Introduction
Non-Hodgkin's intravascular large B-cell lymphoma
(IVL) is an extremely rare subgroup of diffuse large B-cell
lymphoma, accounting for only 1% of the total cases of the disease
(1). According to the latest
histological classification of lymphomas, the World Health
Organization (WHO) classified IVL as an extranodal lymphoma in 2001
(1–3). IVIBCL, together with primary effusion
lymphoma and large B cell lymphoma of the mediastinum, was
classified as a subtype of DLBCL (2,3).
According to the new classification standard (2), IVLBCL belongs to extranodal lymphoma,
which is characterized by diffuse and obliterative proliferation of
lymphoma cells in tissues, organs and lumens of small and medium
vessels (terminal arteries, veins, capillaries and blood sinusoid),
and involvement of different extranodal organs and tissues,
including the central nervous system, skin, lung, kidney, adrenal
gland and bone marrow (2–5). It does not involve lymph nodes and
peripheral blood, and has several clinical manifestations,
including fever, large liver and spleen, hemocytopenia,
disseminated intravascular coagulation and organ damage (2–5). The
reported incidence rate of men and women worldwide is ~1:1, the
median age of onset is ~60 years and the average survival time is
~6-9 months (1–5). Most patients die within 1 year, and it
is a malignant tumor with poor prognosis (1–5). IVL is
a highly invasive and extremely rare malignant hematological
disease, with poor prognosis and a lack of specificity in the
clinical setting (1,2,4). The
stable immunohistochemical expression of CD34, CD20 and PAX5 is
considered beneficial to the diagnosis and differential diagnosis
of IVL (2,3), which may occur in various organs and
tissues, though are most commonly found in the adrenal gland and
skin tissue (3). As the nomenclature
and biological characteristics of IVL remained unclear for a long
period of time, diagnosis and treatment strategies for IVL were not
standardized in the clinical setting, including imaging and
pathological methodologies (3). The
principle clinical manifestations included: Fever in 13 cases
(76.47%), nephrotic syndrome in four cases (23.53%), hypertension
in six cases (35.29%), elevated serum lactic dehydrogenase in nine
cases (52.94%), increased interleukin 6 expression in seven cases
(41.18%), tumor size ≤2 cm in five cases (29.41%) and tumor size
>2 cm in 12 cases (70.59%). Immunohistochemistry analysis
demonstrated that the positive expression rates of CD34, CD20,
paired box protein PAX5, CD10, Mum-1, Bcl-2, Bcl-6 and c-Myc in the
17 patients were 17/17, 17/17, 17/17, 8/17, 9/17, 7/17, 8/17 and
9/17, respectively. The positive expression rates of Ki-67 were
>60% in all patients, and >70% in 11 patients. No expression
was observed for CD3, CD6, granzyme B, EBER, CD113, CD33, CD117,
CKpan and HMB-45. Fluorescence in situ hybridization (FISH)
analysis demonstrated that of the 17 patients with IVL, seven cases
(41.18%) exhibited c-Myc cleavage, eight cases (47.06%) revealed
Bcl-2 cleavage, seven cases (41.18%) exhibited Bcl-6 cleavage and
16 cases (94.12%) Thus, the present study aimed to improve the
current understanding of IVL and provide an accurate basis for
clinical treatment and prognosis, via HE morphology,
immunohistochemistry, FISH detection and gene rearrangement, by
retrospectively analyzing and summarizing the clinicopathological
and pathologic features, and follow-up data of 17 patients with
IVL.
Materials and methods
Patient data
A total of 17 IVL samples (13 men and 4 women; age
range, 38–82 years; median age, 59 years; mean age, 57.2 years)
were collected following surgical resection at the Yantai
Yuhuangding Hospital (six cases), Shandong Provincial Hospital
(five cases) and the Affiliated Hospital of Qingdao University (six
cases) between January 2000 and December 2018. Diagnoses were
pathologically confirmed by three senior pathologists from the
Department of Pathology, Yantai Yuhuangding Hospital of Qingdao
University (Yantai, China), using a BX53 multi-head light
microscope (Olympus Corporation), set at magnifications of ×4, ×100
and ×200. The clinical data and general findings were acquired from
clinical medical records and specimen delivery forms. The follow-up
information was obtained by telephone, from medical record rooms in
the aforementioned hospitals and from the household registration
department of the Public Security Bureau (Yantai, Qingdao and
Jinan; China).
Immunohistochemistry (IHC)
IVL tissue samples were fixed in 4% neutral
formaldehyde for 6–48 h at room temperature, embedded in paraffin
and cut into 4-µm-thick sections, prior to staining with
hematoxylin for 90 sec at room temperature and eosin for 3 sec at
room temperature. For each case, representative wax blocks were
selected for histochemical staining, using the detailed steps of
immunohistochemistry, as follows: The sections were deparaffinized
with xylene at room temperature for 10 min and washed twice with
buffer for 3 min. The sections were incubated with hydrogen
peroxide at room temperature for 10–15 min to inhibit endogenous
peroxidase activity, washed twice with buffer for 5 min and
subsequently incubated with ultra V block (Guangzhou Anbiping
Pharmaceutical Technology Co., Ltd.) at room temperature for 5 min.
Tissue sections were re-washed twice with buffer for 5 min, prior
to incubation with primary antibody dilution (Guangzhou Anbiping
Pharmaceutical Technology Co., Ltd.) at 37°C for 1–2 h, and
subsequently washed twice with buffer for 5 min. Subsequently,
tissue sections were incubated with primary antibody enhancer
(Guangzhou Anbiping Pharmaceutical Technology Co., Ltd.) at room
temperature for 20 min, and washed twice with buffer for 5 min.
This was followed by incubation with enzyme labeled secondary
antibody (Guangzhou Anbiping Pharmaceutical Technology Co., Ltd.)
at room temperature for 30 min, and sections were subsequently
washed twice with buffer for 5 min. DAB Plus Chromogen (1-2 drops)
was added to 1 ml DAB Plus Substrate (both purchased from Guangzhou
Anbiping Pharmaceutical Technology Co., Ltd.). The mixture was
added to the slides and incubated at 37°C for 3–15 min. The slides
were flushed with distilled water for 3 min, and counterstained
with hematoxylin (10:100) for 4 min and lithium carbonate aqueous
solution (5:100) for 10 min at room temperature. Subsequently, the
sections were dehydrated with 85% alcohol, 95% alcohol and 100%
alcohol for 2 min each at room temperature. The slides were flushed
with xylene at room temperature for 2 min and subsequently mounted
onto coverslips, using mounting medium (all purchased from
Guangzhou Anbiping Pharmaceutical Technology Co., Ltd.) Cells were
observed under the BX53 multi-head light microscope (Olympus
Corporation; magnification, ×100). Antibodies against CD34 (1:200;
cat. no. MAB-0532), CD20 (1:200; cat. no. MAB-0594), PAX-5 (1:400;
cat. no. MAB-0332), CD10 (1:200; cat. no. MAB-0542), Interferon
regulatory factor 4 (IRF4; 1:200; cat. no. MAB-2232), B-cell
lymphoma 2 (Bcl-2; 1:400; cat. no. MAB-2532), B-cell lymphoma 6
(Bcl-6; 1:200; cat. no. MAB-0323), c-Myc (1:200; cat. no.
