Introduction
Pancreatic adenocarcinoma (PAAD) is a type of
malignant tumor that primarily originates from ductal
adenocarcinomas of the glandular epithelium, and causes 331,000
deaths worldwide every year due to there being few symptoms before
the disease reaches an advanced stage. Therefore, PAAD has a poor
prognosis and a high relative incidence rate (1). Data between 1974 and 2013 has
demonstrated that the overall increase in the incidence of PAAD is
associated with risk factors such as smoking, obesity and diabetes
in the USA (2). In recent decades
the proportion of patients with metastatic PAAD with survival time
>1 year has increased significantly, but the proportion of
deaths within 2 months is still considerable (50.6%; P<0.001)
(3). The median overall survival
(OS) is ~16 months after resection in patients with non-metastatic
pancreatic ductal adenocarcinoma, but the resectability rate is
also low, <10% (4). Despite the
common use of surgical resection and adjuvant or palliative
chemotherapy, improvements in OS are negligible among all patients,
as the majority of patients only receive supportive treatment, and
most preoperative chemoradiotherapy is associated with high
postoperative morbidity and mortality (5,6).
Therefore, novel effective diagnosis and treatment options are
still required.
Previous studies have demonstrated that drugs that
inhibit specific targets may improve the therapeutic effectiveness
and overcome the resistance of pancreatic cancer to the majority of
standard therapies (7–9). The expression of RNA binding protein in
PAAD cells has been demonstrated to change the expression of mRNA,
subsequently altering the entire transcriptome and proteome, which
suggests that the gene regulatory mechanism monitors the
proto-oncogenic signaling pathway (10,11). As
the currently published research is limited by small sample sizes,
application of different technology platforms, constant discovery
of novel mRNAs and different methods of processing and analyzing
data, the common disadvantage in mRNA expression profiling research
is a lack of consistency, resulting in non-specific and insensitive
biomarkers. Thus, there is an urgent requirement for the
identification of differential genes that may provide clues to
detect PAAD early and improve the OS time of the patients.
The Cancer Genome Atlas (TCGA; http://www.cancer.gov/) and Gene Expression Omnibus
(GEO; http://www.ncbi.nlm.nih.gov/pmc/) are open access
databases, which can be used to analyze the whole genome and
epigenome of the selected pancreatic cancer types. Using
large-scale parallel sequencing technology, it is possible to
reveal the previously unclear molecular mechanisms, determine the
tissue-specific changes and provide clues for PAAD staging,
pathological grading and drug target determination of the disease
in order to improve the current understanding of PAAD and enable
effective treatment decisions (12–14).
However, to the best of our knowledge, previous studies on PAAD
diagnostic markers have not been comprehensive or only analyzed the
genes associated with a poor prognosis (15,16).
Traditional experimental methods can only recognize a single gene
or a limited number of genes at the same time; thus, the previous
research progress on biomarkers of pancreatic cancer has not yet
been translated into a significant improvement in OS rates or a
reduction in mortality. For example, previous studies have
demonstrated that KRAS is the most common mutated gene in
pancreatic ductal adenocarcinoma (17), and KRAS mutation can be used as a
marker of pancreatic cancer (18).
However, inhibitors targeting KRAS gene have not been successful in
treatment (19).
The present study aimed to identify hub genes that
were highly associated with PAAD development and prognosis in order
to improve the current understanding of the molecular basis of PAAD
and direct new therapeutic techniques.
Materials and methods
Microarray data
The present study downloaded seven publicly
available gene expression profiles (GSE15471, GSE16515, GSE28735,
GSE32676, GSE55643, GSE62165 and GSE62452) (20–26) from
the GEO database, which met the following criteria: i) Included
human pancreas samples; ii) Contained both pancreatic cancer and
normal (or adjacent) samples; iii) The sample size was ≥30; iv) The
sample size of the case and control group was >15 samples/group.
Table I presents the details of the
seven datasets. In total, 566 samples were analyzed in the present
study.
 | Table I.Details of pancreatic cancer studies
and associated microarray data sets from the Gene Expression
Omnibus database. |
Table I.
Details of pancreatic cancer studies
and associated microarray data sets from the Gene Expression
Omnibus database.
| Author, year | Dataset | Platform | Samples, n
(tumor/control) | (Refs.) |
|---|
| Badea et al,
2008 | GSE15471 | [HG-U133_Plus_2]
Affymetrix Human Genome U133 Plus 2.0 Array | 78 (39/39) | (20) |
| Pei et al,
2009 | GSE16515 | [HG-U133_Plus_2]
Affymetrix Human Genome U133 Plus 2.0 Array | 52 (16/36) | (21) |
| Zhang et al,
2012 | GSE28735 | [HuGene-1_0-st]
Affymetrix Human Gene 1.0 ST Array [transcript (gene) version] | 90 (45/45) | (22) |
| Donahue et
al, 2012 | GSE32676 | [HG-U133_Plus_2]
Affymetrix Human Genome U133 Plus 2.0 Array | 32 (25/7) | (23) |
| Lunardi et
al, 2014 | GSE55643 | Agilent-014850
Whole Human Genome Microarray 4×44K G4112F | 53 (45/8) | (24) |
| Janky et al,
2016 | GSE62165 | [HG-U219]
Affymetrix Human Genome U219 Array | 131 (118/13) | (25) |
| Yang et al
2016 | GSE62452 | [HuGene-1_0-st]
Affymetrix Human Gene 1.0 ST Array | 130 (69/61) | (26) |
Integration of the microarray
data
The present study used the ‘limma’ package (limma:
Data analysis, linear models and differential expression for
microarray data. URL http://bioinf.wehi.edu.au/limma) of R 3.6.1 software
http://www.R-project.org/) to normalize and
log2 transform the matrix files of each GEO dataset, and
identify the GEO-DEGs between normal pancreatic tissue and
pancreatic cancer tissue in each GEO dataset. ‘RobustRankAggreg’
(RRA; http://CRAN.R-project.org/package=RobustRankAggreg)
was used to integrate the GEO-DEGs from each dataset. |log
fold-change (FC)|>1, P-value <0.05 and adjusted P-value
<0.05 were used as the thresholds of statistical significance
for the GEO-DEGs.
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analysis of the DEGs
In order to further investigate the roles of
GEO-DEGs in the development of PAAD, the present study used the
Database for Annotation, Visualization and Integrated Discovery
(DAVID; http://david.ncifcrf.gov/) to
annotate and analyze the functions of GEO-DEGs to determine the
biological processes, molecular functions, cellular components and
signaling pathways associated with these GEO-DEGs. A false
discovery rate (FDR) <0.05 was considered to indicate a
statistically significant difference.
Protein-protein interaction (PPI) and
modular analysis
A gene network can be used to analyze the
association between proteins and genes, and further clarify the
specific association between genes and diseases (27). The present study used the Search Tool
for the Retrieval of Interacting Genes/Proteins (STRING; http://string-db.org/) database, which is an online
tool for assessing PPI information, to analyze the potential
association between proteins encoded by the GEO-DEGs; the GEO-DEGs
with a minimum required interaction score >0.9 were selected,
and disconnected nodes were removed from the network. Cytoscape
3.7.2 (https://cytoscape.org/) is a type of
software that can graphically display, analyze and edit the
network, as well as add annotation information, and it was used in
the present study to complete the visualization of the PPI network
and calculate the correlation degrees of DEGs. the top 10 genes
with the highest degrees in the PPI network were regarded as key
genes. Molecular complex detection (MCODE) is a plug-in of
Cytoscape 3.7.2 that uses the inherent associations between
proteins in the network in order to identify gene clusters (highly
interconnected regions); module analysis of the PPI network was
performed using MCODE (degree cutoff=2; node score cutoff=0.2;
k-core=2 and Max. Depth=100). The functional enrichment analysis of
each module was performed using DAVID. The expression of the 10 hub
genes in PAAD and adjacent normal pancreatic tissues can be
downloaded from GEPIA database (http://gepia.cancer-pku.cn), which integrates the
relevant information of TCGA database and GTEx database (http://commonfund.nih.gov/GTEx/).
Prognostic gene signature
construction
The survival time, OS data and status of patients
with PAAD were obtained from TCGA. In the repository group of TCGA
data portal, the following steps were carried out: selection of
‘Pancreatic Ductal Adenocarcinoma’; choosing the TCGA-PAAD project
and ‘transcribe profiling’ in the ‘data category’; ‘gene expression
quantification’ in the ‘data type’ and ‘HTSeq-counts’ in the
‘workflow type’. Data were downloaded on 18th December 2019. The
data of 171 patients with PAAD were used to build a prognostic
signature by integrating gene expression and survival information.
