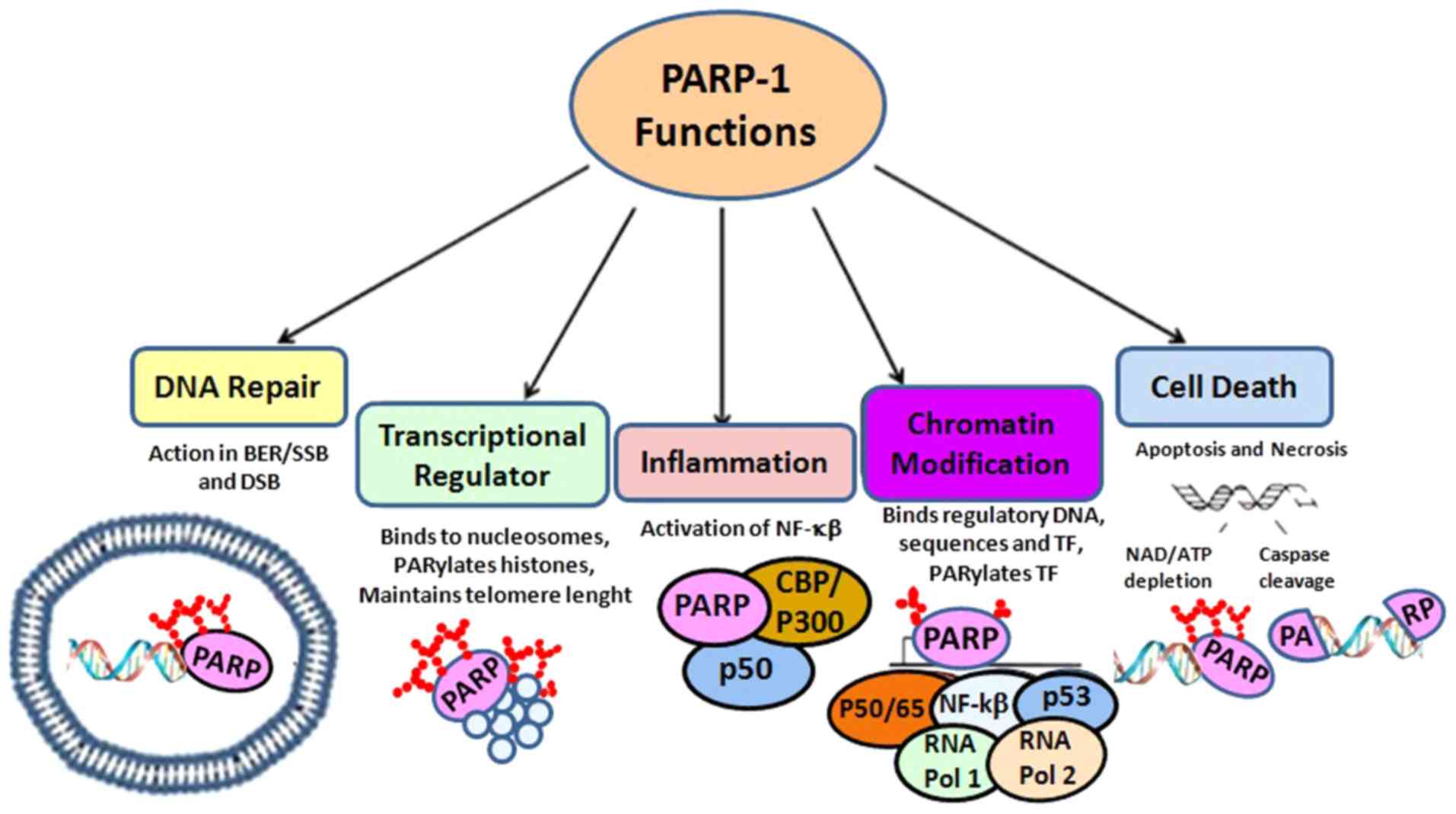
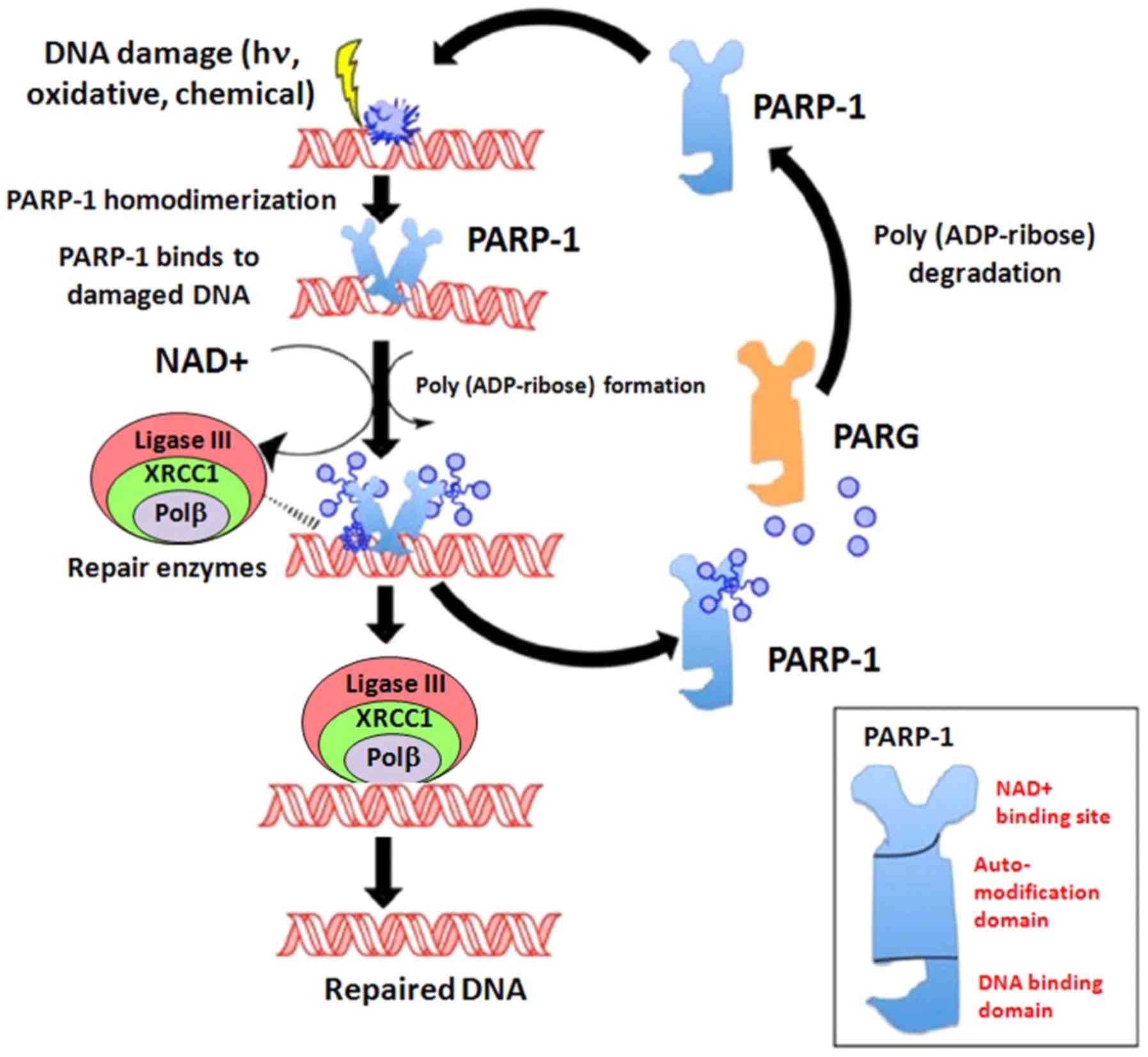
|
1
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R; Gynecologic Oncology Group, : Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
gynecologic oncology group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade- Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, Kurzeder C, et al: A phase 3 trial of bevacizumab in
ovarian cancer. N Engl J Med. 365:2484–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Incorporation of bevacizumab in the primary treatment of
ovarian cancer. N Engl J Med. 365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ranieri G, Ferrari C, Di Palo A, Marech I,
Porcelli M, Falagario G, Ritrovato F, Ramunni L, Fanelli M, Rubini
G and Gadaleta CD: Bevacizumab-based chemotherapy combined with
regional deep capacitive hyperthermia in metastatic cancer
patients: A pilot study. Int J Mol Sci. 18:14582017. View Article : Google Scholar
|
|
5
|
Ranieri G, Patruno R, Ruggieri E,
Montemurro S, Valerio P and Ribatti D: Vascular endothelial growth
factor (VEGF) as a target of bevacizumab in cancer: From the
biology to the clinic. Curr Med Chem. 13:1845–1857. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gadducci A and Guerrieri ME: PARP
inhibitors alone and in combination with other biological agents in
homologous recombination deficient epithelial ovarian cancer: From
the basic research to the clinic. Crit Rev Oncol Hematol.
