Introduction
Hallmarks of glioma
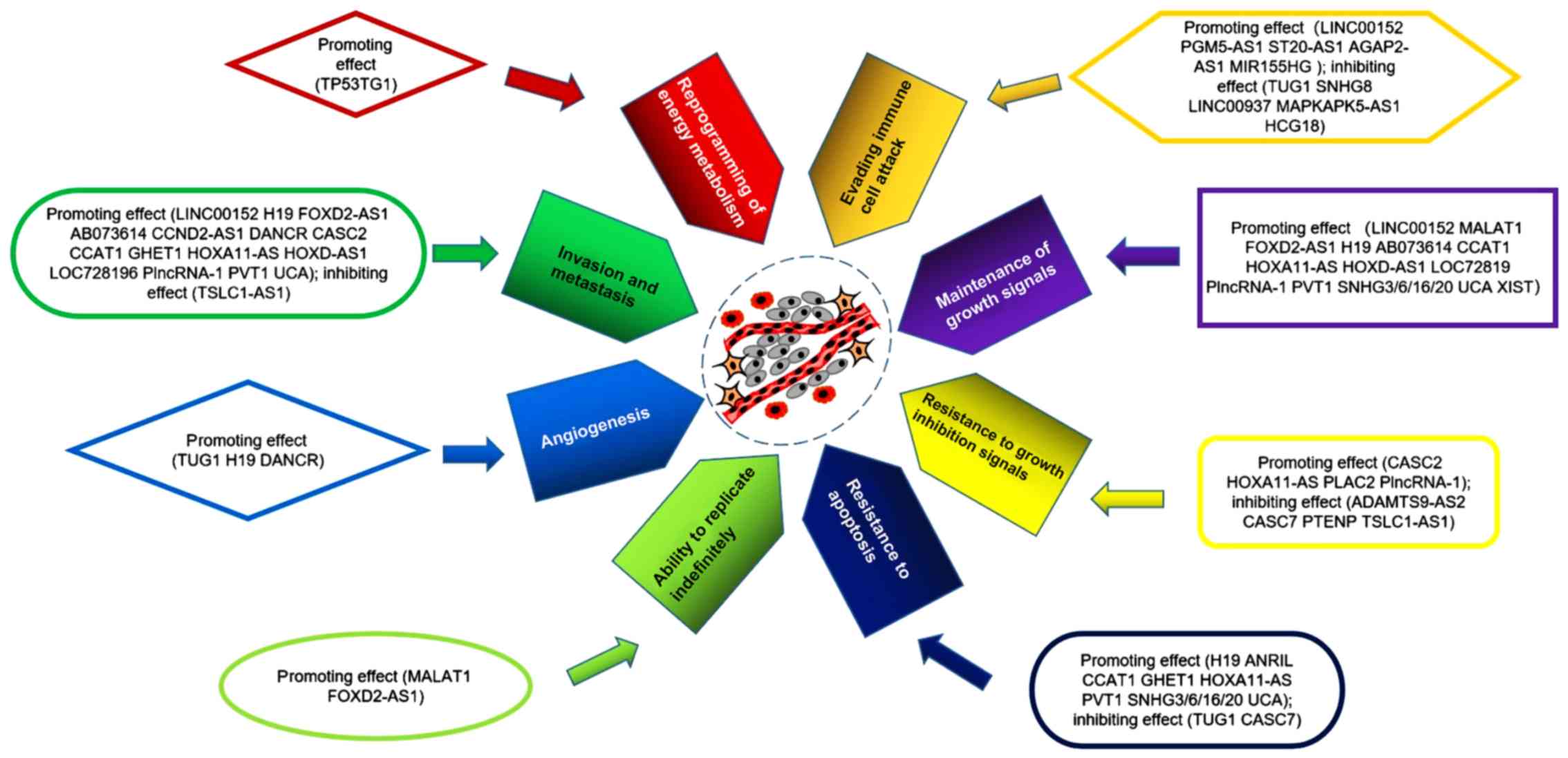
The eight hallmarks of cancer are also hallmarks of
gliomas (1) and are as follows: i)
Maintenance of growth signals; ii) resistance to growth inhibition
signals; iii) resistance to apoptosis; iv) ability to replicate
indefinitely; v) angiogenesis; vi) invasion; vii) reprogramming of
energy metabolism; and viii) evading immune cell attack (1,2). In
addition to these eight hallmarks, the tumor microenvironment (TME)
is another feature of gliomas (1–3). The
composition and role of the TME have been extensively studied, and
it has been demonstrated that the TME serves an important role when
tumors acquire the aforementioned hallmarks during tumor
progression (1–3). The blood-brain barrier (BBB) provides
the brain with immune resistance through physical protection
(3,4). Although gliomas usually change the
basal lamina of their internal blood vessels and destroy the
endothelial barrier, which increases endothelial permeability
through the high expression of vascular endothelial growth factor
(VEGF) (5–8), the BBB serves a specific role in immune
resistance in the early stage of glioma (5–8); thus,
the TME in gliomas is different from that in other parts of the
body.
Functions of lncRNAs in cancer
Only ~2% of the genes in the human genome encode
proteins, whereas the remainder constitute non-coding RNAs (ncRNAs)
(9,10). ncRNAs can be divided into two types:
Long ncRNAs (lncRNAs, >200 nt) and short nc RNAs (<200 nt)
(11). lncRNAs account for ~80% of
all ncRNAs (12) and can be divided
into three categories according to their function: i) Functional
lncRNAs, which function through their own sequences; ii)
non-functional lncRNAs; and iii) lncRNAs that affect transcription
independently of their sequence (13,14). The
human genome encodes ~28,000 distinct lncRNAs, a number of which
remain to be explored (15). lncRNAs
have been demonstrated to serve important roles in the development
of cancer, as the expression of a number of lncRNAs is abnormal in
various cancer types (16–18). lncRNAs can be involved in cell cycle
changes, escape from apoptosis and invasion, and can cause various
types of cancer, such as glioma, and lung, liver, breast,
colorectal, ovarian and prostate cancer (12,19–22). In
addition, maternally expressed 3 (MEG3), a class of tumor
suppressor lncRNA, was the first lncRNA group discovered to possess
tumor suppressor functions (22). In
conclusion, the roles of lncRNAs in cancer are complex and
diverse.
Roles of lncRNAs in the hallmarks of
glioma
Increasing numbers of studies have demonstrated that
lncRNAs serve important roles in the hallmarks of glioma (23–25).
lncRNAs promote or inhibit the development of glioma through a
variety of pathways; lncRNAs serve a number of roles, and multiple
lncRNAs are involved in the hallmarks of glioma (Table I; Fig.
1), which are described in this section.
 | Table I.Roles of different lncRNAs in glioma
hallmarks. |
Table I.
