Introduction
Breast cancer is the most common types of cancer and
the second leading cause of cancer-associated death in females
worldwide, and accounts for ~500,000 deaths every year (1). Over 90% of breast cancer-associated
deaths are primarily the result of metastasis (2). Despite recent advances in the diagnosis
and treatment, 20–30% of patients with early-stage breast cancer
remain at high risk of recurrence and metastasis (3). The bone has been identified as one of
the predominant metastatic sites in patients with breast cancer and
bone metastases account for 60–80% of patients with metastatic
breast cancer (MBC) (4,5). Furthermore, the bone is the most common
site of initial distant metastatic spread in patients with breast
cancer (6). Bone-only metastasis
(BOM) is defined as a metastatic disease limited to the bone at the
initial diagnosis of MBC. BOM is reported to occur in 25–40% of
patients with MBC (4,6,7). Bone
metastases are frequently complicated by skeletal-related events
(SREs), including bone pain, pathological fractures, spinal cord
compression and hypercalcemia, all of which are associated with a
decreased survival time and quality of life (8–10).
Patients with BOM have unique clinical
characteristics and prognostic outcomes compared with patients with
other types of MBC. Hormone receptor (HR)+, human
epidermal growth factor receptor 2 (HER2)− and a low or
intermediate histologic grade preferentially metastasize to the
bone rather than the viscera (11–13).
Patients with BOM exhibit longer survival times compared with
patients with visceral metastasis or bone metastases combined with
visceral metastases (13,14). Previous studies determined that the
median overall survival (OS) of patients with BOM from diagnosis of
metastasis was 52–59 months (7,15,16).
Bone metastases from breast cancer are incurable and
their clinical management is challenging. Bisphosphonates and
denosumab have been used successfully to reduce the frequency of
SREs of bone metastasis (17).
Regrettably, bisphosphonate treatment is not able prevent the
occurrence and development of bone metastasis (18). Palliative radiotherapy is effective
in relieving bone pain caused by bone metastasis (19). Endocrine therapy, chemotherapy and
sequential therapy (chemotherapy followed by endocrine therapy) may
be used as alternative systemic therapies for these patients.
However, it remains elusive which treatment method is able to
prolong the survival time of patients with BOM the most. In
addition, the current knowledge regarding prognostic factors for
predicting outcomes among patients with BOM in China is
limited.
The aim of the present retrospective study was to
compare the clinical characteristics and prognosis between patients
with BOM and non-BOM MBC. Outcomes were also compared among
patients with different molecular subtypes with BOM or non-BOM.
Survival and SREs of patients with BOM and different numbers of
bone metastases and first-line treatment approaches were analyzed
and the distribution of secondary metastatic sites in patients with
BOM was determined. The conclusions of the present study may
improve the current understanding of BOM and direct future
guidelines for diagnosis and treatment selection. To the best of
our knowledge, the present study is so far the largest study based
on real-world data comparing the clinicopathological
characteristics and prognosis of patients with breast cancer and
BOM and non-BOM in China.
Patients and methods
Patient selection
Patients with MBC treated at Tianjin Medical
University Cancer Institute and Hospital (Tianjin, China) between
January 2008 and December 2017 were retrospectively analyzed. All
patients were assigned to either the BOM or non-BOM group. Patients
with the bone as the first and only site of metastasis at the time
of MBC diagnosis were assigned to the BOM group. Patients with
non-skeletal metastases (including non-skeletal combined with bone
metastases) at the time of MBC diagnosis were assigned to the
non-BOM group. A total of 1,290 patients were diagnosed with MBC;
208 (16.1%) had BOM and 1,082 (83.9%) had non-skeletal metastasis.
Patients were required to have at least 6 months of follow-up. The
median follow-up period was 26 months.
The inclusion criteria were as follows: ⅰ) Primary
unilateral breast cancer that was pathologically diagnosed; ⅱ)
female patients; ⅲ) metastatic disease pathologically diagnosed or
diagnosed using an imaging technique [e.g. bone scan, CT, MRI or
positron emission tomography (PET)/CT]; and ⅳ) relatively complete
clinicopathological data and survival data. The exclusion criteria
were as follows: ⅰ) Primary bilateral breast cancer; ⅱ) male
patients; ⅲ) early breast cancer; ⅳ) coexistence of another
malignancy; or ⅳ) patients with incomplete data. The present study
was performed in accordance with all relevant guidelines, and the
procedures were approved by the Tianjin Medical University Ethical
Committee (Tianjin, China). The requirement of informed consent was
waived due to the retrospective study design.
Tumor subtype assessment and
evaluation
To diagnose patients with metastatic disease, all
patients included in the present study were examined using a
whole-body imaging technique (e.g. B-ultrasound, CT, MRI or PET).
Patients with a single bone metastasis were diagnosed by bone
biopsy. Tumor types were classified as infiltrating ductal
carcinoma or others. The clinical stages were classified according
to the American Joint Committee on Cancer TNM staging system 7th
edition (20). The expression status
of progesterone receptor (PR), estrogen receptor (ER) and HER2 was
assessed by initial immunohistochemical (IHC) analysis of a biopsy
of the breast carcinoma. Breast cancer was divided into three major
molecular subtypes: HR+/HER2−,
HER2+ and HR−/HER2−.
HR+ was defined as ER or PR ≥1% by IHC (20). Regarding HER2, IHC 1+ was considered
as negative and IHC 3+ was considered as positive, while IHC 2+ was
inconclusive and fluorescence in situ hybridization (FISH)
was used to make a decision (21).
In the case of discordance between IHC and FISH results, the FISH
result was prioritized. All other samples were classified as
negative on analysis. In addition, triple-negative breast cancer
(TNBC) was negative for HR and HER2.
Bone metastasis characteristics
The location of bone metastasis was determined by
examining clinical records and imaging reports at the time of BOM
diagnosis. Skull, sternum, rib, spine and pelvic bones were
classified as axial bones. Forearm, arms, hands, thighs, shanks and
foot bones were classified as appendicular bones. Single bone
metastasis was defined as a solitary metastatic lesion confined to
a single bone. Multiple bone metastases were defined as ≥2 lesions,
including >1 lesion in the same bone. Bone metastasis is
incurable; it is almost impossible to remove tumors in a
metastasized bone and then tumors tend to recur or metastasize to
other organs (22). The
metastasis-free interval (MFI) was defined as the interval between
the first diagnosis of BOM and time of the second presentation with
metastatic disease.
Treatment modalities
Data on first-line treatment characteristics for 144
patients with BOM and HR+/HER2− status,
including administration of chemotherapy, endocrine therapy or
sequential therapy (chemotherapy followed by endocrine therapy),
were obtained. A total of 36 patients received chemotherapy, 33
endocrine therapy and 75 sequential therapy.
Statistical analysis
Categorical variables used to quantify clinical
characteristics are presented as frequencies and proportions. The
mean and a Student's t-test were used to compare the age
differences between the BOM and non-BOM groups. Pearson's
χ2 tests and Fisher's exact tests were used to compare
the differences between two groups. The primary endpoints of the
present study were disease-free survival (DFS), progression-free
survival (PFS) and OS from the time-point of diagnosis of BOM. DFS
was defined as the time interval from the diagnosis of breast
cancer to recurrence or metastasis. PFS was defined as the time
interval from diagnosis of metastasis to progression, death or the
last follow-up date, whichever occurred first. OS was defined as
the time interval from diagnosis of metastasis to death or to the
last follow-up date if patients were alive, whichever occurred
first. DFS, PFS and OS curves were drawn using the Kaplan-Meier
method and compared among groups using a log-rank test. Univariate
analysis was performed by logistic regression. Cox proportional
hazards model was used for multivariate analysis and to estimate
the hazard ratio and 95% CIs. All statistical tests used were
two-sided. P<0.05 was considered to indicate a statistically
significant difference. SPSS version 25.0 (IBM Corp.) and GraphPad
Prism version 7 were used to analyze the data.
Results
Patient characteristics
The median age at breast cancer diagnosis in the BOM
and non-BOM groups was 48 years (range, 24–72 and 21–78 years,
respectively). The median ages at MBC diagnosis in the BOM and
non-BOM groups were 50 years (range, 24–78 years) and 52 years
(range, 25–80 years), respectively. The clinical and pathological
characteristics of the patients in the two groups are presented in
Table I. In total, 1,290 patients
with MBC were enrolled in the present study, including 208 (16.1%)
patients in the BOM group and 1,082 (83.9%) patients in the non-BOM
group. Compared with the non-BOM group, patients with BOM were more
frequently diagnosed as clinical stage IV in the first instance
(P<0.001), as well as HR+ (P<0.001) and
HER2− (P=0.03). The metastatic lesions in non-BOM
patients included the lungs (60.7%), liver (42.4%) and brain
(4.6%).
 | Table I.Patient characteristics at the
initial diagnosis of breast cancer in the BOM (n=208) and non-BOM
(n=1,082) groups. |
Table I.
