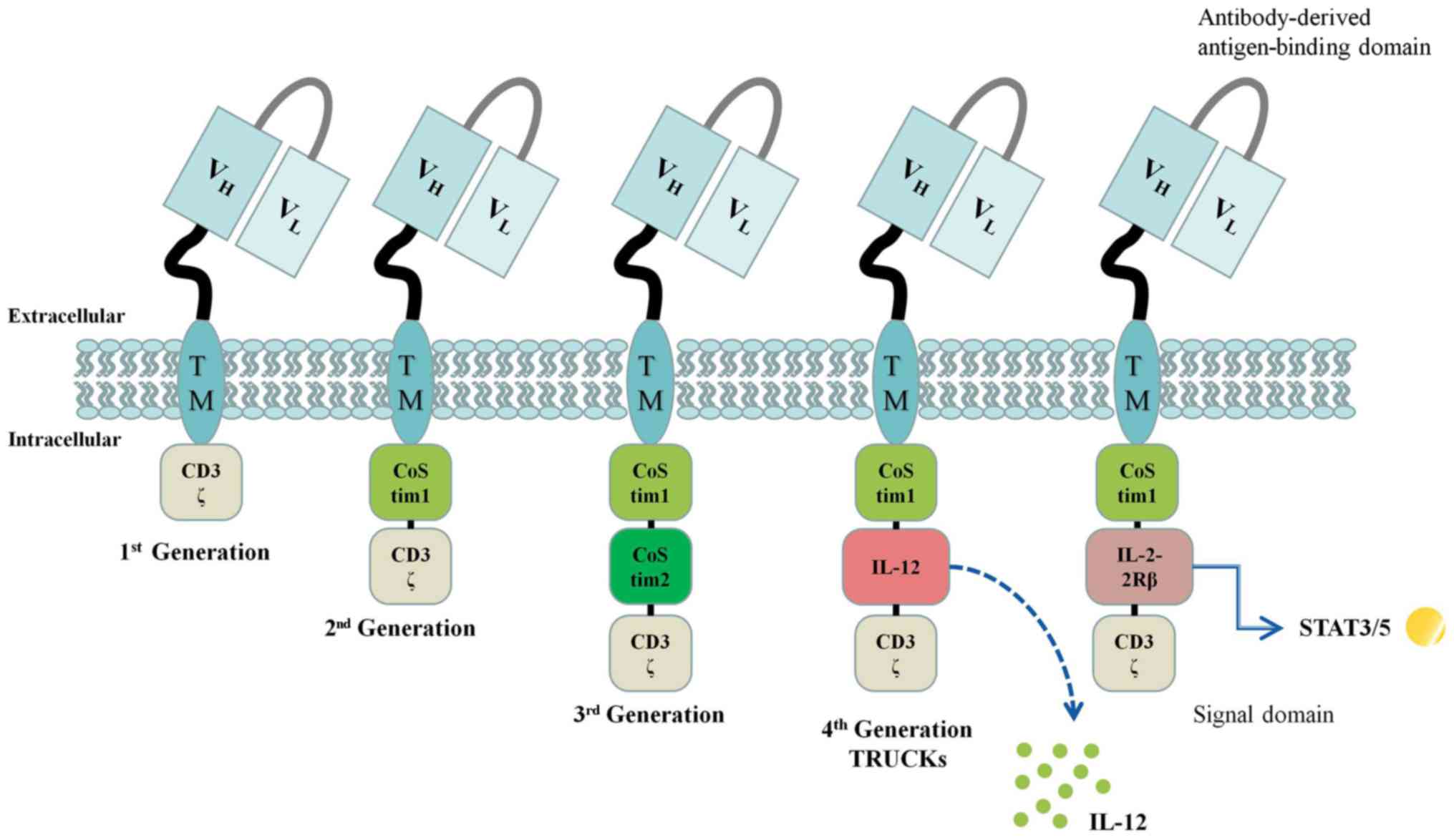
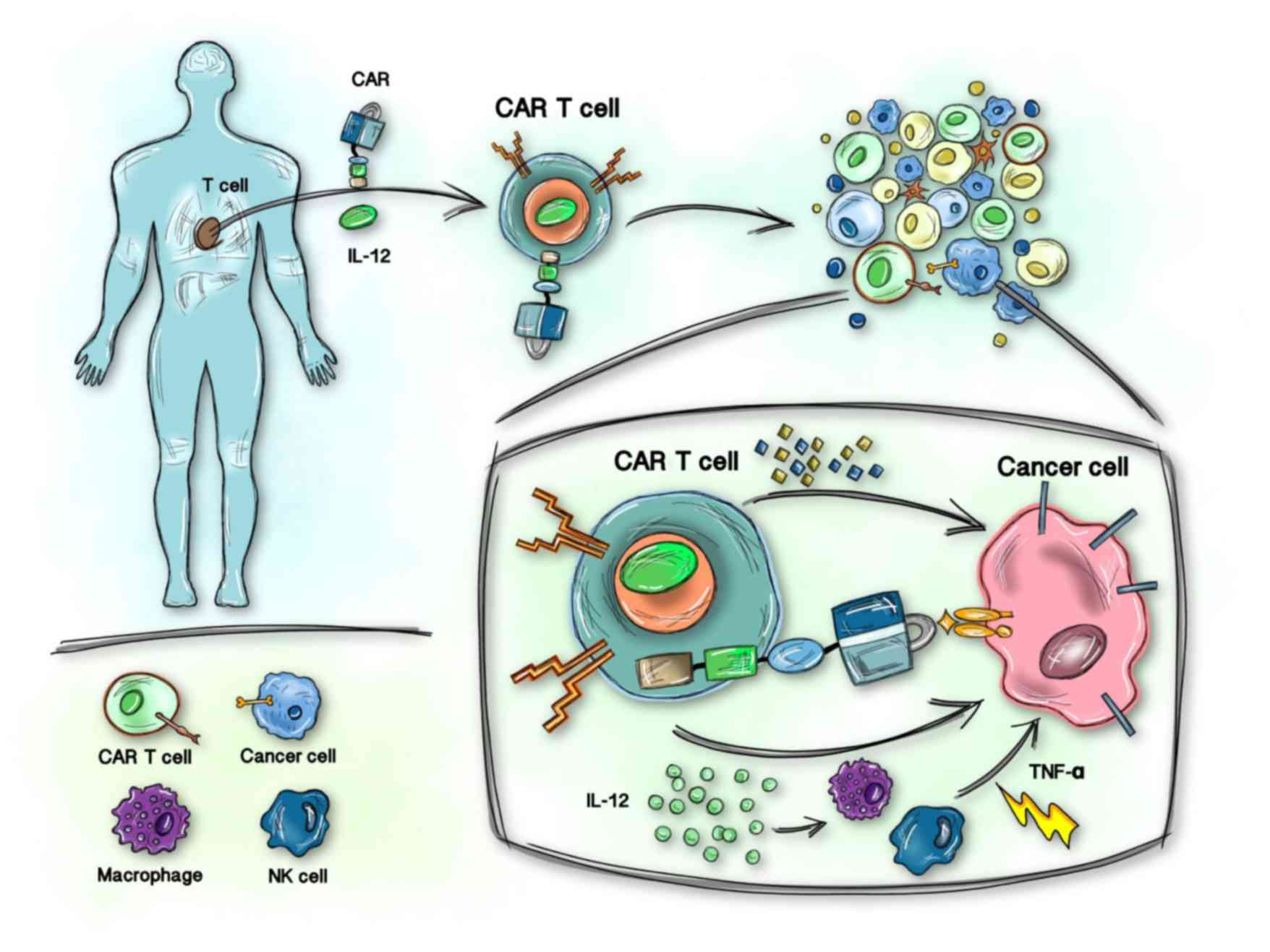
|
1
|
Katz AJ, Chia VM, Schoonen WM and Kelsh
MA: Acute lymphoblastic leukemia: an assessment of international
incidence, survival, and disease burden. Cancer Causes Control.
26:1627–1642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noone AM HN, Krapcho M, et al: SEER Cancer
Statistics Review, 1975–2015, National Cancer Institute. Bethesda,
MD: based on
November 2017 SEER data submission, posted to the SEER web site.