Roles of different lncRNAs in glioma
hallmarks.
| lncRNA | Expression in
glioma | Maintenance of
growth signals | Resistance to
growth inhibition signals | Resistance to
apoptosis | Ability to
replicate indefinitely | Angiogenesis | Invasion | Reprogramming of
energy metabolism | Evading immune cell
attack | (Refs.) |
|---|
| H19 | ↑ | + |
| + |
| + | + |
|
| (21,52,69,
72,73,91) |
| XIST | ↑ | + |
|
|
|
|
|
|
| (23,24) |
| HOXD-AS1 | ↑ | + |
|
|
|
| + |
|
| (25) |
| CCAT1 | ↑ | + |
| + |
|
| + |
|
| (26) |
| LOC728196 | ↑ | + |
|
|
|
| + |
|
| (27) |
| PLAC2 | ↓ |
| – |
|
|
|
|
|
| (31) |
| PTENP1 | ↓ |
| – |
|
|
|
|
|
| (32) |
| HOXA11-AS | ↑ | + | + | + |
|
| + |
|
| (33,76) |
| Plncrna-1 | ↑ | + | + |
|
|
| + |
|
| (34–37) |
| ADAMTS9-AS2 | ↓ |
| – |
|
|
|
|
|
| (38) |
| CASC2 | ↑ |
| + |
|
|
| + |
|
| (39) |
| ANRIL | ↑ |
|
| + |
|
|
|
|
| (41) |
| SNHG3/6/16/2O | ↑ | + |
| + |
|
|
|
|
| (47–50) |
| TUG1 | ↓ |
|
| – |
|
|
|
| – | (51,82) |
| CASC7 | ↓ |
| – | – |
|
|
|
|
| (53) |
| GHET1 | ↑ |
|
| + |
|
| + |
|
| (54) |
| MALAT1 | ↑ | + |
|
| + |
|
|
|
| (56,57) |
| FOXD2-AS1 | ↑ | + |
|
| + |
| + |
|
| (59–61) |
| TUG1 | ↑ |
|
|
|
| + |
|
|
| (62) |
| PVT1 | ↑ | + |
| + |
|
| + |
|
| (64–66,93) |
| AB073614 | ↑ | + |
|
|
|
| + |
|
| (67,68) |
| TSLC1-AS1 | ↓ |
| – |
|
|
| – |
|
| (69) |
| DANCR | ↑ |
|
| + |
|
| + |
|
| (71,75) |
| CCND2-AS1 | ↑ |
|
|
|
|
| + |
|
| (72) |
| UCA | ↑ | + |
| + |
|
| + |
|
| (78) |
| TP53TG1 | ↑ |
|
|
|
|
|
| + |
| (79) |
| LINC00152 | ↑ | + |
|
|
|
| + |
| + | (82,89) |
| PGM5-AS1 | ↑ |
|
|
|
|
|
|
| + | (83) |
| ST20-AS1 | ↑ |
|
|
|
|
|
|
| + | (83) |
| AGAP2-AS1 | ↑ |
|
|
|
|
|
|
| + | (83) |
| MIR155HG | ↑ |
|
|
|
|
|
|
| + | (83) |
| SNHG8 | ↓ |
|
|
|
|
|
|
| – | (83) |
| LINC00937 | ↓ |
|
|
|
|
|
|
| – | (83) |
| MAPKAPK5-AS1 | ↓ |
|
|
|
|
|
|
| – | (83) |
| HCG18 | ↓ |
|
|
|
|
|
|
| – | (83) |
Roles of lncRNAs in the maintenance of
growth signals
The expression of the lncRNA X-inactive specific
transcript (XIST) is increased in glioma compared with that in
normal controls, and XIST can directly bind microRNA (miR)-29c to
inhibit its expression (23,24). In glioma, the XIST/miR-29c axis
induces an increase in the protein levels of MutS Homolog 6,
transcription factor specificity protein 1 and O -methylguanine-DNA
methyltransferase through mutations in the mismatch repair pathway,
which promotes cell proliferation and reduces the temozolomide
(TMZ)-induced inhibition of cell proliferation (23). XIST also promotes angiogenesis in
glioma by targeting miR-137 (24).
The lncRNA HOXD cluster antisense RNA 1 (HOXD-AS1)
acts as a miR-204 sponge to promote the proliferation, migration
and invasion of glioma cells, and to confer cisplatin resistance
(25). miR-204 directly inhibits the
expression of its target oncogenes high-mobility group box-1 and
RAB22A, a member of the Ras oncogene family, in various
malignancies, such as glioma, cervical cancer and breast cancer
(25).
In glioma, the expression of the lncRNA colon
cancer-associated transcript-1 (CCAT1) is increased, whereas the
expression of miR-181b is decreased compared with that in normal
brain tissues from patients with cerebral trauma (26). Additionally, studies have
demonstrated that the expression of these two RNAs is negatively
correlated in glioma, and that miR-181b inhibits the expression of
fibroblast growth factor receptor 3 (FGFR3) and platelet-derived
growth factor receptor (PDGFR) by directly binding to the
3′-untranslated regions (UTRs) of these genes (26). Thus, CCAT1 may be a competitive
endogenous RNA (ceRNA) of miR-181b, which increases the expression
of FGFR3 and PDGFR by regulating miR-181b, thus increasing cell
proliferation (26).
In glioma, the expression of the lncRNA LOC728196 is
increased, whereas the expression of miR-513c is decreased compared
with adjacent normal tissues, demonstrating a negative correlation,
and high expression of LOC728196 increases the proliferation of
glioma cells (27). A previous study
has also demonstrated that LOC728196 reduces the targeted
inhibition of transcription factor 7 (TCF7) by binding to miR-513c
(27). TCF7 is involved in the
Wnt/β-catenin signaling pathway to leads to excessive activation of
the pathway, which leads to the development of malignant tumors
(28,29).
Another study demonstrated that cell proliferation
is inhibited in glioma U87 cells following small nucleolar RNA host
gene (SNHG)20-knockdown; in addition, a decrease in the levels of
cyclin A1 and an increase in the levels of p21 were observed in U87
cells of SNHG20 siRNA group compared with the NC siRNA group
(30). Overall, XIST, HOXD-AS1,
CCAT1, LOC728196 and SNHG20 increased expression in glioma compared
with normal brain tissue or brain tissue adjacent to the tumor and
promoted the maintenance of growth signals.
Roles of lncRNAs in resistance to
growth inhibition signals
The lncRNA placenta-specific protein 2 (PLAC2) is
expressed at lower levels in glioma compared with those in normal
brain tissue (31). The study also
has demonstrated that PLAC2 binds to STAT1 and inhibits the
expression of the ribosomal protein L36 by binding to its promoter
in the nucleus (31). In addition,
cytoplasmic PLAC2 inhibits the nuclear translocation of STAT1 and
inhibits cell proliferation (31).
The lncRNA PTEN pseudogene 1 (PTENP1) is a tumor
suppressor, the expression of which is decreased in glioma; its
overexpression inhibits cell proliferation and invasion, which may
be achieved by promoting the expression of p21 and inhibiting the
p38 mitogen-activated protein kinase (MAPK) pathway (32).
The expression of the lncRNA homeobox A11 antisense
(HOXA11-AS) in glioma tissues and cells, which has been
demonstrated to be significantly increased compared with normal
brain tissue (33), promotes
proliferation by upregulating cyclin-dependent kinase 2/4, cyclin
D1 and cyclin E, and promotes the downregulation of p16, p21, p27
and retinoblastoma tumor suppressor protein (33).
Prostate cancer-upregulated lncRNA 1 (PlncRNA-1)
promotes cancer progression by regulating androgen receptors and
activating transforming growth factor-β1 (34–36).
Recent a study reported that PlncRNA-1 is significantly upregulated
in glioma and indicates a poor survival prognosis (37). PlncRNA-1 has also been demonstrated
to promote the proliferation and invasiveness of glioma, which is
achieved by upregulating the notch pathway-related proteins
Notch-1, Jagged-1 and Hes family bhlh transcription factor 1
(37).