Patient characteristics at the
initial diagnosis of breast cancer in the BOM (n=208) and non-BOM
(n=1,082) groups.
| Characteristic | BOM group | non-BOM group | P-value |
|---|
| Median age at
primary diagnosis (range), years | 48 (24–72) | 48 (21–78) | 0.978 |
| Median age at
metastasis or recurrence (range), years | 50 (24–78) | 52 (25–80) | 0.262 |
| Menopausal status,
n (%) |
|
| 0.062 |
|
Premenopause | 116 (55.8) | 527 (48.7) |
|
|
Postmenopause | 92
(44.2) | 555 (51.3) |
|
| Lymph node status,
n (%) |
|
| 0.838 |
|
Negative | 61
(29.3) | 325 (30.0) |
|
|
Positive | 147 (70.7) | 757 (70.0) |
|
| Tumor stage, n
(%) |
|
| 0.392 |
| T1 | 53
(25.5) | 329 (30.4) |
|
| T2 | 103 (49.5) | 515 (47.6) |
|
| T3 | 34
(16.3) | 141 (13.0) |
|
| T4 | 18
(8.7) | 97
(9.0) |
|
| Clinical stage, n
(%) |
|
| <0.001 |
| I | 24
(11.5) | 151 (14.0) |
|
| II | 75
(36.1) | 447 (41.3) |
|
|
III | 61
(29.3) | 382 (35.3) |
|
| IV | 48
(23.1) | 102 (9.4) |
|
| Tumor type, n
(%) |
|
| 0.273 |
|
Invasive ductal carcinoma | 170 (81.7) | 917 (84.8) |
|
|
Others | 38
(18.3) | 165 (15.2) |
|
| HR status, n
(%) |
|
| <0.001 |
|
Negative | 39
(18.7) | 422 (39.0) |
|
|
Positive | 169 (81.3) | 660 (61.0) |
|
| HER2 status, n
(%) |
|
|
|
|
Negative | 171 (82.2) | 814 (75.2) | 0.030 |
|
Positive | 37
(17.8) | 268 (24.8) |
|
| Molecular type, n
(%) |
|
| <0.001 |
|
HR+/HER2− | 144 (69.2) | 538 (49.7) |
|
|
HR/HER2+ | 40
(19.3) | 268 (24.8) |
|
|
HR−/HER2− | 24
(11.5) | 276 (25.5) |
|
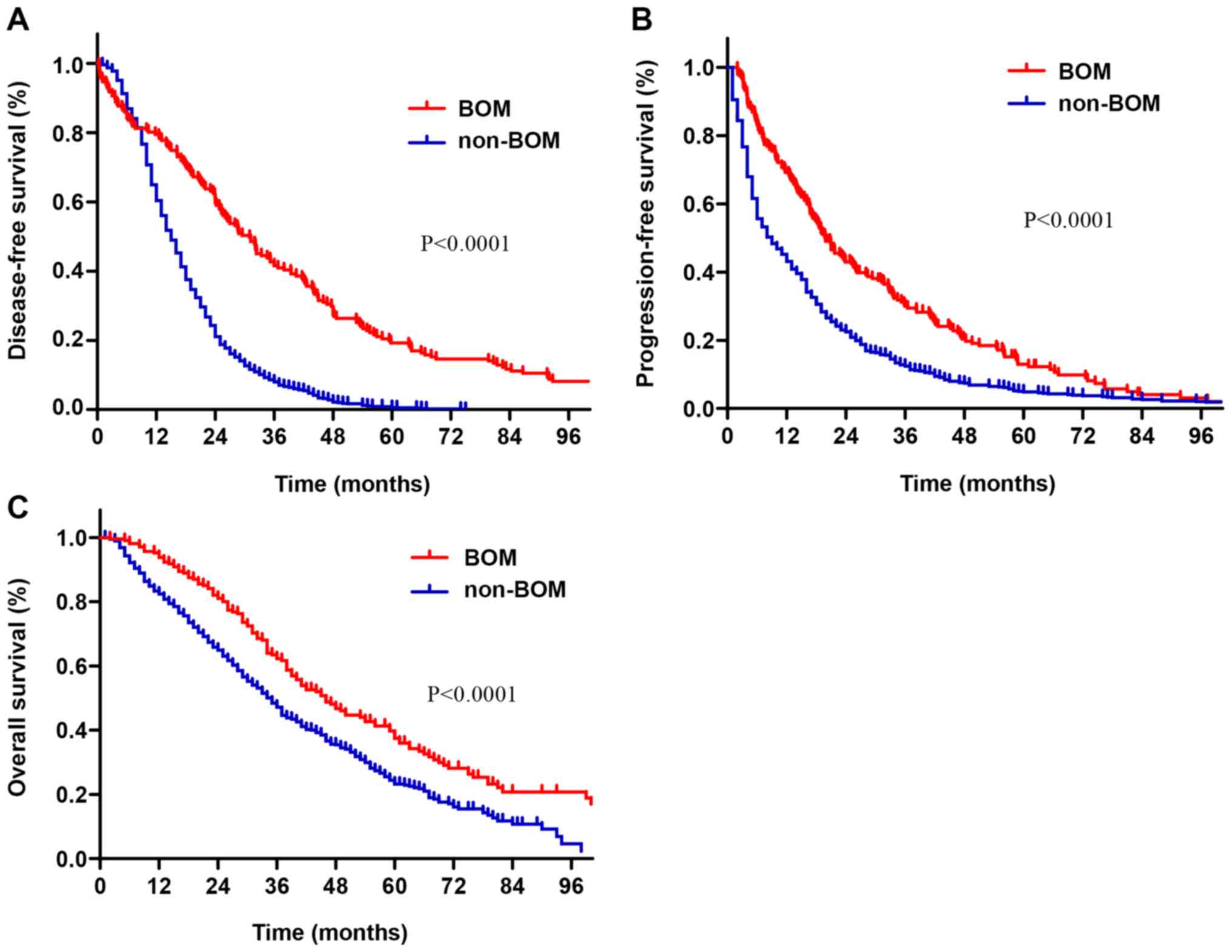
The survival time of patients with BOM was longer
than that of patients with non-BOM (Fig.
1). The median DFS time of patients with BOM was 31 months,
while that of patients with non-BOM was 15 months (P<0.001;
Fig. 1A). The median PFS in patients
with BOM and non-BOM was 19.9 and 9.0 months, respectively
(P<0.001; Fig. 1B). The median OS
in the BOM and non-BOM groups was 45 months (95% CI: 38.1-51.9) and
35 months (95% CI:32.7-37.2), respectively (P<0.001; Fig. 1C).
Prognostic factors for patients with
BOM
Univariate analyses indicated that the tumor stage,
HR status, HER2 status, molecular subtypes and the number of bone
metastases were associated with PFS (P<0.05; Table II). HR status, molecular subtypes,
location of bone metastasis and the number of bone metastases were
associated with OS (P<0.05; Table
II). Additionally, bisphosphonates therapy and radiotherapy
were not associated with PFS or OS in patients with BOM.
 | Table II.Univariate analyses of the influence
of clinical factors on the PFS and OS of patients with BOM. |
Table II.
Univariate analyses of the influence
of clinical factors on the PFS and OS of patients with BOM.