MAB-7932), Ki-67 (1:200; cat. no. MAB-1132), CD3 (1:200; cat. no.
MAB-0212), CD56 (1:400; cat. no. MAB-0582), granzyme B (GrB; 1:600;
cat. no. MAB-6332), EBER (1:200; cat. no. MAB-8232), CD13 (1:200;
cat. no. MAB-2132), CD33 (1:200; cat. no. MAB-2132), CD117 (1:200;
cat. no. MAB-9932), CKpan (1:200; cat. no. MAB-8742) and HMB-45
(1:400; cat. no. MAB-5332), and kits (cat. no. MAB-00153) were
purchased from Guangzhou Anbiping Pharmaceutical Technology Co.,
Ltd., using known positive sections as the positive control. The
experimental steps were performed according to the protocol for
each kit. CD34, CD20, CD10, CD3, cdl3, CD33, CD56 and Bcl-2 were
all membrane-positive, while Pax-5, mum-1, c-Myc, Ki-67, EBER,
cdll7 and bcl-6 were expressed in the nucleus, and GrB, CKpan and
HMB-45 in the cytoplasm.
Fluorescence in situ hybridization
(FISH)
Tissue samples were cut into 3–4 um-thick sections
and heated at 60°C for 2 h. Sections were deparaffinized with
xylene at room temperature for 10 min and incubated with 3%
H2O2 methanol solution in a humidified box at
room temperature for 15 min to inhibit endogenous peroxidase
activity, and subsequently washed three times with distilled water,
for 3 min each time. Tissue sections were incubated with proteinase
K (Guangzhou Anbiping Pharmaceutical Technology Co., Ltd.) in a
humidified box at room temperature for 15 min, and subsequently
washed three times with PBS (pH 7.4) specialized for in situ
hybridization, for 5 min each time. Sections were then washed twice
with distilled water, for 3 min each time. The pre-hybridizing
solution (40:100, Guangzhou Anbiping Pharmaceutical Technology Co.,
Ltd.) was added and the box was incubated at 38°C for 3 h.
Subsequently, hybridizing solution (40:100, Guangzhou Anbiping
Pharmaceutical Technology Co., Ltd.) was added and the wet box was
incubated at 38°C for 3 h to block the non-specific background
staining. Following removal of excess liquid, the hybridizing
liquid (40:100, Guangzhou Anbiping Pharmaceutical Technology Co.,
Ltd.) was added, the tissues were overlaid with special FISH
coverslips (Guangzhou Anbiping Pharmaceutical Technology Co.,
Ltd.), and the humidified box was incubated at 38°C overnight, then
washed twice with 2X SSC buffer (Guangzhou Anbiping Pharmaceutical
Technology Co., Ltd.) at 37°C, for 5 min each time. Sections were
subsequently washed with 0.5X SSC buffer for 15 min at 37°C, and
then re-washed twice with 0.2X SCC buffer, for 5 min each time,
prior to adding blocking buffer (Guangzhou Anbiping Pharmaceutical
Technology Co., Ltd.) at −20°C for 30 min.
c-Myc, Bcl-2 and Bcl-6 fracture probes and kits
(cat. no. MCB-00153) from Guangzhou Anbiping Pharmaceutical
Technology Co., Ltd., were used to confirm the cleavage of c-Myc,
Bcl-2 and Bcl-6. Multicopy patient samples were used as positive
controls, while normal lymph nodes were used as negative controls.
The experiment was performed according to the manufacturers'
protocols. In the case where separation of red and green signals
was >2 signal points, it was considered a cleavage. A total of
200 tumor nuclear signals were recorded in the high-power visual
field, and the ratio of isolated signaling cells to counting cells
was ≥30%, suggesting that the gene was broken. If the ratio of
isolated signaling cells to counting cells was <10%, it
suggested that the gene was not broken. When the ratio of isolated
signaling cells to counting cells was ≥10% and <30%, a further
200 counting cells were added. The ratio of isolated signaling
cells to counting cells was then recalculated and was confirmed to
be positive if ≥15%, and negative if <15%.
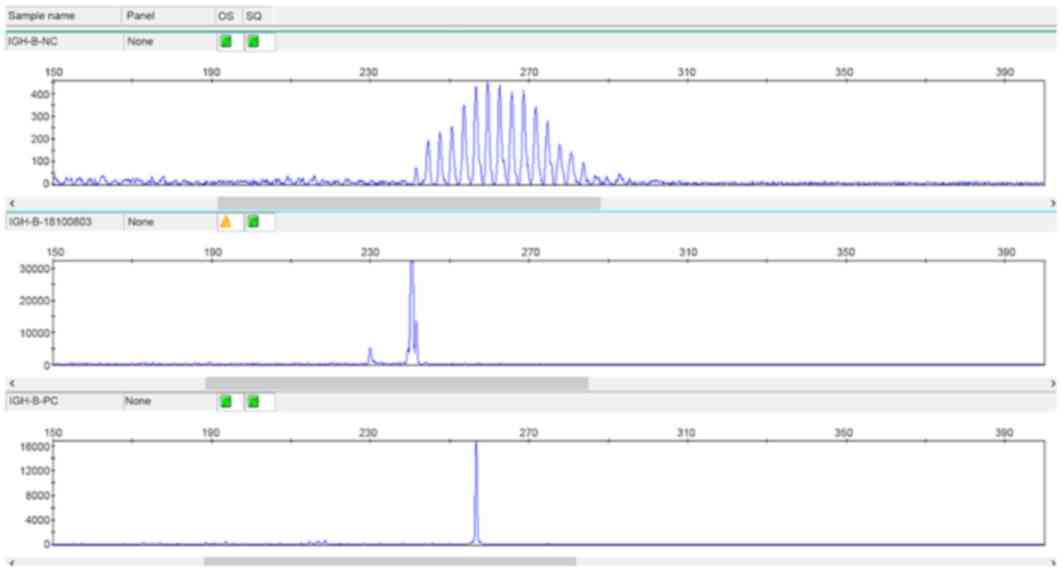
Gene rearrangement detection
DNA was extracted from the tissue samples using
nucleic acid extraction buffer (Amoy Diagnostics Co., Ltd.) to
detect gene rearrangement of the immunoglobulin heavy chain (IgH).
The detection fragments were selected according to the van Krieken
method (4) and PCR capillary
electrophoresis was performed according to the instructions of the
Gene Rearrangement Detection kit (Shanghai Yuanqi Co., Ltd.). The
following thermocycling conditions were used for PCR: Initial
denaturation at 95°C for 7 min, low temperature annealing at 60°C
for 45 sec and 72°C for 90 sec for a total of 40 cycles, and
elongation at 72°C for 10 min. The total reaction system was 20 µl
and the denatured products were analyzed via capillary
electrophoresis on the gene analyze r (room temperature extension).
Results were interpreted according to the instructions on the kit
purchased from Amoy Diagnostics Co., Ltd.