April. 2018
|
|
3
|
Inaba H, Greaves M and Mullighan CG: Acute
lymphoblastic leukaemia. Lancet. 381:1943–1955. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pui CH, Nichols KE and Yang JJ: Somatic
and germline genomics in paediatric acute lymphoblastic leukaemia.
Nat Rev Clin Oncol. 16:227–240. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hunger SP and Mullighan CG: Acute
lymphoblastic leukemia in children. N Engl J Med. 373:1541–1552.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maude SL, Noelle F, Shaw PA, Richard A,
Barrett DM, Bunin NJ, Anne C, Gonzalez VE, Zhaohui Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brudno JN, Somerville RP, Shi V, Rose JJ,
Halverson DC, Fowler DH, Geabanacloche JC, Pavletic SZ, Hickstein
DD, Lu TL, et al: Allogeneic T cells that express an anti-CD19
chimeric antigen receptor induce remissions of B-cell malignancies
that progress after allogeneic hematopoietic stem-cell
transplantation without causing graft-versus-host disease. J Clin
Oncol. 34:1112–1121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kebriaei P, Singh H, Huls MH, Figliola MJ,
Bassett R, Olivares S, Jena B, Dawson MJ, Kumaresan PR, Su S, et
al: Phase I trials using Sleeping Beauty to generate CD19-specific
CAR T cells. J Clin Invest. 126:3363–3376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gardner R, Wu D, Cherian S, Fang M, Hanafi
L, Finney O, Smithers H, Jensen MC, Riddell SR, Maloney DG and
Turtle CJ: Acquisition of a CD19 negative myeloid phenotype allows
immune escape of MLL-rearranged B-ALL from CD19 CAR-T cell therapy.
Blood. 127:2406–2410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turtle CJ, Hanafi LA, Berger C, Gooley TA,
Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto
TM, et al: CD19 CAR-T cells of defined
CD4+:CD8+ composition in adult B cell ALL
patients. J Clin Invest. 126:2123–2138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fry TJ, Shah NN, Orentas RJ,
Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S,
Delbrook C, Yates B, et al: CD22-targeted CAR T cells induce
remission in B-ALL that is naive or resistant to CD19-targeted CAR
immunotherapy. Nat Med. 24:20–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fuerst ML: CD22 CAR T-Cell therapy induces
remissions in young ALL patients. Oncol Times. 39:352017.
View Article : Google Scholar
|
|
13
|
Prasad V: Immunotherapy:
Tisagenlecleucel-the first approved CAR-T-cell therapy:
Implications for payers and policy makers. Nat Rev Clin Oncol.
15:11–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma F, Ho JY, Du H, Xuan F, Wu X, Wang Q,
Wang L, Liu Y, Ba M, Wang Y, et al: Evidence of long-lasting
anti-CD19 activity of engrafted CD19 chimeric antigen
receptor-modified T cells in a phase I study targeting pediatrics
with acute lymphoblastic leukemia. Hematol Oncol. 37:601–608. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai B, Guo M, Wang Y, Zhang Y, Yang J, Guo
Y, Dai H, Yu C, Sun Q, Qiao J, et al: Co-infusion of
haplo-identical CD19-chimeric antigen receptor T cells and stem
cells achieved full donor engraftment in refractory acute
lymphoblastic leukemia. J Hematol Oncol. 9:1312016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Zhang J, Wang M, Fu G, Li Y, Pei L,
Xiong Z, Qin D, Zhang R, Tian X, et al: Treatment of acute
lymphoblastic leukaemia with the second generation of CD19 CAR-T
containing either CD28 or 4-1BB. Br J Haematol. 181:360–371. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weng J, Lai P, Qin L, Lai Y, Jiang Z, Luo
C, Huang X, Wu S, Shao D, Deng C, et al: A novel generation 1928zT2
CAR T cells induce remission in extramedullary relapse of acute
lymphoblastic leukemia. J Hematol Oncol. 11:252018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao X, Yuan T, Meng JX, Jiang YY, Cao YQ,
Li Q, Sun R and Zhao MF: Analysis on poor efficacy factors in the
treatment of recurrent/refractory B-cell lymphoma with CD19 CAR-T
cells. Zhonghua Yi Xue Za Zhi. 100:593–598. 2020.(In Chinese).