|
|
| PFS, % |
| OS, % |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Number | 3 years | 5 years | P-value | 3 years | 5 years | P-value |
|---|
| Age at primary
diagnosis, years |
|
|
| 0.246 |
|
| 0.244 |
|
<35 | 25 | 14.4 | 4.8 |
| 49.9 | 21.8 |
|
|
35-60 | 153 | 31.9 | 14.7 |
| 64.4 | 41.3 |
|
|
≥60 | 30 | 29.2 | 7.8 |
| 56.6 | 38.2 |
|
| Menopausal status
at primary diagnosis |
|
|
| 0.879 |
|
| 0.740 |
|
Premenopause | 116 | 27.1 | 12.9 |
| 59.0 | 33.6 |
|
|
Postmenopause | 92 | 32.6 | 11.0 |
| 65.2 | 46.2 |
|
| Lymph node status
at primary diagnosis |
|
|
| 0.412 |
|
| 0.785 |
|
Negative | 61 | 31.7 | 16.4 |
| 69.2 | 41.4 |
|
|
Positive | 147 | 29.2 | 11.3 |
| 59.1 | 37.7 |
|
| Tumor stage at
primary diagnosis |
|
|
| 0.040 |
|
| 0.114 |
| T1 | 53 | 43.7 | 20.7 |
| 67.4 | 50.3 |
|
| T2 | 103 | 20.6 | 13.2 |
| 59.6 | 37.0 |
|
| T3 | 34 | 28.4 | 11.8 |
| 61.3 | 29.6 |
|
| T4 | 18 | 39.9 | 0 |
| 62.2 | 23.3 |
|
| Clinical stage at
primary diagnosis |
|
|
| 0.337 |
|
| 0.476 |
| I | 24 | 39.4 | 24.6 |
| 72.2 | 49.4 |
|
| II | 75 | 23.7 | 17.9 |
| 66.5 | 37.7 |
|
|
III | 61 | 28.3 | 7.7 |
| 51.5 | 34.1 |
|
| IV | 48 | 37.0 | 5.9 |
| 64.1 | 37.6 |
|
| Pathologic type at
primary diagnosis |
|
|
| 0.118 |
|
| 0.149 |
|
Invasive ductal carcinoma | 170 | 27.7 | 12.3 |
| 59.2 | 37.3 |
|
|
Others | 38 | 40.4 | 15.9 |
| 75.3 | 49.6 |
|
| HR status at
primary diagnosis |
|
|
| <0.001 |
|
| <0.001 |
|
Negative | 39 | 9.6 | 4.8 |
| 30.5 | 13.1 |
|
|
Positive | 169 | 34.7 | 14.9 |
| 69.3 | 45.3 |
|
| HER2 status at
primary diagnosis |
|
|
| 0.032 |
|
| 0.168 |
|
Negative | 171 | 32.3 | 13.5 |
| 66.5 | 42.4 |
|
|
Positive | 37 | 19.5 | 10.4 |
| 40.0 | 25.4 |
|
| Molecular subtype
at primary diagnosis |
|
|
| 0.001 |
|
| <0.001 |
|
HR+/HER2− | 144 | 36.0 | 14.9 |
| 71.9 | 45.4 |
|
|
HR/HER2+ | 40 | 20.4 | 12.8 |
| 42.8 | 26.4 |
|
|
HR−/HER2− | 24 | 10.0 | 0 |
| 33.5 | 16.8 |
|
| Bone
radiotherapy |
|
|
| 0.977 |
|
| 0.353 |
| No | 131 | 29.3 | 12.4 |
| 59.2 | 36.3 |
|
|
Yes | 77 | 30.4 | 13.6 |
| 66.7 | 42.3 |
|
| Bisphosphonate
therapy |
|
|
| 0.967 |
|
| 0.386 |
| No | 23 | 30.4 | 13.0 |
| 51.1 | 33.7 |
|
|
Yes | 185 | 30.0 | 13.0 |
| 63.0 | 40.2 |
|
| Location of bone
metastases at BOM diagnosis |
|
|
| 0.123 |
|
| 0.003 |
|
Axial | 164 | 28.1 | 11.8 |
| 63.4 | 40.1 |
|
|
Appendicular | 7 | 71.4 | 53.6 |
| 85.7 | 71.4 |
|
| Axial
and appendicular | 37 | 30.4 | 9.1 |
| 48.7 | 24.7 |
|
| Number of bone
metastases at BOM diagnosis |
|
|
| 0.035 |
|
| 0.004 |
|
Single | 60 | 43.7 | 17.5 |
| 77.0 | 51.7 |
|
|
Multiple | 148 | 24.4 | 11.2 |
| 56.1 | 33.3 |
|
Multivariate analysis using Cox regression indicated
that the HR status and the number of bone metastases were
independent factors affecting the PFS of patients with BOM.
HR− and multiple bone metastases were significantly
associated with shorter PFS of patients with BOM (Table III). HR status, the number of bone
metastases and location of bone metastases were independent factors
affecting OS of patients with BOM. HR−, multiple bone
metastases, as well as the combination of axial and appendicular
bone metastases, were significantly associated with shorter OS of
patients with BOM (Table IV).
 | Table III.Multivariate analysis of prognostic
factors affecting progression-free survival of patients with
BOM. |
Table III.
Multivariate analysis of prognostic
factors affecting progression-free survival of patients with
BOM.
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| HR status at breast
cancer diagnosis |
|
|
|
|
Negative | 1.00 | Reference | – |
|
Positive | 0.63 | 0.40-0.92 | 0.027 |
| Number of bone
metastases at BOM diagnosis |
|
|
|
|
Multiple | 1.00 | Reference | – |
|
Single | 0.64 | 0.45-0.91 | 0.013 |
 | Table IV.Multivariate analysis of prognostic
factors affecting overall survival of patients with BOM. |
Table IV.
Multivariate analysis of prognostic
factors affecting overall survival of patients with BOM.
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| HR status at breast
cancer diagnosis |
|
|
|
|
Negative | 1.00 | Reference | – |
|
Positive | 0.54 | 0.32-0.90 | 0.020 |
| Number of
metastases at BOM diagnosis |
|
|
|
|
Multiple | 1.00 | Reference | – |
|
Single | 0.62 | 0.40-0.96 | 0.031 |
| Location of bone
metastasis at BOM diagnosis |
|
|
|
| Axial
and appendicular | 1.00 | Reference | – |
|
Axial | 0.65 | 0.41-1.02 | 0.062 |
|
Appendicular | 0.07 | 0.01-0.57 | 0.013 |
Prognostic factors for non-BOM
patients
Univariate analysis indicated that age at diagnosis
of metastasis, tumor stage, HR status and molecular subtypes were
associated with OS of non-BOM patients (P<0.05; Table V). In the multivariate analysis, age
at diagnosis of metastasis, HR status and tumor stage were
independent factors affecting the OS of non-BOM patients (Table VI). In non-BOM patients,
HR−, age ≤35 years at diagnosis of metastasis or stage
T3-4 were associated with poor prognosis.
 | Table V.Univariate analyses of prognostic
factors affecting overall survival of patients with non-bone-only
metastasis. |
Table V.
Univariate analyses of prognostic
factors affecting overall survival of patients with non-bone-only
metastasis.
|
|
| Overall survival,
% |
|
|---|
|
|
|
|
|
|---|
| Characteristic | N (%) | 3 years | 5 years | P-value |
|---|
| Age at diagnosis of
metastasis, years |
|
|
| 0.008 |
|
≤35 | 85
(7.9) | 36.0 | 8.0 |
|
|
>35 | 997 (92.1) | 48.1 | 23.9 |
|
| Lymph node
status |
|
|
| 0.077 |
|
Negative | 325 (30.0) | 50.7 | 26.4 |
|
|
Positive | 757 (70.0) | 45.5 | 21.7 |
|
| Tumor stage |
|
|
| 0.007 |
|
T1-2 | 844 (78.0) | 48.8 | 25.1 |
|
|
T3-4 | 238 (22.0) | 41.0 | 15.5 |
|
| Clinical stage |
|
|
| 0.205 |
| I | 151 (14.0) | 49.4 | 26.1 |
|
| II | 447 (41.3) | 49.6 | 25.7 |
|
|
III | 382 (35.3) | 44.1 | 19.2 |
|
| IV | 102 (9.4) | 42.1 | 14.1 |
|
| Pathologic
type |
|
|
| 0.437 |
|
Invasive ductal carcinoma | 909 (84.0) | 45.7 | 24.6 |
|
|
Others | 173 (16.0) | 54.3 | 18.6 |
|
| HR status |
|
|
| <0.001 |
|
Negative | 422 (39.0) | 36.4 | 15.4 |
|
|
Positive | 660 (61.0) | 54.3 | 28.6 |
|
| HER2 status |
|
|
| 0.943 |
|
Negative | 814 (75.2) | 47.5 | 23.2 |
|
|
Positive | 268 (24.8) | 46.2 | 23.6 |
|
| Molecular
subtype |
|
|
| <0.001 |
|
HR+/HER2− | 538 (49.7) | 54.2 | 28.0 |
|
|
HR/HER2+ | 268 (24.8) | 46.2 | 23.6 |
|
|
HR−/HER2− | 276 (25.5) | 34.5 | 14.7 |
|
 | Table VI.Multivariate analysis of overall
survival of patients with non-bone-only metastasis. |
Table VI.
Multivariate analysis of overall
survival of patients with non-bone-only metastasis.