The lncRNA ADAMTS9-antisense RNA 2 (ADAMTS9-AS2) is
a tumor suppressor that inhibits the migration of glioma cells and
is negatively regulated by DNA methyltransferase-1, which results
in its decreased expression in glioma (38).
The lncRNA cancer susceptibility 2 (CASC2) exerts
tumor inhibitory effects and is significantly decreased in glioma
compared with normal brain tissue (39). CASC2 can directly bind miR-181a to
upregulate PTEN, inhibit tumor proliferation and increase the
sensitivity of glioma cells to TMZ (39).
In summary, PLAC2, PTENP1, ADAMTS9-AS2 and CASC2
inhibits resistance to growth inhibition signals and are decreased
in glioma. Increased HOXA11-AS and PlncRNA-1 expression in glioma
and promotes resistance to growth inhibition signals.
Roles of lncRNAs in the resistance to
apoptosis
In glioma, the expression of lncRNA antisense
non-coding RNA in the INK4 locus (ANRIL) is negatively associated
with the expression of the anticancer gene miR-203a (40). Studies have demonstrated that ANRIL
promotes carcinogenesis by affecting the expression of miR-203a,
and that knockdown of ANRIL inhibits twist-related protein 1 and
c-jun transcription by inhibiting the phosphorylation of the AKT
signaling pathway components, which promotes apoptosis (40–42).
c-myc is an oncogene that inhibits the expression of p21 (40); p21 is a tumor suppressor gene that
can promote stagnation of the tumor cell cycle and inhibit the
expression of bcl-2 (41,42), an anti-apoptotic gene that inhibits
apoptosis by inhibiting the activity of apoptosis-related caspase
proteins (43). A number of studies
have reported that ANRIL can inhibit the expression of c-myc,
thereby promoting cell cycle arrest in tumor cells and increasing
caspase-3/8/9 activity, which promotes apoptosis (40–44).
The lncRNAs SNHG3/6/16/20 are primarily expressed in
the nucleus and are increased in glioma compared with normal brain
tissue (45–50). SNHG3 recruits enhancer of zeste
homologue 2 (EZH2) to the promoter region of Kruppel-like factor 2
(KLF2) and p21 through sponging EZH2 (45). EZH2 binds to the KLF2 and p21
promoters to inhibit their expression (45,46),
which inhibits apoptosis and increases cell proliferation (47). In transfected normal astrocytes with
an SNHG6 overexpression vector, the development of malignant
phenotypes is observed, which indicates that SNHG6 induces the
transformation of cells with a normal phenotype to those with a
malignant phenotype. In addition, knockdown of SNHG6 promotes cell
proliferation by enhancing the mRNA and protein levels of p21 in
glioma cells compared with negative control-transfected glioma
cells (48). Additionally, in
SNHG6-knockdown glioma cells, the apoptosis-related proteins
caspase-3 and caspase-9 are activated, promoting apoptosis
(48). SNHG16 upregulates the
expression of protein arginine methyltransferase 5 (PRMT5) through
sponging miR-4518 to promote the relevant functions of PRMT5 in
glioma (49). In addition, SNHG16
inhibits apoptosis by regulating the expression of p21, bcl-2 and
the components of the PI3K/AKT pathway (49). In SNHG20-knockout glioma cells,
inhibition of the PTEN/PI3K/AKT signaling pathway and increased
apoptosis were observed compared with negative control-transfected
glioma cells, suggesting that SNHG20 may regulate apoptosis through
the PTEN/PI3K/AKT signaling pathway (50).
The lncRNA taurine upregulated 1 (TUG1) is a
tumor-inhibiting lncRNA, the expression of which is decreased in
glioma. TUG1 activates caspase-3/9 and inhibits the bcl-2-mediated
anti-apoptosis pathway to promote apoptosis in glioma cells
(51). Notably, another study has
reported that TUG1 is upregulated in glioblastoma, that it induces
the expression of vascular endothelial growth factor A (VEGFA) and
that it promotes tumor angiogenesis through sponging miR-299
(52).
In glioma, the expression of lncRNA CASC7 is
significantly decreased compared with normal brain tissues
(53). A previous study demonstrated
that CASC7 inhibits glioma progression, promotes apoptosis and
inhibits cell proliferation by inactivating the Wnt/β-catenin
signaling pathway (53).
Additionally, increased caspase-3 activity was also observed in
glioma cell lines that overexpressed CASC7 (53).
The expression of the lncRNA gastric carcinoma
proliferation enhancing transcript 1 (GHET1) is increased in glioma
compared with normal brain tissues, and may inhibit apoptosis by
downregulating the Numb protein, which in turn downregulates p53
and upregulates matrix metalloproteinase 2/9 (54).
The expression of the lncRNA urothelial
cancer-associated 1 (UCA1) is increased in glioma compared with
normal brain tissues, where it inhibits miR-182 expression
(55). Notably,
apoptosis-stimulating protein inhibitor of p53 (iASPP) and its
3′-UTR are inhibited by binding miR-182; thus, UCA1 may increase
the expression of iASPP and inhibit apoptosis (55). In summary, ANRIL, SNHG3/6/16/20 and
GHET1 increased expression in glioma compared with normal brain
tissue and promoted the resistance to apoptosis. TUG1 and CASC7
decreased expression in glioma and promoted apoptosis.
Roles of lncRNAs in indefinite
replication
Telomerase reverse transcriptase (TERT) is a
catalytic subunit of telomerase that serves an important role in
the replication of telomeres (56).
A previous study has demonstrated that in BRL-3A cells,
metastasis-associated lung adenocarcinoma transcript 1 (MALAT1)
regulates telomerase activity by regulating TERT (57). In glioma, MALAT1 expression is
increased and promotes glioma cell proliferation compared with
normal brain tissues (58); thus,
MALAT1 is likely to have a positive effect on telomerase levels in
glioma cells, although no relevant studies have been published to
date.
A previous study has demonstrated that FOXD2
adjacent opposite strand RNA 1 (FOXD2-AS1) upregulates TERT
expression in thyroid cancer (59).
FOXD2-AS1 is also expressed in glioma, and its expression level is
higher compared with that in normal brain tissue. Additionally,
FOXD2-AS1 promotes glioma cell proliferation and migration, and
inhibit apoptosis (60,61). In summary, MALAT1 and FOXD2-AS1
increased expression in glioma compared with normal brain tissue or
brain tissue adjacent to the tumor and promotes indefinite
replication.
Roles of lncRNAs in angiogenesis
In glioma cells, TUG1 expression is increased
compared with normal astrocytes, and knockdown of TUG1 reduces the
expression of VEGFA (52). The study
also demonstrated that TUG1 can be used as a ceRNA of miR-299
(52). Notably, miR-299 inhibits
VEGFA expression by targeting its 3′-UTR; thus, TUG1 may promote
angiogenesis by inhibiting miR-299 and increasing VEGF expression
in glioma cells (52). Another study
has demonstrated that overexpression of H19 in glioma cells also
has an angiogenic effect similar to that of TUG1 (62).
Roles of lncRNA in invasion
The lncRNA plasmacytoma variant translocation 1
(PVT1) is highly expressed in glioma and can sponge miR-128-3p to
inhibit its activity. miR-128-3p inhibits gremlin 1 (GREM1) protein
expression by binding to the 3′-UTR of GREM1 mRNA (63). Studies have demonstrated that PVT1
regulates the bone morphogenetic protein (BMP) signaling pathway
through the miR-128-3p/GREM1 axis, and can upregulate BMP2 and BMP4
(63), promote cell proliferation
and invasion, and inhibit apoptosis in glioma (64). In addition, knockdown of PVT1
negatively regulates miR-424 to inhibit cell activity and the
invasiveness of glioma (65). PVT1
also promotes connective tissue growth factor and angiopoietin 2
expression by sponging and degrading miR-26b (66).
