Introduction
Prostate cancer (PCa) is one of the most common
malignant tumors in men worldwide (1). According to estimates in 2019 among the
10 leading types of cancer in the United States, PCa was the
disease with the highest number of new cases in men
(174,650/870,970 new cancer cases; 20%) and the second leading
cause of cancer-associated death in men (31,620/321,670 deaths;
10%) (2). Despite significant
advances in diagnostics, surgical techniques and adjuvant therapies
over the past few decades, the morbidity and mortality rates of PCa
have continued to rise rapidly (3).
PCa is more prone to bone metastasis compared with cancer
originating in any other tissue, which leads to excessive
osteolysis and osteogenesis (4,5). Most
patients with PCa inevitably develop castration-resistant PCa
(CRPC) within two years, which is a more aggressive type of PCa
(6). Bone metastases occur in the
vast majority of patients with CRPC, resulting in less favorable
prognosis and a significant increase in mortality; therefore, it is
the most common cause of death in patients with PCa (7). A lack of effective treatment strategies
is a major limitation for the treatment of recurrent and metastatic
PCa (8). Therefore, improving the
understanding of the molecular mechanisms underlying PCa
progression is required for the effective treatment of patients
with PCa.
Long non-coding RNAs (lncRNAs) are defined as
transcripts >200 nucleotides in length with no apparent protein
coding function (9). The role of
lncRNAs in various biological processes, such as cell
differentiation, proliferation and apoptosis in various diseases,
including cancer, has received increasing attention (10–12). The
colon cancer associated transcript 2 (CCAT2) gene has been reported
to be upregulated in various types of human cancer, including
gastric cancer, non-small cell lung cancer, esophageal cancer
(13–17), hepatocellular carcinoma (18,19) and
other tumors (20–22), and it is associated with the
occurrence and metastasis of cancer, as well as poor prognosis.
Previous studies have demonstrated that lncRNA CCAT2 is localized
in the 8q24 genomic region, which has been identified as the first
reproducible genetic risk site (23,24).
However, to the best of our knowledge, the biological function and
related molecular mechanisms underlying CCAT2 in PCa have not been
previously studied.
Wnt is a cysteine-rich glycoprotein that is secreted
by cells into the extracellular matrix (25). Activation of the Wnt/β-catenin
signaling pathway alters receptor-mediated signaling, primarily by
inhibiting the degradation of β-catenin in cells, which causes
cytoplasmic β-catenin to accumulate and transfer to the nucleus,
thereby affecting the transcriptional activity of the target gene
(26,27). Alterations to the activity of the
Wnt/β-catenin signaling pathway are often associated with
inflammatory responses, cell proliferation and differentiation,
transcriptional activity and cell membrane structure, which in turn
affect the progression of various types of cancer, neuronal
diseases, bone diseases and developmental disorders (28–30). An
increasing number of studies have reported that abnormal
Wnt/β-catenin signaling is associated with the development and
progression of a variety of malignancies (31,32).
It has also been reported that by activating the
Wnt/β-catenin signaling pathway, lncRNA CCAT2 affects oral squamous
cell carcinoma, ovarian cancer and renal cell carcinoma (33), as well as non-small cell lung cancer,
breast cancer, esophageal cancer and glioma (15,17,33–37).
However, to the best of our knowledge, no studies have previously
examined the relationship between CCAT2 and the Wnt/β-catenin
signaling pathway in PCa, and its potential regulatory mechanism.
The results of the present study indicated that CCAT2 regulated the
expression of transcription factor 7-like 2 (TCF7L2) by targeting
microRNA (miR)-217, and regulated the Wnt/β-catenin signaling
pathway to control PCa. Therefore, the present study suggested that
CCAT2 may display a pivotal role during PCa carcinogenesis, and may
serve as an early diagnostic and molecular therapeutic target for
PCa.
Materials and methods
Patient samples
PCa tissues and adjacent normal prostate tissues
(5-cm from the resection margin and pathologically confirmed) were
obtained from 18 male patients (mean age, 60.8 years; age range,
50–72 years) who attended The Fifth Hospital of Wuhan (Wuhan,
China) between March 2018 and November 2018. The patients had not
received local or systemic treatment before surgery. Following
prostatectomy, all specimens were collected within 20 min and
immediately stored in liquid nitrogen until RNA extraction. The
present study was approved by the Evaluation Committee of The Fifth
Hospital of Wuhan (approval no. 2018-k-003). The present study
carefully followed the ethical and procedural rules of clinical
research, and obtained written informed consent from each patient
before surgery.
Cell culture
The human PCa PC3 and DU145 cell lines, and human
prostate epithelial RWPE-1 cell line were purchased from American
Type Culture Collection. PC3 and DU145 cells were cultured in DMEM
(HyClone; Cytiva) containing 10% fetal bovine serum (HyClone;
Cytiva), 100 µg/ml penicillin and 100 µg/ml streptomycin. RWPE-1
cells were cultured in keratinocyte serum-free medium (cat. no.
10724; Gibco; Thermo Fisher Scientific, Inc.) containing 50 µg/ml
bovine pituitary extract L-glutamine and 5 ng/ml epidermal growth
factor. All cells were cultured at 37°C with 5% CO2.