Statistical analysis
An Excel database of patients was established, and
statistical analysis was performed using SPSS software (version
17.0; SPSS, Inc.). The association between clinicopathological
features and the expression levels of Ki-67, c-Myc, Bcl-6 and Bcl-2
was evaluated using Fisher's exact test. Survival curves were
generated and the survival rate was calculated using the
Kaplan-Meier method. Multivariate Cox regression analysis was
performed and the log-rank test was used to determine the
independent risk factors affecting the survival of patients.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological data
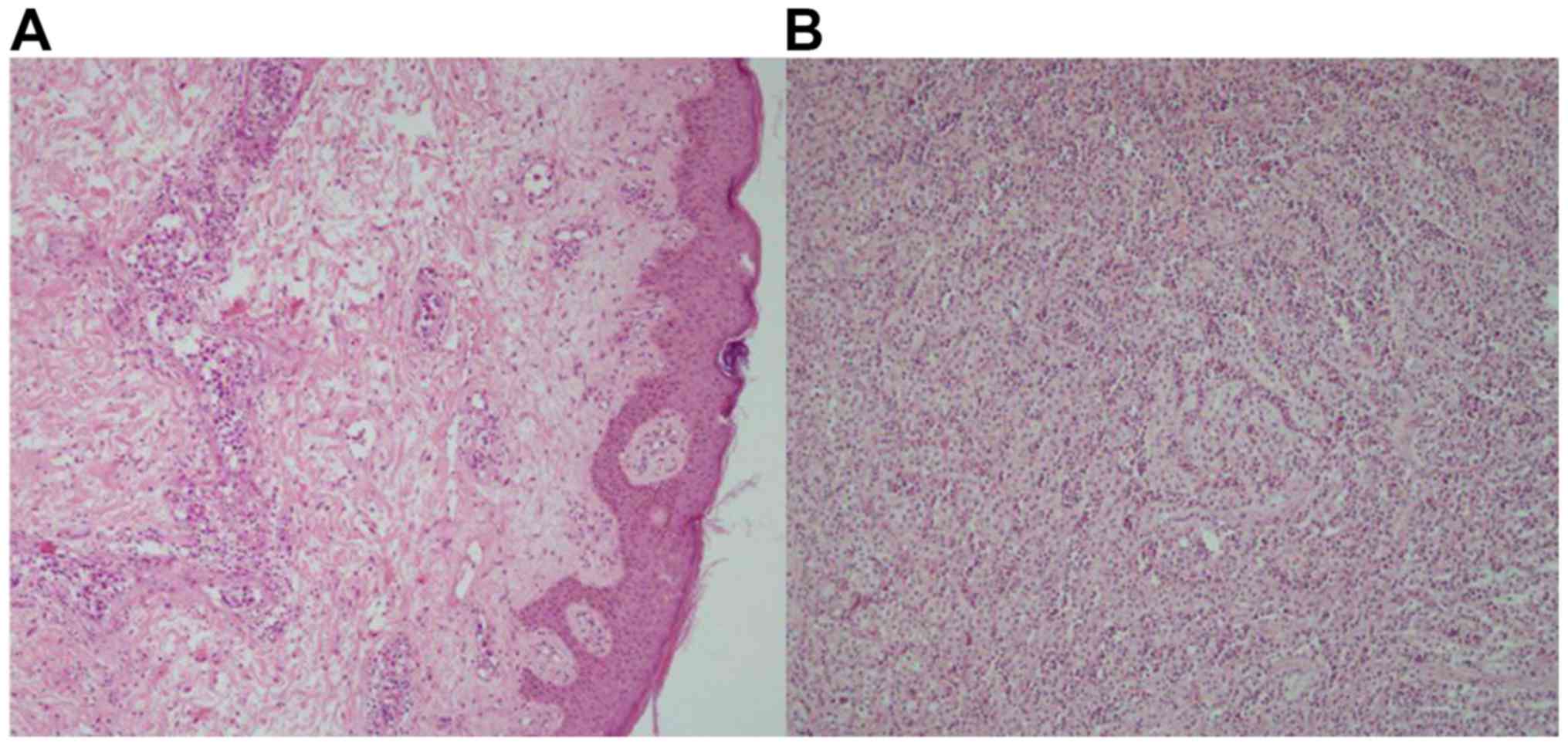
Of the 17 patients with IVL, 13 cases (76.47%)
occurred in the adrenal gland (Fig.
1A), while four cases (23.53%) occurred on the skin (Fig. 1B). The principle clinical
manifestations included: Fever in 13 cases (76.47%), nephrotic
syndrome in four cases (23.53%) and hypertension in six cases
(35.29%).
IHC analysis
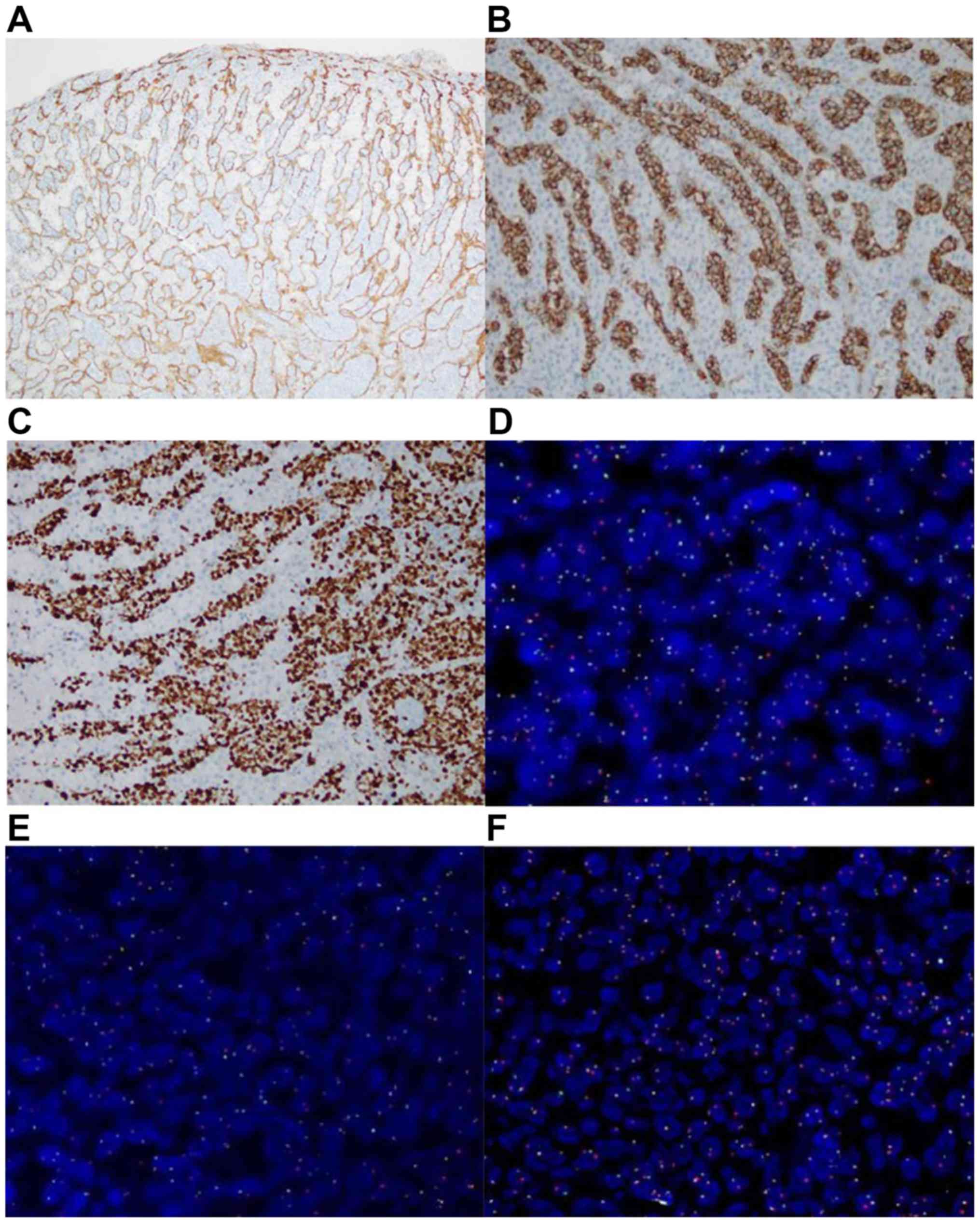
The positive expression rates of CD34 (Fig. 2A), CD20 (Fig. 2B), paired box protein PAX5 (Fig. 2C), CD10, IRF4, Bcl-2, Bcl-6 and c-Myc
in the 17 patients with IVL were 17/17, 17/17, 17/17, 8/17, 9/17,
7/17, 8/17 and 9/17, respectively. The positive expression rates of
Ki-67 were >60%. Conversely, the expression rates of CD3, CD56,
GrB, EBER, CD13, CD33, CD117, CKpan and HMB-45 were negative (data
not shown).