114:153–165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sunada S, Nakanishi A and Miki Y:
Crosstalk of DNA double-strand break repair pathways in
poly(ADP-ribose) polymerase inhibitor treatment of breast cancer
susceptibility gene 1/2-mutated cancer. Cancer Sci. 109:893–899.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bitler BG, Watson ZL, Wheeler LJ and
Behbakht K: Behbakht K: PARP inhibitors: Clinical utility and
possibilities of overcoming resistance. Gynecol Oncol. 147:695–704.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alsop K, Fereday S, Meldrum C, deFazio A,
Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, et
al: BRCA mutation frequency and patterns of treatment response in
BRCA mutation-positive women with ovarian cancer: A report from the
Australian ovarian cancer study group. J Clin Oncol. 30:2654–2663.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hennessy BT, Timms KM, Carey MS, Gutin A,
Meyer LA, Flake DD II, Abkevich V, Potter J, Pruss D, Glenn P, et
al: Somatic mutations in BRCA1 and BRCA2 could expand the number of
patients that benefit from poly (ADP ribose) polymerase inhibitors
in ovarian cancer. J Clin Oncol. 28:3570–3576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Richter S, Haroun I, Graham TC, Eisen A,
Kiss A and Warner E: Variants of unknown significance in BRCA
testing: Impact on risk perception, worry, prevention and
counseling. Ann Oncol. 24 (Suppl 8):viii69–viii74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frank TS, Deffenbaugh AM, Reid JE, Hulick
M, Ward BE, Lingenfelter B, Gumpper KL, Scholl T, Tavtigian SV,
Pruss DR and Critchfield GC: Clinical characteristics of
individuals with germline mutations in BRCA1 and BRCA2: Analysis of
10,000 individuals. J Clin Oncol. 20:1480–1490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Foulkes WD, Metcalfe K, Sun P, Hanna WM,
Lynch HT, Ghadirian P, Tung N, Olopade OI, Weber BL, McLennan J, et
al: Estrogen receptor status in BRCA1- and BRCA2-related breast
cancer: The influence of age, grade, and histological type. Clin
Cancer Res. 10:2029–2034. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhattacharjee S and Nandi S: Rare genetic
diseases with defects in DNA repair: Opportunities and challenges
in orphan drug development for targeted cancer therapy. Cancers
(Basel). 10:2982018. View Article : Google Scholar
|
|
15
|
Andor N, Maley CC and Ji HP: Genomic
instability in cancer: Teetering on the limit of tolerance. Cancer
Res. 77:2179–2185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Connor MJ: Targeting the DNA damage
response in cancer. Mol Cell. 60:547–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Langelier MF, Eisemann T, Riccio AA and
Pascal JM: PARP family enzymes: Regulation and catalysis of the
poly(ADP-ribose) posttranslational modification. Curr Opin Struct
Biol. 53:187–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grimaldi G and Corda D: ADP-ribosylation
and intracellular traffic: An emerging role for PARP enzymes.