The lncRNA AB073614 is highly expressed in glioma
and is used as an independent indicator of poor prognosis for
patients with glioma (67). AB073614
promotes the epithelial-mesenchymal transition (EMT), proliferation
and migration of glioma cells (68).
The lncRNA TSLC1-AS1 exerts an antitumor effect, and
its expression is decreased in glioma compared with normal brain
tissues (69). TSLC1-AS1 is the
antisense transcript of the tumor suppressor TSLC1, and their
expression levels are positively correlated in glioma (69). TSLC1-AS1 overexpression inhibits the
proliferation, migration and invasiveness of glioma U87 cells
(69). In contrast, knockdown of
TSLC1-AS1 exerts the opposite effect; for example, in knockdown
experiments, double-stranded TSLC1-AS1 small interfering (si)RNA
significantly reduces the expression of TSLC1-AS1 and TSLC1 and
promotes the proliferation, migration and invasiveness of glioma
U87 cells compared with negative control-transfected glioma cells
(69). Additionally, in a study
where the sense and antisense strand of TSLC1-AS1 siRNA were
transfected into glioma cells, it was demonstrated that the
expression levels of TSLC1-AS1 and TSLC1 were decreased in glioma
cells transfected with the sense strand, but not the antisense
strand (69). The aforementioned
study also demonstrated that increased TSLC1-AS1 expression
upregulated the tumor suppressors neurofibromin 1, von
Hippel-Lindau and phosphoinositide-3-kinase regulatory subunit 1,
and inhibited the expression of the oncogene B-Raf proto-oncogene
(69).
CCND2-antisense RNA 1 (CCND2-AS1), H19 and
differentiation antagonizing non-protein coding RNA (DANCR) can
activate the Wnt/β-catenin signaling pathway to promote EMT,
further increase the resistance of glioma cells to TMZ, and promote
cell proliferation and migration (70–72). The
activation of H19 may be mediated by miR-675 (73). In addition, H19 reduces the
inhibition of miR-140 on iASPP through sponging miR-140,
upregulating iASPP expression, and promoting tumor proliferation
and invasion (74). Additionally,
previous studies have demonstrated that in osteosarcoma and
hepatocellular carcinoma, DANCR binds miR-33a-5p and miR-15b-5p to
promote tumor growth (75,76).
HOXA11-AS promotes the invasiveness of glioma by
binding miR-130a-5p to reduce the inhibition of miR-130a-5p on
high-mobility group protein B2 (77).
Another study has demonstrated that UCA1 reduces the
inhibitory effect of miR-204-5p on zinc finger E-box binding
homeobox 1 (ZEB1) through sponging miR-204-5p, which upregulates
ZEB1 and further increases the invasiveness of glioma and EMT
(78).
In summary, increased expression of PVT1, AB073614,
CCND2-AS1, H19, DANCR, HOXA11-AS and UCA1 in glioma compared with
normal brain tissue promotes invasion. TSLC1-AS1 decreased
expression in glioma and inhibits invasion.
Roles of lncRNAs in the reprogramming
of energy metabolism
The expression of TP53 target 1 (TP53TG1) is
significantly higher in human glioma compared with that in normal
brain tissue, and the expression of TP53TG1 is also increased in
the conditions of glucose deprivation (79). Under low glucose conditions, the
increased expression of TP53TG1 upregulates the expression of
glucose-regulated protein 78 and isocitrate dehydrogenase (IDH)1,
and downregulates the expression of pyruvate kinase 2 (PKM2)
(79). Glucose deprivation promotes
the production of reactive oxygen species (ROS) and ROS-mediated
cell death. The pentose phosphate pathway (PPP) produces NADPH,
which detoxifies ROS (80). IDH
promotes NADPH production, whereas PKM2 inhibits the PPP (80,81).
Therefore, TP53TG1 may increase the tolerance of glioma cells to
glucose deprivation by changing the energy metabolism pathway of
the tumor.
Roles of lncRNAs in evading immune
cell attack
LINC00152 has been demonstrated to serve an
immune-related role in glioma, although the specific mechanism has
not yet been determined (82).
Additionally, another study reported nine immunologically related
lncRNAs in anaplastic glioma: Phosphoglucomutases 5-antisense RNA 1
(PGM5-AS1), ST20-antisense RNA 1 (ST20-AS1), ankyrin repeat and PH
domain 2-antisense RNA 1 (AGAP2-AS1), MIR155 host gene (MIR155HG),
SNHG8, LINC00937, TUG1, MAPK activated protein kinase 5-antisense
RNA 1 (MAPKAPK5-AS1) and HLA complex group 18 (HCG18). The former
four are risk-related genes, and the latter four are protective
genes (83). Notably, a number of
studies have demonstrated that TUG1, an lncRNA, is increased in
glioma and that it promotes glioma-associated angiogenesis,
although other studies have reported that its expression is
decreased and that it regulates immune functions and reduces
apoptosis (51,52,83). In
addition, TUG1 has been demonstrated to be upregulated in certain
types of tumors, such as oesophageal squamous cell carcinoma, but
downregulated in others, such as non-small cell lung cancer
(84,85).
Roles of lncRNAs in glioma
subpopulations
Roles of lncRNAs in glioma stem cells
(GSCs)
The expression of the lncRNA nuclear paraspeckle
assembly transcript 1 (NEAT1) is increased in GSCs compared with
astrocytes, and it has been demonstrated that NEAT1 exerts an
effect of mutual inhibition with miR let-7e (86). let-7e is a tumor suppressor that can
target and downregulate neuroblastoma ras (NRAS) to inhibit the
malignant behavior of GSCs; thus, NEAT1 upregulates NRAS to promote
the proliferation, invasion and apoptosis inhibition of GSCs by
sponging let-7e (86). Additionally,
another study has demonstrated that NEAT1 enhances the effects of
GSCs on glioma through the miR-107/CDK6 pathway (87).
The expression of the lncRNA hypoxia-inducible
factor 1-α-antisense RNA 2 (HIF1A-AS2) is upregulated in GSCs and
has been demonstrated to be beneficial to the resistance of GSCs to
hypoxia (88). HIF1A-AS2 interacts
with insulin-like growth factor 2 mRNA-binding protein 2 and
ATP-dependent RNA helicase A to enhance the expression of these two
proteins and to promote the adaptation of GSCs to a hypoxic
environment (88).
The expression of lncRNA linc00152 is upregulated in
GSCs compared with astrocytes and can negatively regulate the
levels of miR-103a-3p by sponging it (89). miR-103a-3p targets and upregulates
FEZ family zinc finger protein 1, promoting the expression of cell
division cycle 25A, which is an oncogene that promotes malignant
behavior of GSCs by activating the PI3K/AKT pathway (89).
lncRNA growth arrest-specific 5 (GAS5) is decreased
in GSCs, where it exerts an antitumor effect (90). GAS5 promotes the expression of the
transcription factor forkhead box O1 (FOXO1) through sponging
miR-196a-5p; FOXO1 subsequently inhibits the malignant biological
behavior of GSCs by upregulating phosphotyrosine interaction domain
containing 1 and migration and invasion inhibitory protein
(91).