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| Age at recurrence
or metastasis (≤35 vs. >35 years) | 1.36 | 1.03-1.79 | 0.027 |
| HR status (negative
vs. positive) | 1.55 | 1.32-0.81 | 0.027 |
| Tumor stage (T3-4
vs. T1-2) | 1.21 | 1.00-1.46 | 0.048 |
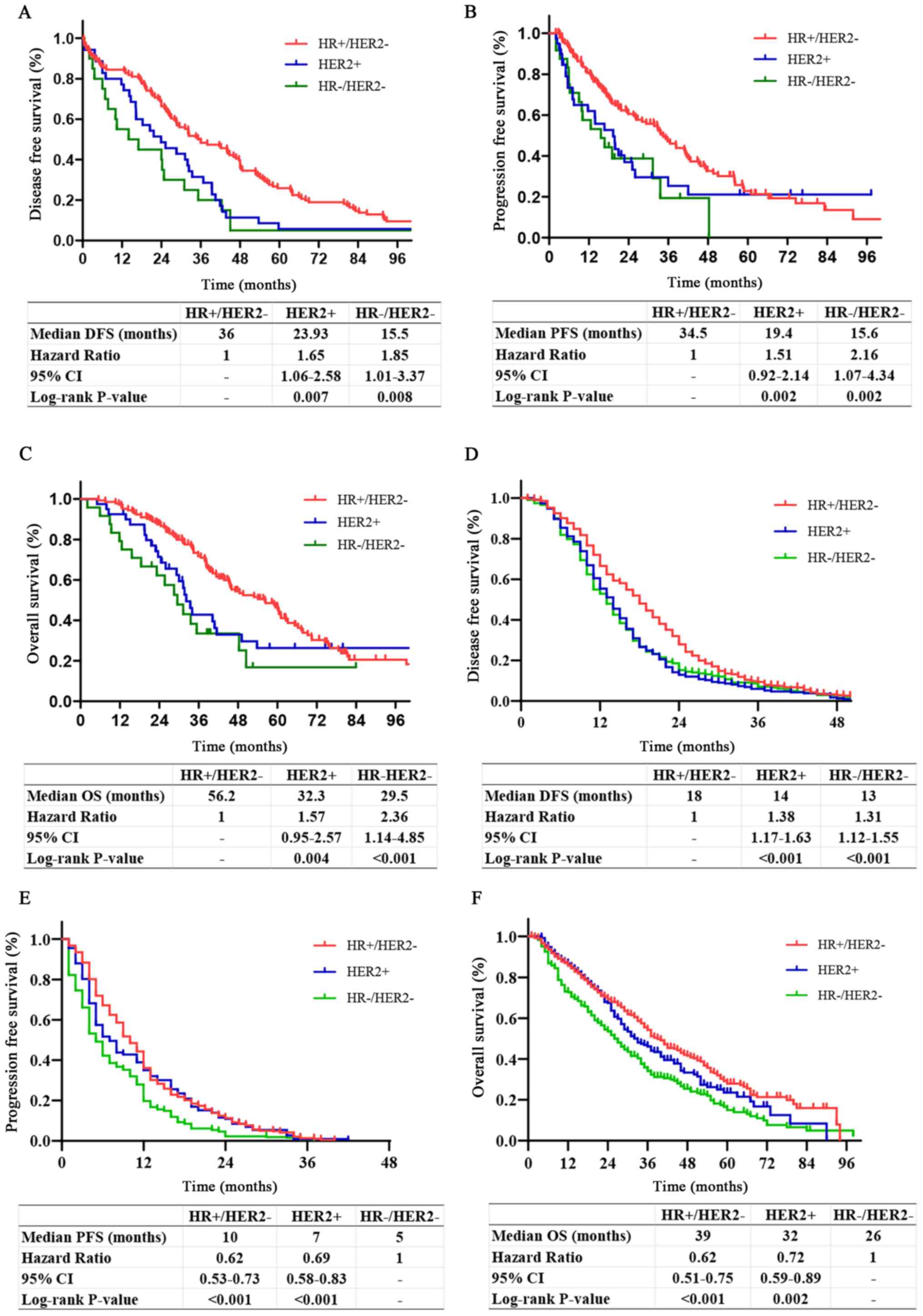
Survival analysis of patients with BOM
and non-BOM with different molecular subtypes
Survival analyses of patients with BOM with
different molecular subtypes indicated that compared with patients
with HER2+ or HR−/HER2−, those
with HR+/HER2− had significantly improved
DFS, PFS and OS. There was no significant difference in DFS, PFS or
OS between patients with HER2+ and
HR−/HER2− (P=0.25, 0.83 and 0.25,
respectively; Fig. 2A-C).
Survival analyses of non-BOM patients with different
molecular subtypes suggested that patients with
HR+/HER2− and HER2+ had
significantly longer PFS and OS compared with those with
HR−/HER2−. There was no significant
difference in PFS or OS of patients with HER2+ and
HR+/HER2− (P=0.12 and P=0.28, respectively).
Patients with HR+/HER2− had significantly
longer DFS compared with patients with HER2+ or
HR−/HER2−. There was no significant
difference in the DFS of patients with HER2+ and
HR−/HER2− (P=0.61; Fig. 2D-F).
Treatment outcomes
First-line treatment approaches for patients with
BOM and HR+/HER2− status included
chemotherapy, endocrine therapy and sequential therapy. Of the
total 144 patients with HR+/HER2− BOM, 36
received chemotherapy, 33 received endocrine therapy and 75
received sequential therapy. The proportion of patients receiving
taxane or anthracyclines was 91.7% (33/36) in the chemotherapy
group and 96% (72/75) in the sequential therapy group. Among
patients receiving endocrine or sequential therapy, the proportions
of those receiving aromatase inhibitors was 63.4% (21/33) and 78.7%
(59/75), the proportions of those receiving tamoxifen was 15.2%
(5/33) and 8% (6/75), the proportions of those receiving
fulvestrant was 12.1% (4/33) and 13.3% (10/75), and the proportions
of those receiving palbociclib were 9.1% (3/33) and 0%.
Premenopausal patients with endocrine therapy were all treated by
ovarian function suppression. It is important to note that Chinese
patients have different national conditions and medical policies to
face. The medical insurance coverage rate in China is 97.5%, but
patients have varying medical expenses according to different types
of insurance (23). Palbociclib was
approved by the Chinese Food and Drug Administration on July 31,
2018 (24); despite its approval,
the high medical costs have limited its use in China. Only a small
number of patients in the present study received palbociclib due to
its late availability and high medical costs. Most patients
preferred chemotherapy because it was relatively cheaper and was
covered by health insurance. Table
VII presents the different treatments applied for patients with
HR+/HER2− BOM with different characteristics
at diagnosis of metastasis. Patients with multiple bone metastases
were more likely to receive sequential therapy (P<0.001).
 | Table VII.Characteristics at initial diagnosis
of metastasis in patients with hormone receptor-positive/human
epidermal growth factor receptor 2-negative bone-only
metastasis. |
Table VII.
Characteristics at initial diagnosis
of metastasis in patients with hormone receptor-positive/human
epidermal growth factor receptor 2-negative bone-only
metastasis.