TCGA-PAAD dataset was normalized and analyzed with the ‘edgeR’
package (https://bioconductor.org/packages/edgeR/) of the R
software. FDR<0.05 and |logFC|>2 were used as the criteria to
screen TCGA-DEGs. The expression values of DEGs in TCGA were
analyzed by univariate Cox regression, and the genes associated
with OS were determined. To further evaluate the relative
contribution of these prognostic gene markers to the survival
prediction of patients, multivariate Cox regression analysis was
constructed with the top 12 genes with P<0.05 in the univariate
analysis. A risk score model was constructed by linear combination
of the prognostic gene expression markers and their regression
coefficients (β) from the multivariate Cox proportional hazards
regression analysis as previously described (28). According to the median risk score
(1.069), patients were divided into high-risk (median risk score
≥1.069) and a low-risk (median risk score <1.069) groups. The
‘survival’ package (https://www.rdocumentation.org/packages/survival/) of
R software was used to analyze the survival of the patients in the
high- and low-risk groups. A time-dependent receiver operating
characteristic (ROC) curve was constructed using the ‘survivalROC’
package (https://cran.r-project.org/web/packages/survivalROC/index.html)
to analyze the predictive accuracy of patient OS obtained using the
risk score model.
Statistical analysis
Data are presented as mean ± SD unless otherwise
shown. The associations between gene expression levels and
clinicopathological features were analyzed by two-sided Pearson's
χ2 test using IBM SPSS version 20.0 (IBM, Corp.). The
regression analysis of univariate and multivariate Cox proportional
hazards analysis was completed using the ‘survival’ package of R
software. The Kaplan-Meier method was used to calculate the
survival rates of the patients in the low- and high-risk groups.
The P-value between two groups was obtained by log-rank test and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of DEGs
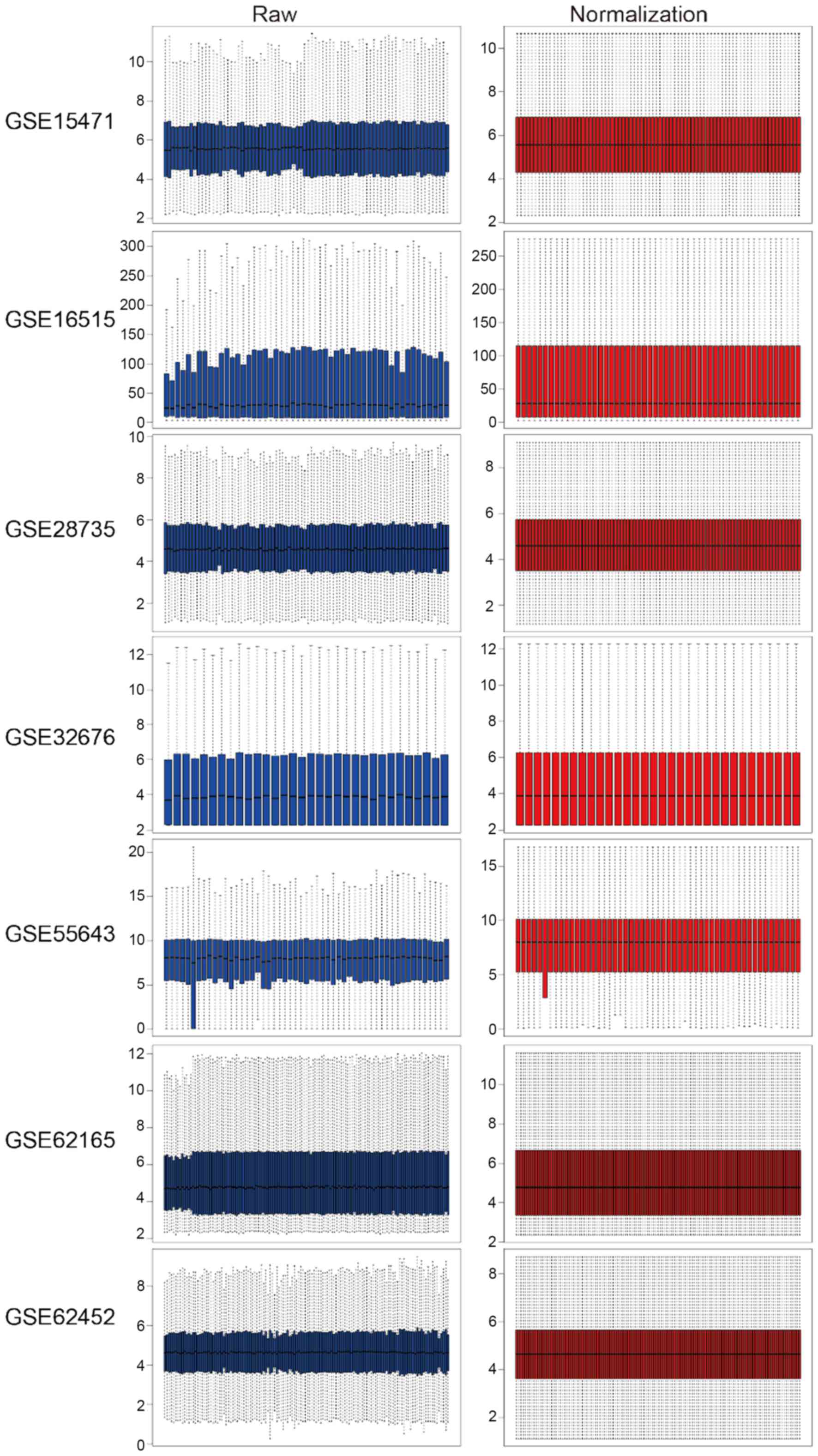
The present study downloaded the raw data and
platform information from the GEO database and reannotated them,