PubMed/NCBI
|
|
19
|
Zhang J, Yang F, Qiu HY, Wu Q, Kong DQ,
Zhou J, Han Y and Wu DP: Anti-CD19 chimeric antigen receptors T
cells for the treatment of relapsed or refractory E2A-PBX1 positive
acute lymphoblastic leukemia: Report of three cases. Leuk Lymphoma.
60:1454–1461. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kochenderfer JN, Dudley ME, Feldman SA,
Wilson WH, Spaner DE, Irina M, Maryalice SS, Phan GQ, Hughes MS,
Sherry RM, et al: B-cell depletion and remissions of malignancy
along with cytokine-associated toxicity in a clinical trial of
anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood.
119:2709–2720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Richman SA, Nunez-Cruz S, Moghimi B, Li
LZ, Gershenson ZT, Mourelatos Z, Barrett DM, Grupp SA and Milone
MC: High-affinity GD2-specific CAR T cells induce fatal
encephalitis in a preclinical neuroblastoma model. Cancer Immunol
Res. 6:36–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Curran KJ, Pegram HJ and Brentjens RJ:
Chimeric antigen receptors for T cell immunotherapy: Current
understanding and future directions. J Gene Med. 14:405–415. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai H, Wang Y, Lu X and Han W: Chimeric
antigen receptors modified T-cells for cancer therapy. J Natl
Cancer Inst. 108:djv4392016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tokarew N, Ogonek J, Endres S, von
Bergwelt-Baildon M and Kobold S: Teaching an old dog new tricks:
Next-generation CAR T cells. B J Cancer. 120:26–37. 2019.
View Article : Google Scholar
|
|
26
|
Kenderian SS, Ruella M, Gill S and Kalos
M: Chimeric antigen receptor T-cell therapy to target hematologic
malignancies. Cancer Res. 74:6383–6389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chmielewski M, Kopecky C, Hombach AA and
Abken H: IL-12 release by engineered T cells expressing chimeric
antigen receptors can effectively Muster an antigen-independent
macrophage response on tumor cells that have shut down tumor
antigen expression. Cancer Res. 71:5697–5706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scheuermann RH and Racila E: CD19 Antigen
in Leukemia and Lymphoma Diagnosis and Immunotherapy. Leuk
Lymphoma. 18:385–397. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abate-Daga D and Davila ML: CAR models:
Next-generation CAR modifications for enhanced T-cell function. Mol
Ther Oncolytics. 3:160142016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maude SL, Laetsch TW, Buechner J, Rives S,
Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers
GD, et al: Tisagenlecleucel in children and young adults with
B-cell lymphoblastic leukemia. N Engl J Med. 378:439–448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gardner RA, Finney O, Annesley C, Brakke
H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S,
Kelly-Spratt KS, et al: Intent-to-treat leukemia remission by CD19
CAR T cells of defined formulation and dose in children and young
adults. Blood. 129:3322–3331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zuo YX, Jia YP, Wu J, Wang JB, Lu AD, Dong
LJ, Chang LJ and Zhang LP: Chimeric antigen receptors T cells for
treatment of 48 relapsed or refractory acute lymphoblastic leukemia
children: Long term follow-up outcomes. Zhonghua Xue Ye Xue Za Zhi.
40:270–275. 2019.(In Chinese). PubMed/NCBI
|
|
34
|
Barba P, Sampol A, Calbacho M, Gonzalez J,
Serrano J, Martínez-Sánchez P, Fernández P, García-Boyero R, Bueno
J and Ribera JM: Clofarabine-based chemotherapy for
relapsed/refractory adult acute lymphoblastic leukemia and
lymphoblastic lymphoma. The Spanish experience. Am J Hematol.
