Introduction
Breast cancer is a major health problem, it
accounted for 11.6% of all new cancer cases and 6.6% of all cancer
deaths among women worldwide in 2018 and its etiology has thus far
remained unidentified (1). According
to epidemiological studies, environmental factors are influencing
the increase in the incidence of breast cancer risk (2). Components as chemicals, including
pesticides, are agents that produce deleterious effects on wildlife
and humans (3–13).
Pesticides constitute a diverse class of xenobiotics
used to protect crops and increase agricultural production. Among
them, the organophosphorus pesticides (OPs) are the most commonly
active insecticide used in the world (3,4). There
is evidence that chronic exposure to these compounds can have
adverse effects on human health. Notably, pesticide exposure has
been associated with an increased incidence of various diseases
such as non-Hodgkin's lymphoma, multiple myeloma, soft tissue
sarcoma, lung sarcoma, and several types of cancer, including
pancreas, stomach, liver, bladder, and gallbladder cancer, as well
as Alzheimer's and Parkinson's disease. Studies seeking to monitor
people exposed to pesticides have shown several indicators of DNA
damage, such as chromosomal aberrations, sister chromatid
exchanges, micronuclei, and single-cell gel electrophoresis, among
others (14–16). Previous studies have associated the
increase of cancer risk with long-term exposure to several OPs in
different countries, including in the USA (5,6,8,9), Canada
(7), and Italy (10) by both occupational and
non-occupational exposures (11–13). For
instance, malathion has been used to control a variety of pests in
crops (17,18). Furthermore, this pesticide has been
associated with certain adverse effects, such as obesity and type 2
diabetes mellitus (19–21) and carcinogenicity (18).
The present review aimed to identify markers that
exhibit properties of cell transformation based on a model that
helps to define whether malathion or other substances affect the
mammary gland and that may complement traditional markers for
breast cancer in humans.
Data collection methods
For this review article, a thorough search on
MEDLINE (through PubMed), Web of Science, and SCOPUS was performed
from inception to May 2019 to identify studies addressing EMT as
markers for the long-term effect of environmental pesticides.
Experimental induced rat mammary gland
cancer model
Experimental mammary gland induced cancer models
allow the ability to study the cellular and molecular mechanisms
involved in breast carcinogenesis and gain an insight into the
factors controlling cell proliferation of the normal mammary
epithelium, both essential for an improved understanding of breast
cancer initiation. An experimental rat mammary gland induced-cancer
model provided an opportunity to study not only cancer initiation
but also to address questions on which markers to use facing
exposure to environmental substances over long time exposure. To
the best of our knowledge, Calaf et al was the first group
to examine the association between OPs and breast cancer using both
in vivo (22–26) and in vitro systems (27–32) and
by a pesticide, specifically, malathion. Epithelial rat mammary
gland cells underwent a stepwise transformation into malignant ones
through an increase of structures called terminal end buds,
important targets associated with rat mammary carcinogenesis
(22,33), these studies indicated that malathion
alone induced changes exclusively at the duct level, increasing in
size and number of cells per mm2 with time. Such ducts
defined as ductal in the proliferation stage, since one notable
finding was that as time progressed those structures were
transformed into mammary gland tumors that revealed a similarity to
ductal carcinomas in comparison with controls, as reported by the
World Health Organization (34).
The administration of an endogenous substance such
as estrogen increased the average number of lobules per
mm2 of rat mammary glands in comparison with control and
malathion at 30, 124, 240 and 400 days after 5 days of treatment
(24). The combination of the
pesticide malathion and estrogen synergistically increased the
number of such lobules following such treatment. Furthermore,
markers for cancer detection such as c-myc, c-fos, CYP, and mutant
p53 were up-regulated following 124 and 240 days after 5 days of
treatment by the effect of malathion and estrogen. On the other
hand, a combination of malathion and estrogen sharply induced
pathological lesions in lung alveolar parenchyma, bronchiolar
epithelial, and lymphatic tissues in comparison with control
animals or animals treated with either substance alone (25). Thus, these results indicated an
increase in the risk of rodent lung tumor formation by
environmental exposure and endogenous substances. Furthermore, the
same combination induced pathological lesions in glomeruli and
convoluted tubules of the kidney indicating malignant
transformation by exposure to environmental and endogenous
substances (26). Thus, rat kidney
tissues were analyzed for histomorphological and immunocytochemical
alterations by the effect of malathion and 17β-estradiol
(estrogen). The animals were injected malathion, estrogen, and the
combination of both substances for 5 days and sacrificed 30, 124,
240 and 400 days after treatments. The results indicated that
Vimentin protein expression was increased in convoluted tubules of
animals treated with a combination of malathion and estrogen after
240 days of 5-day treatment and malignant proliferation was
observed in the hilium zone. In summary, the combination of
malathion and estrogen-induced pathological lesions in glomeruli,
convoluted tubules, atypical cell proliferation, and malignant
proliferation in the hilium zone and immunocytochemical alterations
in comparison with control animals or animals treated with either
substance alone concluding that an increased risk of kidney
malignant transformation could be due to the exposure to
environmental and endogenous substances (26).
On the other hand, malathion alone significantly
increased the number of ducts of mammary glands in the
proliferation stage. Whereas, the results indicated that there was
no rat mammary tumor formation following the injection of atropine
alone or in combination with the pesticide. A marker for cancer
detection such as mutant p53 was up-regulated following treatment
of malathion and down-regulated by atropine after 10 and 20 days.