| Characteristic | Chemotherapy, n
(%) | Endocrine therapy,
n (%) | Sequential therapy,
n (%) |
|---|
| Total patients | 36
(25) | 33 (22.9) | 75 (52.1) |
| Age at metastasis
diagnosis, years |
|
|
|
|
<35 | 3
(8.3) | 6
(18.2) | 0
(0.0) |
|
35-60 | 25 (69.4) | 25 (75.6) | 60 (80.0) |
|
≥60 | 8
(22.2) | 2
(6.1) | 15 (20.0) |
| Location of bone
metastasis |
|
|
|
|
Axial | 0
(0.0) | 2
(5.7) | 2
(3.4) |
|
Appendicular | 29 (80.9) | 25 (80.0) | 59 (79.3) |
| Axial
and appendicular | 7
(19.1) | 6
(14.3) | 14 (17.2) |
| Number of bone
metastases |
|
|
|
|
Single | 8
(22.2) | 21 (63.6) | 12 (16.0) |
|
Multiple | 28 (77.8) | 12 (36.4) | 63 (84.0) |
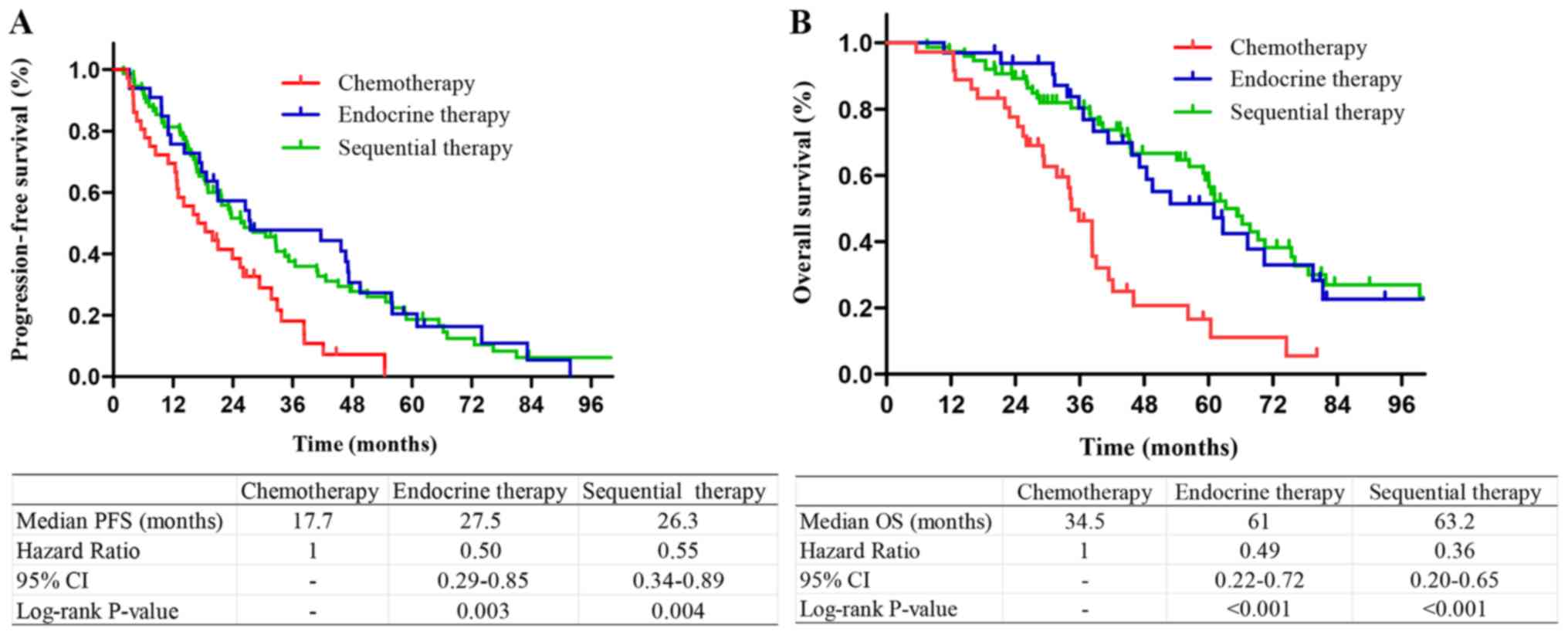
Among the patients with
HR+/HER2− tumors, those who received
sequential therapy or endocrine therapy had longer PFS and OS
compared with those who received chemotherapy. There was no
significant difference in the survival of patients who received
sequential therapy and endocrine therapy (P=0.53 and 0.48,
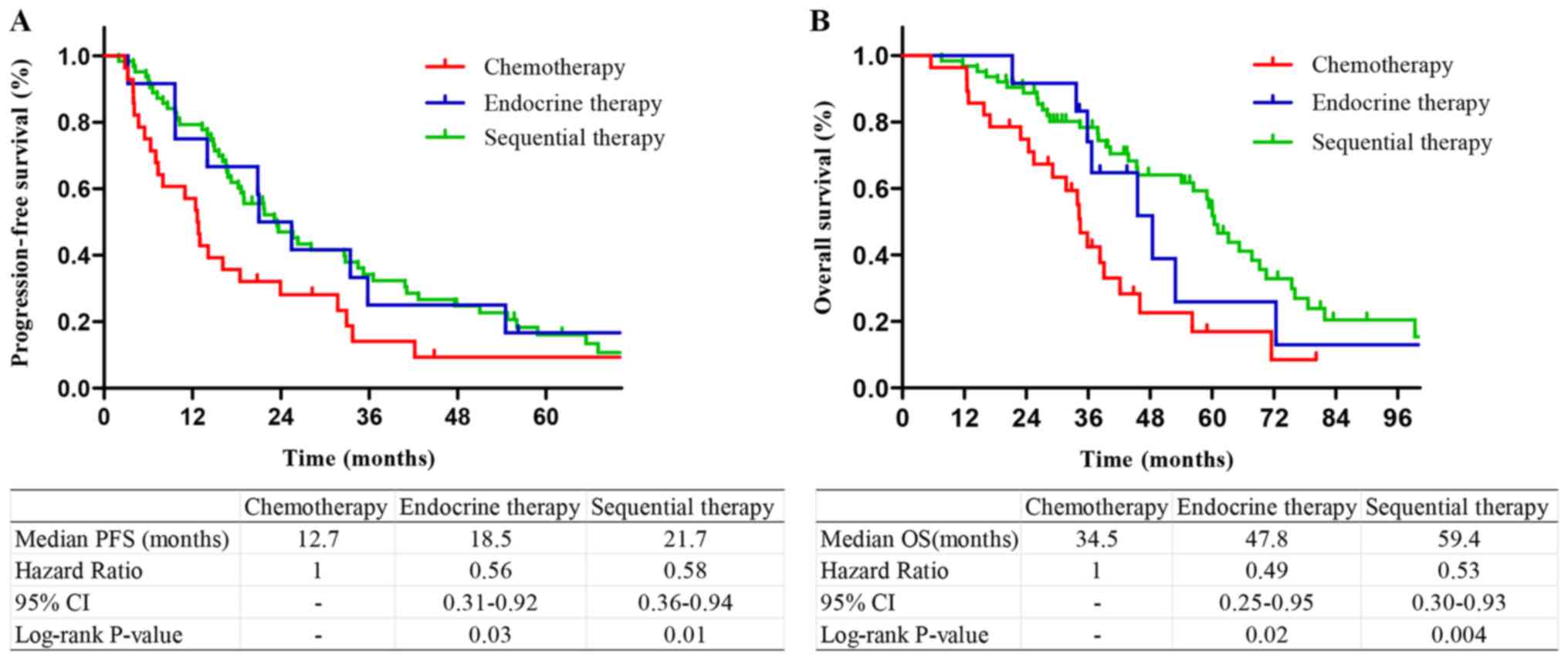
respectively; Fig. 3). Among the
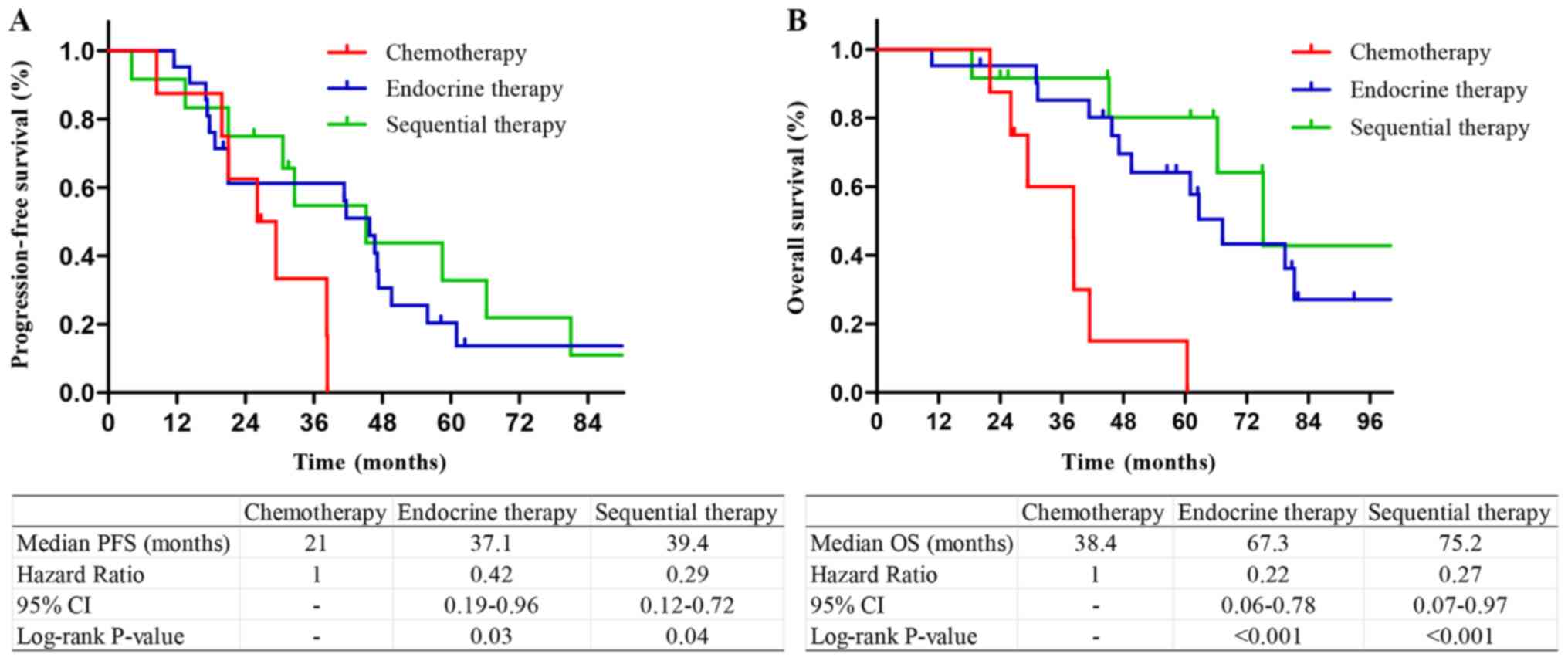
patients with a single bone metastasis, patients who received
endocrine therapy and sequential therapy had longer PFS and OS
compared with those who received chemotherapy. There was no
significant difference in PFS or OS between sequential therapy and
endocrine therapy (P=0.28 and 0.12, respectively; Fig. 4). Among the patients with multiple
bone metastases, patients who received endocrine therapy or
sequential therapy had longer PFS and OS compared with those who
received chemotherapy. There was no difference in PFS and OS
between sequential therapy and endocrine therapy (P=0.43 and 0.33,
respectively; Fig. 5).