87:631–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang C, Zhang X and Chen XH:
Interleukin-2 priming chemotherapy: A strategy to improve the
remission of refractory/relapsed T cell acute lymphoblastic
leukemia. Med Hypotheses. 81:878–880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morgan RA, Gray D, Lomova A and Kohn DB:
Hematopoietic stem cell gene therapy: Progress and lessons learned.
Cell Stem Cell. 21:574–590. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maus MV, Grupp SA, Porter DL and June CH:
Antibody-modified T cells: CARs take the front seat for hematologic
malignancies. Blood. 123:2625–2635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mahadeo KM, Khazal SJ, Abdel-Azim H,
Fitzgerald JC, Taraseviciute A, Bollard CM, Tewari P, Duncan C,
Traube C, McCall D, et al: Management guidelines for paediatric
patients receiving chimeric antigen receptor T cell therapy. Nat
Rev Clin Oncol. 16:45–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sermer D and Brentjens R: CAR T-cell
therapy: Full speed ahead. Hematol Oncol. 37 (Suppl 1):S95–S100.
2019. View Article : Google Scholar
|
|
40
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19–28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6:224ra2252014. View Article : Google Scholar
|
|
41
|
Norelli M, Camisa B, Barbiera G, Falcone
L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C,
Cristofori P, et al: Monocyte-derived IL-1 and IL-6 are
differentially required for cytokine-release syndrome and
neurotoxicity due to CAR T cells. Nat Med. 24:739–748. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Neelapu SS, Tummala S, Kebriaei P, Wierda
W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, et
al: Chimeric antigen receptor T-cell therapy-assessment and
management of toxicities. Nat Rev Clin Oncol. 15:47–62. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu Y, Sun J, Wu Z, Yu J, Cui Q, Pu C,
Liang B, Luo Y, Shi J, Jin A, et al: Predominant cerebral cytokine
release syndrome in CD19-directed chimeric antigen
receptor-modified T cell therapy. J Hematol Oncol. 9:702016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gust J, Hay KA, Hanafi LA, Li D, Myerson
D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, et al:
Endothelial activation and blood-brain barrier disruption in
neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells.
Cancer Discov. 7:1404–1419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Acharya UH, Dhawale T, Yun S, Jacobson CA,
Chavez JC, Ramos JD, Appelbaum J and Maloney DG: Management of
cytokine release syndrome and neurotoxicity in chimeric antigen
receptor (CAR) T cell therapy. Expert Rev Hematol. 12:195–205.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chavez JC, Jain MD and Kharfan-Dabaja MA:
Cytokine release syndrome and neurologic toxicities associated with
chimeric antigen receptor T-cell therapy: A comprehensive review of
emerging grading models. Hematol Oncol Stem Cell Ther. 13:1–6.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee DW, Santomasso BD, Locke FL, Ghobadi
A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, et
al: ASTCT consensus grading for cytokine release syndrome and
neurologic toxicity associated with immune effector cells. Biol
Blood Marrow Transplant. 25:625–638. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Traube C, Silver G, Kearney J, Patel A,
Atkinson TM, Yoon MJ, Halpert S, Augenstein J, Sickles LE, Li C and
Greenwald B: Cornell Assessment of Pediatric Delirium: A valid,
rapid, observational tool for screening delirium in the PICU*. Crit
Care Med. 42:656–663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Silver G, Kearney J, Traube C and Hertzig
M: Delirium screening anchored in child development: The Cornell
Assessment for Pediatric Delirium. Palliat Support Care.
13:1005–1011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hombach AA and Abken H: Shared target
antigens on cancer cells and tissue stem cells: go or no-go for CAR
T cells? Expert Rev Clin Immunol. 13:151–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hucks G and Rheingold SR: The journey to
CAR T cell therapy: The pediatric and young adult experience with
relapsed or refractory B-ALL. Blood Cancer J. 9:102019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Porter DL, Hwang WT, Frey NV, Lacey SF,
Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, et al:
Chimeric antigen receptor T cells persist and induce sustained
remissions in relapsed refractory chronic lymphocytic leukemia. Sci
Transl Med. 7:303ra1392015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Perez EE, Orange JS, Bonilla F, Chinen J,
Chinn IK, Dorsey M, El-Gamal Y, Harville TO, Hossny E, Mazer B, et
al: Update on the use of immunoglobulin in human disease: A review
of evidence. J Allergy Clin Immunol. 139:S1–S46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Finney OC, Brakke HM, Rawlings-Rhea S,
Hicks R, Doolittle D, Lopez M, Futrell RB, Orentas RJ, Li D,
Gardner RA and Jensen MC: CD19 CAR T cell product and disease
attributes predict leukemia remission durability. J Clin Invest.