Biochem Soc Trans. 47:357–370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ricks TK, Chiu HJ, Ison G, Kim G, McKee
AE, Kluetz P and Pazdur R: Successes and challenges of PAPR
inhibitors in cancer therapy. Front Oncol. 5:2222015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dziadkowiec KN, Gasiorowska E,
Nowak-Markwitz E and Jankowska A: PARP inhibitors: Review of
mechanisms of action and BRCA1/2 mutation targeting. Prz
Menopauzalny. 15:215–219. 2016.PubMed/NCBI
|
|
21
|
Davar D, Beumer JH, Hamieh L and TawbiH:
Role of PARP inhibitors in cancer biology and therapy. Curr Med
Chem. 19:3907–3921. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhattacharjee S and Nandi S: Choices have
consequences: The nexus between DNA repair pathways and genomic
instability in cancer. Clin Transl Med. 5:452016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhattacharjee S and Nandi S: DNA damage
response and cancer therapeutics through the lens of the fanconi
anemia DNA repair pathway. Cell Commun Signal. 15:412017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghosh S, Lu Y, Katz A, Hu Y and Li R:
Tumor suppressor BRCA1 inhibits a breast cancer-associated promoter
of the aromatase gene (CYP19) in human adipose stromal cells. Am J
Physiol Endocrinol Metab. 292:E246–E252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhattacharjee S and Nandi S: Synthetic
lethality in DNA repair network: A novel avenue in targeted cancer
therapy and combination therapeutics. IUBMB Life. 69:929–937. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen A: PARP inhibitors: Its role in
treatment of cancer. Chin J Cancer. 30:463–471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ranieri G: Biological basis of tumor
angiogenesis and therapeutic intervention: Past, present, and
future. Int J Mol Sci. 19:16552018. View Article : Google Scholar
|
|
28
|
Prat J; FIGO Committee on Gynecologic
Oncology, : FIGO's staging classification for cancer of the ovary,
fallopian tube, and peritoneum: Abridged republication. J Gynecol
Oncol. 26:87–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin-Oliva D, Aguilar-Quesada R, O'valle
F, Muñoz-Gámez JA, Martínez-Romero R, García Del Moral R, Ruiz de
Almodóvar JM, Villuendas R, Piris MA and Oliver FJ: Inhibition of
poly(ADP-ribose) polymerase modulates tumor-related gene
expression, including hypoxia-inducible factor-1 activation, during
skin carcinogenesis. Cancer Res. 66:5744–5756. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mizukami Y, Kohgo Y and Chung DC: Hypoxia
inducible factor-1 independent pathways in tumor angiogenesis. Clin
Cancer Res. 13:5670–5674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zimna A and Kurpisz M: Hypoxia-inducible
factor-1 in physiological and pathophysiological angiogenesis:
Applications and therapies. Biomed Res Int. 2015:5494122015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Balamurugan K: HIF-1 at the crossroads of
hypoxia, inflammation, and cancer. Int J Cancer. 138:1058–1066.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Del Rivero J and Kohn EC: PARP inhibitors:
The cornerstone of DNA repair-targeted therapies. Oncology
(Williston Park). 31:265–273. 2017.PubMed/NCBI
|
|
34
|
Wei W, Li Y, Lv S, Zhang C and Tian Y:
PARP-1 may be involved in angiogenesis in epithelial ovarian
cancer. Oncol Lett. 12:4561–4567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tentori L, Lacal PM, Muzi A, Dorio AS,
Leonetti C, Scarsella M, Ruffini F, Xu W, Min W, Stoppacciaro A, et
al: Poly(ADP-ribose) polymerase (PARP) inhibition or PARP-1 gene
deletion reduces angiogenesis. Eur J Cancer. 43:2124–2133. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benafif S and Hall M: An update on PARP
inhibitors for the treatment of cancer. Onco Targets Ther.
8:519–528. 2015.PubMed/NCBI
|
|
37
|
Oplustilova L, Wolanin K, Mistrik M,
Korinkova G, Simkova D, Bouchal J, Lenobel R, Bartkova J, Lau A,
O'Connor MJ, et al: Evaluation of candidate biomarkers to predict
cancer cell sensitivity or resistance to PARP-1 inhibitor
treatment. Cell Cycle. 11:3837–3850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arnaudeau C, Lundin C and Helleday T: DNA
double-strand breaks associated with replication forks are
predominantly repaired by homologous recombination involving an
exchange mechanism in mammalian cells. J Mol Biol. 307:1235–1245.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tutt AN, Lord CJ, McCabe N, Farmer H,
Turner N, Martin NM, Jackson SP, Smith GC and Ashworth A:
Exploiting the DNA repair defect in BRCA mutant cells in the design
of new therapeutic strategies for cancer. Cold Spring Harb Symp
Quant Biol. 70:139–148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Domchek SM, Aghajanian C, Shapira-Frommer
R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G,
Fried G, Stemmer SM, et al: Efficacy and safety of olaparib
monotherapy in germline BRCA1/2 mutation carriers with advanced
ovarian cancer and three or more lines of prior therapy. Gynecol
Oncol. 140:199–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sakai W, Swisher EM, Karlan BY, Agarwal
MK, Higgins J, Friedman C, Villegas E, Jacquemont C, Farrugia DJ,
Couch FJ, et al: Secondary mutations as a mechanism of cisplatin
resistance in BRCA2-mutated cancers. Nature. 451:1116–1120. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lord CJ and Ashworth A: PARP inhibitors:
Synthetic lethality in the clinic. Science. 355:1152–1158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fong PC, Boss DS, Yap TA, Tutt A, Wu P,
Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et
al: Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA
mutation carriers. N Engl J Med. 361:123–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fong PC, Yap TA, Boss DS, Carden CP,
Mergui-Roelvink M, Gourley C, De Greve J, Lubinski J, Shanley S,
Messiou C, et al: Poly(ADP)-ribose polymerase inhibition: Frequent
durable responses in BRCA carrier ovarian cancer correlating with
platinum-free interval. J Clin Oncol. 28:2512–2519. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Audeh MW, Carmichael J, Penson RT,
Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN,
Oaknin A, Loman N, et al: Oral poly(ADP-ribose) polymerase
inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and
recurrent ovarian cancer: A proof-of-concept trial. Lancet.