Association between SREs and
characteristics of bone metastases
Of the 208 patients with BOM, 131 (63%) were
complicated by SREs; SREs were significantly associated with the
number of bone metastases (P<0.001; Table VIII). The incidence of SREs in
patients with multiple bone metastases was significantly higher
compared with that in patients with a single bone metastasis. The
location of bone metastasis had no impact on the incidence of SREs.
For patients with HR+/HER2− tumors, there was
no significant difference in the incidence of SREs among the
different treatments.
 | Table VIII.Associations between SREs and
characteristics of bone metastasis. |
Table VIII.
Associations between SREs and
characteristics of bone metastasis.
|
| SREs |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Yes, n (%) | No, n (%) | Total, n | χ2 | P-value |
|---|
| Number of bone
metastasis |
|
|
| 28.316 | <0.001 |
|
Single | 21
(16) | 39 (50.6) | 60 |
|
|
|
Multiple | 110 (84) | 38 (49.4) | 148 |
|
|
| Location of bone
metastasis |
|
|
| 0.314 | 0.855 |
|
Appendicular | 5
(3.8) | 2
(2.6) | 7 |
|
|
|
Axial | 102 (77.9) | 62 (80.5) | 164 |
|
|
|
Appendicular and axial | 24
(18.3) | 13 (16.9) | 37 |
|
|
|
HR+/HER2-BOM treatment |
|
|
| 0.013 | 0.990 |
|
Chemotherapy | 22
(25.3) | 14 (24.6) | 36 |
|
|
|
Endocrine therapy | 20
(23) | 13 (22.8) | 33 |
|
|
|
Sequential therapy | 45
(51.7) | 30 (52.6) | 75 |
|
|
Distribution of the secondary distant
metastatic site
Of the 208 patients with BOM, 109 (52.4%) developed
a secondary distant metastasis and the median MFI was 21 months
(95% CI: 17.2-24.8 months). Furthermore, of the 109 patients with a
second distant metastasis, 25.7% had an MFI within 1 year and 56.0%
had an MFI within 2 years. The most common metastatic site was the
liver (51.4%), followed by the lung (30.3%) and brain (13.8%). The
proportions of HR+/HER2− and HER2+
patients with liver metastasis were 76.8 and 16.1%, respectively.
The high-risk period of liver or lung metastasis was the first
three years after diagnosis of bone metastasis and the high-risk
period of brain metastasis was the first 2 years (Fig. 6).
Discussion
The present study was the largest real-world
analysis comparing clinicopathological characteristics, survival
and prognostic factors of patients with BOM and non-BOM MBC
published to date, to the best of our knowledge, and may be of
great significance for guiding clinical treatment and prediction of
prognosis. Understanding the time and location of the second
metastatic site may help guide the follow-up and reexamination of
patients with BOM. The proportion of patients with clinical stage
IV in the BOM group was significantly higher compared with that in
the non-BOM group. A previous study indicated that the clinical
stage was an independent risk factor for the incidence of bone
metastasis in patients with breast cancer (25). The bone is the most common metastatic
site of de novo MBC (26,27). The
‘seed-and-soil hypothesis’ highlights the bone as the preferred
site of metastatic development (28). In agreement with other studies
(13,29), the HR status and molecular subtypes
were identified as important factors affecting the presence of BOM.
HR+ status and in particular
HR+/HER2− (or luminal A) carcinoma was more
likely to develop BOM (11,12,29–31).
Patients with HER2+ or HR−/HER2−
carcinoma were more likely to develop visceral and cerebral
metastases, which was similar to the results of earlier studies
(31–35).
Prior studies indicated that the prognosis of
patients with BOM was better compared with that of breast cancer
patients with visceral or brain metastasis (36–38). The
results of the present study were in agreement with this, as the
DFS, PFS and OS of patients with BOM were significantly longer
compared with those of patients with non-BOM. Patients with non-BOM
had metastases, which were highly invasive and more prone to
progression. Recent studies have reported median OS times of
patients with BOM of 51–59 months (7,15,16),
which was longer than the median OS time of the cohort of the
present study. A reason for this difference may be that patients in
developed countries have comparatively better access to healthcare
and receive increased medical attention, which may prevent
recurrence or metastasis. To a certain extent, Chinese patients
have limited access to the latest drugs and high medical costs skew
comparisons between cohorts from different countries.
In the present study, HR+ primary tumors
were significantly and independently associated with improved
prognosis for patients with BOM, in agreement with previous studies
(15,16,39). The
ER status was closely associated with the histological grade;
highly differentiated tumors tended to have higher expression
levels of ER (40), which may be due
to improved prognosis of ER+ patients. The number and
the location of bone metastases were independent prognostic factors
affecting PFS and OS of patients with BOM in the present study.
These results were similar to those of previous studies (15,41,42), in
terms of multiple bone metastases, as well as combined axial and
appendicular bone metastases being associated with unfavorable
prognosis of patients with BOM. The location of bone metastasis had
no impact on the incidence of SREs in this study. Patients with
combined appendicular and axial metastases at BOM diagnosis had a
significantly greater odds of experiencing bone pain compared with
patients with metastases confined to axial locations (42), decreasing the quality of life of
patients. The present study suggested that patients with
appendicular bone metastasis had improved prognosis. Specifically,
there were only 7 patients with BOM with appendicular bone
metastasis alone, all of which were a solitary bone metastasis at
the time-point of BOM diagnosis, and 3 patients received sequential
therapy and 2 patients received endocrine therapy. It is probably
due to the small number of patients with appendicular bone
metastasis and the superior treatment received that these patients
had a longer survival time. Another previous retrospective study on
91 patients with bone metastases suggested that bisphosphonates
significantly prolonged OS of patients with breast cancer (39). However, bisphosphonates therapy was
not associated with PFS or OS in patients with BOM in the present
study. There may have been a selection bias in the previous study,
as patients in good physical condition or those expected to survive
for longer were more likely to receive bisphosphonates. In patients
with MBC, bisphosphonates are able to prevent or delay SREs, but
cannot prolong OS (18,43–45). To
clarify, denosumab had not been marketed in the Chinese mainland by
the end of the present study, and thus, none of the patients in the
present study were treated with denosumab for bone
preservation.
Non-BOM patients aged ≤35 years at diagnosis of
metastasis exhibited poorer prognosis compared with patients aged
>35 years. This may have been due to the carcinomas in younger
patients being more invasive and aggressive. It remains elusive
whether age at primary diagnosis influences outcomes. Purushotham
et al (46) indicated a
decrease in the risk of distant metastasis with increasing age.
Hung et al (47) determined
that patients aged <35 years had a significantly higher risk of
brain metastasis. Chen et al (38) suggested that older age significantly
contributed to poor prognosis. In the present study, there were no
associations between age at primary diagnosis and prognosis of
patients with breast cancer. Patients with HR− status
had a particularly high risk and poor prognosis, similar to
previous studies (48–50). Laohavinij et al (51) indicated that T4 was significantly
associated with poor prognosis of patients with MBC. In patients of
the present study with non-BOM, T3-4 vs. T1-2 carcinoma was
associated with poor prognosis.
In the present study, the DFS of both patients with
BOM and non-BOM with HR+/HER2− status was
significantly longer, which was similar to previous studies
(7,33). In a previous study, the median
follow-up time was 80.8 months and the luminal A subtype had the
highest 10-year DFS rate compared with luminal B, luminal/HER2,
HER2 enriched and TNBC (52). In
agreement with earlier studies (7,29),
patients of the present study with BOM and
HR+/HER2− had improved PFS and OS compared
with patients with HER2+ or
HR−/HER2−. A previous large retrospective
study indicated that the luminal A subtype had the highest 10-year
survival rate, followed by luminal B and luminal/HER2, while HER2
enriched and TNBC had the worst survival (52), which was similar to the results of
the present study. Amongst the patients with non-BOM in the present
study, the PFS and OS of the HR+/HER2− and
HER2+ types was superior to that of
HR−/HER2− cases. Patients with
HER2+ breast cancer had relatively improved survival.
Studies suggested that among patients with MBC, those with
HER2+ tumors had the best prognostic outcomes, while
those with HR−/HER2− tumors had the worst
prognosis (12,53), possibly due to advances in anti-HER2
molecular targeted drugs.