129:2123–2132. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bonifant CL, Jackson HJ, Brentjens RJ and
Curran KJ: Toxicity and management in CAR T-cell therapy. Mol Ther
Oncolytics. 3:160112016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zah E, Lin MY, Silva-Benedict A, Jensen MC
and Chen YY: T cells expressing CD19/CD20 bispecific chimeric
antigen receptors prevent antigen escape by malignant B Cells.
Cancer Immunol Res. 4:498–508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Di Stasi A, Tey SK, Dotti G, Fujita Y,
Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG,
Grilley B, et al: Inducible apoptosis as a safety switch for
adoptive cell therapy. N Engl J Med. 365:1673–1683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Howard SC, Jones DP and Pui CH: The tumor
lysis syndrome. N Engl J Med. 364:1844–1854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Minoia F, Bovis F, Davi S, Insalaco A,
Lehmberg K, Shenoi S, Weitzman S, Espada G, Gao YJ, Anton J, et al:
Development and initial validation of the macrophage activation
syndrome/primary hemophagocytic lymphohistiocytosis score, a
diagnostic tool that differentiates primary hemophagocytic
lymphohistiocytosis from macrophage activation syndrome. J Pediatr.
189:72–78.e3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Henter JI, Horne A, Arico M, Egeler RM,
Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski
J and Janka G: HLH-2004: Diagnostic and therapeutic guidelines for
hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer.
48:124–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang Z, Wu Z, Liu Y and Han W: New
development in CAR-T cell therapy. J Hematol Oncol. 10:532017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ruella M and Maus MV: Catch me if you can:
Leukemia escape after CD19-directed T cell immunotherapies. Comput
Struct Biotechnol J. 14:357–362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X,
Lin YH, Wu YN, Deng ZL, Zhang YL, Liu SH, et al: High efficacy and
safety of low-dose CD19-directed CAR-T cell therapy in 51
refractory or relapsed B acute lymphoblastic leukemia patients.
Leukemia. 31:2587–2593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sotillo E, Barrett DM, Black KL, Bagashev
A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, et
al: Convergence of acquired mutations and alternative splicing of
CD19 enables resistance to CART-19 immunotherapy. Cancer Discov.
5:1282–1295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ruella M, Xu J, Barrett DM, Fraietta JA,
Reich TJ, Ambrose DE, Klichinsky M, Shestova O, Patel PR,
Kulikovskaya I, et al: Induction of resistance to chimeric antigen
receptor T cell therapy by transduction of a single leukemic B
cell. Nat Med. 24:1499–1503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Grupp SA, Kalos M, Barrett D, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mejstríková E, Hrusak O, Borowitz MJ,
Whitlock JA, Brethon B, Trippett TM, Zugmaier G, Gore L, von
Stackelberg A and Locatelli F: CD19-negative relapse of pediatric
B-cell precursor acute lymphoblastic leukemia following
blinatumomab treatment. Blood Cancer J. 7:6592017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu X, Sun Q, Liang X, Chen Z, Zhang X,
Zhou X, Li M, Tu H, Liu Y, Tu S and Li Y: Mechanisms of relapse
after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and
its prevention and treatment strategies. Front Immunol.