then normalized. The raw and normalized data are presented in
Fig. 1. Under the criteria of
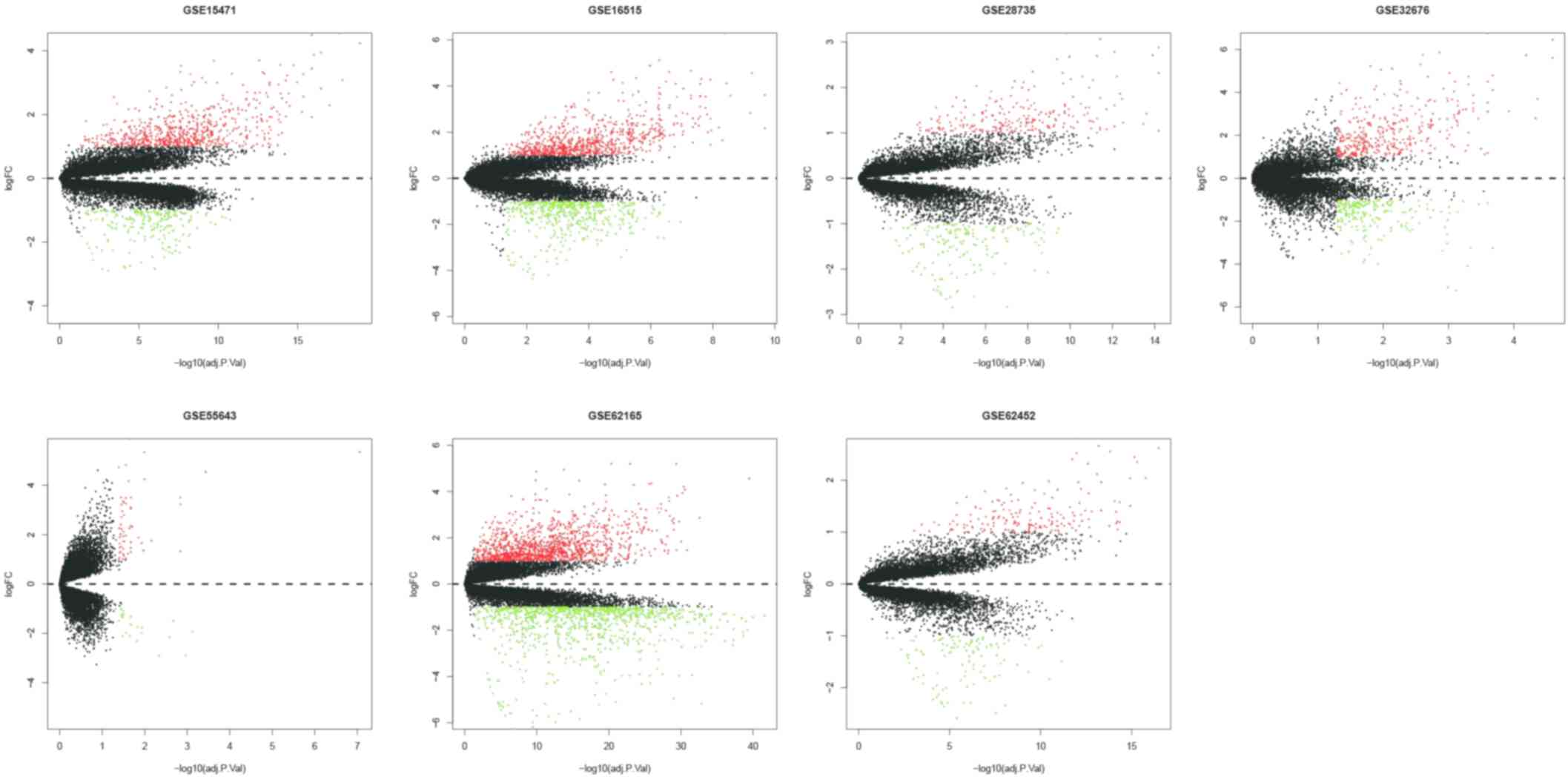
|logFC| >1, P-value <0.05 and adjusted P-value <0.05, the
‘limma’ package was used to screen GEO-DEGs, and the DEGs in each
dataset were obtained. The number of DEGs identified from each
dataset are presented in Fig. 2; a
total of 622 integrated GEO-DEGs were obtained through RRA rank
analysis, including 387 upregulated genes and 235 downregulated
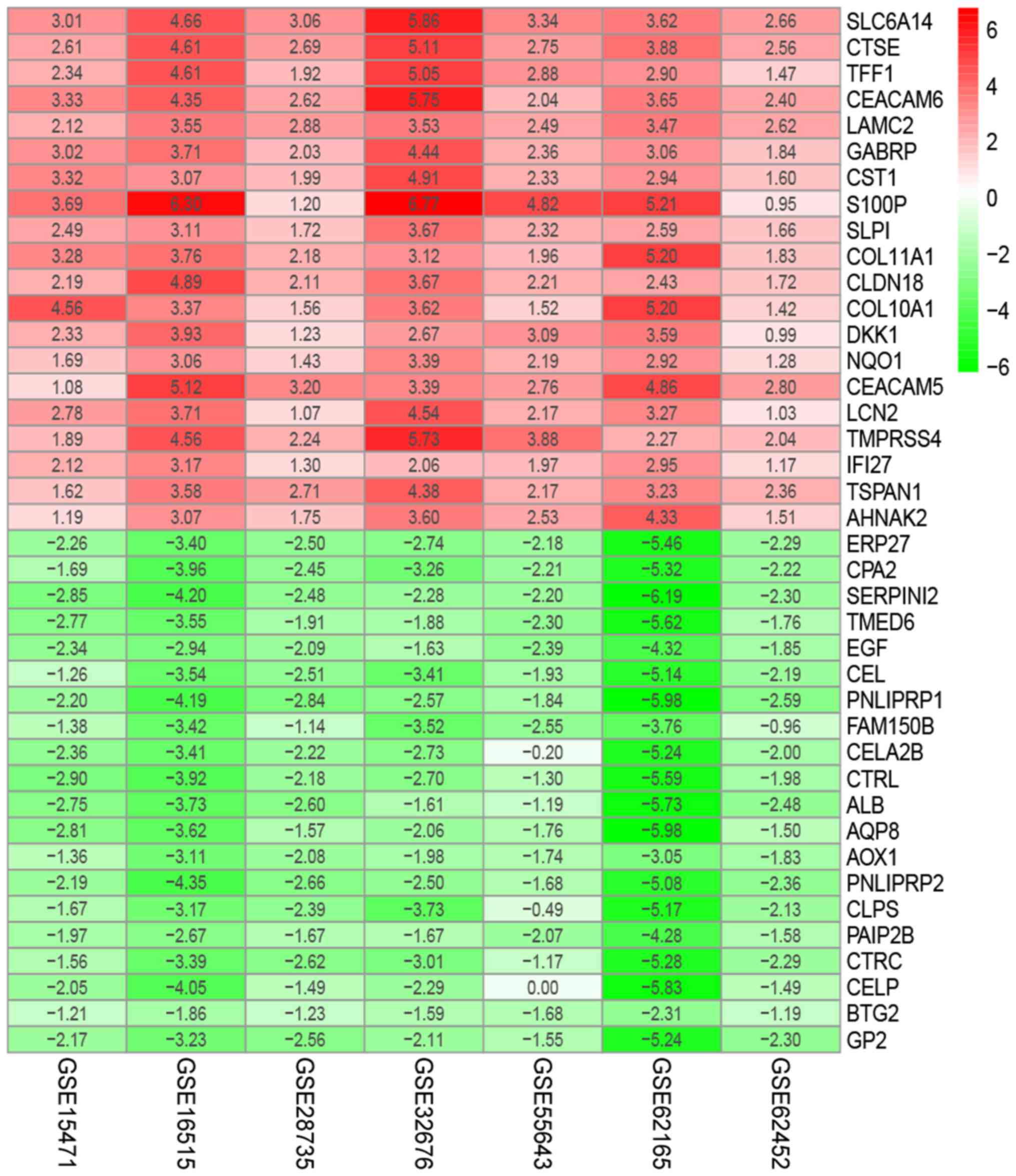
genes (Table SI). The top 20
upregulated genes and the top 20 downregulated genes are presented
in Fig. 3.
Functional enrichment analysis of the
DEGs
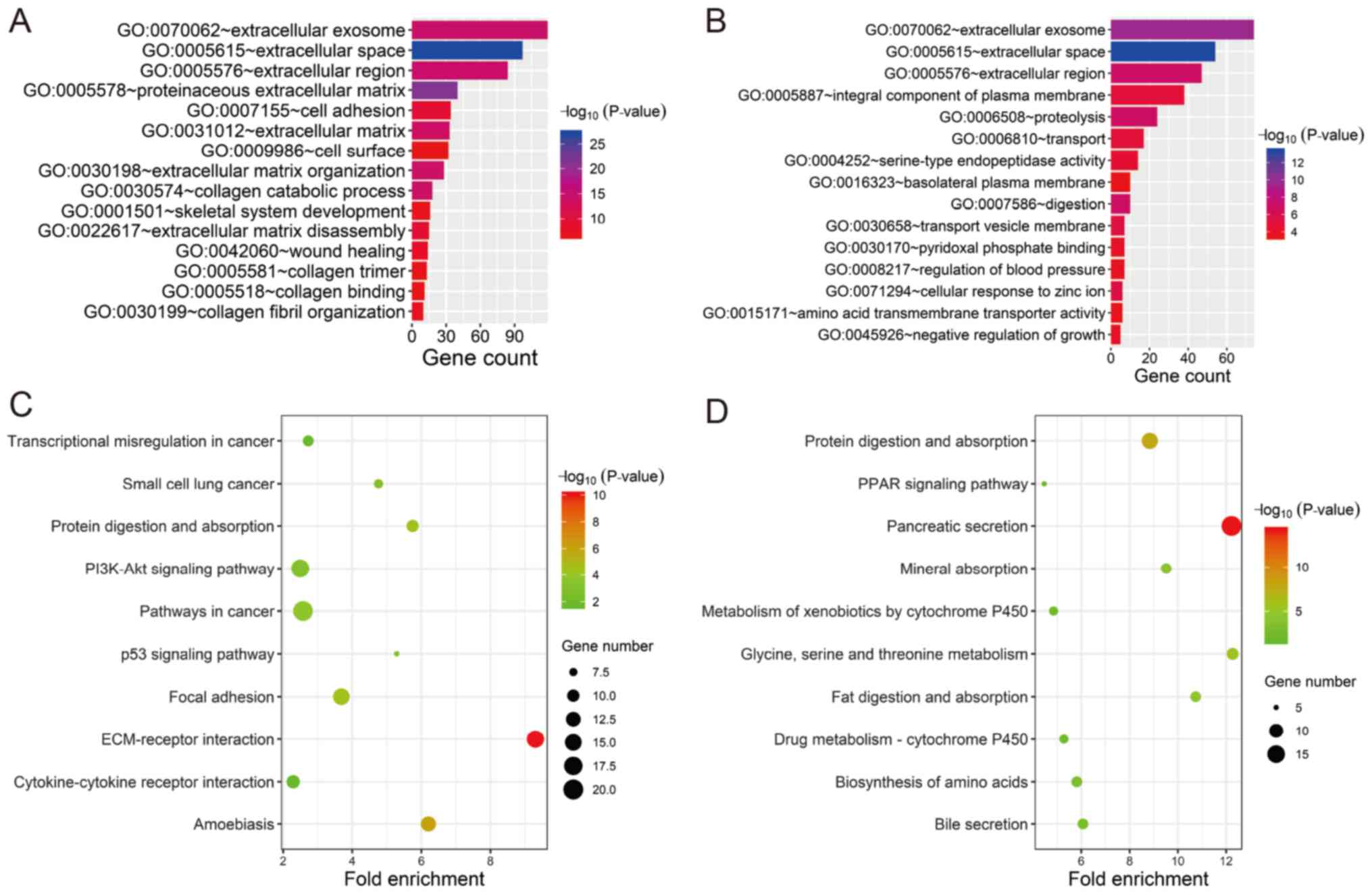
In order to investigate the potential biological
functions of the GEO-DEGs, GO term and KEGG pathway analysis was
performed (Fig. 4). The results
revealed that the 387 upregulated genes were primarily associated
with extracellular structure and composition (‘extracellular
space’, ‘proteinaceous extracellular matrix’, ‘extracellular
region’, ‘extracellular exosome’, ‘extracellular matrix
organization’, ‘extracellular matrix’ and ‘extracellular matrix
disassembly’) and collagen (‘collagen catabolic process’, ‘collagen
fibril organization’, ‘collagen binding and collagen trimer’;
Fig. 4A). The 235 downregulated
genes were primarily associated with extracellular structure and
composition (‘extracellular space’, ‘extracellular exosome’ and
‘extracellular region’) and the membrane (‘transport vesicle
membrane’, ‘integral component of plasma membrane’ and ‘basolateral
plasma membrane’; Fig. 4B). All GO
items of the GEO-DEGs are presented in Table SII. The KEGG pathway analysis
revealed that the upregulated GEO-DEGs were mainly enriched in
‘ECM-receptor interaction’, ‘Focal adhesion’, ‘Pathways in cancer’,
‘PI3K-Akt signaling pathway’ and ‘p53 signaling pathway’ (Fig. 4C), whereas the downregulated GEO-DEGs
were associated with absorption and metabolism (‘Pancreatic
secretion’, ‘Protein digestion and absorption’, ‘Glycine, serine
and threonine metabolism’, ‘Fat digestion and absorption’; Fig. 4D and Table SIII).