Molecular characteristics
FISH analysis demonstrated that of the 17 patients
with IVL, seven cases (41.18%) exhibited c-Myc cleavage (Fig. 2D), eight cases (47.06%) exhibited
Bcl-2 cleavage (Fig. 2E), seven
cases (41.18%) exhibited Bcl-6 cleavage (Fig. 2F) and 16 cases (94.12%) exhibited
positive IgH gene rearrangement (Fig.
3).
Association between Ki-67, c-Myc,
Bcl-2 and Bcl-6 expression and clinicopathological features
The association between expression levels of c-Myc,
Bcl-2 and Bcl-6 and clinicopathological features are presented in
Table I. FISH analysis demonstrated
that c-Myc exhibited statistical significance with regards to sex,
hypertension status and tumor size (all P<0.05), while the
difference in Bcl-6 expression among tumor groups with different
tumor sizes was statistically significant (P<0.05).
 | Table I.Association between expression levels
of Ki-67, c-Myc, Bcl-6 and Bcl-2 and clinicopathological features
in patients with Hodgkin's intravascular large B-cell lymphoma. |
Table I.
Association between expression levels
of Ki-67, c-Myc, Bcl-6 and Bcl-2 and clinicopathological features
in patients with Hodgkin's intravascular large B-cell lymphoma.
|
|
| Ki-67 |
| c-Myc |
| Bcl-6 |
| Bcl-2 |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Feature | Patient, n | >80 | ≤80 | P-value | Positive | Negative | P-value | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| Sex |
|
|
| 0.825 |
|
| 0.006 |
|
| 0.116 |
|
| 0.893 |
|
Female | 4 | 1 | 3 |
| 4 | 0 |
| 3 | 1 |
| 2 | 2 |
|
|
Male | 13 | 4 | 9 |
| 3 | 10 |
| 4 | 9 |
| 6 | 7 |
|
| Age, years |
|
|
| 0.331 |
|
| 0.208 |
|
| 0.208 |
|
| 0.929 |
|
≤60 | 2 | 0 | 2 |
| 0 | 2 |
| 0 | 2 |
| 1 | 1 |
|
|
>60 | 15 | 5 | 10 |
| 7 | 8 |
| 7 | 8 |
| 7 | 8 |
|
| Tumor size, cm |
|
|
| 0.582 |
|
| 0.036 |
|
| 0.001 |
|
| 0.079 |
| ≤2 | 5 | 1 | 4 |
| 4 | 1 |
| 5 | 0 |
| 4 | 1 |
|
|
>2 | 12 | 4 | 8 |
| 3 | 9 |
| 2 | 10 |
| 4 | 8 |
|
| Position |
|
|
| 0.140 |
|
| 0.682 |
|
| 0.682 |
|
| 0.200 |
| Adrenal
gland | 13 | 5 | 8 |
| 5 | 8 |
| 5 | 8 |
| 5 | 8 |
|
|
Skin | 4 | 0 | 4 |
| 2 | 2 |
| 2 | 2 |
| 3 | 1 |
|
| Nephrotic
syndrome |
|
|
| 0.022 |
|
| 0.116 |
|
| 0.452 |
|
| 0.312 |
|
Negative | 13 | 2 | 11 |
| 4 | 9 |
| 6 | 7 |
| 7 | 6 |
|
|
Positive | 4 | 3 | 1 |
| 3 | 1 |
| 1 | 3 |
| 1 | 3 |
|
| Fever |
|
|
| 0.140 |
|
| 0.452 |
|
| 0.452 |
|
| 0.200 |
|
Negative | 4 | 0 | 4 |
| 1 | 3 |
| 1 | 3 |
| 3 | 1 |
|
|
Positive | 13 | 5 | 8 |
| 6 | 7 |
| 6 | 7 |
| 5 | 8 |
|
| Hypertension |
|
|
| 0.793 |
|
| 0.001 |
|
| 0.627 |
|
| 0.064 |
|
Negative | 11 | 3 | 8 |
| 1 | 10 |
| 5 | 6 |
| 7 | 4 |
|
|
Positive | 6 | 2 | 4 |
| 6 | 0 |
| 2 | 4 |
| 1 | 5 |
|