A previous study indicated that the survival rate of
patients with BOM of the HR+/HER2− type
treated with sequential therapy was significantly higher compared
with those treated with chemotherapy or endocrine therapy alone
(16). However, these results are
different from those of the present study, according to which
patients receiving endocrine or sequential therapy had longer PFS
and OS times compared with patients receiving chemotherapy;
however, there was no significant difference between outcomes of
endocrine therapy and sequential therapy. The same results were
obtained in subgroups of patients with a single bone metastasis or
multiple bone metastases. As Chinese patients have limitations
regarding the availability of the latest drugs and affordability of
medical treatments, palbociclib was approved by the Chinese Food
and Drug Administration on July 31, 2018 (24). There were only 3 patients receiving
cyclin-dependent kinase 4/6 inhibitors (palbociclib) in the
endocrine therapy group. In the present study, the impact of the
time of diagnosis was not considered. Among the patients with BOM,
only a small number received fulvestrant and palbociclib as the
first-line endocrine therapy. Of note, certain patients with BOM
may benefit from the rapid development of endocrine drugs in the
future. Furthermore, the present study indicated that the number of
bone metastases should be considered by the clinician when making a
decision on treatment options. Due to limited availability of
drugs, as well as the intense demand for relieving symptoms,
several patients with multiple bone metastases prefer sequential
therapy. However, in the present study, endocrine therapy alone was
as effective as sequential therapy, and sequential therapy may
result in more adverse reactions, including vomiting, leukopenia
and alopecia. Therefore, endocrine therapy may be preferred for
patients with HR+/HER2− BOM.
Earlier studies suggested that major risk factors
for SREs were age, menopausal status, clinical stage, tissue grade
and molecular subtypes (54,55). The number of bone metastases was
significantly associated with SREs in the present study. The
incidence of SREs in patients with multiple bone metastases was
significantly higher compared with that in patients with a single
bone metastasis. Kuchuk et al (55) indicated that patients with ≥5 bone
metastases were more likely to have an SRE than those with <5.
Parkes et al (42) determined
that multiple bone metastases were associated with increased
pain.
Among the patients of the present study with BOM who
developed secondary site metastases, the liver was the most common
site, followed by the lung and the brain. According to previous
studies, the liver was the most frequent organ of metastasis after
bone metastasis in HR+ breast cancer patients (11,56).
Furthermore, patients with HER2+ tumors were more prone
to liver metastasis (32,57). In the present study, the majority of
patients with liver metastasis were of the HR+ or
HER2+ types. Thus, it is necessary to pay increasing
attention to the possibility of liver and lung metastasis in the
first three years after diagnosis, and to brain metastases in the
first two years after diagnosis to detect metastasis as early as
possible.
The present study had certain limitations. The
cohort was based on patients from a single institution and may thus
have different characteristics from those at other centers,
limiting widespread generalizations. The present study was a
retrospective study and there may have been selection bias. The
systemic therapy included various drugs, doses and courses, and
future studies using randomization designs for different treatment
methods are required.
In conclusion, the prognosis of patients with BOM
was better compared with that of non-BOM patients. HR−
tumors, chemotherapy alone, multiple bone metastases, and combined
axial and appendicular bone metastases, were significantly
associated with poor prognosis in patients with BOM. An age at
metastasis or recurrence of ≤35 years, HR− status and a
tumor stage of T3-4 were significantly associated with a poorer
prognosis of patients with non-BOM. For patients with BOM and
HR+/HER2− status, endocrine therapy alone
achieved satisfactory results.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81702636
and 81602340), the Anticancer Key Technologies R&D Program of
Tianjin (grant no. 12ZCDZSY16200), the Natural Science Foundation
of Tianjin (grant no. 18JCYBJC93500) and the Science &
Technology Development Fund of Tianjin Education Commission for
Higher Education (grant no. 2016YD15).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ, JZ, ZL, YW and ZT conceived and designed the
study. ZT provided administrative support and gave final approval
of the version to be published. JZ, ZL, YW and LZ collected,
analyzed and interpreted the data. JZ and LZ drafted and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Committee and requirement of informed consent was waived due
to the retrospective study design. The present study was performed
in accordance with all relevant guidelines and procedures and
approved by the Tianjin Medical University Ethical Committee
(Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schröder J, Fietz T, Köhler A, Petersen V,
Tesch H, Spring L, Fleitz A, Jänicke M and Marschner N; TMK-Group
(Tumour Registry Breast Cancer, : Treatment and pattern of bone
metastases in 1094 patients with advanced breast cancer-Results
from the prospective German Tumour Registry Breast Cancer cohort
study. Eur J Cancer. 79:139–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin X and Mu P: Targeting breast cancer
metastasis. Breast Cancer (Auckl). 9 (Suppl 1):S23–S34. 2015.
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG: Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival,
. An overview of the randomised trials. Lancet. 365:1687–717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manders K, van de Poll-Franse LV, Creemers
GJ, Vreugdenhil G, van der Sangen MJ, Nieuwenhuijzen GA, Roumen RM
and Voogd AC: Clinical management of women with metastatic breast
cancer: A descriptive study according to age group. BMC Cancer.
6:1792006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mulcrone PL, Campbell JP, Clément-Demange
L, Anbinder AL, Merkel AR, Brekken RA, Sterling JA and Elefteriou
F: Skeletal colonization by breast cancer cells is stimulated by an
osteoblast and β2AR-dependent Neo-angiogenic switch. J Bone Miner
Res. 32:1442–1454. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coleman RE and Rubens RD: The clinical
course of bone metastases from breast cancer. Br J Cancer.
55:61–66. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parkes A, Clifton K, Al-Awadhi A, Oke O,
Warneke CL, Litton JK and Hortobagyi GN: Characterization of bone
only metastasis patients with respect to tumor subtypes. NPJ Breast
Cancer. 4:22018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von Moos R, Body JJ, Egerdie B, Stopeck A,
Brown J, Fallowfield L, Patrick DL, Cleeland C, Damyanov D, Palazzo
FS, et al: Pain and analgesic use associated with skeletal-related
events in patients with advanced cancer and bone metastases.
Support Care Cancer. 24:1327–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sathiakumar N, Delzell E, Morrisey MA,
Falkson C, Yong M, Chia V, Blackburn J, Arora T, Brill I and
Kilgore ML: Mortality following bone metastasis and
skeletal-related events among women with breast cancer: A
population-based analysis of U.S. Medicare beneficiaries,
1999–2006. Breast Cancer Res Treat. 131:231–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li BT, Wong MH and Pavlakis N: Treatment
and prevention of bone metastases from breast cancer: A
comprehensive review of evidence for clinical practice. J Clin Med.
3:1–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Molnár IA, Molnár BÁ, Vízkeleti L, Fekete
K, Tamás J, Deák P, Szundi C, Székely B, Moldvay J, Vári-Kakas S,
et al: Breast carcinoma subtypes show different patterns of
metastatic behavior. Virchows Arch. 470:275–283. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gong Y, Liu YR, Ji P, Hu X and Shao ZM:
Impact of molecular subtypes on metastatic breast cancer patients:
A SEER population-based study. Sci Rep. 7:454112017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Solomayer EF, Diel IJ, Meyberg GC, Gollan
C and Bastert G: Metastatic breast cancer: Clinical course,
prognosis and therapy related to the first site of metastasis.
Breast Cancer Res Treat. 59:271–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Plunkett TA, Smith P and Rubens RD: Risk
of complications from bone metastases in breast cancer.
Implications for management. Eur J Cancer. 36:476–82. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahn SG, Lee HM, Cho SH, Lee SA, Hwang SH,
Jeong J and Lee HD: Prognostic factors for patients with bone-only
metastasis in breast cancer. Yonsei Med J. 54:1168–1177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niikura N, Liu J, Hayashi N, Palla SL,
Tokuda Y, Hortobagyi GN, Ueno NT and Theriault RL: Treatment
outcome and prognostic factors for patients with bone-only
metastases of breast cancer: A single-institution retrospective
analysis. Oncologist. 16:155–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eckhardt BL, Francis PA, Parker BS and
Anderson RL: Strategies for the discovery and development of
therapies for metastatic breast cancer. Nat Rev Drug Discov.