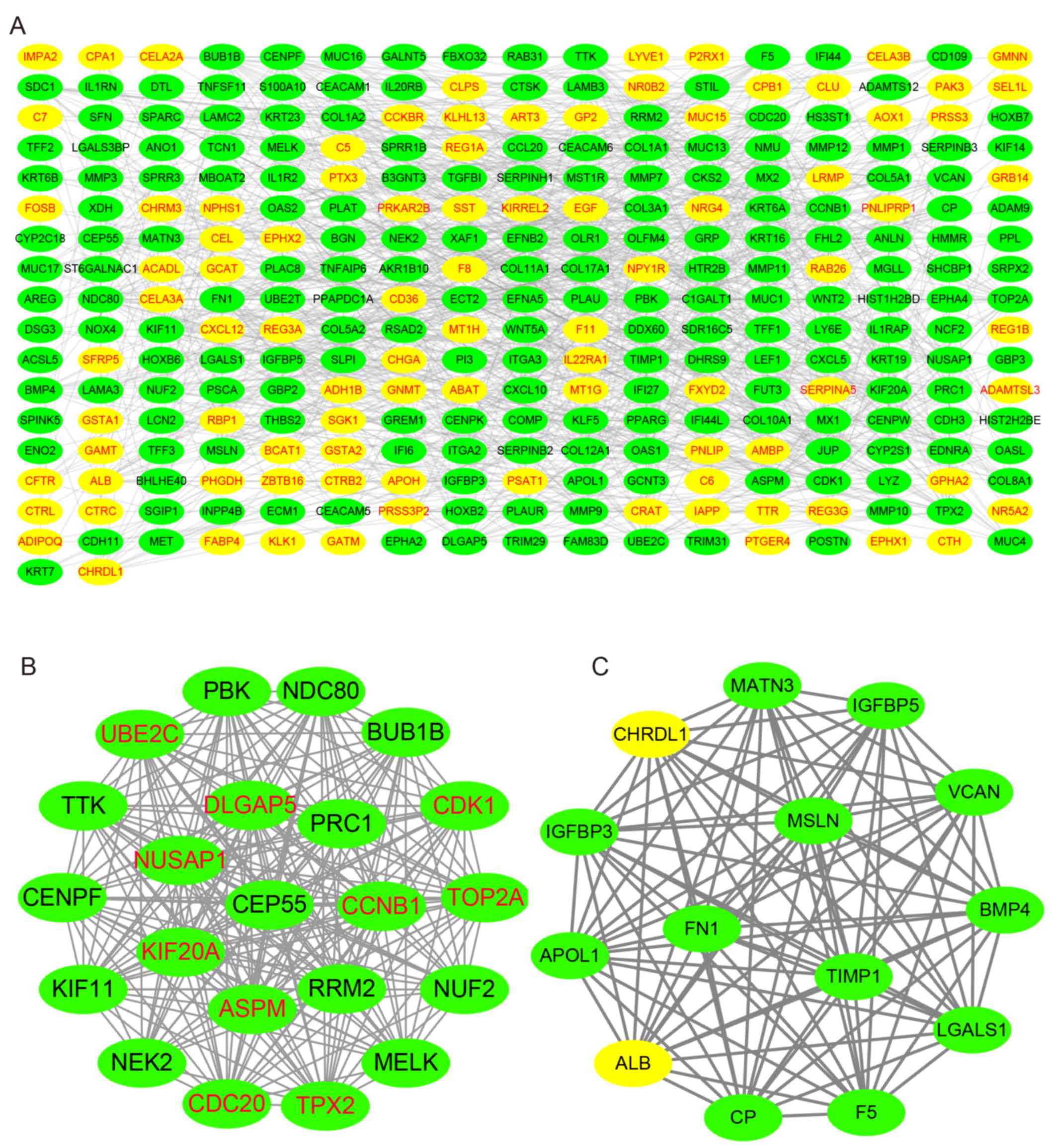
PPI network analysis
Using the STRING database, the present study
analyzed the PPI of the GEO-DEGs, and visualized the PPI network
using Cytoscape software. The PPI network included 291 nodes and
986 edges (Fig. 5A), and the top 10
nodes by degree were cyclin-dependent kinase 1 (CDK1), cyclin B1
(CCNB1), cell division cycle 20 homolog (CDC20), abnormal spindle
microtubule assembly (ASPM), ubiquitin-conjugating enzyme E2 C
(UBE2C), TPX2 microtubule nucleation factor (TPX2), DNA
topoisomerase IIα (TOP2A), nucleolar and spindle-associated protein
1 (NUSAP1), kinesin family member 20A (KIF20A) and discs large
homolog-associated protein 5 (DLGAP5), which were considered to be
hub genes. Subsequently, the present study identified the two
top-ranking modules with scores of 20.875 and 14.000 in MCODE.
Module 1 contained 22 nodes and 219 edges, and module 2 contained
14 nodes and 91 edges (Fig. 5B and
C). As presented in Fig. 6,
compared with normal tissues, the expression levels of the 10 hub
genes were significantly increased in PAAD compared with normal
tissues in TCGA cohort. Of note, all the hub genes were in module
1, which suggested that module 1 may serve an important role in the
PPI network. The KEGG analysis demonstrated that module 1 was
mainly associated with the ‘Cell cycle’ and ‘p53 signaling pathway’
(Table SIV).