| Bone marrow
involvement |
|
|
| 0.079 |
|
| 0.486 |
|
| 0.486 |
|
| 0.457 |
|
Negative | 9 | 1 | 8 |
| 3 | 6 |
| 3 | 6 |
| 5 | 4 |
|
|
Positive | 8 | 4 | 4 |
| 4 | 4 |
| 4 | 4 |
| 3 | 5 |
|
| Metastasis |
|
|
| 0.309 |
|
| 0.263 |
|
| 0.906 |
|
| 0.092 |
|
Negative | 10 | 2 | 8 |
| 3 | 7 |
| 4 | 6 |
| 3 | 7 |
|
|
Positive | 7 | 3 | 4 |
| 4 | 3 |
| 3 | 4 |
| 5 | 2 |
|
| Surgery |
|
|
| 0.536 |
|
| 0.309 |
|
| 0.252 |
|
| 0.707 |
| No | 12 | 3 | 9 |
| 4 | 8 |
| 6 | 6 |
| 6 | 6 |
|
|
Yes | 5 | 2 | 3 |
| 3 | 2 |
| 1 | 4 |
| 2 | 3 |
|
| Chemotherapy |
|
|
| 0.331 |
|
| 0.253 |
|
| 0.208 |
|
| 0.110 |
|
Insensitive | 15 | 5 | 10 |
| 7 | 8 |
| 7 | 8 |
| 6 | 9 |
|
|
Sensitive | 2 | 0 | 2 |
| 0 | 2 |
| 0 | 2 |
| 2 | 0 |
|
| Radiotherapy |
|
|
| 0.218 |
|
| 0.761 |
|
| 0.110 |
|
| 0.110 |
|
Insensitive | 14 | 5 | 9 |
| 6 | 8 |
| 7 | 7 |
| 6 | 9 |
|
|
Sensitive | 3 | 0 | 3 |
| 1 | 2 |
| 0 | 3 |
| 2 | 0 |
|
Association between
clinicopathological features and prognosis
The follow-up period was 7.5–24.8 months, from the
date of diagnosis of the disease (January 2019). The median
follow-up time was 13.6 months. A total of 12 patients (70.6%) died
during the follow-up period. The average survival time was 13.2
months, and the overall survival rates of the 3 years were 75.6,
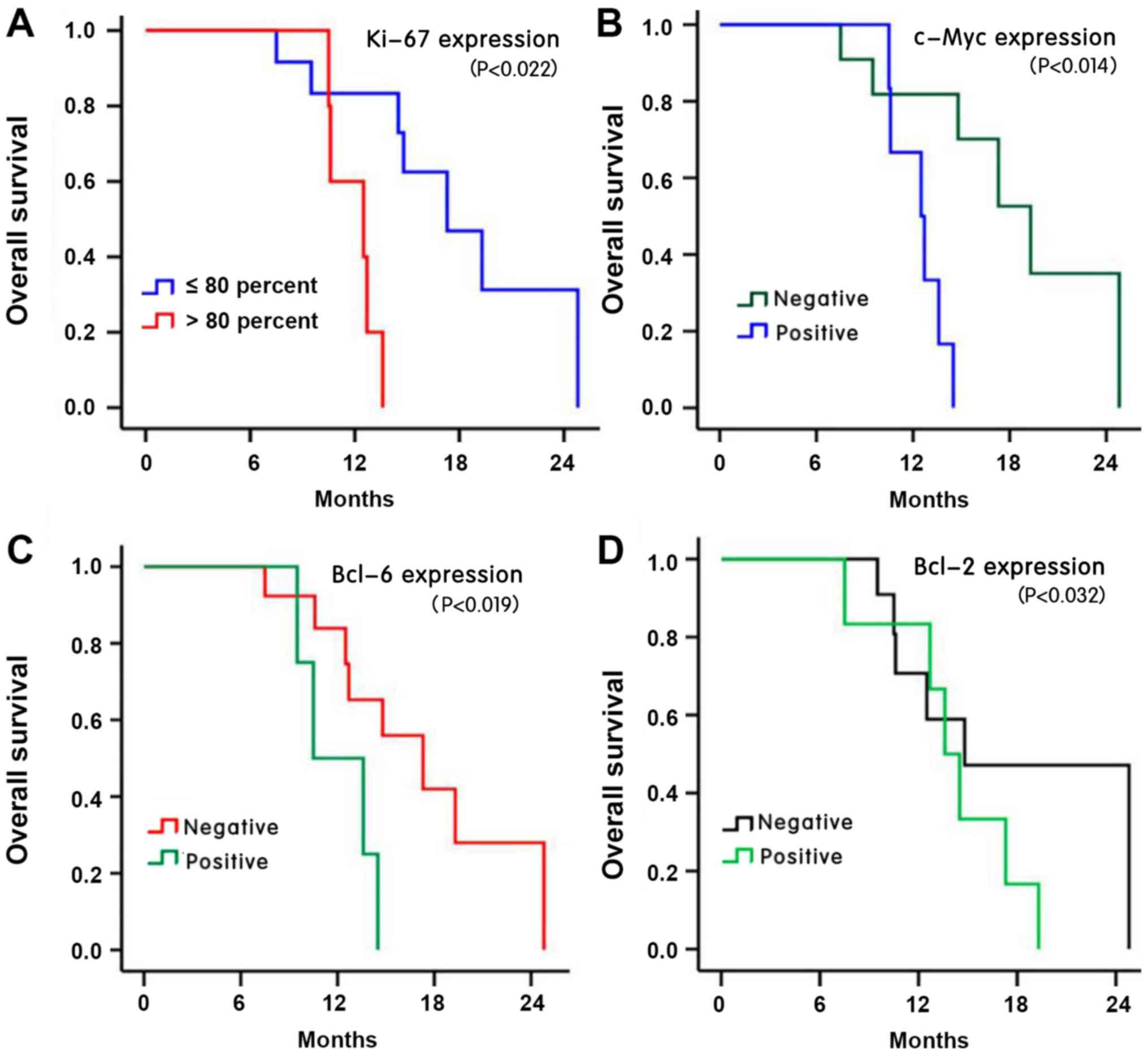
20.6 and 0.00%, respectively. The 1- and 2-year survival rates in
the low Ki-67 expression group (≤80%) were 83.3 and 31.3%,
respectively, which were significantly higher than those (60 and
0%) in the high Ki-67 expression group (>80%) (P<0.05;
Fig. 4A). The survival time of the
positive c-Myc group (the 1- and 2-year survival rates were 66.7
and 0.7%, respectively) was significantly shorter compared with the
negative group (the 1- and year 2-year survival rates were 81.80
and 35.1%, respectively) (P<0.05; Fig. 4B). The survival time of the positive
Bcl-2 group (the 1- and 2-year survival rates were 53.70 and 0.93%,
respectively) was significantly shorter compared with the negative
group (the 1- and year 2-year survival rates were 65.80 and 25.10%,
respectively). Furthermore, the survival time of the positive Bcl-6
group (the 1- and 2-year survival rates were 68.70 and 0.80%,
respectively) was significantly shorter compared with the negative
group (the 1- and year 2-year survival rates were 80.80 and 33.10%,
respectively) (P<0.05; Fig. 4C and
D). The results demonstrated no significant association between
survival time and age, sex, tumor size, tumor location, nephrotic
syndrome, fever, hypertension, metastasis, bone marrow invasion,
radiotherapy, chemotherapy and IgH rearrangement (all P>0.05;
Fig. 5A-L).