376:245–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gelmon KA, Tischkowitz M, Mackay H,
Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M,
Gilks B, et al: Olaparib in patients with recurrent high-grade
serous or poorly differentiated ovarian carcinoma or
triple-negative breast cancer: A phase 2, multicentre, open-label,
nonrandomised study. Lancet Oncol. 12:852–861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kaufman B, Shapira-Frommer R, Schmutzler
RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G,
Stemmer SM, Hubert A, et al: Olapari bmonotherapy in patients with
advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol.
33:244–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott C, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
platinum-sensitive relapsed ovarian cancer. N Engl J Med.
366:1382–1392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott CL, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
patients with platinum-sensitive relapsed serous ovarian cancer: a
preplanned retrospective analysis of outcomes by BRCA status in a
randomized phase 2 trial. Lancet Oncol. 15:852–861. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pujade-Lauraine E, Ledermann JA, Selle F,
Gebski V, Penson RT, Oza AM, Korach J, Huzarski T, Poveda A,
Pignata S, et al: Olaparib tablets as maintenance therapy in
patients with platinum-sensitive, relapsed ovarian cancer and a
BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised,
placebo-controlled, phase 3 trial. Lancet Oncol. 18:1274–1284.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim G, Ison G, McKee AE, Zhang H, Tang S,
Gwise T, Sridhara R, Lee E, Tzou A, Philip R, et al: FDA approval
summary: Olaparib monotherapy in patients with deleterious germline
BRCA-mutated advanced ovarian cancer treated with three or more
lines of chemotherapy. Clin Cancer Res. 21:4257–4261. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Swisher EM, Lin KK, Oza AM, Scott CL,
Giordano H, Sun J, Konecny GE, Coleman RL, Tinker AV, O'Malley DM,
et al: Rucaparib in relapsed, platinum-sensitive high-grade ovarian
carcinoma (ARIEL2 Part 1): An international, multicentre,
open-label, phase 2 trial. Lancet Oncol. 18:75–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Coleman RL, Oza AM, Lorusso D, Aghajanian
C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G,
et al: Rucaparib maintenance treatment for recurrent ovarian
carcinoma after response to platinum therapy (ARIEL3): A
randomised, double-blind, placebo-controlled, phase 3 trial.
Lancet. 390:1949–1961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mirza MR, Monk BJ, Herrstedt J, Oza AM,
Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I,
et al: Niraparib maintenance therapy in platinum-sensitive,
recurrent ovarian cancer. N Engl J Med. 375:2154–2164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Coleman RL, Sill MW, Bell-McGuinn K,
Aghajanian C, Gray HJ, Tewari KS, Rubin SC, Rutherford TJ, Chan JK,
Chen A and Swisher EM: A phase II evaluation of the potent, highly
selective PARP inhibitor veliparib in the treatment of persistent
or recurrent epithelial ovarian, fallopian tube, or primary
peritoneal cancer in patients who carry a germline BRCA1 or BRCA2
mutation-an NRG oncology/gynecologic oncology group study. Gynecol
Oncol. 137:386–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Steffensen KD, Adimi P and Jakobsen A:
Veliparib monotherapy to patients with BRCA germ line mutation and
platinum-resistant or partially platinum-sensitive relapse of
epithelial ovarian cancer: A phase I/II study. Int J Gynecol
Cancer. 27:1842–1849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shen Y, Rehman FL, Feng Y, Boshuizen J,
Bajrami I, Elliott R, Wang B, Lord CJ, Post LE and Ashworth A: BMN
673, a novel and highly potent PARP1/2 inhibitor for the treatment
of human cancers with DNA repair deficiency. Clin Cancer Res.