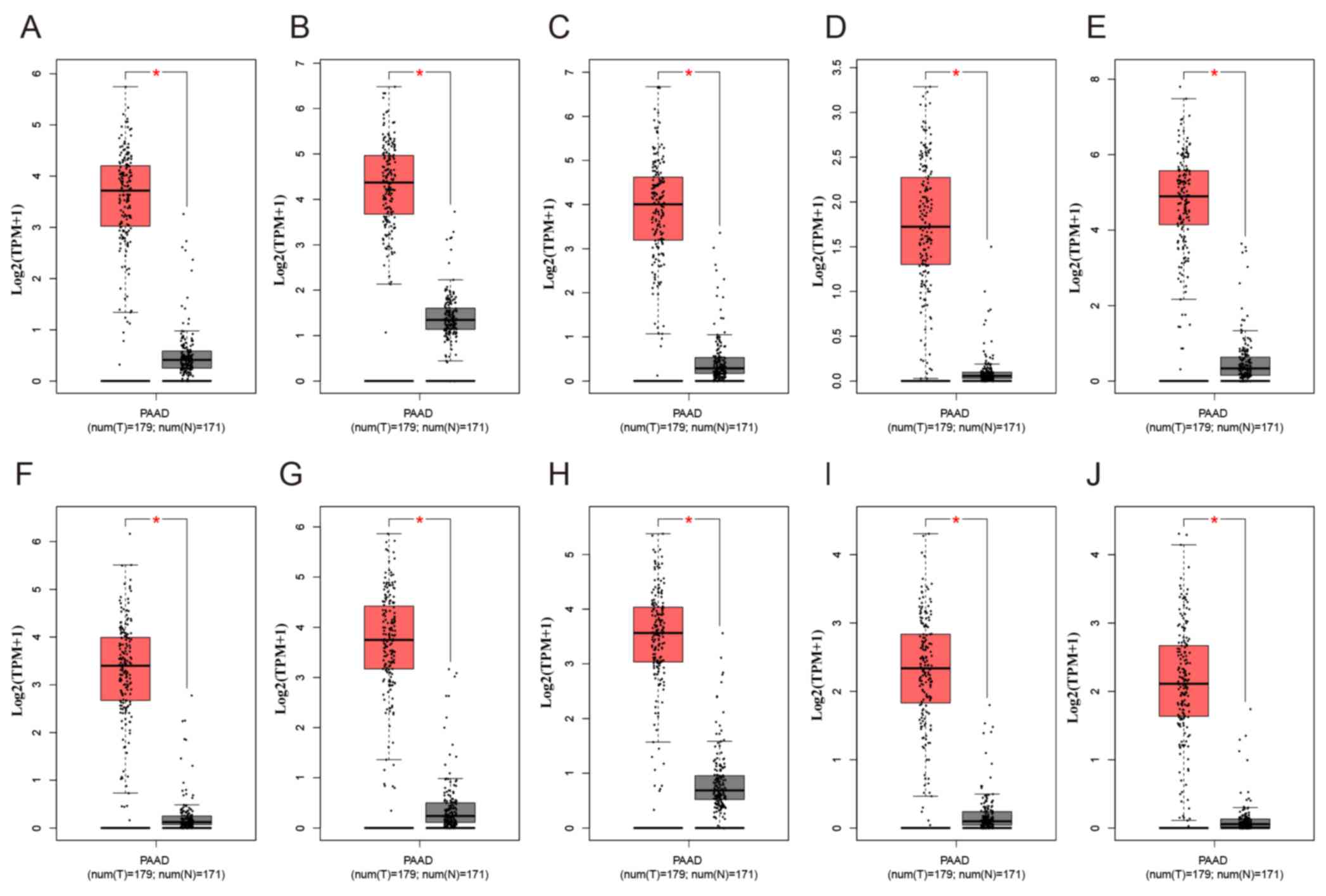 | Figure 6.Expression of the ten differentially
expressed hub genes in PAAD and normal pancreatic tissues from The
Cancer Genome Atlas and Genotype-Tissue Expression datasets.
Expression values of genes are log2-transformed. Expression of (A)
CDK1, (B) CCNB1, (C) CDC20, (D) ASPM, (E) UBE2C, (F) TPX2, (G)
TOP2A, (H) NUSAP1, (I) KIF20A and (J) DLGAP5. PAAD, pancreatic
adenocarcinoma; T, tumour; N, normal. The red boxplot represents
pancreatic tumour tissue, and the black boxplot represents normal
pancreatic tissue. *P<0.05. |
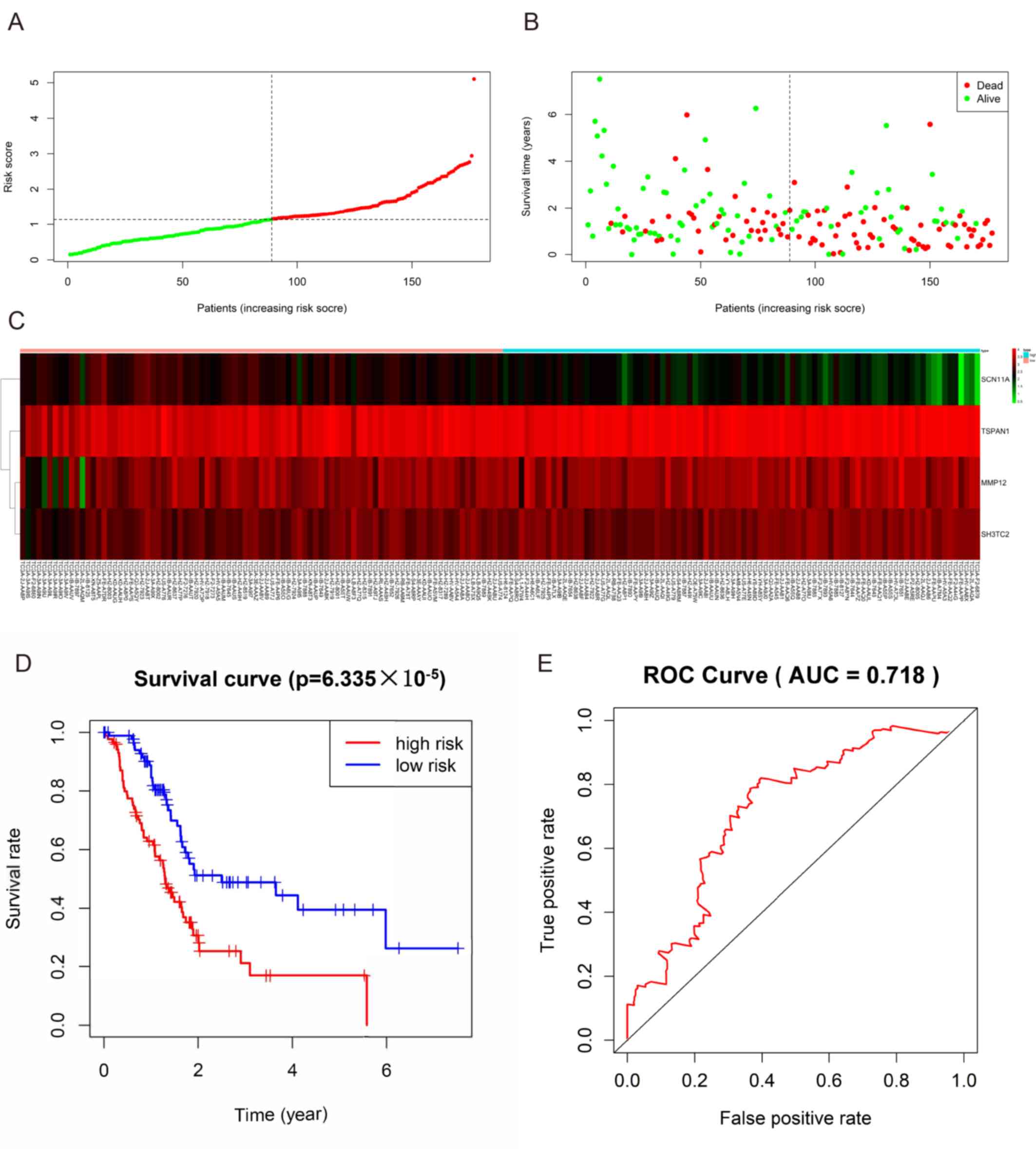
Prognostic gene signature
The present study identified 446 TCGA-DEGs from TCGA
dataset, including 26 upregulated genes and 420 downregulated genes
(Table SV). A total of 281 genes
identified using the univariate Cox regression model were
significantly associated with survival time (P<0.05; Table SVI). In addition, a prognostic gene
characteristic comprising six genes was detected through
multivariate Cox regression analysis, containing matrix
metalloproteinase 12 (MMP12), sodium voltage-gated channel α
subunit 11 (SCN11A), tetraspanin 1 (TSPAN1) and SH3 domain and
tetratricopeptide repeats-containing 2 (SH3TC2). Among them, MMP12,
TSPAN1 and SH3TC2 with hazard ratios (HRs) >1 were identified as
risk prognostic genes, whereas SCN1A with an HR <1 was
considered as a protective prognostic gene (Table II). According to the risk score
model, 86 patients were assigned into the high-risk group, and 85
patients were assigned into the low-risk group (Fig. 7A-C). The survival analysis
demonstrated that the OS rate of the high-risk group was
significantly lower compared with that of the low-risk group
(P=6.335×105; Fig. 7D).
In addition, the 1-, 3- and 5-year OS rates in the high-risk group
were significantly lower than those in the low-risk group (Table III). Time-dependent ROC analysis
based on the risk score model demonstrated good efficiency in
predicting patient survival (area under the curve, 0.718; Fig. 7E).
 | Table II.Prognostic values of the four genes
in patients with pancreatic adenocarcinoma in The Cancer Genome
Atlas cohort. |
Table II.
Prognostic values of the four genes
in patients with pancreatic adenocarcinoma in The Cancer Genome
Atlas cohort.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Gene symbol | HR (95% CI) | P-value | HR (95% CI) | P-value | Coefficient |
|---|
| MMP12 | 1.180
(1.062-1.310) | 0.00199 | 1.134
(1.014-1.270) | 0.028 | 0.126 |
| SCN11A | 0.703
(0.586-0.845) | 0.00017 | 0.776
(0.638-0.945) | 0.011 | −0.253 |
| TSPAN1 | 1.346
(1.140-1.589) | 0.00046 | 1.189
(1.002-1.412) | 0.048 | 0.173 |
| SH3TC2 | 1.299
(1.106-1.524) | 0.00137 | 1.177
(0.981-1.411) | 0.079 | 0.163 |
 | Table III.The OS rates in the high-risk group
and low-risk group. |
Table III.