Atropine is a well-known parasympatholytic alkaloid used as an
antidote for organophosphorus/carbamates poisonings (35).
In vitro studies revealed that either
pesticide parathion or malathion alone or combined with
estrogen-induced malignant transformation, as shown by
anchorage-independent growth capability and invasive
characteristics in comparison with the control as well as genomic
instability, that is the frequency of loss of heterozygosity (LOH)
and microsatellite instability (MSI) in the immortalized human
breast epithelial cell line, MCF-10F. It was found that induced
genomic instability altering p53 and c-Ha-ras protein and gene
expression when treated with parathion or malathion alone and in
combination with estrogen (32). MSI
was found in malathion and estrogen-treated cells with a marker
used for the p53 tumor suppressor gene at loci 17p13.1. The same
combination of substances presented MSI with a marker used for
c-Ha-ras mapped in chromosome 11p14.1, as well as mutations in
c-Ha-ras for codons 12 and 61. LOH was observed in codon 12 in the
presence of estrogen or malathion alone. Parathion alone and
combined with estrogen-induced MSI in codon 61 (32).
Interestingly, malathion was classified as ‘probably
carcinogenic’ to humans (Group 2A) by the International Agency for
Research on Cancer in 2017 (18).
Thereafter, studies have confirmed the increase in risk with OP in
several hormonal-mediated cancers, including breast, thyroid, and
ovary, suggesting potential cancer risk among women (36–39).
Epithelial-to-mesenchymal transition
(EMT)
EMT is a biological process that allows interactions
between the epithelial cells and basement membranes, even in normal
conditions. EMT is a phenotypic change in the epithelial cells
that, under specific conditions, allows cells to acquire a
mesenchymal phenotype characterized by increased motility and one
of these specific conditions is the loss of the adhesion molecule,
E-cadherin, an adhesion protein that induces the EMT process
(40).
Regarding cell-cell interaction, classical cadherins
are transmembrane proteins that participate in
Ca2+-dependent cell adhesion (41,42).
Among them, the E-cadherin is a cell surface protein, functionally
linked to a polarized epithelial phenotype (43,44) and
its intracellular region contains binding sites that interact with
catenins and other regulatory proteins (45,46).
Chen et al (40) investigated the E-cadherin expression
patterns in primary breast cancers and metastatic lymph nodes and
observed that E-cadherin expression was aberrant in invasive ductal
cancer and their corresponding metastatic lymph nodes. E-cadherin
expression in the metastasized lymph node was closely associated
with tumor size and the number of metastasized lymph nodes
(40). Other reports have indicated
that overexpression of Slug suppressed E-cadherin expression, with
an eventual decrease in intercellular adhesion (47,48) and
initiation of the EMT in breast cancer due to the loss of
E-cadherin expression (49,50).
Among other proteins involved in EMT, it should be
mentioned Vimentin as a structural protein that supports and
anchors the position of organelles in the cytosol. It also controls
the transport of low-density lipoprotein (LDL)-derived cholesterol
from a lysosome to the site of esterification (51–53).
Then, the EMT process allows tumor cells to undergo phenotypic
changes the including migratory and invasive capabilities (54). Thus, EMT can be initiated by
overexpression of certain proteins, for instance, Vimentin that is
present in progression from in situ to invasive breast
cancer (55). EMT induces the
expression of other proteins such as fibronectin, N-cadherin
(55,56). This process comprises loss of cell
cohesiveness and reorganization of the cytoskeleton (54). It was observed that there was a
greater level of Vimentin protein expression in the mammary gland
of pesticide-treated rats when compared with the control with
metastatic properties similar to results observed in patients with
breast cancer (57).
Another one is Axl, a cell surface receptor that has
emerged as a key facilitator of immune escape and drug-resistance
in cancer cells, leading to aggressive and metastatic cancer
(58). Axl belongs to the TAM family
of receptor tyrosine kinases. In particular, Axl is the central
regulator of various signaling pathways implicated in cancer
(59) that upon binding with its
ligand, growth arrest-specific protein 6 (Gas6), undergoes
dimerization and autophosphorylation which subsequently activates
the downstream signaling pathways associated with carcinogenesis,
such as the phosphatidylinositol 3-kinase/protein kinase B and the
nuclear factor-κB (NFkB) signaling pathways (60,61).
Besides, Axl is an underlying oncogenic factor that is involved in
the EMT process, which allows epithelial cells to undergo cell
migration and invasion, inducing tumor progression (62).
Main findings
Firstly, the effect of malathion and estrogen was
analyzed in these studies and showed that a combination of both
substances caused malignant phenotypic alterations in mammary gland
tissues and the changes increased progressively with time after
exposure that was greater than control. The present study showed
important histological alterations in the rat mammary gland treated
with malathion, estrogen, and combination of both in comparison to
controls (63).