Roles of lncRNAs in glioma endothelial
cells (GECs)
The expression of lncRNA H19 is significantly
increased in GECs compared with astrocytes, where it upregulates
vasohibin 2 to promote GEC proliferation, migration and tubule
formation by sponging miR-29a (92).
The expression of the lncRNA PVT1 is significantly
increased in GECs compared with astrocytes, where it interacts with
miR-186 and reduces the expression of autophagy-related 7 (Atg7)
and beclin1 by binding to their mRNAs (93); thus, PVT1 may enhance the expression
of the autophagy-related proteins Atg7 and beclin1 through sponging
miR-186. Atg7 and beclin1 induce protective autophagy; therefore,
PVT1 may promote the proliferation, migration and angiogenesis of
GECs by inducing protective autophagy (93).
Expression of the lncRNA SNHG15 is increased in GEC,
where it sponges miR-153 to inhibit its expression (94). miR-153 inhibits the expression of
VEGFA and CDC42 and in turn inhibits their ability to promote
angiogenesis; thus, SNHG15 may upregulate VEGFA and CDC42 to
promote GEC proliferation, migration and tubule formation (94).
For other cell subpopulations in glioma, such as
immune cells, pericytes, fibroblasts and mesenchymal stem cells, no
specific studies on lncRNAs have been published to date.
Conclusions and future perspectives
Numerous studies have demonstrated that lncRNAs
serve an important role in human tumorigenesis. Overall, lncRNAs
are involved in the acquisition of all eight markers of glioma.
Some lncRNAs promote upregulation of hallmarker biomarkers in
glioma, for example XIST, whereas others can inhibit biomarkers,
for example PLAC2, leading to glioma gaining hallmark
characteristics. Some lncRNAs can promote proliferation and
migration in GSCs and GECs. GECs is a part of tumor
microenvironment; however, there are no reports about the effect of
lncRNA on other parts of tumor microenvironment except GECs, to the
best of our knowledge.
The TME is composed of a variety of extracellular
components, such as the extracellular matrix, various hormones,
cytokines and growth factors, and a variety of cell types,
including endothelial cells, stem cells (including mesenchymal stem
cells), immune cells and fibroblasts (95). The TME serves an important role in
the occurrence and development of tumors as it not only promotes
the occurrence, progression and metastasis of tumors, and maintains
the characterization of tumors in favor of various types of the
cells and extracellular components contained in the tumor
microenvironment, but also serves an important role in the
formation of tumor resistance to chemotherapy (95–97). The
TME promotes the formation of tumor drug resistance in a variety of
ways, such as secreting soluble factors, cell adhesion and
participating in the immune response (95). Therefore, TME intervention may serve
an important role in the treatment of tumors. In recent years,
increased attention has been paid to the role of the TME in glioma;
it has been demonstrated that the TME serves an important role in
glioma progression and treatment effects (3), but only a few studies on lncRNAs in
GECs have been published, and no studies have been published on the
function of lncRNAs in fibroblasts, immune cells and pericytes.
Studies of glioma-associated mesenchymal stem cells (gbMSCs) are
also receiving increasing attention; these cells may be divided
into two subgroups (CD90 gbMSCs and CD90 gbMSCs), the proportion of
which in glioma is associated with the survival rate of patients
(98). Our previous studies
demonstrated that CD90 gbMSCs can differentiate into pericytes, and
that CD90 gbMSCs promote the growth of glioma cells, whereas CD90
gbMSCs promote angiogenesis (99–101).
In addition, our previous study also identified specific lncRNAs in
the two subgroups (99), but at
present, no related studies on lncRNAs in gbMSCs have been
reported. Therefore, based on the important role of the TME and
gbMSCs in the development and treatment of glioma, future studies
should focus on how lncRNAs affect these components of glioma.
Acknowledgements
Not applicable.
Funding
This review was supported by the National Natural
Science Foundation of China (grant no. 81572488).
Availability of data and materials
Not applicable.
Authors' contributions
BZX and WX conceived the study, drafted and modified
the manuscript. QZ, YHW, HFW, DYY, NXX, XBJ and HYZ equally
contributed to data collection. PF revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNA
|
long non-coding RNA
|
|
GSC
|
glioma stem cell
|
|
GEC
|
glioma endothelial cell
|
|
TME
|
tumor microenvironment
|
|
ncRNA
|
non-coding RNA
|
|
ceRNA
|
competitive endogenous RNA
|
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The Next Generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fouad YA and Aanei C: Revisiting the
hallmarks of cancer. Am J Cancer Res. 7:1016–1036. 2017.PubMed/NCBI
|
|
3
|
Quail DF and Joyce JA: The
Microenvironmental landscape of brain tumors. Cancer Cell.
31:326–341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weiss N, Miller F, Cazaubon S and Couraud
P: The blood-brain barrier in brain homeostasis and neurological
diseases. Biochim Biophys. 1788:842–857. 2009. View Article : Google Scholar
|
|
5
|
Wolburg H, Wolburg-Buchholz K, Kraus J,
Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote E, Risau W
and Engelhardt B: Localization of claudin-3 in tight junctions of
the blood-brain barrier is selectively lost during experimental
autoimmune encephalomyelitis and human glioblastoma multiforme.
Acta Neuropathol. 105:586–592. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain RK, di Tomaso E, Duda DG, Loeffler
JS, Sorensen AG and Batchelor TT: Angiogenesis in brain tumours.
Nat Rev Neurosci. 8:610–622. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plate KH, Breier G, Weich HA and Risau W:
Vascular endothelial growth factor is a potential tumour
angiogenesis factor in human gliomas in vivo. Nature. 359:845–848.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gavard J and Gutkind JS: VEGF controls
endothelial-cell permeability by promoting the
beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol.
8:1223–1234. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
ENCODE Project Consortium, . Birney E,
Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH,
Weng Z, Snyder M, Dermitzakis ET, et al: Identification and
analysis of functional elements in 1% of the human genome by the
ENCODE pilot project. Nature. 447:799–816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang DQ, Fu P, Yao C, Zhu LS, Hou TY, Chen
JG, Lu Y, Liu D and Zhu LQ: Long Non-coding RNAs, Novel culprits,
or bodyguards in neurodegenerative diseases. Mol Ther Nucleic
Acids. 10:269–276. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rynkeviciene R, Simiene J, Strainiene E,
Stankevicius V, Usinskiene J, Miseikyte Kaubriene E, Meskinyte I,
Cicenas J and Suziedelis K: Non-coding RNAs in Glioma. Cancers
(Basel). 11:172018. View Article : Google Scholar
|
|
13
|
Kornienko AE, Guenzl PM, Barlow DP and
Pauler FM: Gene regulation by the act of long non-coding RNA
transcription. BMC Biol. 11:592013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ulitsky I and Bartel DP: lincRNAs:
Genomics, evolution, and mechanisms. Cell. 154:26–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tragante V, Moore JH and Asselbergs FW:
The ENCODE project and perspectives on pathways. Genet Epidemiol.
38:275–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhan A and Mandal SS: Long noncoding RNAs:
Emerging stars in gene regulation, epigenetics and human disease.