Cells were periodically passaged to maintain exponential growth and
passage two cells were used for subsequent experiments.
Small interfering (si)RNA
transfection
An siRNA targeting CCAT2 (si-CCAT2) and the control
siRNA [si-negative control (NC)] were constructed by Shanghai
GenePharma Co., Ltd. The siRNA sequences used were as follows:
si-CCAT2, 5′-GUGCAACUCUGCAAUUUAAUU-3′; and si-NC,
5′-AATGGACAACTGGTCGTGGAC-3′. A miR-217 mimic (miR-217) and its
corresponding NC (miR-NC), and a miR-217 inhibitor and its
corresponding NC (NC inhibitor) were purchased from Shanghai
GenePharma Co., Ltd. Human TCF7L2 cDNA (1,809 bp; Shanghai
GeneChem Co., Ltd.) was inserted into a pcDNA3.1 plasmid (Shanghai
GeneChem Co., Ltd.) to form TCF7L2 overexpression plasmids.
DU145 and PC3 cells were seeded (5×105 cells/well) into
6-well plates for 1 day prior to transfection. At 70% confluence,
these molecular productions were transfected into DU145 and PC3
cells using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. The transfection concentrations were 10 µmol for
si-CCAT2 or siNC, and 50 nM for miR-217 mimic/inhibitor, their NCs
or TCF7L2 overexpression plasmids. Following incubation at
37°C for 48 h, cells were harvested and used for subsequent
experiments.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
RT-qPCR was used to detect the mRNA expression
levels of CCAT2 in PCa and adjacent non-cancerous tissues, as well
as DU145 and PC3 cells. Total RNA was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Isolated RNA (2
µg) was reverse transcribed into cDNA using a reverse transcription
kit (DRR037A, Takara Biotechnology Co., Ltd.) at 37°C for 15 min
and 85°C for 5 sec. Subsequently, qPCR was performed using SYBR
Green (Takara Biotechnology Co., Ltd.) and the Biosystems 7500
real-time PCR system (Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. The following primer pairs were
used for qPCR: CCAT2 forward, 5′-ACTGGGAATGGAGGAAGA-3′ and reverse,
5′-TGAGAAAGGATTGAGGGAAAAG-3′; TCF7L2 forward,
5′-ACGAGGGCGAACAGGAGGAG-3′ and reverse, 5′-TGGGCGAGAGCGATCCGTTG-3′;
miR-217 forward, 5′-GCGTACTGCATCAGGAACTGATTGGA-3′ and reverse,
5′-GGGCACACAAAGGCAACTTTTGT-3′; and U6 forward,
5′-TGCGGGTGCTCGCTTCGCAGC-3′ and reverse, 5′-CCAGTGCAGGGTCCGAGGT-3′.
The following thermocycling conditions were used for qPCR: An
initial step of 95°C for 60 sec, followed by 40 cycles of
denaturation at 95°C for 20 sec, annealing and extension at 59°C
for 40 sec, and hold at 72°C for 15 sec. Expression levels were
quantified using the 2−ΔΔCq method (38) and normalized to the internal
reference gene U6. RT-qPCR was performed in at least
triplicate.
Cell proliferation assay
Cell proliferation was assessed using the Cell
Counting Kit-8 assay (CCK8; Dojindo Molecular Technologies, Inc.).
DU145 and PC3 cells were seeded (5×103 cells/well) into
96-well plates after transfection and incubated at 37°C with 5%
CO2. Cells were cultured at 37°C for 0, 24, 48, 72 and
96 h. Subsequently, ~20 µl CCK8 reagent was added to each well and
incubated for 2 h at 37°C in the dark. Then, 100 µl dimethyl
sulfoxide was added to dissolve the purple formazan. The absorbance
of each well was measured at a wavelength of 450 nm using a
SpectraMax M3 microplate reader (Molecular Devices, LLC). The CCK-8
assay was performed in triplicate.
Flow cytometry and cell cycle
analysis
DU145 and PC3 cells (3×105 cells/ml) were
transfected/treated with si-CCAT2, si-NC or si-CCAT2 + 20 mmol/l
lithium chloride (LiCl; Sigma-Aldrich Merck KGaA) at 37°C for 24 h.
After 48 h, cells were centrifuged at 1,000 × g for 5 min at room
temperature, washed with PBS and fixed with pre-cooled 70% ethanol
at 4°C overnight. Subsequently, cells were stained at room
temperature for 30 min with propidium iodide (PI; Sigma-Aldrich;
Merck KGaA) staining buffer containing 500 µg/ml PI and 100 µg/ml
RNase A at 37°C for 30 min in the dark. Cell cycle distribution was
measured using a FACSCalibur flow cytometer (BD Biosciences), and
cell cycle profiles were generated using ModFit software (v3.0; BD
Biosciences). Cell cycle distribution is presented as the
percentage of cells in the G0/G1, S and
G2/M phases. Flow cytometry was performed in
triplicate.