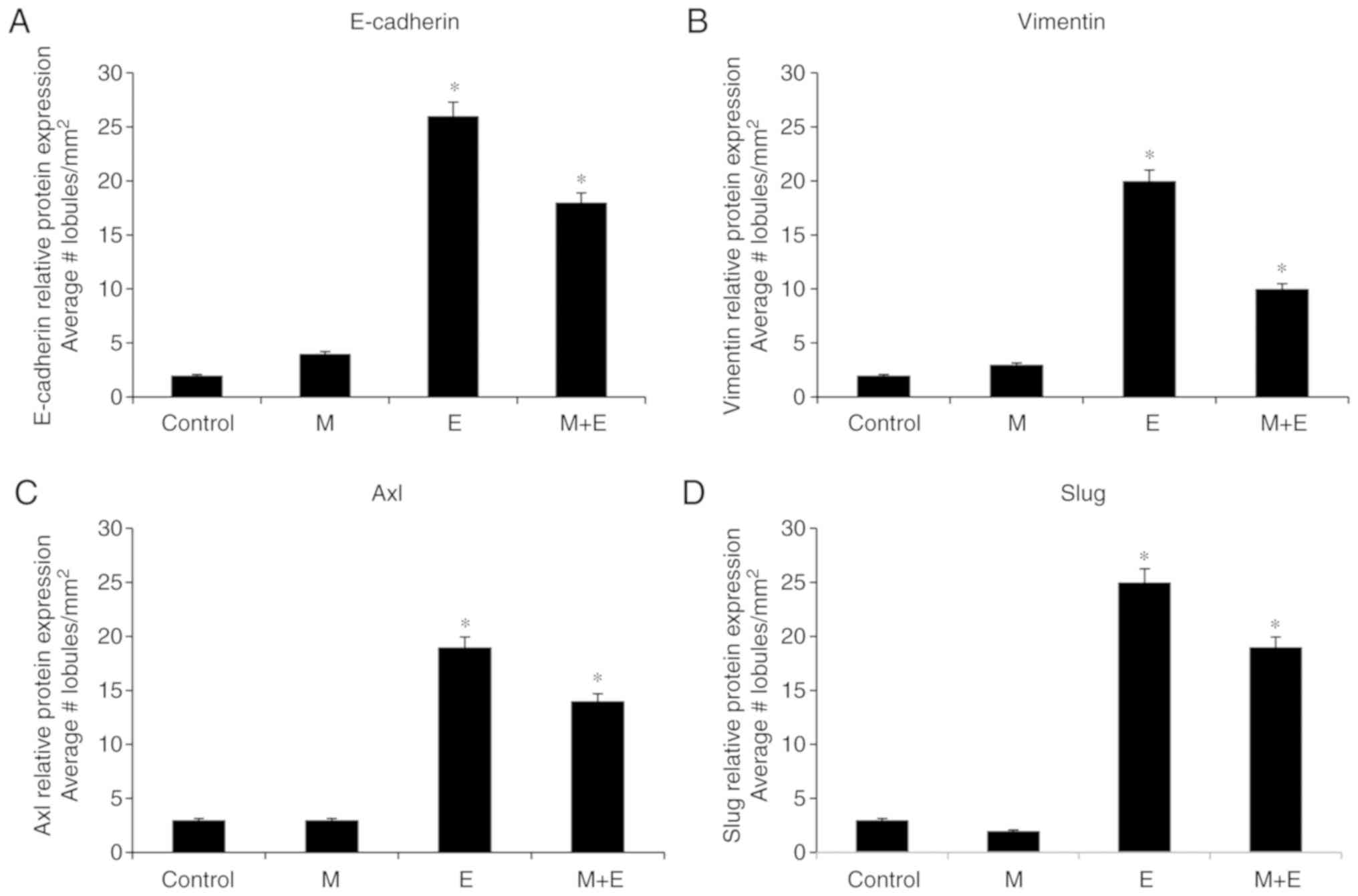
Graphs of Fig. 1A-D
correspond to the quantification of the effect of malathion (M),
estrogen (E), and combinations of both substances (M + E) in
comparison to control on (A) E-cadherin, (B) Vimentin, (C) Axl, and
(D) Slug protein expression in lobules of rat mammary gland
(lobules/mm2). The results indicated that malathion
alone did not change the number of lobules in sections
immune-stained when compared with control, estrogen, and
combinations of both substances. However, malathion and a
combination with estrogen increased the number of lobules in the
stage of proliferation in comparison with the control. Results were
expressed as the average ± standard error (SE) of the mean.
Comparison between groups was determined by ANOVA and Dunnett´s
test using STATA 12 software (StataCorp LP) and P≤0.05 was
considered to indicate a statistically significant difference. The
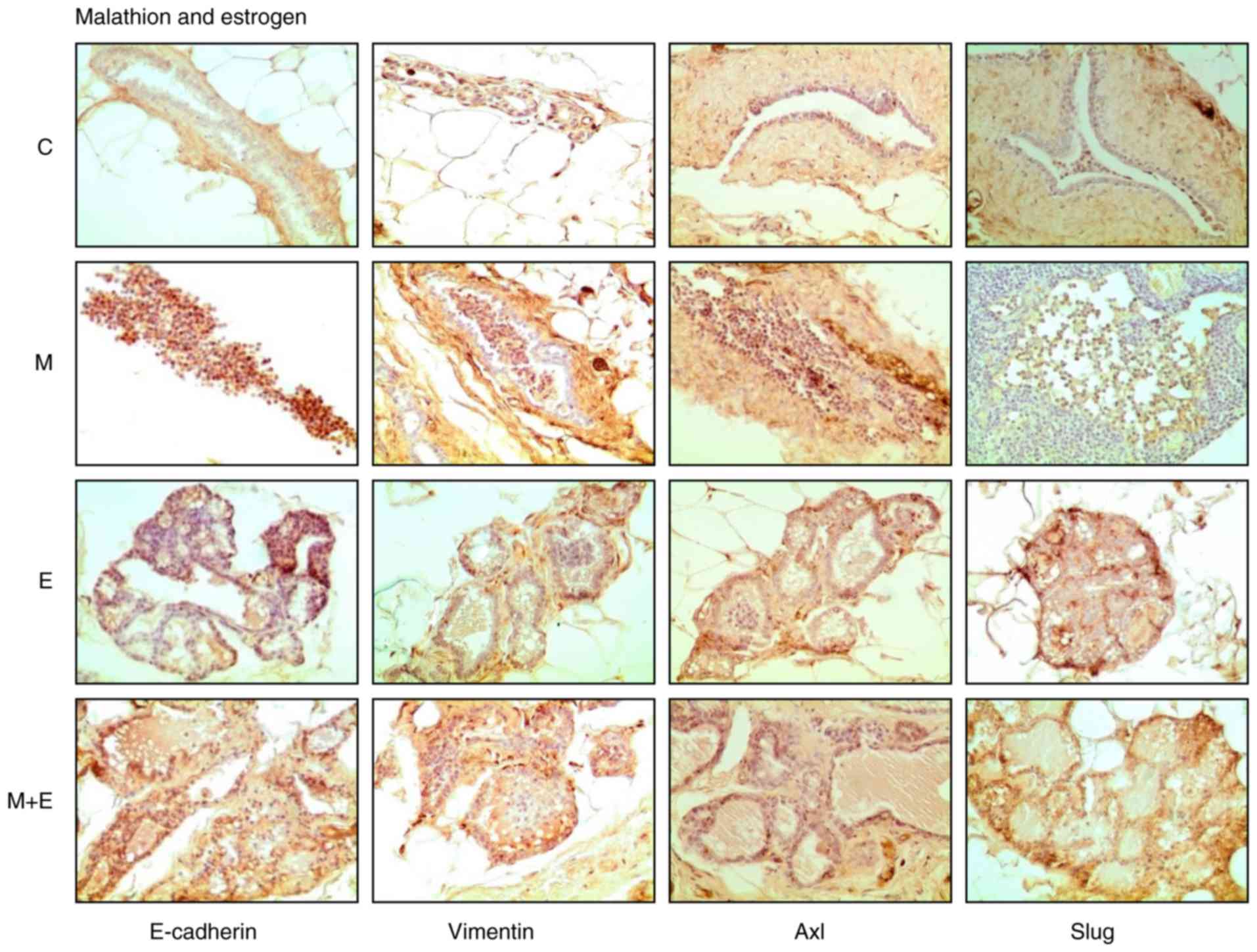
stimulatory effect of estrogen was analyzed, and Fig. 2 shows the cross-section of
representative images of mammary gland structures immune-stained
with E-cadherin, Vimentin, Axl, and Slug by the effect of malathion