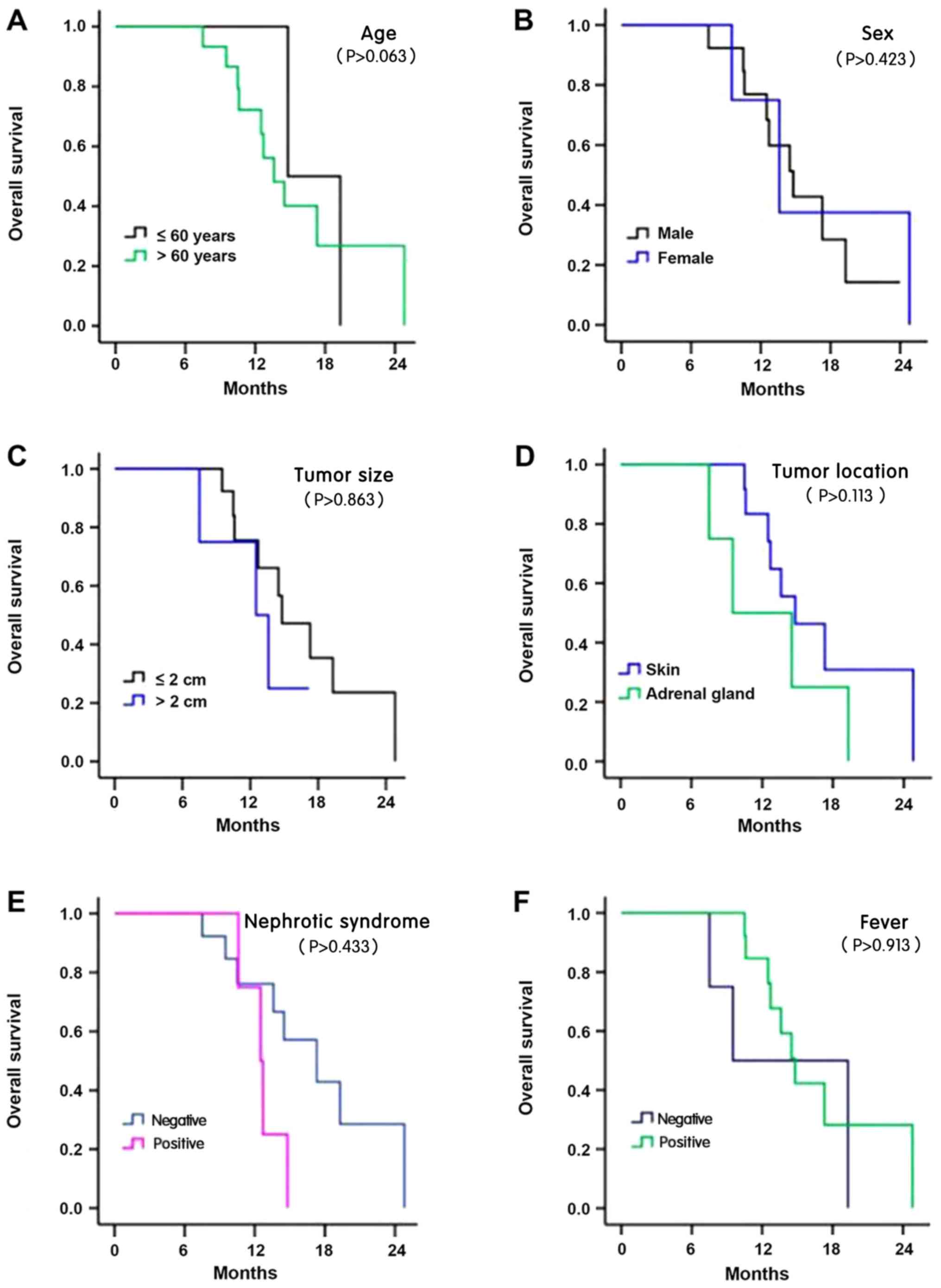 | Figure 5.Survival analysis of patients with
Hodgkin's intravascular large B-cell lymphoma. The Kaplan-Meier
curves of (A) different age, (B) sex, (C) tumor size, (D) tumor
location, and (E) nephrotic syndrome, and with or without (F)
fever. The Kaplan-Meier curves with or without (G) hypertension,
(H) metastasis, (I) bone marrow invasion, (J) radiotherapy, (K)
chemotherapyand, and (L) immunoglobulin heavy chain
rearrangement. |
Clinicopathological features
The clinicopathological features were included as
variables in the Cox regression model analysis, which demonstrated
that the prognosis of the patients was associated with the Ki-67
proliferation index, c-Myc cleavage and Bcl-6 cleavage.
Multivariate regression analysis demonstrated that the Ki-67
proliferation index was an independent risk factor for prognosis
(survival time), as presented in Table
II.
 | Table II.Univariate and Multivariate Cox
regression analysis on the prognosis of patients with Hodgkin's
intravascular large B-cell lymphoma. |
Table II.
Univariate and Multivariate Cox
regression analysis on the prognosis of patients with Hodgkin's
intravascular large B-cell lymphoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Feature | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Ki-67 proliferation
(>80 vs. ≤80) | 7.610
(1.413–40.989) | 0.018 | 7.610
(1.413–40.989) | 0.018 |
| c-Myc expression
(Positive vs. Negative) | 7.146
(1.373–37.184) | 0.019 | 20.783
(3.782–27.451) | 1.657 |
| Bcl-6 expression
(Positive vs. Negative) | 4.138
(1.022–16.747) | 0.046 | 29.126
(14.300–46.900) | 0.845 |
Discussion
IVL is a rare subtype of diffuse large B-cell
lymphoma, characterized by selective growth of lymphoma cells in
the small vascular cavity. It was first reported by Pfleger and
Tappeiner in 1959 as hemangiomatosis proliferation syndrome, and is
thought to have originated from the endothelium (1,2). In
1982, Ansell et al detected immunoglobulins on the surface
of tumor cells, suggesting that this was the origin of lymphocytes.
In 1985, leukocyte common antigen was detected on the surface of
tumor cells, and in 1986, Wick et al confirmed its
lymphoma-like characteristics. Other historical appellations
include hemangioendothelioma proliferation syndrome, malignant
hemangioendothelioma, malignant endothelial proliferation,
intravascular lymphoma, angiophilic endothelial (intravascular)
lymphoma (Kiel's classification), angiophilic large cell lymphoma
(Luke-Collins's classification) and diffuse large B-cell lymphoma
(2–4). According to the recent WHO
classification (2), IVL is a rare
subtype of extranodal diffuse large B-cell lymphoma and has
independent disease entity (2–8). Due to
the fact that proliferating tumor cells only invade the small
vessels and blood capillaries of different organs, they demonstrate
a diversity and non-specificity of clinical symptoms, and the cause
of disease still remains unclear (7–9). As
there is no lymph node enlargement, no detection of abnormal
peripheral blood and no notable abnormality in the bone marrow in
the early stages of disease, early diagnosis was difficult, and the
majority of cases were diagnosed in the late stages of disease or
during autopsy (1).
Fluorodeoxyglucose (18F-FDG) PET/CT ha been reported to
be beneficial for early diagnosis (9). As the incidence of the disease has
increased in previous years, researchers in Japan have proposed the
concept of ‘Asian variants’, based on the pathological features of
large intravascular B-cell lymphoma (8). It has previously been reported that
that the majority of tumors occur in middle-aged men, primarily
aged 40–80 years, 80% of which are >60 years of age (8). Among the 17 patients with IVL in the
present study, 13 were men and four were women, aged 38–82 years
(median age, 59 years; mean age, 57.2 years), indicating that the
male patients had a younger age of onset, which was consistent with
previous findings (7–9). A previous study demonstrated that the
majority of patients in Europe and the United States possess tumors
involved in the central nervous system and skin, which primarily
invade the kidney, lung, adrenal gland, skin and soft tissue, and
rarely involve the lymph nodes. Asian patients were dominated by
hemophilic syndrome and bone marrow invasion (10). Of the patients in the present study,
13 cases were observed in the adrenal gland, while four cases were
reported on the skin, and eight cases included bone marrow
invasion, which was consistent with previous findings (2,8). The
clinical symptoms of each patient were not uniform, obvious or
typical, and the majority of symptoms were observed following
physical examination. The final diagnoses were confirmed following
histopathological analysis. A previous study demonstrated that
there are often symptoms of fever of unknown origin, weight loss or
systemic inflammation of the whole body for a long period of time.