The OS rates in the high-risk group
and low-risk group.
|
| OS (95% CI) |
|
|
|
| Years | High-risk group
(%) | Low-risk group
(%) |
|---|
| 1 | 62.9
(53.3-74.2) | 88.7
(82.0-96.0) |
| 3 | 21.1
(11.8-37.7) | 48.9
(37.4-63.8) |
| 5 | 16.9
(8.2-35.0) | 39.5
(26.6-58.9) |
Discussion
The present study obtained 622 GEO-DEGs (387
upregulated and 235 downregulated) from seven datasets on GEO and
identified 10 hub genes from the PPI network, including CDK1,
CCNB1, CDC20, ASPM, UBE2C, TPX2, TOP2A, NUSAP1, KIF20A and DLGAP5.
However, as the GEO datasets did not provide survival data, these
genes were not incorporated into the prognostic risk signature.
CDK1, also termed cell division control protein 2,
which is highly expressed in cancer cells, exhibits a vital
function in the transition from G2 stage in mitosis
(29). It is one of the potential
radiosensitization targets to inhibit the cell cycle-dependent
sensitization of PAAD cells, but it may also aggravate the toxicity
of normal tissues; thus, the mechanism of CDK1 in cancer cells
requires further study (29,30). CCNB1/CDK1-mediated phosphorylation of
the mitochondrial substrate provides effective biological energy
for cell G2/M transformation and upregulates
mitochondrial respiration to promote successful cell cycle
progression (31). CDK1 plays an
important role in cell cycle progression and antitumor activity.
Its dysfunction or hyperactivity leads to cell different
transformation, tumor invasion and other pathological states
(31,32).
CDC20 is a cell cycle regulator that coordinates the
mitotic process by promoting the orderly degradation of mitotic the
anaphase-promoting complex/cyclosome substrates (33). The average level of CDC20 in PDAC was
20 times higher than that in normal pancreas and pancreatitis. The
high expression of CDC20 was associated with poor differentiation,
and the high expression of CDC20 significantly reduced the 5-year
recurrence-free survival rate, and had the trend of shortening the
total survival period (34).
ASPM is a novel Wnt and stemness regulatory factor
in PAAD (35). Mechanistically, its
protein subtype ASPM-iI, which colocalizes with disheveled-2 and
active β-catenin as well as the stemness marker aldehyde
dehydrogenase-1, is indispensable for the Wnt activity, stemness
and the tumorigenicity of PAAD cells (36). Therefore, ASPM-iI staining as a novel
Wnt-associated marker of cancer stemness can not only predict the
outcome and survival time of patients with resected PAAD, but also
may guide future targeted therapies (36).
UBE2C is involved in tumorigenesis by regulating
cell cycle, apoptosis, metastasis and transcription (37). UBE2C gene knockdown downregulated the
expression of vimentin, an mesenchymal marker, and up-regulated the
expression of E-cadherin, an epithelial marker, to promote EMT in
lung cancer cells (38). In
addition, mouse experiments have demonstrated that UBE2C gene
knockout can significantly inhibit tumor growth in vivo
(39).
A previous study has demonstrated that TPX2
expression in pancreatic tumors is higher than that in their normal
counterparts (40). Targeting TPX2
using small interfering (si)RNAs can effectively inhibit the
proliferation of pancreatic cancer cells in tissue culture, induce
apoptosis, and inhibit the growth of pancreatic tumors in nude mice
(41). TPX2 gene knockout also
increases the sensitivity of pancreatic cancer cells to paclitaxel
therapy (41).
A high amplification rate of TOP2A is present in
multiple different types of malignancy, including PAAD (42). As a co-activator of β-catenin, TOP2A
can activate the EMT process and directly target microRNA-139 to
drive the malignant progress of pancreatic cancer (43).
Nucleolar and spindle associated protein 1(NUSAP1)
is located in dynamic spindle microtubules at metaphase and
anaphase of mitosis with a unique chromosomal central pattern. It
interacts with SUMO E3 ligase complex in the process of chromosome
separation near overlapping microtubules (44). NUSAP1 siRNA (L-004754-00) inhibited
the drug resistance of human prostate cancer cells induced by
NUSAP1, which suggested that NUSAP1 may be used as a biomarker of
the antitumor activity of galiellalactone (45); however, to the best of our knowledge,
there are currently few studies that focus on NUSAP1 in PAAD.
KIF20A, which is highly expressed in pancreatic
cancer and other malignant tumors, but not expressed in
non-cancerous tissues, is a tumor-associated antigen and a
potential target for tumor immunotherapy (46,47). It
has been reported that kif20a-66 is well-tolerated as an
immunotherapy for advanced pancreatic cancer and can effectively
induce cytotoxic T lymphocytes (48).
DLGAP5 has the unique function of stabilizing
spindle formation and surviving microtubule attack caused by
docetaxel in androgen-regulated prostate cancer cell (LNCaP) cycle
system (49). Thus, DLGAP5 may be
involved in spindle stability in other malignant tumors.
KEGG analysis in the present study revealed that
these 10 genes were mainly associated with the ‘Cell cycle’ and
‘p53 signaling pathway’. P53-protein tyrosine phosphatase
non-receptor type 14 (PTPN14)-Yap pathway is a tumor suppressor
mechanism mediated by p53. The p53 transcription activation domain
2 mutant is a ‘super tumor suppressor’, with an enhanced ability to
restrain pancreatic cancer cell proliferation and to transactivate
select p53 target genes (including ptpn14) (50). With the successive inactivation of
tumor suppressor genes, the proliferation of tumor cells is
increased, and remains at high levels in metastatic tumors, which
may be caused by cell cycle regulatory gene variants. This leads to
cell cycle disorder, which is characteristic of various cancer
subtypes (51). Various target genes
affect the cycle, senescence and apoptosis of pancreatic cancer
cells through the p53 pathway (52,53),
which is consistent with the results of the present study in the
biological function analysis.
The aforementioned results indicated that these 10
hub genes may serve a role in PAAD development. Focusing on these
10 hub genes may provide ideas and directions for revealing the
molecular mechanism of pancreatic cancer and developing the
corresponding therapeutic drugs.
The present study also identified four genes
associated with PAAD prognosis (MMP12, SCN11A, TSPAN1 and SH3TC2),
which were used to construct a prognostic gene signature. It has
been reported that SCN11A is upregulated in breast and prostate
cancer compared with adjacent normal tissues (54,55), but
its molecular nature and association with pancreatic cancer
function have remained elusive. In the present study, SCN1A was
considered to be a protective prognostic gene; thus, its role in
pancreatic cancer requires further investigation. The other three
prognostic genes MMP12, TSPAN1 and SH3TC2 were considered to be
risk prognostic genes, implying malignant phenotypes. MMPs are
associated with the invasion and metastasis of tumor cells. It
promotes tumor cells to degrade the components of the extracellular
matrix, separate from the primary site, migrate to the distal site
and invade the surrounding tissue to induce metastasis (56). When MMP12, a member of MMP family, is
inhibited, the invasion and metastasis of human pancreatic cancer
is inhibited, which prolongs the survival period and exhibits
antimetastatic effects in situ in a mouse model (57). However, to the best of our knowledge,
the physiological function of MMP12 has not yet been described
completely.
A previous study demonstrated that the increased
TSPAN1 in pancreatic cancer tissues was associated with the
clinicopathological features and survival rate of patients with
PAAD (52). siRNA targeting TSPAN1
significantly inhibits the proliferation of PAAD cells, increases
apoptosis, and decreases cell migration and invasion, therefore
this may be a potential strategy for the treatment of human PAAD
(58,59). Previous studies have suggested that
an SH3TC2 variant allele is associated with the cause of
Charcot-Marie-Tooth neuropathy (60,61).
Although the expression and functions of SH3TC2 in cancer have
rarely been described, it remains reasonable to identify it as a
prognostic biomarker due to its significance in the present
signature model.
The results of the present study demonstrated that
10 hub genes may be involved in the occurrence and progression of
PAAD, and these 10 hub genes were highly expressed in PAAD tissues.
Therefore, further research may focus on the reasons for the high
expression of these genes to develop corresponding drugs to reduce
or inhibit the expression of these genes, which may improve the
treatment of PAAD. In addition, according to the survival
information of patients with PAAD in TCGA database, four prognostic
signature genes were identified. By detecting the expression of
these four genes, the risk of PAAD could be predicted in advance.