ChemMedChem. 9:1932–1956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan J, Yue H, Zhang M, Luo J, Liu L, Wu
W, Xiao T, Chen X, Chen X, Zhang D, et al: Transcriptional
profiling analysis and functional prediction of long noncoding RNAs
in cancer. Oncotarget. 16:72016.
|
|
18
|
Tsai M, Spitale RC and Chang HY: Long
intergenic noncoding RNAs: New links in cancer progression. Cancer
Res. 71:3–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie H, Ma H and Zhou D: Plasma HULC as a
promising novel biomarker for the detection of hepatocellular
carcinoma. Biomed Res Int. 2013:1361062013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parasramka MA, Maji S, Matsuda A, Yan IK
and Patel T: Long non-coding RNAs as novel targets for therapy in
hepatocellular carcinoma. Pharmacol Ther. 161:67–78. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Yang F, Yuan J, Yuan S, Zhou W,
Huo X, Xu D, Bi H, Wang F and Sun S: Epigenetic activation of the
MiR-200 family contributes to H19-mediated metastasis suppression
in hepatocellular carcinoma. Carcinogenesis. 34:577–586. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai M, Li S and Qin X: Colorectal
neoplasia differentially expressed: A long noncoding RNA with an
imperative role in cancer. Onco Targets Ther. 11:3755–3763. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du P, Zhao H, Peng R, Liu Q, Yuan J, Peng
G and Liao Y: LncRNA-XIST interacts with miR-29c to modulate the
chemoresistance of glioma cell to TMZ through DNA mismatch repair
(MMR) pathway. Biosci Rep. 37:BSR201706962017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu H, Xue Y, Wang P, Liu X, Ma J, Zheng J,
Li Z, Li Z, Cai H and Liu Y: Knockdown of long non-coding RNA XIST
increases blood-tumor barrier permeability and inhibits glioma
angiogenesis by targeting miR-137. Oncogenesis. 6:e3032017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou H, Ma Y, Zhong D and Yang L:
Knockdown of lncRNA HOXD-AS1 suppresses proliferation, migration
and invasion and enhances cisplatin sensitivity of glioma cells by
sponging miR-204. Biomed Pharmacother. 112:1086332019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui B, Li B, Liu Q and Cui Y: lncRNA CCAT1
promotes glioma tumorigenesis by sponging miR-181b. J Cell Biochem.
118:4548–4557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang O, Huang Y, Wu H, Zheng B, Lin J and
Jin P: LncRNA LOC728196/miR-513c axis facilitates glioma
carcinogenesis by targeting TCF7. Gene. 679:119–125. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wallmen B, Schrempp M and Hecht A:
Intrinsic properties of Tcf1 and Tcf4 splice variants determine
cell-type-specific Wnt/β-catenin target gene expression. Nucleic
Acids Res. 40:9455–9469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li X, Xue L and Peng Q: Tunicamycin
inhibits progression of glioma cells through downregulation of the
MEG-3-regulated wnt/β-catenin signaling pathway. Oncol Lett.
15:8470–8476. 2018.PubMed/NCBI
|
|
30
|
Li X, Shen F, Huang L, Hui L, Liu R, Ma Y
and Jin B: lncRNA small nucleolar RNA host gene 20 predicts poor
prognosis in glioma and promotes cell proliferation by silencing
P21. Oncotargets Ther. 12:805–814. 2019. View Article : Google Scholar
|
|
31
|
Hu Y, Kang C, Zhao J, Nie Y, Zheng L, Li
H, Li X, Wang Q and Qiu Y: LncRNA PLAC2 down-regulates RPL36
expression and blocks cell cycle progression in glioma through a
mechanism involving STAT1. J Cell Mol Med. 22:497–510. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu S, Xu L, Li L, Luo D, Zhao H, Li D and
Peng B: Overexpression of lncRNA PTENP1 suppresses glioma cell
proliferation and metastasis in vitro. Onco Targets Ther.
12:147–156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Q, Zhang J, Liu Y, Zhang W, Zhou J,
Duan R, Pu P, Kang C and Han L: A novel cell cycle-associated
lncRNA, HOXA11-AS, is transcribed from the 5-prime end of the HOXA
transcript and is a biomarker of progression in glioma. Cancer
Lett. 373:251–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui Z, Ren S, Lu J, Wang F, Xu W, Sun Y,
Wei M, Chen J, Gao X, Xu C, et al: The prostate cancer-up-regulated
long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation
through reciprocal regulation of androgen receptor. Urol Oncol.
31:1117–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fang Z, Xu C, Li Y, Cai X, Ren S, Liu H,
Wang Y, Wang F, Chen R, Qu M, et al: A feed-forward regulatory loop
between androgen receptor and PlncRNA-1 promotes prostate cancer
progression. Cancer Lett. 374:62–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin Y, Cui Z, Li X, Jin X and Peng J:
Upregulation of long non-coding RNA PlncRNA-1 promotes
proliferation and induces epithelial-mesenchymal transition in
prostate cancer. Oncotarget. 8:26090–26099. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Yan Y, Zhang C, Wei W, Ai X, Pang
Y and Bian Y: Upregulation of lncRNA PlncRNA-1 indicates the poor
prognosis and promotes glioma progression by activation of Notch
signal pathway. Biomed Pharmacother. 103:216–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yao J, Zhou B, Zhang J, Geng P, Liu K, Zhu
Y and Zhu W: A new tumor suppressor LncRNA ADAMTS9-AS2 is regulated
by DNMT1 and inhibits migration of glioma cells. Tumour Biol.
35:7935–7944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liao Y, Shen L, Zhao H, Liu Q, Fu J, Guo
Y, Peng R and Cheng L: LncRNA CASC2 Interacts With miR-181a to
modulate glioma growth and resistance to TMZ Through PTEN pathway.
J Cell Biochem. 118:1889–1899. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dorasamy MS, Choudhary B, Nellore K,
Subramanya H and Wong P: Dihydroorotate dehydrogenase Inhibitors
Target c-Myc and arrest melanoma, myeloma and lymphoma cells at
S-phase. J Cancer. 8:3086–3098. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang M, Xia P, Hou T, Qi Z, Liao S and
Yang X: MicroRNA-190b inhibits tumor cell proliferation and induces
apoptosis by regulating Bcl-2 in U2OS osteosarcoma cells.
Pharmazie. 72:279–282. 2017.PubMed/NCBI
|
|
42
|
Liu Z, Liu H, Yuan X, Wang Y, Li L, Wang
G, Song J, Shao Z and Fu R: Downregulation of Pim-2 induces cell
cycle arrest in the G0/G1 phase via the p53-non-dependent p21
signaling pathway. Oncol Lett. 15:4079–4086. 2018.PubMed/NCBI
|
|
43
|
Ju X, Yu H, Liang D, Jiang T, Liu Y, Chen
L, Dong Q and Liu X: LDR reverses DDP resistance in ovarian cancer
cells by affecting ERCC-1, Bcl-2, Survivin and Caspase-3
expressions. Biomed Pharmacother. 102:549–554. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dai W, Tian C and Jin S: Effect of lncRNA
ANRIL silencing on anoikis and cell cycle in human glioma via
microRNA-203a. Onco Targets Ther. 11:5103–5109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Seward S, Semaan A, Qazi AM, Gruzdyn OV,
Chamala S, Bryant CC, Kumar S, Cameron D, Sethi S, Ali-Fehmi R, et
al: EZH2 blockade by RNA interference inhibits growth of ovarian
cancer by facilitating re-expression of p21(waf1/cip1) and by
inhibiting mutant p53. Cancer Lett. 336:53–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Taniguchi H, Jacinto FV, Villanueva A,
Fernandez AF, Yamamoto H, Carmona FJ, Puertas S, Marquez VE,
Shinomura Y, Imai K and Esteller M: Silencing of Kruppel-like
factor 2 by the histone methyltransferase EZH2 in human cancer.