19:5003–5015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
de Bono J, Ramanathan RK, Mina L, Chugh R,
Glaspy J, Rafii S, Kaye S, Sachdev J, Heymach J, Smith DC, et al:
Phase I, dose-escalation, two-part trial of the PARP inhibitor
talazoparib in patients with advanced germline BRCA1/2 mutations
and selected sporadic cancers. Cancer Discov. 7:620–629. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
LaFargue CJ, Dal Molin GZ, Sood AK and
Coleman RL: Exploring and comparing adverse events between PARP
inhibitors. Lancet Oncol. 20:e15–e28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rucaparib, . LiverTox: Clinical and
research information on drug-induced liver injury [Internet].
(Bethesda (MD)). National Institute of Diabetes and Digestive and
Kidney Diseases. Jun 1–2012-2017.
|
|
61
|
Olaparib, . LiverTox: Clinical and
research information on drug-induced liver injury [Internet].
(Bethesda (MD)). National Institute of Diabetes and Digestive and
Kidney Diseases. Jun 1–2012-2017.
|
|
62
|
Niraparib, . LiverTox: Clinical and
research information on drug-induced liver injury [Internet].
(Bethesda (MD)). National Institute of Diabetes and Digestive and
Kidney Diseases. Jun 20–2012-2017.
|
|
63
|
Zhou J, Feng L and Zhang X: Risk of severe
hematologic toxicities in cancer patients treated with PARP
inhibitors: A meta-analysis of randomized controlled trials. Drug
Des Devel Ther. 11:3009–3017. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Alecu I, Milenkova T and Turner SR: Risk
of severe hematologic toxicities in cancer patients treated with
PARP inhibitors: Results of monotherapy and combination therapy
trials. Drug Des Devel Ther. 12:347–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu Y, Meng J and Wang G: Risk of selected
gastrointestinal toxicities associated with poly (ADP-ribose)
polymerase (PARP) inhibitors in the treatment of ovarian cancer: A
meta-analysis of published trials. Drug Des Devel Ther.
12:3013–3019. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Moore KN, Mirza MR and Matulonis UA: The
poly (ADP ribose) polymerase inhibitor niraparib: Management of
toxicities. Gynecol Oncol. 149:214–220. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hsiang YH and Liu LF: Identification of
mammalian DNA topoisomerase I as an intracellular target of the
anticancer drug camptothecin. Cancer Res. 48:1722–1726.
1988.PubMed/NCBI
|
|
68
|
Staker BL, Hjerrild K, Feese MD, Behnke
CA, Burgin AB Jr and Stewart L: The mechanism of topoisomerase I
poisoning by a camptothecin analog. Proc Natl Acad Sci USA.
99:15387–15392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
ten Bokkel Huinink W, Gore M, Carmichael
J, Gordon A, Malfetano J, Hudson I, Broom C, Scarabelli C, Davidson
N, Spanczynski M, et al: Topotecan versus paclitaxel for the
treatment of recurrent epithelial ovarian cancer. J Clin Oncol.
15:2183–2193. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
D'Onofrio G, Tramontano F, Dorio AS, Muzi
A, Maselli V, Fulgione D, Graziani G, Malanga M and Quesada P:
Poly(ADP-ribose) polymerase signaling of topoisomerase 1-dependent
DNA damage in carcinoma cells. Biochem Pharmacol. 81:194–202. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Patel AG, Flatten KS, Schneider PA, Dai
NT, McDonald JS, Poirier GG and Kaufmann SH: Enhanced killing of
cancer cells by poly(ADP-ribose) polymerase inhibitors and
topoisomerase I inhibitors reflects poisoning of both enzymes. J
Biol Chem. 287:4198–4210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wahner Hendrickson AE, Menefee ME,
Hartmann LC, Long HJ, Northfelt DW, Reid JM, Boakye-Agyeman F,
Kayode O, Flatten KS, Harrell MI, et al: A phase I clinical trial
of the Poly(ADP-ribose) polymerase inhibitor veliparib and weekly
topotecan in patients with solid tumors. Clin Cancer Res.
24:744–752. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hjortkjær M, Kanstrup H, Jakobsen A and
Steffensen KD: Veliparib and topotecan for patients with
platinum-resistant or partially platinum-sensitive relapse of
epithelial ovarian cancer with BRCA negative or unknown BRCA
status. Cancer Treat Res Commun. 14:7–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bi Y, Verginadis II, Dey S, Lin L, Guo L,
Zheng Y and Koumenis C: Radiosensitization by the PARP inhibitor
olaparib in BRCA1-proficient and deficient high-grade serous
ovarian carcinomas. Gynecol Oncol. 150:534–544. 2018. View Article : Google Scholar : PubMed/NCBI
|