These results may provide clues for further investigating the
pathogenesis of PAAD and to establish a new risk classification and
prognosis assessment model. However, there are limitations to the
present study, as it was performed based on data analysis,
experimental results of this prediction in PAAD are required. As
the present study identified hub genes in a public database,
further experimental research is required to demonstrate the
molecular pathogenesis and signal transduction mechanism of these
genes in PAAD.
In conclusion, using the datasets of multi-gene
expression profiles and the comprehensive bioinformatics analysis,
the present study identified 10 hub genes that may be responsible
for the pathogenesis of PAAD. In addition, the present study also
constructed a four-gene prediction model that performed well in
predicting 1-, 3- and 5-year OS, and thus may be used as a
prognosis marker for patients with PAAD. The present study may be
helpful to improve the current understanding of the potential
carcinogenesis or progress of PAAD, as well as for the prognostic
prediction and molecular targeted management of PAAD.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
None.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81873190).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LES, XS and ZZZ advance the research direction and
method. LES, XS, QX and NBC conceived and designed the study. LES,
KCN and XS drafted the manuscript, analyzed the data, developed the
algorithm and interpreted the results. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gordon-Dseagu VL, Devesa SS, Goggins M and
Stolzenberg-Solomon R: Pancreatic cancer incidence trends: Evidence
from the surveillance, epidemiology and end results (SEER)
population-based data. Int J Epidemiol. 47:427–439. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Golan T, Sella T, Margalit O, Amit U,
Halpern N, Aderka D, Shacham-Shmueli E, Urban D and Lawrence YR:
Short- and long-term survival in metastatic pancreatic
adenocarcinoma, 1993-2013. J Natl Compr Canc Netw. 15:1022–1027.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mackay MT, van Erning FN, van der Geest
LG, Koerkamp BG, van Laarhoven MH, Bonsing BA, Wilmink JW, van
Santvoort HC, de Vos-Geelen Jd, van Eijck CH, et al: Association of
the location of pancreatic ductal adenocarcinoma (head, body, tail)
with tumor stage, treatment, and survival: A population-based
analysis. Pancreatology. 18 (Suppl):S1322018. View Article : Google Scholar
|
|
5
|
Latenstein AEJ, van der Geest LGM, Bonsing
BA, Groot Koerkamp B, Haj Mohammad N, de Hingh IHJT, de Meijer VE,
Molenaar IQ, van Santvoort HC, van Tienhoven G, et al: Nationwide
trends in incidence, treatment and survival of pancreatic ductal
adenocarcinoma. Eur J Cancer. 125:83–93. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mokdad AA, Minter RM, Yopp AC, Porembka
MR, Wang SC, Zhu H, Augustine MM, Mansour JC, Choti MA and Polanco
PM: Comparison of overall survival between preoperative
chemotherapy and chemoradiotherapy for resectable pancreatic
adenocarcinoma. J Natl Compr Canc Netw. 16:1468–1475. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moore MJ and Stathis A: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jun I, Park HS, Piao H, Han JW, An MJ, Yun
BG, Zhang X, Cha YH, Shin YK, Yook JI, et al: ANO9/TMEM16J promotes
tumourigenesis via EGFR and is a novel therapeutic target for
pancreatic cancer. Br J Cancer. 117:1798–1809. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chio IIC, Jafarnejad SM, Ponz-Sarvise M,
Park Y, Rivera K, Palm W, Wilson J, Sangar V, Hao Y, Öhlund D, et
al: NRF2 promotes tumor maintenance by modulating mrna translation
in pancreatic cancer. Cell. 166:963–976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jain A, Brown SZ, Thomsett HL, Londin E
and Brody JR: Evaluation of post-transcriptional gene regulation in
pancreatic cancer cells: Studying RNA binding proteins and their
mRNA targets. Methods Mol Biol. 1882:239–252. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uchida S, Kinoh H, Ishii T, Matsui A,
Tockary TA, Takeda KM, Uchida H, Osada K, Itaka K and Kataoka K:
Systemic delivery of messenger RNA for the treatment of pancreatic
cancer using polyplex nanomicelles with a cholesterol moiety.
Biomaterials. 82:221–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hutter C and Zenklusen JC: The cancer
genome atlas: Creating lasting value beyond its data. Cell.
173:283–285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ho J, Li X, Zhang L, Liang Y, Hu W, Yau
JC, Chan H, Gin T, Chan MT, Tse G and Wu WK: Translational genomics
in pancreatic ductal adenocarcinoma: A review with re-analysis of
TCGA dataset. Semin Cancer Biol. 55:70–77. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barrett T: NCBI GEO: Mining millions of
expression profiles-database and tools. Nucleic Acids Res.
33:D562–D566. 2004. View Article : Google Scholar
|
|
15
|
Li C, Zeng X, Yu H, Gu Y and Zhang W:
Identification of hub genes with diagnostic values in pancreatic
cancer by bioinformatics analyses and supervised learning methods.
World J Surg Oncol. 16:2232018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang H, Wei P, Chang P, Li Y, Yan D, Liu
C, Hassan M and Li D: Genetic polymorphisms associated with
pancreatic cancer survival: A genome-wide association study. Int J
Cancer. 141:678–686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sivakumar S, de Santiago I, Chlon L and
Markowetz F: Master regulators of oncogenic KRAS response in
pancreatic cancer: An integrative network biology analysis. PLoS
Med. 14:e10022232017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muzumdar MD, Chen PY, Dorans KJ, Chung KM,
Bhutkar A, Hong E, Noll EM, Sprick MR, Trumpp A and Jacks T:
Survival of pancreatic cancer cells lacking KRAS function. Nat
Commun. 8:10902017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Badea L, Herlea V, Dima SO, Dumitrascu T
and Popescu I: Combined gene expression analysis of whole-tissue
and microdissected pancreatic ductal adenocarcinoma identifies
genes specifically overexpressed in tumor epithelia.
Hepatogastroenterology. 55:2016–2027. 2008.PubMed/NCBI
|
|
21
|
Pei H, Li L, Fridley BL, Jenkins GD,
Kalari KR, Lingle W, Petersen G, Lou Z and Wang L: FKBP51 affects
cancer cell response to chemotherapy by negatively regulating akt.
Cancer Cell. 16:259–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang G, Schetter A, He P, Funamizu N,
Gaedcke J, Ghadimi BM, Ried T, Hassan R, Yfantis HG, Lee DH, et al:
DPEP1 inhibits tumor cell invasiveness, enhances chemosensitivity
and predicts clinical outcome in pancreatic ductal adenocarcinoma.
PLoS One. 7:e315072012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Donahue TR, Tran LM, Hill R, Li Y,
Kovochich A, Calvopina JH, Patel SG, Wu N, Hindoyan A, Farrell JJ,
et al: Integrative survival-based molecular profiling of human
pancreatic cancer. Clin Cancer Res. 18:1352–1363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lunardi S, Jamieson NB, Lim SY, Griffiths
KL, Carvalho-Gaspar M, Al-Assar O, Yameen S, Carter RC, McKay CJ,
Spoletini G, et al: IP-10/CXCL10 induction in human pancreatic
cancer stroma influences lymphocytes recruitment and correlates
with poor survival. Oncotarget. 5:11064–11080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Janky R, Binda MM, Allemeersch J, Van den
Broeck A, Govaere O, Swinnen JV, Roskams T, Aerts S and Topal B:
Prognostic relevance of molecular subtypes and master regulators in
pancreatic ductal adenocarcinoma. BMC Cancer. 16:6322016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang S, He P, Wang J, Schetter A, Tang W,
Funamizu N, Yanaga K, Uwagawa T, Satoskar AR, Gaedcke J, et al: A
novel MIF signaling pathway drives the malignant character of
pancreatic cancer by targeting NR3C2. Cancer Res. 76:3838–3850.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nie K, Shi L, Wen Y, Pan J, Li P, Zheng Z
and Liu F: Identification of hub genes correlated with the
pathogenesis and prognosis of gastric cancer via bioinformatics
methods. Minerva Med. 111:213–225. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu L, Lin J and He H: Identification of
potential crucial genes associated with the pathogenesis and
prognosis of endometrial cancer. Front Genet. 10:3732019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prevo R, Pirovano G, Puliyadi R, Herbert
KJ, Rodriguez-Berriguete G, O'Docherty A, Greaves W, McKenna WG and
Higgins GS: CDK1 inhibition sensitizes normal cells to DNA damage
in a cell cycle dependent manner. Cell Cycle. 17:1513–1523. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei D, Parsels LA, Karnak D, Davis MA,
Parsels JD, Marsh AC, Zhao L, Maybaum J, Lawrence TS, Sun Y and
Morgan MA: Inhibition of protein phosphatase 2A radiosensitizes
pancreatic cancers by modulating CDC25C/CDK1 and homologous
recombination repair. Clin Cancer Res. 19:4422–4432. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Fan M, Candas D, Zhang TQ, Qin L,
Eldridge A, Wachsmann-Hogiu S, Ahmed KM, Chromy BA, Nantajit D, et
al: Cyclin B1/Cdk1 coordinates mitochondrial respiration for
cell-cycle G2/M progression. Dev Cell. 29:217–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Levasseur MD, Thomas C, Davies OR, Higgins
JM and Madgwick S: Aneuploidy in oocytes is prevented by sustained
CDK1 activity through degron masking in cyclin B1. Dev Cell.