(M), estrogen (E), and combination of both substances (M + E) in
comparison to control (C).
Secondly, complementary, Calaf (63) studied E-cadherin, Slug, Axl, and
Vimentin to analyze the effect of malathion and atropine as its
antagonist that confirmed the malignant phenotypic alterations in
rat mammary gland tissues. The changes increased progressively with
time after exposure in comparison with control reaching mammary
gland tumor formation. Graphs of Fig.
3A-D correspond to the quantification of the intensity of
E-cadherin, Vimentin, Axl, and Slug protein expressions of ducts in
the proliferation stage (dsp/mm2) by the effect of
malathion (M), atropine (A), and combination of both substances
(M+A) in comparison to control of rat mammary glands. The main
findings indicated that the malathion-treated group had
significantly (P<0.05) higher E-cadherin (Fig. 3A), Vimentin (Fig. 3B), Axl (Fig. 3C) and Slug (Fig. 3D) protein expression than the
control, atropine, and combination of both. Interestingly, the
malathion effect was counteracted by the effect of atropine
providing evidence that a pesticide affects the mammary gland
carcinogenesis as shown in an experimental induced mammary gland
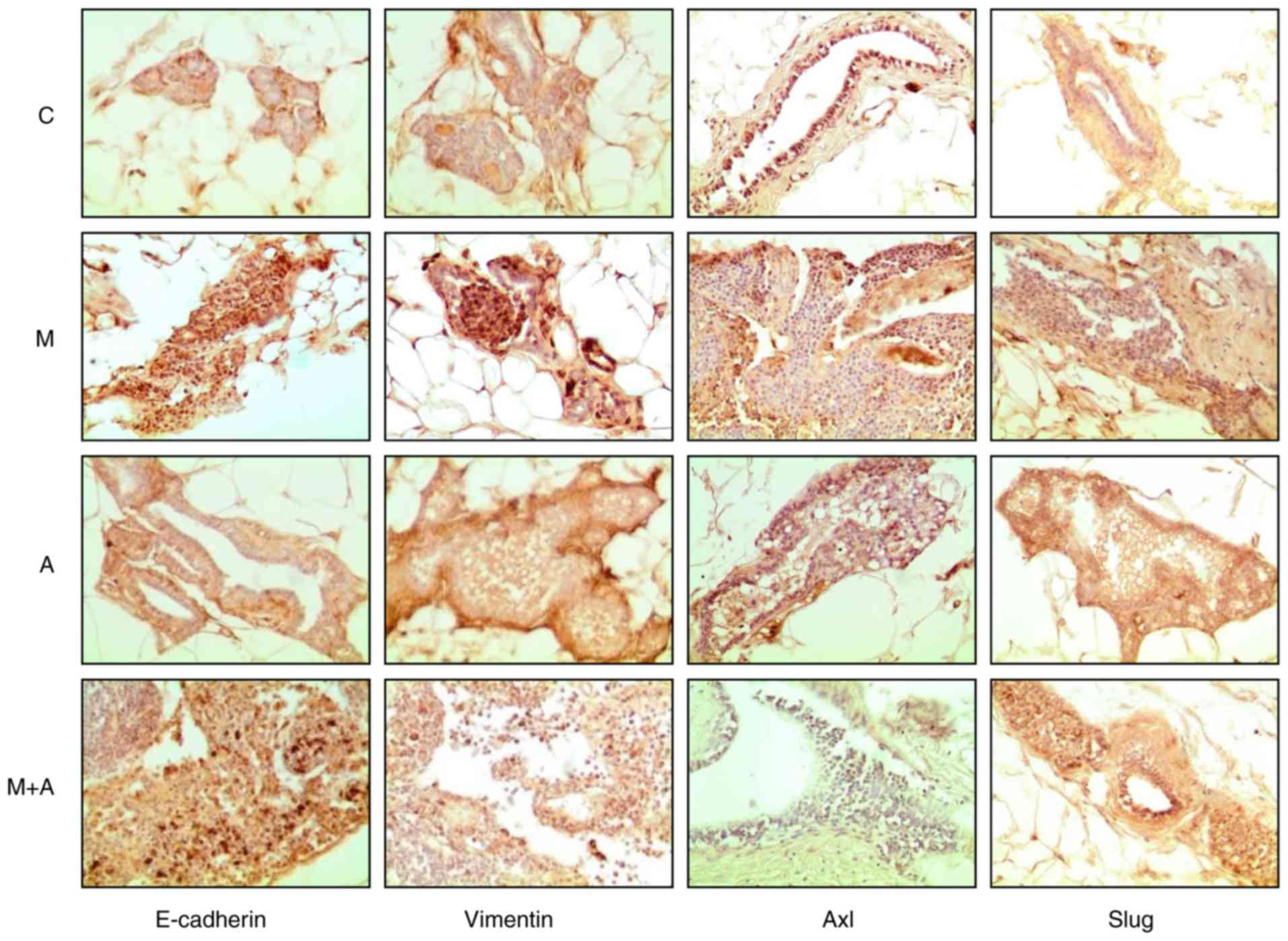
cancer model. Fig. 4 displays the
cross-section of representative images showing the effect malathion
(M), atropine (A), and combination of both substances (M+A) in
comparison to control (C) on E-cadherin, Vimentin, Axl, and Slug
protein expressions of ducts in stage of proliferation
(dsp/mm2) of rat mammary glands.
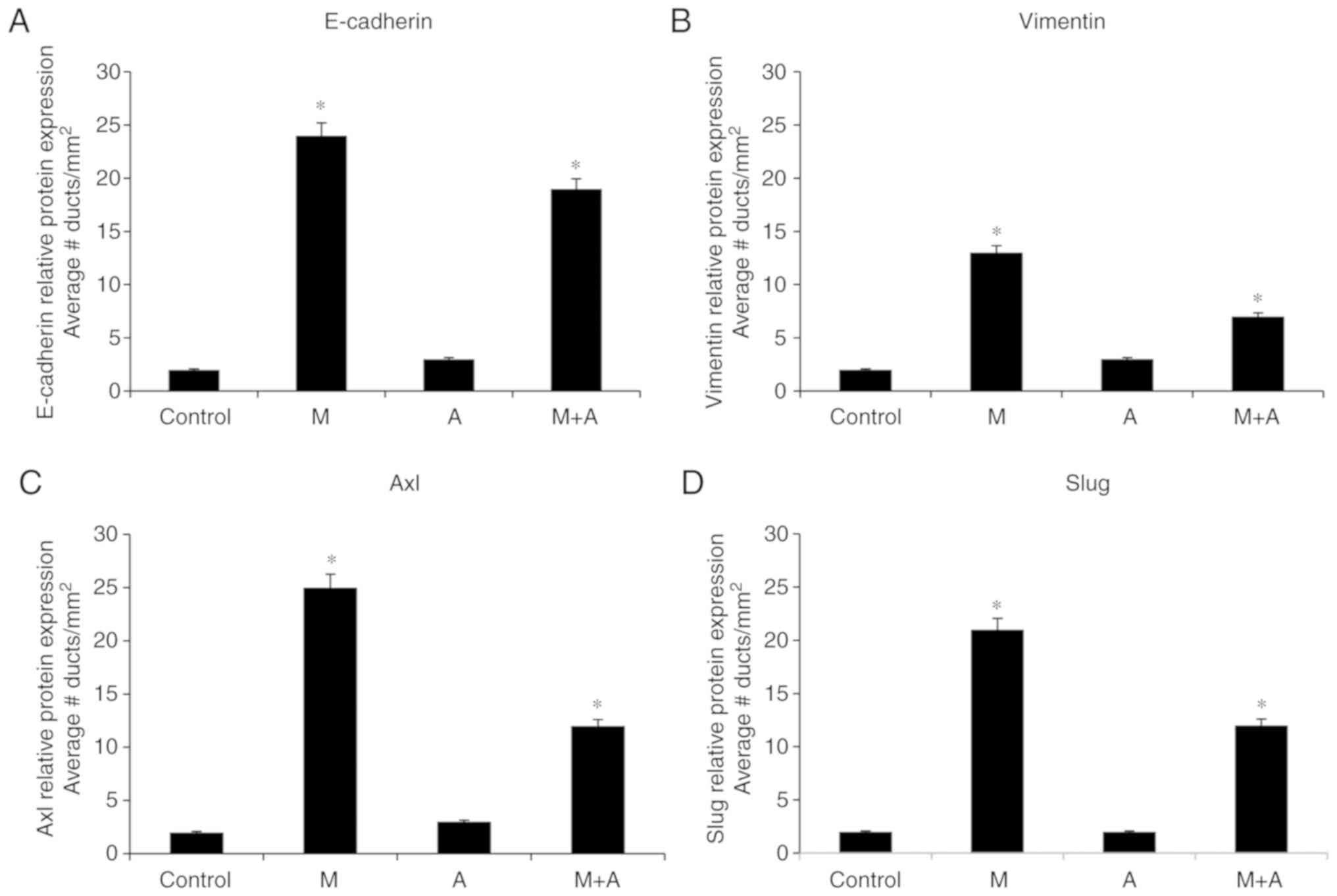 | Figure 3.Graphs correspond to the
quantification of the effect of M, A and a combination of both
substances (M + A) in comparison to control on (A) E-cadherin
(sc-8426), (B) Vimentin (sc-6260), (C) Axl (sc-166269) and (D) Slug
(sc-166476) protein expressions of ducts in stage of proliferation
(dsp/mm2) of rat mammary glands. (P<0.05). (All
antibodies from Santa Cruz Biotechnology, Inc.). Results scored
according to a scale from 0 to 30 points. The intensity of protein
expression ratings: None (0 points), weak (10 points), slight (15
points), moderate (20 points), and intense (30 points). Structures
were graded as 0 when the morphology of normal structure was
present and there were no cells in the proliferative ducts. Results
expressed as the average ± standard error (SE) of the mean.
