notch1 and HES1 were highly expressed in osteosarcoma patients.
Notch1 and HES1 as indicators exhibited a good diagnostic efficacy,
as shown by ROC curve analysis, and Notch1 and HES1 expression were
strongly associated with the occurrence and development of
osteosarcoma. Thus, they may prove to be efficient markers for the
diagnosis and prognosis of patients with osteosarcoma. These
findings may provide future reference and insight into future
studies on osteosarcoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LF, BL, DY and TZ were involved in the conception
and design of the study. LF, BL, DY, BW and TZ were responsible for
data collection and analysis. LF, BW and TZ were responsible for
the interpretation of the data and for drafting the manuscript. LF
and TZ made revisions from a critical perspective for important
intellectual content. All authors have read and confirmed the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shandong Cancer Hospital. Signed written informed
consents were obtained from the patients and/or parents or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlick R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Pediatric and adolescent osteosarcoma. Cancer
Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamora A, Talbot J, Bougras G, Amiaud J,
Leduc M, Chesneau J, Taurelle J, Stresing V, Le Deley MC, Heymann
MF, et al: Overexpression of smad7 blocks primary tumor growth and
lung metastasis development in osteosarcoma. Clin Cancer Res.
20:5097–5112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pece S, Serresi M, Santolini E, Capra M,
Hulleman E, Galimberti V, Zurrida S, Maisonneuve P, Viale G and Di
Fiore PP: Loss of negative regulation by Numb over Notch is
relevant to human breast carcinogenesis. J Cell Biol. 167:215–221.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Zhang J, Ma D, Zhang L, Si M, Yin H
and Li J: Curcumin inhibits proliferation and invasion of
osteosarcoma cells through inactivation of Notch-1 signaling. FEBS
J. 279:2247–2259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Diévart A, Beaulieu N and Jolicoeur P:
Involvement of Notch1 in the development of mouse mammary tumors.
Oncogene. 18:5973–5981. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garber K: Notch emerges as new cancer drug
target. J Natl Cancer Inst. 99:1284–1285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang SC, Lin XL, Wang HY, Qin YJ, Chen L,
Li J, Jia JS, Shen HF, Yang S, Xie RY, et al: Hes1 triggers
epithelial-mesenchymal transition (EMT)-like cellular marker
alterations and promotes invasion and metastasis of nasopharyngeal
carcinoma by activating the PTEN/AKT pathway. Oncotarget.
6:36713–36730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Candy PA, Phillips MR, Redfern AD, Colley
SM, Davidson JA, Stuart LM, Wood BA, Zeps N and Leedman PJ:
Notch-Induced transcription factors are predictive of survival and
5-fluorouracil response in colorectal cancer patients. Br J Cancer.
109:1023–1030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farnie G, Clarke RB, Spence K, Pinnock N,
Brennan K, Anderson NG and Bundred NJ: Novel cell culture technique
for primary ductal carcinoma in situ: Role of Notch and epidermal
growth factor receptor signaling pathways. J Natl Cancer Inst.
99:616–627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Konishi J, Kawaguchi KS, Vo H, Haruki N,
Gonzalez A, Carbone DP and Dang TP: Gamma-secretase inhibitor
prevents Notch3 activation and reduces proliferation in human lung
cancers. Cancer Res. 67:8051–8057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bielack S, Carrle D and Casali PG; ESMO
Guidelines Working Group, : Osteosarcoma: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20 (Suppl 4):S137–S139. 2009. View Article : Google Scholar
|
|
15
|
Ando K, Heymann MF, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sampson VB, Yoo S, Kumar A, Vetter NS and
Kolb EA: MicroRNAs and potential targets in osteosarcoma: Review.
Front Pediatr. 3:692015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao H, Yao Y, Wang Z, Lin F, Sun Y and
Chen P: Therapeutic effect of pirarubicin-based chemotherapy for
osteosarcoma patients with lung metastasis. J Chemother.
22:119–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He A, Yang X, Huang Y, Feng T, Wang Y, Sun
Y, Shen Z and Yao Y: CD133(+) CD44(+) cells mediate in the lung
metastasis of osteosarcoma. J Cell Biochem. 116:1719–1729. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Daw NC, Chou AJ, Jaffe N, Rao BN, Billups
CA, Rodriguez- Galindo C, Meyers PA and Huh WW: Recurrent
osteosarcoma with a single pulmonary metastasis: A
multi-institutional review. Br J Cancer. 112:278–282. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Faisham WI, Mat Saad AZ, Alsaigh LN, Nor
Azman MZ, Kamarul Imran M, Biswal BM, Bhavaraju VM, Salzihan MS,
Hasnan J, Ezane AM, et al: Prognostic factors and survival rate of
osteosarcoma: A single-institution study. Asia Pac J Clin Oncol.
13:e104–e110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Ma L and Wei G: Comment on Fu et
al.: A systematic review of p53 as a biomarker of survival in
patients with osteosarcoma. Tumour Biol. 35:5049–5050. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin SH, Jeong HJ, Han I, Cho HS and Kim
HS: Osteosarcoma and chondrosarcoma of the shoulder: Site-Specific
comparative analysis. Orthopedics. 36:e179–e185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miele L: Notch signaling. Clin Cancer Res.
12:1074–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiba S: Notch signaling in stem cell
systems. Stem Cells. 24:2437–2447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Villanueva A, Alsinet C, Yanger K, Hoshida
Y, Zong Y, Toffanin S, Rodriguez-Carunchio L, Solé M, Thung S,
Stanger BZ and Llovet JM: Notch signaling is activated in human
hepatocellular carcinoma and induces tumor formation in mice.
Gastroenterology. 143:1660–1669.e7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bocchicchio S, Tesone M and Irusta G:
Convergence of wnt and notch signaling controls ovarian cancer cell
survival. J Cell Physiol. 234:22130–22143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lai XX, Li G, Lin B and Yang H:
Interference of Notch 1 inhibits the proliferation and invasion of
breast cancer cells: Involvement of the β-catenin signaling
pathway. Mol Med Rep. 17:2472–2478. 2018.PubMed/NCBI
|
|
29
|
Takebayashi K, Sasai Y, Sakai Y, Watanabe
T, Nakanishi S and Kageyama R: Structure, chromosomal locus, and
promoter analysis of the gene encoding the mouse helix-loop-helix
factor HES-1. Negative autoregulation through the multiple N box
elements. J Biol Chem. 269:5150–5156. 1994.PubMed/NCBI
|
|
30
|
Sasai Y, Kageyama R, Tagawa Y, Shigemoto R
and Nakanishi S: Two mammalian helix-loop-helix factors
structurally related to Drosophila hairy and enhancer of split.
Genes Dev. 6:2620–2634. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishibashi M, Ang SL, Shiota K, Nakanishi
S, Kageyama R and Guillemot F: Targeted disruption of mammalian
hairy and Enhancer of split homolog-1 (HES-1) leads to
up-regulation of neural helix-loop-helix factors, premature
neurogenesis, and severe neural tube defects. Genes Dev.
9:3136–3148. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohtsuka T, Ishibashi M, Gradwohl G,
Nakanishi S, Guillemot F and Kageyama R: Hes1 and Hes5 as notch
effectors in mammalian neuronal differentiation. EMBO J.
18:2196–2207. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang P, Yang Y, Zweidler-McKay PA and
Hughes DP: Critical role of notch signaling in osteosarcoma
invasion and metastasis. Clin Cancer Res. 14:2962–2969. 2008.
View Article : Google Scholar : PubMed/NCBI
|