Oncogene. 31:1988–1994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fei F, He Y, He S, He Z, Wang Y, Wu G and
Li M: LncRNA SNHG3 enhances the malignant progress of glioma
through silencing KLF2 and p21. Biosci Rep. 38:BSR201804202018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cai G, Zhu Q, Yuan L and Lan Q: LncRNA
SNHG6 acts as a prognostic factor to regulate cell proliferation in
glioma through targeting p21. Biomed Pharmacother. 102:452–457.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu YF, Cai XL, Li ZZ, Lv J, Xiang YA, Chen
JJ, Chen WJ, Sun WY, Liu XM and Chen JB: LncRNA SNHG16 Functions as
an oncogene by sponging MiR-4518 and Up-regulating PRMT5 expression
in glioma. Cell Physiol Biochem. 45:1975–1985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guo LP, Zhang ZJ, Li RT, Li HY and Cui YQ:
Influences of LncRNA SNHG20 on proliferation and apoptosis of
glioma cells through regulating the PTEN/PI3K/AKT signaling
pathway. Eur Rev Med Pharmacol Sci. 23:253–261. 2019.PubMed/NCBI
|
|
51
|
Li J, Zhang M, An G and Ma Q: LncRNA TUG1
acts as a tumor suppressor in human glioma by promoting cell
apoptosis. Exp Biol Med (Maywood). 241:644–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cai H, Liu X, Zheng J, Xue Y, Ma J, Li Z,
Xi Z, Li Z, Bao M and Liu Y: Long non-coding RNA taurine
upregulated 1 enhances tumor-induced angiogenesis through
inhibiting microRNA-299 in human glioblastoma. Oncogene.
36:318–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gong X, Liao X and Huang M: LncRNA CASC7
inhibits the progression of glioma via regulating Wnt/β-catenin
signaling pathway. Pathol Res Pract. 215:564–570. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ni W, Luo L, Zuo P, Li R, Xu X, Wen F and
Hu D: lncRNA GHET1 down-regulation suppresses the cell activities
of glioma. Cancer Biomark. 23:9–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
He Z, Wang Y, Huang G, Wang Q, Zhao D and
Chen L: The lncRNA UCA1 interacts with miR-182 to modulate glioma
proliferation and migration by targeting iASPP. Arch Biochem
Biophys 623–624. 1–8. 2017. View Article : Google Scholar
|
|
56
|
Horn S, Figl A, Rachakonda PS, Fischer C,
Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al:
TERT promoter mutations in familial and sporadic melanoma. Science.
339:959–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tan Y, Tang L, OuYang W, Jiang T, Zhang H
and Li S: β-catenin-coordinated lncRNA MALAT1 up-regulation of
ZEB-1 could enhance the telomerase activity in HGF-mediated
differentiation of bone marrow mesenchymal stem cells into
hepatocytes. Pathol Res Pract. 215:546–554. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fu Z, Luo W, Wang J, Peng T, Sun G, Shi J,
Li Z and Zhang B: Malat1 activates autophagy and promotes cell
proliferation by sponging miR-101 and upregulating STMN1, RAB5A and
ATG4D expression in glioma. Biochem Biophys Res Commun.
492:480–486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu X, Fu Q, Li S, Liang N, Li F, Li C,
Sui C, Dionigi G and Sun H: LncRNA FOXD2-AS1 Functions as a
Competing Endogenous RNA to Regulate TERT Expression by Sponging
miR-7-5p in Thyroid Cancer. Front Endocrinol (Lausanne).
10:2072019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dong H, Cao W and Xue J: Long noncoding
FOXD2-AS1 is activated by CREB1 and promotes cell proliferation and
metastasis in glioma by sponging miR-185 through targeting AKT1.
Biochem Biophys Res Commun. 508:1074–1081. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ni W, Xia Y, Bi Y, Wen F, Hu D and Luo L:
FoxD2-AS1 promotes glioma progression by regulating
miR-185-5P/HMGA2 axis and PI3K/AKT signaling pathway. Aging (Albany
NY). 11:1427–1439. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jiang X, Yan Y, Hu M, Chen X, Wang Y, Dai
Y, Wu D, Wang Y, Zhuang Z and Xia H: Increased level of H19 long
noncoding RNA promotes invasion, angiogenesis, and stemness of
glioblastoma cells. J Neurosurg. 2016:129–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yan K, Wu Q, Yan DH, Lee CH, Rahim N,
Tritschler I, DeVecchio J, Kalady MF, Hjelmeland AB and Rich JN:
Glioma cancer stem cells secrete Gremlin1 to promote their
maintenance within the tumor hierarchy. Gene Dev. 28:1085–1100.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fu C, Li D, Zhang X, Liu N, Chi G and Jin
X: LncRNA PVT1 Facilitates Tumorigenesis and progression of glioma
via regulation of MiR-128-3p/GREM1 Axis and BMP signaling pathway.
Neurotherapeutics. 15:1139–1157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Han Y, Li X, Yan J, Ma C, Zheng X, Zhang
J, Zhang D, Meng C, Zhang Z, Ji X, et al: Knockdown of LncRNA PVT1
inhibits glioma progression by regulating miR-424 expression. Oncol
Res. 27:681–690. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zheng J, Hu L, Cheng J, Xu J, Zhong Z,
Yang Y and Yuan Z: lncRNA PVT1 promotes the angiogenesis of
vascular endothelial cell by targeting miR26b to activate
CTGF/ANGPT2. Int J Mol Med. 42:489–496. 2018.PubMed/NCBI
|
|
67
|
Hu L, Lv Q, Chen S, Sun B, Qu Q, Cheng L,
Guo Y, Zhou H and Fan L: Up-Regulation of long Non-coding RNA
AB073614 predicts a poor prognosis in patients with glioma. Int J
Environ Res Public Health. 13:4332016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li J, Wang Y and Song Y: Knockdown of long
noncoding RNA AB073614 inhibits glioma cell proliferation and
migration via affecting epithelial-mesenchymal transition. Eur Rev
Med Pharmaco. 20:3997–4002. 2016.
|
|
69
|
Qin X, Yao J, Geng P, Fu X, Xue J and
Zhang Z: LncRNA TSLC1-AS1 is a novel tumor suppressor in glioma.
Int J Clin Exp Patho. 7:3065–3072. 2014.
|
|
70
|
Jia L, Tian Y, Chen Y and Zhang G: The
silencing of LncRNA-H19 decreases chemoresistance of human glioma
cells to temozolomide by suppressing epithelial-mesenchymal
transition via the Wnt/β-catenin pathway. Onco Targets Ther.
11:313–321. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li J and Zhou L: Overexpression of lncRNA
DANCR positively affects progression of glioma via activating
Wnt/β-catenin signaling. Biomed Pharmacother. 102:602–607. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang H, Wei D, Wan L, Yan S and Sun Y:
Highly expressed lncRNA CCND2-AS1 promotes glioma cell
proliferation through Wnt/β-catenin signaling. Biochem Bioph Res
Commun. 482:1219–1225. 2017. View Article : Google Scholar
|
|
73
|
Zhang T and Wang Y, Zeng F, Cao H, Zhou H
and Wang Y: LncRNA H19 is overexpressed in glioma tissue, is
negatively associated with patient survival, and promotes tumor
growth through its derivative miR-675. Eur Rev Med Pharmaco.