Disease progression results in more central system injuries,
including nephrotic syndrome, hypertension, leukopenia and
pancytopenia, increased levels of serum lactic dehydrogenase and
interleukin 6 (IL-6), recurrence and even death. Furthermore, the
diversity of clinical symptoms (alongside atypical symptoms) can
lead to delayed diagnosis (10,11). In
the present study, there were 13 cases of fever, six cases of
hypertension, four cases of nephrotic syndrome, nine cases of
increased serum lactic dehydrogenase, seven cases of increased IL-6
expression, eight cases of bone marrow invasion and seven cases of
distant metastasis. Fever, hypertension, nephrotic syndrome,
increased serum lactic dehydrogenase, increased IL-6 expression,
bone marrow invasion and distant metastasis were analyzed via
univariate, multivariate and survival analysis, and were
demonstrated to not be statistically significant. A larger sample
size is required for future studies.
In the present study Fisher's exact test was used to
determine the difference between c-Myc, Bcl-2 and Bcl-6 expression
and clinicopathological features. FISH analysis revealed that the
presence of c-Myc, regardless of sex, hypertension status or
different tumor size, Bcl-6 was also significantly different
between the groups, with regards to tumor size. Multivariate
analysis demonstrated that the Ki-67 proliferation index was an
independent risk factor for prognosis (survival time). The positive
rate of Ki-67 was >60%, among which the rates in 11 cases were
>70%, and the rates in five cases were >80%. High Ki-67
expression had certain clinical significance for the diagnosis and
prognosis of IVL. The Ki67 proliferation index of different types
of tumor has a certain guiding significance for the prognosis of
patients, including IVL (10–12). The
1- and 2-year survival rates of the low Ki-67 expression group were
83.30 and 31.30%, respectively, which was significantly higher
compared with the high expression group (60.00 and 0.00%,
respectively) To the best of our knowledge, the effect of Ki-67
expression on survival rate has not yet been investigated, thus
further verification is required by increasing the sample size. It
has been reported that when one, two or three of the FISH-c-Myc,
Bcl-2 or Bcl-6 proteins break at the same time, it suggests poor
prognosis (11–13), which is inconsistent with the results
of the present study. This may be due to the small sample size used
in the present study. Previous studies have reported that IgH heavy
chain gene clonal rearrangement was is observed in IVL tumor cells
(12,13), and chromosome translocation has also
been exhibited in a few cases (13).
Deficiency of the adhesion molecules, CD29 (B1 integrin) and CD54
(ICAM) in IVL tumor cells may be associated with their inability to
break free from blood vessels. These adhesion molecules, which are
involved in the migration of intravascular leukocytes, have been
considered a factor in intravascular localization and can assist in
diagnosis (14–17). In the present study, the
rearrangement rate of large B-cell lymphoma was >85%, while 16
cases in this group were rearranged, which was consistent with the
currently published literature. However, the molecular mechanism of
IVL generation may be multifaceted and requires further
investigation.
The present study was not without limitations.
Firstly, the sample size used was too small. However, as IVL is an
extremely rare disease it was difficult to attain sufficient
samples. Regardless, a total of 17 cases of IVL were successfully
collected, analyzed and summarized over a 10-year period, aiming to
improve the understanding of the disease, and to provide a basis
for clinical diagnosis and treatment.
IVL has a unique histopathological manifestation,
and is primarily located in small vessels of organs or tissues
(including arterioles, venules and capillaries, some of which are
in medium vessels) filled with a large number of heteromorphic
lymphocytic-like cells. The majority of tumor cells are round,
large in volume, or irregular slightly nuclei; the chromatin is
often aggregated, and small nucleolar and mitotic images can be
observed (16–18). In the present study, the tissue
originated from the adrenal gland and the skin, and the vasculature
was observed to be CD34(+), CD20(+) and PAX-5(+).
IVL is characterized by the selective proliferation
of tumor cells in the lumen of blood vessels (particularly
capillaries), the majority of which are derived from NK/T cells.
NK/T cell lymphomas account for only 10–15% of IVLs.
Morphologically, vascular dilatation, a large lumen, distribution
of mononuclear cells with large nuclei and 1–2 small nucleoli may
be observed. Many heteromorphic lymphocytic-like cells are present
(which are large with a rich cytoplasm, with round, oval or
irregular nuclei with dense chromatin) and thrombi may be present
in the vascular cavity (16–19). Immunophenotypic CD3(+), CD56(+),
GrB(+) and EBER(+) in situ hybrids have also been observed
(14). However, in the present
study, immunophenotypic CD3, CD56, GrB and EBER expression in
situ was negative.
Intravascular lymphoid retention is a benign
lymphoid lesion with chronic inflammatory changes and fibrosis that
may lead to local lymphatic compression and lymphoid retention. The
morphology of the tissue was similar to that of IVL, demonstrating
a single T cell that was immunophenotypical; however, the
heteromorphism of the cells was not notable, exhibiting isolated
lesions and no marked malignant clinical manifestations.
Immunophenotyping confirmed that the vessels were dilated lymphoid
vessels (2,12,14,17,18). The
lymphocytes were primarily T cells, and the Ki-67-positive index
was generally <10% (15–19). Ki-67 expression in all patients of
the present study was >60%, of which 11 cases were >70%. In
anaplastic large cell lymphoma or other types of DLBCL, primary
lesions in the blood infiltrate the surrounding tissues. However,
intravascular lesions are only part of the tumor, while IVL cells
only selectively proliferate in the blood vessels (11,19). The
immunophenotypes of the two can be distinguished by the
distribution of tumor cells (20,21). The
clinical manifestations of extramedullary leukemia are similar to
those of IVL, and include fever, hepatosplenomegaly and multiple
organ failure; however, the peripheral hemogram of leukemia is
abnormal, whereas the peripheral blood image of IVL is not
(16–18,20). A
variety of myeloid antigens are expressed (6,18,22);
however, no tumor-cell expression of CD33, CD34 or CDll7 was noted
in the present study. Intravascular metastases, such as metastatic
tumors and metastatic melanoma, are common primary lesions in
vascular metastasis. Immunolabelling CKpan, HMB-45 and CD20 is
useful for differential diagnosis (23–26);
however, the expression of CKpan, HMB-45 and CD20 was not
immunohistochemically identified in the present study.
In conclusion, the results of the present study
demonstrated that IVL acts invasively and that the course of the
disease progresses rapidly. The prognosis of the majority of
patients was poor; the mean survival time was 6–9 months, whereas
the survival time following non-active treatment was only 3–5
months, and the response to chemotherapy and radiotherapy was poor.
Only two of the patients were sensitive to chemotherapy, while
three cases were sensitive to radiotherapy. Previous studies have
demonstrated that a mesna/ifosfamide, mitoxantrone and etoposide
regimen is an effective treatment for recurrent and refractory
non-Hodgkin's lymphoma (13,14,19,27). It
has some advantages in improving survival outcome and the adverse
reactions of the digestive tract (26,27);
however, whether the regime is also effective against IVL has not
yet been fully investigated.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YL, YM and HZ were major contributors of data
collection, data analysis and manuscript writing. XZ and JS were
responsible for manuscript preparation, study design, data analysis
and article finalization. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Yantai Yuhuangding Hospital (Yantai, China; approval
no. 2019-42681) and written informed consent was provided by all
participants.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Khojeini EV and Song JY: Intravascular
large B-cell lymphoma presenting as interstitial lung disease. Case
Rep Pathol. 2014:9280652014.PubMed/NCBI
|
|
2
|
Sabattini E, Bacci F, Sagramoso C and
Pileri SA: WHO classification of tumours of haematopoietic and
lymphoid tissues in 2008: an overview. Pathologica. 102:83–87.