11:479–497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Erdogan B and Cicin I: Medical treatment
of breast cancer bone metastasis: From bisphosphonates to targeted
drugs. Asian Pac J Cancer Prev. 15:1503–1510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sze WM, Shelley M, Held I and Mason M:
Palliation of metastatic bone pain: Single fraction versus
multifraction radiotherapy-a systematic review of the randomised
trials. Cochrane Database Syst Rev. 2002:CD0047212004.
|
|
20
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian XL, Wen HY, Yang YL, Gu F, Guo XJ,
Liu FF, Zhang L, Zhang XM and Fu L: Assessment of dual-probe Her-2
fluorescent in situ hybridization in breast cancer by the 2013
ASCO/CAP guidelines produces more equivocal results than that by
the 2007 ASCO/CAP guidelines. Breast Cancer Res Treat. 159:31–39.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tiedemann K, Hussein O and Komarova SV:
Role of altered metabolic microenvironment in osteolytic
metastasis. Front Cell Dev Biol. 8:4352020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong X, Zhang Z, Ren J, Zhang J, Pan X,
Zhang L, Gong S and Jin S: Impact of universal medical insurance
system on the accessibility of medical service supply and
affordability of patients in China. PLoS One. 13:e01932732018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu X, Li T, Wang B, Zhang J, Yu X and Shao
Z: Comparison of 4th ESO-ESMO international consensus guidelines
for advance breast cancer and Chinese anti-cancer association
committee of Breast Cancer Society guideline. Breast. 45:36–42.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamashiro H, Takada M, Nakatani E, Imai S,
Yamauchi A, Tsuyuki S, Matsutani Y, Sakata S, Wada Y, Okamura R, et
al: Prevalence and risk factors of bone metastasis and skeletal
related events in patients with primary breast cancer in Japan. Int
J Clin Oncol. 19:852–862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hölzel D, Eckel R, Bauerfeind I, Baier B,
Beck T, Braun M, Ettl J, Hamann U, Harbeck N, Kiechle M, et al:
Survival of de novo stage IV breast cancer patients over three
decades. J Cancer Res Clin Oncol. 143:509–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miao H, Hartman M, Bhoo-Pathy N, Lee SC,
Taib NA, Tan EY, Chan P, Moons KG, Wong HS, Goh J, et al:
Predicting survival of de novo metastatic breast cancer in Asian
women: Systematic review and validation study. PLoS One.
9:e937552014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diessner J, Wischnewsky M, Stüber T, Stein
R, Krockenberger M, Häusler S, Janni W, Kreienberg R, Blettner M,
Schwentner L, et al: Evaluation of clinical parameters influencing
the development of bone metastasis in breast cancer. BMC Cancer.
16:3072016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Zhu W, Biskup E, Yang W, Yang Z,
Wang H, Qiu X, Zhang C and Hu G and Hu G: Incidence, risk factors
and prognostic characteristics of bone metastases and
skeletal-related events (SREs) in breast cancer patients: A
systematic review of the real world data. J Bone Oncol. 11:38–50.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park HS, Kim S, Kim K, Yoo H, Chae BJ, Bae
JS, Song BJ and Jung SS: Pattern of distant recurrence according to
the molecular subtypes in Korean women with breast cancer. World J
Surg Oncol. 10:42012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leone BA, Vallejo CT, Romero AO,
Machiavelli MR, Pérez JE, Leone J and Leone JP: Prognostic impact
of metastatic pattern in stage IV breast cancer at initial
diagnosis. Breast Cancer Res Treat. 161:537–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Metzger-Filho O, Sun Z, Viale G, Price KN,
Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates
AS, Goldhirsch A and Cardoso F: Patterns of Recurrence and outcome
according to breast cancer subtypes in lymph node-negative disease:
Results from international breast cancer study group trials VIII
and IX. J Clin Oncol. 31:3083–3090. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soni A, Ren Z, Hameed O, Chanda D, Morgan
CJ, Siegal GP and Wei S: Breast cancer subtypes predispose the site
of distant metastases. Am J Clin Pathol. 143:471–478. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Beca F, Santos R, Vieira D, Zeferino L,
Dufloth R and Schmitt F: Primary relapse site pattern in women with
triple-negative breast cancer. Pathol Res Pract. 210:571–575. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Domchek SM, Younger J, Finkelstein DM and
Seiden MV: Predictors of skeletal complications in patients with
metastatic breast carcinoma. Cancer. 89:363–368. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lipton A: Should bisphosphonates be
utilized in the adjuvant setting for breast cancer. Breast Cancer
Res Treat. 122:627–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen MT, Sun HF, Zhao Y, Fu WY, Yang LP,
Gao SP, Li LD, Jiang HL and Jin W: Comparison of patterns and
prognosis among distant metastatic breast cancer patients by age
groups: A SEER population-based analysis. Sci Rep. 7:92542017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
James JJ, Evans AJ, Pinder SE, Gutteridge
E, Cheung KL, Chan S and Robertson JF: Bone metastases from breast
carcinoma: Histopathological-radiological correlations and
prognostic features. Br J Cancer. 89:660–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Williams MR, Todd JH, Ellis IO, Dowle CS,
Haybittle JL, Elston CW, Nicholson RI, Griffiths K and Blamey RW:
Oestrogen receptors in primary and advanced breast cancer: An eight
year review of 704 cases. Br J Cancer. 55:67–73. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koizumi M, Yoshimoto M, Kasumi F and Ogata
E: Comparison between solitary and multiple skeletal metastatic
lesions of breast cancer patients. Ann Oncol. 14:1234–1240. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Parkes A, Warneke CL, Clifton K, Al-Awadhi
A, Oke O, Pestana RC, Alhalabi O, Litton JK and Hortobagyi GN:
Prognostic factors in patients with metastatic breast cancer with
bone-only metastases. Oncologist. 23:1282–1288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Henry DH, Costa L, Goldwasser F, Hirsh V,
Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A,
Vadhan-Raj S, et al: Randomized, double-blind study of denosumab
versus zoledronic acid in the treatment of bone metastases in
patients with advanced cancer (excluding breast and prostate
cancer) or multiple myeloma. J Clin Oncol. 29:1125–1132. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Coleman RE: Impact of Bone-targeted
treatments on skeletal morbidity and survival in breast cancer.
Oncology (Williston Park). 30:695–702. 2016.PubMed/NCBI
|
|
45
|
Biskup E, Cai F and Vetter M: Bone
targeted therapies in advanced breast cancer. Swiss Med Wkly.
147:W144402017.PubMed/NCBI
|
|
46
|
Purushotham A, Shamil E, Cariati M, Agbaje
O, Muhidin A, Gillett C, Mera A, Sivanadiyan K, Harries M, Sullivan
R, et al: Age at diagnosis and distant metastasis in breast
cancer-a surprising inverse relationship. Eur J Cancer.
50:1697–1705. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hung MH, Liu CY, Shiau CY, Hsu CY, Tsai
YF, Wang YL, Tai LC, King KL, Chao TC, Chiu JH, et al: Effect of
age and biological subtype on the risk and timing of brain
metastasis in breast cancer patients. PLoS One. 9:e893892014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ren Z, Li Y, Hameed O, Siegal GP and Wei
S: Prognostic factors in patients with metastatic breast cancer at
the time of diagnosis. Pathol Res Pract. 210:301–306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jung SY, Rosenzweig M, Sereika SM, Linkov
F, Brufsky A and Weissfeld JL: Factors associated with mortality
after breast cancer metastasis. Cancer Causes Control. 23:103–112.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cossetti RJ, Tyldesley SK, Speers CH,
Zheng Y and Gelmon KA: Comparison of breast cancer recurrence and
outcome patterns between patients treated from 1986 to 1992 and
from 2004 to 2008. J Clin Oncol. 33:65–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Laohavinij S, Paul V and Maneenil K:
Survival and prognostic factors of metastatic breast cancer. J Med
Assoc Thai. 100 (Suppl 1):S16–S26. 2017.PubMed/NCBI
|
|
52
|
Ignatov A, Eggemann H, Burger E and
Ignatov T: Patterns of breast cancer relapse in accordance to
biological subtype. J Cancer Res Clin Oncol. 144:1347–1355. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Press DJ, Miller ME, Liederbach E, Yao K
and Huo D: De novo metastasis in breast cancer: Occurrence and
overall survival stratified by molecular subtype. Clin Exp
Metastasis. 34:457–465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yamashiro H, Takada M, Nakatani E, Imai S,
Yamauchi A, Tsuyuki S, Matsutani Y, Sakata S, Wada Y, Okamura R, et
al: Prevalence and risk factors of bone metastasis and skeletal
related events in patients with primary breast cancer in Japan. Int
J Clin Oncol. 19:852–862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kuchuk I, Hutton B, Moretto P, Ng T,
Addison CL and Clemons M: Incidence, consequences and treatment of
bone metastases in breast cancer patients-Experience from a single
cancer Centre. J Bone Oncol. 2:137–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ma R, Feng Y, Lin S, Chen J, Lin H, Liang
X, Zheng H and Cai X: Mechanisms involved in breast cancer liver
metastasis. J Transl Med. 13:642015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wei S and Siegal GP: Metastatic
organotropism: An intrinsic property of breast cancer molecular
subtypes. Adv Anat Pathol. 24:78–81. 2017. View Article : Google Scholar : PubMed/NCBI
|