10:26642019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Maude SL, Teachey DT, Rheingold SR, Shaw
PA, Aplenc R, Barrett DM, Barker CS, Callahan C, Frey NV,
Nazimuddin F, et al: Sustained remissions with CD19-specific
chimeric antigen receptor (CAR)-modified T cells in children with
relapsed/refractory ALL. J Clin Oncol. 34:30112016. View Article : Google Scholar
|
|
70
|
Orlando EJ, Han X, Tribouley C, Wood PA,
Leary RJ, Riester M, Levine JE, Qayed M, Grupp SA, Boyer M, et al:
Genetic mechanisms of target antigen loss in CAR19 therapy of acute
lymphoblastic leukemia. Nat Med. 24:1504–1506. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hamieh M, Dobrin A, Cabriolu A, van der
Stegen SJC, Giavridis T, Mansilla-Soto J, Eyquem J, Zhao Z,
Whitlock BM, Miele MM, et al: CAR T cell trogocytosis and
cooperative killing regulate tumour antigen escape. Nature.
568:112–116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zolov SN, Rietberg SP and Bonifant CL:
Programmed cell death protein 1 activation preferentially inhibits
CD28.CAR-T cells. Cytotherapy. 20:1259–1266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zou F, Lu L, Liu J, Xia B, Zhang W, Hu Q,
Liu W, Zhang Y, Lin Y, Jing S, et al: Engineered triple inhibitory
receptor resistance improves anti-tumor CAR-T cell performance via
CD56. Nat Commun. 10:41092019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zheng S, Asnani M and Thomas-Tikhonenko A:
Escape from all-CARTaz: Leukemia immunoediting in the age of
chimeric antigen receptors. Cancer J. 25:217–222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cho FN, Chang TH, Shu CW, Ko MC, Liao SK,
Wu KH, Yu MS, Lin SJ, Hong YC, Chen CH, et al: Enhanced
cytotoxicity of natural killer cells following the acquisition of
chimeric antigen receptors through trogocytosis. PLoS One.
9:e1093522014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jiang H, Li C, Yin P, Guo T, Liu L, Xia L,
Wu Y, Zhou F, Ai L, Shi W, et al: Anti-CD19 chimeric antigen
receptor-modified T-cell therapy bridging to allogeneic
hematopoietic stem cell transplantation for relapsed/refractory
B-cell acute lymphoblastic leukemia: An open-label pragmatic
clinical trial. Am J Hematol. 94:1113–1122. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Schneider D, Xiong Y, Wu D, Nlle V,
Schmitz S, Haso W, Kaiser A, Dropulic B and Orentas RJ: A tandem
CD19/CD20 CAR lentiviral vector drives on-target and off-target
antigen modulation in leukemia cell lines. J Immunother Cancer.
5:422017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ruella M, Barrett DM, Kenderian SS,
Shestova O, Hofmann TJ, Perazzelli J, Klichinsky M, Aikawa V,
Nazimuddin F, Kozlowski M, et al: Dual CD19 and CD123 targeting
prevents antigen-loss relapses after CD19-directed immunotherapies.
J Clin Invest. 126:3814–3826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ruella M, Kenderian SS, Shestova O,
Klichinsky M, Melenhorst JJ, Wasik MA, Lacey SF, June CH and Gill
S: Kinase inhibitor ibrutinib to prevent cytokine-release syndrome
after anti-CD19 chimeric antigen receptor T cells for B-cell
neoplasms. Leukemia. 31:246–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Dolnikov A, Shen S, Klamer G, Joshi S, Xu
N, Yang L, Micklethwaite K and O'Brien TA: Antileukemic potency of
CD19-specific T cells against chemoresistant pediatric acute
lymphoblastic leukemia. Exp Hematol. 43:1001–1014.e5. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lowe KL, Mackall CL, Norry E, Amado R,
Jakobsen BK and Binder G: Fludarabine and neurotoxicity in
engineered T-cell therapy. Gene Ther. 25:176–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang Y, Jiang H, Luo H, Sun Y, Shi B, Sun
R and Li Z: An IL-4/21 inverted cytokine receptor improving CAR-T
cell potency in immunosuppressive solid-tumor microenvironment.
Front Immunol. 10:16912019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ninomiya S, Narala N, Huye L, Yagyu S,
Savoldo B, Dotti G, Heslop HE, Brenner MK, Rooney CM and Ramos CA:
Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells
and is downregulated by lymphodepleting drugs. Blood.
125:3905–3916. 2015. View Article : Google Scholar : PubMed/NCBI
|