Comparison between control and treated groups made by ANOVA and
Dunnet´s test. *P<0.05. M, malathion; E, estrogen. |
Conclusions and future perspectives
In summary, we used several markers associated with
EMT, including E-cadherin, Slug, Axl, and Vimentin. It was observed
the counteracted effect of an antagonist such as atropine, or the
stimulatory effect of estrogen as an endogenous factor in ductal
and lobular rat mammary gland carcinomas, respectively. The present
review demonstrated that EMT such as Slug as well as E-cadherin,
Vimentin, and Axl could be suitable biological markers for breast
cancer progression, besides the traditional cancer markers.
Alteration of these markers may serve as a predicting factor to
indicate whether a person has altered ducts or lobules in breast
tissues indicated in biopsies of persons exposed to OPs or other
environmental substances.
Acknowledgements
The authors would like to thank Mrs. Georgina Vargas
Marchant, Mrs. Guiliana Rojas and Mr. Leodán A. Crispin, (Instituto
de Alta Investigación, Universidad de Tarapacá), for providing
technical support.
Funding
The current study was supported by a grant from
Universidad de Tarapacá, Convenio de Desempeño (grant no. UTA1117)
to GMV, FONDECYT (grant no. 1200656) to GMC. Fondecyt (grant no.
1161219) to FA, Conicyt Fondap (grant no. 15130011) to FA, and
FONDECYT (Postdoctoral grant no. 3190744) to JPM.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GMC wrote the manuscript, and JPM, FA and TCB edited
and reviewed the manuscript and agreed to be accountable for all
aspects of the revision in ensuring that the accuracy or integrity
of any part of the work was appropriately conducted. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knower KC, To SQ, Leung YK, Ho SM and
Clyne CD: Endocrine disruption of the epigenome: A breast cancer
link. Endocr Relat Cancer. 21:T33–T55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grube A, Donaldson D, Kiely T and Wu L:
Pesticide industry sales and usage: 2006 and 2007 market estimates.
US Environmental Protection Agency. (Washington DC).
2011.Aug.
2018
|
|
4
|
EPA, . Mosquito control: Malathion. US
Environmental Protection Agency. 2014.Aug.
2018
|
|
5
|
IARC, . Agents classified by the IARC
monographs. 1-122:International Agency for Research on Cancer,
World Health Organization. (Lyon, France). 2018.Aug.
2018
|
|
6
|
De Roos AJ, Zahm SH, Cantor KP,
Weisenburger DD, Holmes FF, Burmeister LF and Blair A: Integrative
assessment of multiple pesticides as risk factors for non-Hodgkin's
lymphoma among men. Occup Environ Med. 60:E112003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McDuffie HH, Pahwa P, McLaughlin JR,
Spinelli JJ, Fincham S, Dosman JA, Robson D, Skinnider LF and Choi
NW: Non-Hodgkin's lymphoma and specific pesticide exposures in men:
Cross-canada study of pesticides and health. Cancer Epidemiol
Biomarkers Prev. 10:1155–1163. 2001.PubMed/NCBI
|
|
8
|
Mills PK and Yang R: Prostate cancer risk
in California farm workers. J Occup Environ Med. 45:249–258. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pesatori AC, Sontag JM, Lubin JH, Consonni
D and Blair A: Cohort mortality and nested case-control study of
lung cancer among structural pest control workers in Florida
(United States). Cancer Causes Control. 5:310–318. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miligi L, Costantini AS, Bolejack V,
Veraldi A, Benvenuti A, Nanni O, Ramazzotti V, Tumino R, Stagnaro
E, Rodella S, et al: Non-Hodgkin's lymphoma, leukemia, and
exposures in agriculture: Results from the Italian multicenter
case-control study. Am J Ind Med. 44:627–636. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klaasen C: Nonmetallic environmental
toxicants: Air pollutants, solvents and vapors, and pesticides. In:
The Pharmacological Basis of Therapeutics. Goodman RT, Gilman A,
Nies AS and Taylor P: Pergamon Press Inc; New York: pp. 1615–1635.