20:4891–4897. 2016.
|
|
74
|
Zhao H, Peng R, Liu Q, Liu D, Du P, Yuan
J, Peng G and Liao Y: The lncRNA H19 interacts with miR-140 to
modulate glioma growth by targeting iASPP. Arch Biochem Biophys.
610:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jiang N, Wang X, Xie X, Liao Y, Liu N, Liu
J, Miao N, Shen J and Peng T: lncRNA DANCR promotes tumor
progression and cancer stemness features in osteosarcoma by
upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 405:46–55.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang Y, Hou N, Wang X, Wang L, Chang S, He
K, Zhao Z, Zhao X, Song T and Huang C: miR-15b-5p induces
endoplasmic reticulum stress and apoptosis in human hepatocellular
carcinoma, both in vitro and in vivo, by suppressing Rab1A.
Oncotarget. 6:16227–16238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xu C, Xiao L, Liu Y, Chen L, Zheng S, Zeng
E and Li D: The lncRNA HOXA11-AS promotes glioma cell growth and
metastasis by targeting miR-130a-5p/HMGB2. Eur Rev Med Pharmaco.
23:241–252. 2019.
|
|
78
|
Liang C, Yang Y, Guan J, Lv T, Qu S, Fu Q
and Zhao H: LncRNA UCA1 sponges miR-204-5p to promote migration,
invasion and epithelial-mesenchymal transition of glioma cells via
upregulation of ZEB1. Pathol Res Pract. 214:1474–1481. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen X, Gao Y, Li D, Hao B and Cao Y:
LncRNA-TP53TG1 participated in the stress response under glucose
deprivation in glioma. J Cell Biochem. 118:4897–4904. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Anastasiou D, Poulogiannis G, Asara JM,
Boxer MB, Jiang J, Shen M, Bellinger G, Sasaki AT, Locasale JW,
Auld DS, et al: Inhibition of pyruvate kinase M2 by reactive oxygen
species contributes to cellular antioxidant responses. Science.
334:1278–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang
P, Yu W, Li Z, Gong L, Peng Y, et al: Glioma-derived mutations in
IDH1 dominantly inhibit IDH1 catalytic activity and induce
HIF-1alpha. Science. 324:261–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang W, Wu F, Zhao Z, Wang K, Huang R,
Wang H, Lan Q, Wang J and Zhao J: Long noncoding RNA LINC00152 is a
potential prognostic biomarker in patients with high-grade glioma.
CNS Neurosci Ther. 24:957–966. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang W, Zhao Z, Yang F, Wang H, Wu F,
Liang T, Yan X, Li J, Lan Q, Wang J and Zhao J: An immune-related
lncRNA signature for patients with anaplastic gliomas. J
Neurooncol. 136:263–271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang EB, Yin DD, Sun M, Kong R, Liu XH,
You LH, Han L, Xia R, Wang KM, Yang JS, et al: P53-regulated long
non-coding RNA TUG1 affects cell proliferation in human non-small
cell lung cancer, partly through epigenetically regulating HOXB7
expression. Cell Death Dis. 5:e12432014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xu Y, Wang J, Qiu M and Xu L, Li M, Jiang
F, Yin R and Xu L: Upregulation of the long noncoding RNA TUG1
promotes proliferation and migration of esophageal squamous cell
carcinoma. Tumour Biol. 36:1643–1651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gong W, Zheng J, Liu X, Ma J, Liu Y and
Xue Y: Knockdown of NEAT1 restrained the malignant progression of
glioma stem cells by activating microRNA let-7e. Oncotarget.
7:62208–62223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yang X, Xiao Z, Du X, Huang L and Du G:
Silencing of the long non-coding RNA NEAT1 suppresses glioma
stem-like properties through modulation of the miR-107/CDK6
pathway. Oncol Rep. 37:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mineo M, Ricklefs F, Rooj AK, Lyons SM,
Ivanov P, Ansari KI, Nakano I, Chiocca EA, Godlewski J and Bronisz
A: The long Non-coding RNA HIF1A-AS2 facilitates the maintenance of
mesenchymal glioblastoma Stem-like cells in hypoxic niches. Cell
Rep. 15:2500–2509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yu M, Xue Y, Zheng J, Liu X, Yu H, Liu L,
Li Z and Liu Y: Linc00152 promotes malignant progression of glioma
stem cells by regulating miR-103a-3p/FEZF1/CDC25A pathway. Mol
Cancer. 16:1102017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Coccia EM, Cicala C, Charlesworth A,
Ciccarelli C, Rossi GB, Philipson L and Sorrentino V: Regulation
and expression of a growth arrest-specific gene (gas5) during
growth, differentiation, and development. Mol Cell Biol.
12:3514–3521. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhao X, Liu Y, Zheng J, Liu X, Chen J, Liu
L, Wang P and Xue Y: GAS5 suppresses malignancy of human glioma
stem cells via a miR-196a-5p/FOXO1 feedback loop. Biochim Biophys
Acta Mol Cell Res. 1864:1605–1617. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jia P, Cai H, Liu X, Chen J, Ma J, Wang P,
Liu Y, Zheng J and Xue Y: Long non-coding RNA H19 regulates glioma
angiogenesis and the biological behavior of glioma-associated
endothelial cells by inhibiting microRNA-29a. Cancer Lett.
381:359–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ma Y, Wang P, Xue Y, Qu C, Zheng J, Liu X,
Ma J and Liu Y: PVT1 affects growth of glioma microvascular
endothelial cells by negatively regulating miR-186. Tumour Biol.
39:13933953382017. View Article : Google Scholar
|
|
94
|
Ma Y, Xue Y, Liu X, Qu C, Cai H, Wang P,
Li Z, Li Z and Liu Y: SNHG15 affects the growth of glioma
microvascular endothelial cells by negatively regulating miR-153.
Oncol Rep. 38:3265–3277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wu T and Dai Y: Tumor microenvironment and
therapeutic response. Cancer Lett. 387:61–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang Q, Hu B, Hu X, Kim H, Squatrito M,
Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, et al: Tumor
evolution of Glioma-intrinsic gene expression subtypes associates
with immunological changes in the microenvironment. Cancer Cell.
32:42–56.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Shahar T, Rozovski U, Hess KR, Hossain A,
Gumin J, Gao F, Fuller GN, Goodman L, Sulman EP and Lang FF:
Percentage of mesenchymal stem cells in high-grade glioma tumor
samples correlates with patient survival. Neuro Oncol. 19:660–668.
2017.PubMed/NCBI
|
|
99
|
Yi D, Xiang W, Zhang Q, Cen Y, Su Q, Zhang
F, Lu Y, Zhao H and Fu P: Human Glioblastoma-derived mesenchymal
stem cell to pericytes transition and angiogenic capacity in
glioblastoma microenvironment. Cellular physiology and
Biochemistry. 46:279–290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang Q, Yi D, Xue B, Wen WW, Lu YP,
Abdelmaksou A, Sun MX, Yuan DT, Zhao HY, Xiong NX, et al: CD90
determined two subpopulations of glioma-associated mesenchymal stem
cells with different roles in tumour progression. Cell Death Dis.
9:11012018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang Q, Xiang W, Yi D, Xue B, Wen W,
Abdelmaksoud A, Xiong N, Jiang X, Zhao H and Fu P: Current status
and potential challenges of mesenchymal stem cell-based therapy for
malignant gliomas. Stem Cell Res Ther. 9:2282018. View Article : Google Scholar : PubMed/NCBI
|