2010.PubMed/NCBI
|
|
3
|
Ferreri AJ, Dognini GP, Campo E, Willemze
R, Seymour JF, Bairey O, Martelli M, De Renz AO, Doglioni C,
Montalbán C, et al: Variations in clinical presentation, frequency
of hemophagocytosis and clinical behavior of intravascular lymphoma
diagnosed in different geographical regions. Haematologica.
92:486–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hope CB and Pincus LB: Primary cutaneous
B-cell lymphomas with large cell predominance - primary cutaneous
follicle center lymphoma, diffuse large B-cell lymphoma, leg type
and intravascular large B-cell lymphoma. Semin Diagn Pathol.
34:85–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lazzari I, Galetti C, Corvalli G, Bernardi
R, Gianotti G, Sagramoso C and Calogero P: Intravascular large
B-cell lymphoma as a cause of terminal acute respiratory distress
syndrome: atypical presentation of a rare disease. Aging Clin Exp
Res. 30:97–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murase T, Yamaguchi M, Suzuki R, Okamoto
M, Sato Y, Tamaru J, Kojima M, Miura I, Mori N, Yoshino T and
Nakamura S: Intravascular large B-cell lymphoma (IVLBCL): A
clinicopathologic study of 96 cases with special reference to the
immnophenotypic heterogeneity of CD5. Blood. 109:478–485. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimada K, Kosugi H, Narimatsu H, Shimada
S, Suzuki T, Ito M, Kinoshita T, Mori N and Naoe T: Sustainde
remission after rituximab-containing chemotherapy for intravascular
large B-cell lymphoma. J Clin Exp Hematol. 48:25–28. 2008.
View Article : Google Scholar
|
|
8
|
Chen Y, Ding C, Lin Q, Yang K, Li Y and
Chen S: Primary intravascular large B-cell lymphoma of the lung: A
review and case report. J Thorace Dis. 6:E242–E245. 2014.
|
|
9
|
Liu CL, Lai N, Zhou Y, Li S, Chen R and
Zhang N: Intravascular large B-cell lymphoma confirmed by lung
biopsy. Int J Clin Exp Pathol. 7:6301–6306. 2014.PubMed/NCBI
|
|
10
|
Kohan AA, Paganini L, Biedak P, Arma JI,
Dalurzo MC and Garcia-Monaco RD: Pulmonary intravascular lymphoma
detected by FDG PET-CT: A case report. Rev Esp Med Nucl Imagen Mol.
32:318–320. 2013.PubMed/NCBI
|
|
11
|
Bhargava P, Siddiqui F, Aggarwal B, Moore
BE and Elble RJ: A unique case of intravascular lymphoma mimicking
encephalomyeloradiculo neuropathy. Neurologist. 20:18–21. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colavolpe C, Ebbo M, Trousse D, Khibri H,
Franques J, Chetaille B, Coso D, Ouvrier MJ, Gastaud L, Guedj E and
Schleinitz N: FDG-PET/CT is a pivotal imaging modality to diagnose
rare intravascular large B-cell lymphoma: Case report and review of
literature. Hematol Oncol. 33:99–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nixon BK, Kussick SJ, Carlon MJ and Rubin
BP: Intravascular large B-cell lymphoma involving hemangiomas: An
unusual presentation of a rare neoplasm. Mod Pathol. 18:1121–1126.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cerroni L, Massone C, Kutzner H, Mentzel
T, Umbert P and Kerl H: Intravascular large T-cell or NK-cell
lymphoma: A rare variant of intravascular large cell lymphoma with
frequent cytotoxic phenotype and association with Epstein-Barr
virus infection. Am J Surg Pathol. 32:891–898. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ardighieri L, Lonardi S, Vermi W, Medicina
D, Cerroni L and Facchetti F: Intralymphatic atypical T-cell
proliferation in a cutaneous hemangioma. J Cutan Pathol.
37:497–503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baum CL, Stone MS and Liu V: Atypical
intravascular CD30+ T cell proliferation following trauma in a
healthy 17-year-old male: First reported case of a potential
diagnostic pitfall and literature review. J Cutan Pathol.
36:350–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie JL, Shi Y, Zhou XG, Jin Y, Zheng XD
and Wei XJ: Intralymphatic accumulation of lymphocytes mimicking
intravascular lymphomatosis. Zhonghua Bing Li Xue Za Zhi.
39:518–521. 2010.(In Chinese). PubMed/NCBI
|
|
18
|
Katayama K, Tomoda K, Ohya T, Asada H,
Ohbayashi C and Kimura H: Ground-glass opacities and a solitary
nodule on chest in intravascular large B-cell lymphoma. Respirol
Case Rep. 3:108–111. 2015.PubMed/NCBI
|
|
19
|
Yu H, Chen G, Zhang RX and Jin X: Primary
intravascular large B-cell lymphoma of lung: A report of one case
and review. Diagn Pathol. 7:702012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maekawa T, Kornime M, Murata S, Fukushima
N and Ohtsuki M: Random skin biopsy of patients with intravascular
large B-cell lymphoma associated with thrombocytopenia and
coagulation abnormalities: Proposal of a modified biopsy method. J
Dermatol. 42:318–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahasneh T, Harrington Z, Williamson J,
Alkhawaja D, Duflou J and Shin JS: Intravascular large B-cell
lymphoma complicated by invasive pulmonary aspergillosis: A rare
presentation. Respirol Case Rep. 2:67–69. 2014.PubMed/NCBI
|
|
22
|
Willemze R, Jaffe ES, Burg G, Cerroni L,
Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL,
Duncan LM, et al: WHO-EORTC classification for cutaneous lymphomas.
Blood. 105:3768–3785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wenxue W: Progress in diagnosis and
treatment of intravascular lymphomas. Modern Oncol. 14:1624–1627.
2006.
|
|
24
|
Chen M, Qiu B, Kong J and Chen J:
Angiotropic T cell lymphoma. Chin Med J (Engl). 111:762–764.
1998.PubMed/NCBI
|
|
25
|
Kobayashi H, Abe Y, Miura D, Narita K,
Kitadate A, Takeuchi M and Matsue K: Limited efficacy of high-dose
methotrexate in patients with neurolymphomatosis. Int J Hematol.
109:286–291. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donald JS, Barnthouse N and Chen DL: Rare
variant of intravascular Large B-cell lymphoma with hemophagocytic
syndrome. Clin Nucl Med. 43:e125–e126. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ogasawara T, Ebata N, Hamasaki J, Marshall
S, Kawauchi K, Ohshima K, Mori N and Sakura H: BCL2, BCL6, and
MYC-positive intravascular large B-cell lymphoma presenting with
bilateral adrenal gland lesions. Rinsho Ketsueki. 60:570–576.
2019.(In Japanese). PubMed/NCBI
|