48:672–684. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu H: Cdc20: A WD40 activator for a cell
cycle degradation machine. Mol Cell. 27:3–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang DZ, Ma Y, Ji B, Liu Y, Hwu P,
Abbruzzese JL, Logsdon C and Wang H: Increased CDC20 expression is
associated with pancreatic ductal adenocarcinoma differentiation
and progression. J Hematol Oncol. 5:152012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang WY, Hsu CC, Wang TY, Li CR, Hou YC,
Chu JM, Lee CT, Liu MS, Su JJ, Jian KY, et al: A gene expression
signature of epithelial tubulogenesis and a role for ASPM in
pancreatic tumor progression. Gastroenterology. 145:1110–1120.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsu CC, Liao WY, Chan TS, Chen WY, Lee CT,
Shan YS, Huang PJ, Hou YC, Li CR and Tsai KK: The differential
distributions of ASPM isoforms and their roles in Wnt signaling,
cell cycle progression, and pancreatic cancer prognosis. J Pathol.
249:498–508. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu G, Zhao J, Pan B, Ma G and Liu L:
UBE2C overexpression in melanoma and its essential role in G2/M
transition. J Cancer. 10:2176–2184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin D, Guo J, Wu Y, Du J, Wang X, An J, Hu
B, Kong L, Di W and Wang W: UBE2C, directly targeted by
miR-548e-5p, increases the cellular growth and invasive abilities
of cancer cells interacting with the EMT marker protein zinc finger
E-box binding homeobox 1/2 in NSCLC. Theranostics. 9:2036–2055.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang X, Yin L, Yang L, Zheng Y, Liu S,
Yang J, Cui H and Wang H: Silencing ubiquitin-conjugating enzyme 2C
inhibits proliferation and epithelial-mesenchymal transition in
pancreatic ductal adenocarcinoma. FEBS J. 286:4889–4909. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gomes-Filho SM, Dos Santos EO, Bertoldi
ER, Scalabrini LC, Heidrich V, Dazzani B, Levantini E, Reis EM and
Bassères DS: Aurora A kinase and its activator TPX2 are potential
therapeutic targets in KRAS-induced pancreatic cancer. Cell Oncol
(Dordr). 43:445–460. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Warner SL, Stephens BJ, Nwokenkwo S,
Hostetter G, Sugeng A, Hidalgo M, Trent JM, Han H and Von Hoff DD:
Validation of TPX2 as a potential therapeutic target in pancreatic
cancer cells. Clin Cancer Res. 15:6519–6528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heestand GM, Schwaederle M, Gatalica Z,
Arguello D and Kurzrock R: Topoisomerase expression and
amplification in solid tumours: Analysis of 24,262 patients. Eur J
Cancer. 83:80–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pei YF, Yin XM and Liu XQ: TOP2A induces
malignant character of pancreatic cancer through activating
β-catenin signaling pathway. Biochim Biophys Acta Mol Basis Dis.
1864:197–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mills CA, Suzuki A, Arceci A, Mo JY,
Duncan A, Salmon ED and Emanuele MJ: Nucleolar and
spindle-associated protein 1 (NUSAP1) interacts with a SUMO E3
ligase complex during chromosome segregation. J Biol Chem.
292:17178–17189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Garrido-Rodríguez M, Ortea I, Calzado MA,
Muñoz E and García V: SWATH proteomic profiling of prostate cancer
cells identifies NUSAP1 as a potential molecular target for
galiellalactone. J Proteomics. 193:217–229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Taniuchi K, Furihata M and Saibara T:
KIF20A-mediated RNA granule transport system promotes the
invasiveness of pancreatic cancer cells. Neoplasia. 16:1082–1093.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Imai K, Hirata S, Irie A, Senju S, Ikuta
Y, Yokomine K, Harao M, Inoue M, Tomita Y, Tsunoda T, et al:
Identification of HLA-A2-restricted CTL epitopes of a novel
tumour-associated antigen, KIF20A, overexpressed in pancreatic
cancer. Br J Cancer. 104:300–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Asahara S, Takeda K, Yamao K, Maguchi H
and Yamaue H: Phase I/II clinical trial using HLA-A24-restricted
peptide vaccine derived from KIF20A for patients with advanced
pancreatic cancer. J Transl Med. 11:2912013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hewit K, Sandilands E, Martinez RS, James
D, Leung HY, Bryant DM, Shanks E and Markert EK: A functional
genomics screen reveals a strong synergistic effect between
docetaxel and the mitotic gene DLGAP5 that is mediated by the
androgen receptor. Cell Death Dis. 19:10692018. View Article : Google Scholar
|
|
50
|
Mello SS, Valente LJ, Raj N, Seoane JA,
Flowers BM, McClendon J, Bieging-Rolett KT, Lee J, Ivanochko D,
Kozak MM, et al: A p53 super-tumor suppressor reveals a tumor
suppressive p53-Ptpn14-yap axis in pancreatic cancer. Cancer Cell.
32:460–473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Connor AA, Denroche RE, Jang GH, Lemire M,
Zhang A, Chan-Seng-Yue M, Wilson G, Grant RC, Merico D, Lungu I, et
al: Integration of genomic and transcriptional features in
pancreatic cancer reveals increased cell cycle progression in
metastases. Cancer Cell. 35:267–282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jiang W, Zhao S, Jiang X, Zhang E, Hu G,
Hu B, Zheng P, Xiao J, Lu Z, Lu Y, et al: The circadian clock gene
Bmal1 acts as a potential anti-oncogene in pancreatic cancer by
activating the p53 tumor suppressor pathway. Cancer Lett.
371:314–325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang H, Zhang X, Li X, Meng WB, Bai ZT,
Rui SZ, Wang ZF, Zhou WC and Jin XD: Effect of CCNB1 silencing on
cell cycle, senescence, and apoptosis through the p53 signaling
pathway in pancreatic cancer. J Cell Physiol. 234:619–631. 2019.
View Article : Google Scholar
|
|
54
|
Fraser SP, Diss JK, Chioni A, Mycielska
ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, et
al: Voltage-Gated sodium channel expression and potentiation of
human breast cancer metastasis. Clin Cancer Res. 11:5381–5389.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Diss JK, Archer SN, Hirano J, Fraser SP
and Djamgoz MB: Expression profiles of voltage-gated Na(+) channel
alpha-subunit genes in rat and human prostate cancer cell lines.
Prostate. 48:165–178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Stellas D and Patsavoudi E: Inhibiting
matrix metalloproteinases, an old story with new potentials for
cancer treatment. Anticancer Agents Med Chem. 12:707–717. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fujisawa T, Rubin B, Suzuki A, Patel PS,
Gahl WA, Joshi BH and Puri RK: Cysteamine suppresses invasion,
metastasis and prolongs survival by inhibiting matrix
metalloproteinases in a mouse model of human pancreatic cancer.
PLoS One. 7:e344372012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tian J, Zhang R, Piao H, Li X, Sheng W,
Zhou J, Dong M, Zhang X, Yan X, Shang W, et al: Silencing Tspan1
inhibits migration and invasion, and induces the apoptosis of human
pancreatic cancer cells. Mol Med Rep. 18:3280–3288. 2018.PubMed/NCBI
|
|
59
|
Hou FQ, Lei XF, Yao JL, Wang YJ and Zhang
W: Tetraspanin 1 is involved in survival, proliferation and
carcinogenesis of pancreatic cancer. Oncol Rep. 34:3068–3076. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lupski JR, Gonzaga-Jauregui C, Yang Y,
Bainbridge MN, Jhangiani S, Buhay CJ, Kovar CL, Wang M, Hawes AC,
Reid JG, et al: Exome sequencing resolves apparent incidental
findings and reveals further complexity of SH3TC2 variant alleles
causing charcot-marie-tooth neuropathy. Genome Med. 5:572013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Stendel C, Roos A, Kleine H, Arnaud E,
Özçelik M, Sidiropoulos PN, Zenker J, Schüpfer F, Lehmann U, Sobota
RM, et al: SH3TC2, a protein mutant in charcot-marie-tooth
neuropathy, links peripheral nerve myelination to endosomal
recycling. Brain. 133:2462–2474. 2010. View Article : Google Scholar : PubMed/NCBIPubMed/NCBI
|