1990
|
|
12
|
EPA, . Registration review; Draft
malathion human health risk assessment. US Environmental Protection
Agency. (Washington DC). 2016.Aug.
2018
|
|
13
|
EPA, . Revised Chlorpyrifos Human Health
Risk Assessment. US Environmental Protection Agency. (Washington
DC). 2014.http://www.regulations.gov/Aug. 2018
|
|
14
|
Bernieri T, Moraes MF, Ardenghi PG and
Basso da Silva L: Assessment of DNA damage and cholinesterase
activity in soybean farmers in southern Brazil: High versus low
pesticide exposure. J Environ Sci Health B. 55:355–360. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kapka-Skrzypczak L, Cyranka M, Skrzypczak
M and Kruszewski M: Biomonitoring and biomarkers of organophosphate
pesticides exposure-state of the art. Ann Agric Environ Med.
18:294–303. 2011.PubMed/NCBI
|
|
16
|
Shah HK, Sharma T and Banerjee BD:
Organochlorine pesticides induce inflammation, ROS production, and
DNA damage in human epithelial ovary cells: An in vitro study.
Chemosphere. 246:1256912020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guyton KZ, Loomis D, Grosse Y, El
Ghissassi F, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H
and Straif K; International Agency for Research on Cancer Monograph
Working Group, IARC, Lyon, France, : Carcinogenicity of
tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate.
Lancet Oncol. 16:490–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
IARC, . Some organophosphate insecticides
and herbicides. IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans. 112:International Agency for Research
on Cancer. (Lyon, France). 2017.https://monographs.iarc.fr/iarc-monographs-on-the-evaluation-of-carcinogenic-risks-to-humans-4/July.
2017
|
|
19
|
Elobeid MA, Padilla MA, Brock DW, Ruden DM
and Allison DB: Endocrine disruptors and obesity: An examination of
selected persistent organic pollutants in the NHANES 1999–2002
data. Int J Environ Res Public Health. 7:2988–3005. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Everett CJ, Frithsen IL, Diaz VA, Koopman
RJ, Simpson WM Jr and Mainous AG III: Association of a
polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and
DDT with diabetes in the 1999–2002 national health and nutrition
examination Survey. Environ Res. 103:413–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beard J; Australian Rural Health Research
Collaboration, : DDT and human health. Sci Total Environ.
355:78–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calaf GM, Parra E and Garrido F: Cell
proliferation and tumor formation induced by eserine, an
acetylcholinesterase inhibitor, in rat mammary gland. Oncol Rep.
17:25–33. 2007.PubMed/NCBI
|
|
23
|
Calaf GM and Garrido F: Catechol estrogens
as biomarkers for mammary gland cancer. Int J Oncol. 39:177–183.
2011.PubMed/NCBI
|
|
24
|
Calaf GM and Echiburu-Chau C: Synergistic
effect of malathion and estrogen on mammary gland carcinogenesis.
Oncol Rep. 28:640–646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Echiburu-Chau C and Calaf GM: Rat lung
cancer induced by malathion and estrogen. Int J Oncol. 33:603–611.
2008.PubMed/NCBI
|
|
26
|
Alfaro-Lira S, Pizarro-Ortiz M and Calaf
GM: Malignant transformation of rat kidney induced by environmental
substances and estrogen. Int J Environ Res Public Health.
9:1630–1648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calaf GM and Roy D: Human drug metabolism
genes in parathion-and estrogen-treated breast cells. Int J Mol
Med. 20:875–881. 2007.PubMed/NCBI
|
|
28
|
Calaf GM and Roy D: Gene and protein
expressions induced by 17beta-estradiol and parathion in cultured
breast epithelial cells. Mol Med. 13:255–265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calaf GM and Roy D: Gene expression
signature of parathion-transformed human breast epithelial cells.
Int J Mol Med. 19:741–750. 2007.PubMed/NCBI
|
|
30
|
Calaf GM and Roy D: Cancer genes induced
by malathion and parathion in the presence of estrogen in breast
cells. Int J Mol Med. 21:261–268. 2008.PubMed/NCBI
|
|
31
|
Calaf GM and Roy D: Cell adhesion proteins
altered by 17beta estradiol and parathion in breast epithelial
cells. Oncol Rep. 19:165–169. 2008.PubMed/NCBI
|
|
32
|
Calaf GM, Echiburu-Chau C and Roy D:
Organophosphorous pesticides and estrogen induce transformation of
breast cells affecting p53 and c-Ha-ras genes. Int J Oncol.
35:1061–1068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cabello G, Valenzuela M, Vilaxa A, Duran
V, Rudolph I, Hrepic N and Calaf G: A rat mammary tumor model
induced by the organophosphorous pesticides parathion and
malathion, possibly through acetylcholinesterase inhibition.
Environ Health Perspect. 109:471–479. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoon Tan P, Ellis I, Allison K, Brogi E,
Fox SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al:
The 2019 WHO classification of tumours of the breast.
Histopathology. Feb 13–2020.(Epub ahead of print). PubMed/NCBI
|
|
35
|
Taylor P: Anticholinesterase agents. In:
The Pharmacological Basis of Therapeutics. Goodman RT, Gilman A,
Nies AS and Taylor P: Pergamon Press Inc.; New York: pp. 131–147.
1990, PubMed/NCBI
|
|
36
|
Omran OM and Omer OH: The effects of
alpha-lipoic acid on breast of female albino rats exposed to
malathion: Histopathological and immunohistochemical study. Pathol
Res Pract. 211:462–469. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nilsson E, Klukovich R, Sadler-Riggleman
I, Beck D, Xie Y, Yan W and Skinner MK: Environmental toxicant
induced epigenetic transgenerational inheritance of ovarian
pathology and granulosa cell epigenome and transcriptome
alterations: Ancestral origins of polycystic ovarian syndrome and
primary ovarian insufiency. Epigenetics. 13:875–895. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Skinner MK, Manikkam M, Tracey R,
Guerrero-Bosagna C, Haque M and Nilsson EE: Ancestral
dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic
transgenerational inheritance of obesity. BMC Med. 11:2282013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kao CC, Que DE, Bongo SJ, Tayo LL, Lin YH,
Lin CW, Lin SL, Gou YY, Hsu WL, Shy CG, et al: Residue levels of
organochlorine pesticides in breast milk and its associations with
cord blood thyroid hormones and the Offspring's neurodevelopment.
Int J Environ Res Public Health. 16:14382019. View Article : Google Scholar
|
|
40
|
Chen L, Jian W, Lu L, Zheng L, Yu Z and
Zhou D: Elevated expression of E-cadherin in primary breast cancer
and its corresponding metastatic lymph node. Int J Clin Exp Med.
8:11752–11758. 2015.PubMed/NCBI
|
|
41
|
Gumbiner BM: Regulation of
cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol.
6:622–634. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tepass U: Genetic analysis of cadherin
function in animal morphogenesis. Curr Opin Cell Biol. 11:540–548.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wheelock MJ and Jensen PJ: Regulation of
keratinocyte intercellular junction organization and epidermal
morphogenesis by E-cadherin. J Cell Biol. 117:415–425. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jeanes A, Gottardi CJ and Yap AS:
Cadherins and cancer: How does cadherin dysfunction promote tumor
progression? Oncogene. 27:6920–6929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shapiro L and Weis WI: Structure and
biochemistry of cadherins and catenins. Cold Spring Harb Perspect
Biol. 1:a0030532009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Perez-Moreno M and Fuchs E: Catenins:
Keeping cells from getting their signals crossed. Dev Cell.
11:601–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Alves CC, Carneiro F, Hoefler H and Becker
KF: Role of the epithelial-mesenchymal transition regulator Slug in
primary human cancers. Front Biosci (Landmark Ed). 14:3035–3050.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim J, Bae S, An S, Park JK, Kim EM, Hwang
SG, Kim WJ and Um HD: Cooperative actions of p21WAF1 and p53 induce
Slug protein degradation and suppress cell invasion. EMBO Rep.
15:1062–1068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen J, Imanaka N, Chen J and Griffin JD:
Hypoxia potentiates Notch signaling in breast cancer leading to
decreased E-cadherin expression and increased cell migration and
invasion. Br J Cancer. 102:351–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hajra KM, Chen DY and Fearon ER: The SLUG
zinc-finger protein represses E-cadherin in breast cancer. Cancer
Res. 62:1613–1618. 2002.PubMed/NCBI
|
|
51
|
Wong SHM, Fang CM, Chuah LH, Leong CO and
Ngai SC: E-cadherin: Its dysregulation in carcinogenesis and
clinical implications. Crit Rev Oncol Hematol. 121:11–22. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu T, Zhang X, Shang M, Zhang Y, Xia B,
Niu M, Liu Y and Pang D: Dysregulated expression of Slug, vimentin,
and E-cadherin correlates with poor clinical outcome in patients
with basal-like breast cancer. J Surg Oncol. 107:188–194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Myong NH: Loss of E-cadherin and
acquisition of vimentin in epithelial-mesenchymal transition are
noble indicators of uterine cervix cancer progression. Korean J
Pathol. 46:341–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: Role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Calaf GM, Balajee AS, Montalvo-Villagra
MT, Leon M, Daniela NM, Alvarez RG, Roy D, Narayan G and
Abarca-Quinones J: Vimentin and Notch as biomarkers for breast
cancer progression. Oncol Lett. 7:721–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Davidsen KT, Haaland GS, Lie MK, Lorens JB
and Engelsen AST: The role of Axl receptor tyrosine kinase in tumor
cell plasticity and therapy resistance. Biomarkers of the Tumor
Microenvironment: Basic Studies and Practical Applications. Akslen
LA and Watnick RS: Springer; Switzerland: pp. 351–376. 2017,
View Article : Google Scholar
|
|
59
|
Meyer AS, Miller MA, Gertler FB and
Lauffenburger DA: The receptor AXL diversifies EGFR signaling and
limits the response to EGFR-targeted inhibitors in triple-negative
breast cancer cells. Sci Signal. 6:ra662013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yanagita M, Arai H, Nakano T, Ohashi K,
Mizuno K, Fukatsu A, Doi T and Kita T: Gas6 induces mesangial cell
proliferation via latent transcription factor STAT3. J Biol Chem.
276:42364–42369. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Linger RM, Keating AK, Earp HS and Graham
DK: TAM receptor tyrosine kinases: Biologic functions, signaling,
and potential therapeutic targeting in human cancer. Adv Cancer
Res. 100:35–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tai KY, Shieh YS, Lee CS, Shiah SG and Wu
CW: Axl promotes cell invasion by inducing MMP-9 activity through
activation of NF-kappaB and Brg-1. Oncogene. 27:4044–4055. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Calaf GM: Carcinogenicity of malathion and
estrogen in an experimental rat mammary gland model. Siberian J
Oncol. 17:5–13. 2018. View Article : Google Scholar
|