Cell apoptosis assay
DU145 and PC3 cells (3×105 cells/ml) were
transfected with si-NC and si-CCAT2. Following transfection for 48
h, cells were collected and washed with PBS. Apoptotic cells were
detected using the Annexin V-FITC/PI Apoptosis Detection kit
(BioTime, Inc.), according to the manufacturer's protocol. Cells
were stained with Annexin V and PI at 37°C for 15 min in the dark.
Apoptotic cells were analyzed using a FACSCalibur flow cytometer
(BD Biosciences) and BD FACSuite software (v1.0; BD
Biosciences).
Migration and invasion assay
The migratory ability of DU145 and PC3 cells
transfected with si-CCAT2 or si-NC was assessed using a 24-well
Transwell chamber (BD Biosciences). For migration assays, PCa cells
(1×105 cells) with serum-free DMEM were plated into the
upper chambers, and DMEM containing 10% FBS (HyClone; Cytiva) was
plated into the lower chambers. Cells were incubated at 37°C with
5% CO2 for 24 h. Following incubation, the upper surface
of the Transwell membrane was wiped to move non-migratory cells.
Migratory cells on the lower surface of the Transwell membranes
were fixed in 4% paraformaldehyde at 4°C for 20 min, followed by
staining with 0.1% crystal violet for 15 min at 25°C. Stained cells
were observed in five random fields using a light microscope
(magnification, ×20). To assess cell invasion, artificial basement
membrane Matrigel® (BD Biosciences) was pre-coated at
37°C overnight on the bottom of the Transwell culture chamber.
Western blot analysis
Total protein was extracted from the transfected
DU145 and PC3 cells using RIPA buffer (Aladdin Industrial
Corporation) supplemented with 1% protease inhibitors (cat. no.
P2850; Sigma-Aldrich; Merck KGaA) on ice for 30 min. Total protein
was quantified using the bicinchoninic acid assay (Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. Equal amounts of protein (40 µg/lane) were separated via
10% SDS-PAGE and transferred onto a 0.22-µm PVDF membrane (EMD
Millipore). Following blocking at room temperature for 1 h with 5%
skim milk, the membrane was incubated overnight at 4°C with primary
antibodies targeted against the following: β-catenin (1:1,000; cat.
no. ab32572; Abcam), Cyclin D1 (1:1,000; cat. no. ab134175; Abcam),
c-Myc (1:1,000; cat. no. ab39688; Abcam), GAPDH (1:1,000; cat. no.
ab8245; Abcam), E-cadherin (1:200; cat. no. ab219332; Abcam),
N-cadherin (1:200; cat. no. ab12221; Abcam), Vimentin (1:1,000;
cat. no. ab137321; Abcam) and TCF7L2 (1:1,000; cat. no. ab76151;
Abcam). Following incubation with the primary antibodies overnight,
the membranes were incubated with appropriate horseradish
peroxidase-conjugated secondary antibodies (goat anti-mouse;
1:2,000; cat. no. abs20001; Absin Bioscience, Inc., or goat
anti-rabbit; 1:5,000; cat. no. ab6721; Abcam) for 2 h at 37°C.
Protein bands were visualized using an enhanced chemiluminescence
reagent (Bio-Rad Laboratories, Inc.). Protein expression levels
were quantified using ImageJ software v1.48 (National Institutes of
Health). The relative quantification was normalized to the control
(such as si-NC).
Bioinformatics prediction and
dual-luciferase reporter assay
The potential targets of CCAT2 and miR-217 were
predicted using RNA Hybrid (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid/)
(39). The wild-type (WT) and mutant
(MUT) miRNA binding site sequence of CCAT2 and TCF7L2 were
constructed by Shanghai GeneChem Co., Ltd. DU145 and PC3 cells
(1×105 cells) were co-transfected with CCAT2-WT,
CCAT2-MUT, TCF7L2-WT or TCF7L2-MUT and miR-217 mimic or NC mimic
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. A
total of 10 ng Renilla (Promega Corporation) was used as the
internal control. After transfection for 48 h at 37°C, luciferase
activities were assessed using a Dual-Luciferase Reporter Assay
System (Promega Corporation) according to the manufacturer's
protocol, and the activity was normalized with Renilla
luciferase activity. The dual-luciferase reporter assay was
performed in triplicate.
TOPflash assay
PCa cells (1×105) were co-transfected
with 250 ng WNT signaling luciferase reporter constructs (TOPflash)
(Shanghai GeneChem Co., Ltd.) and 25 ng Renilla luciferase
vector (Promega Corporation) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. At 48 h post-transfection at 37°C,
luciferase activities were assessed using a Dual-Luciferase
Reporter Assay System (Promega Corporation) according to the
manufacturer's protocol. Firefly luciferase activities were
normalized to Renilla luciferase activities. The TOP values
were calculated as the relative luciferase activity. The TOPflash
assay was performed in triplicate.
Statistical analysis
Statistical analyses were performed using SPSS
software (v21.0; IBM Corp.). Data are expressed as the mean ±
standard deviation of at least three independent experiments.
Differences between the paired data were analyzed by a paired
Student's t-test. Comparisons between different groups were
performed using one-way ANOVA followed by Tukey's multiple
comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
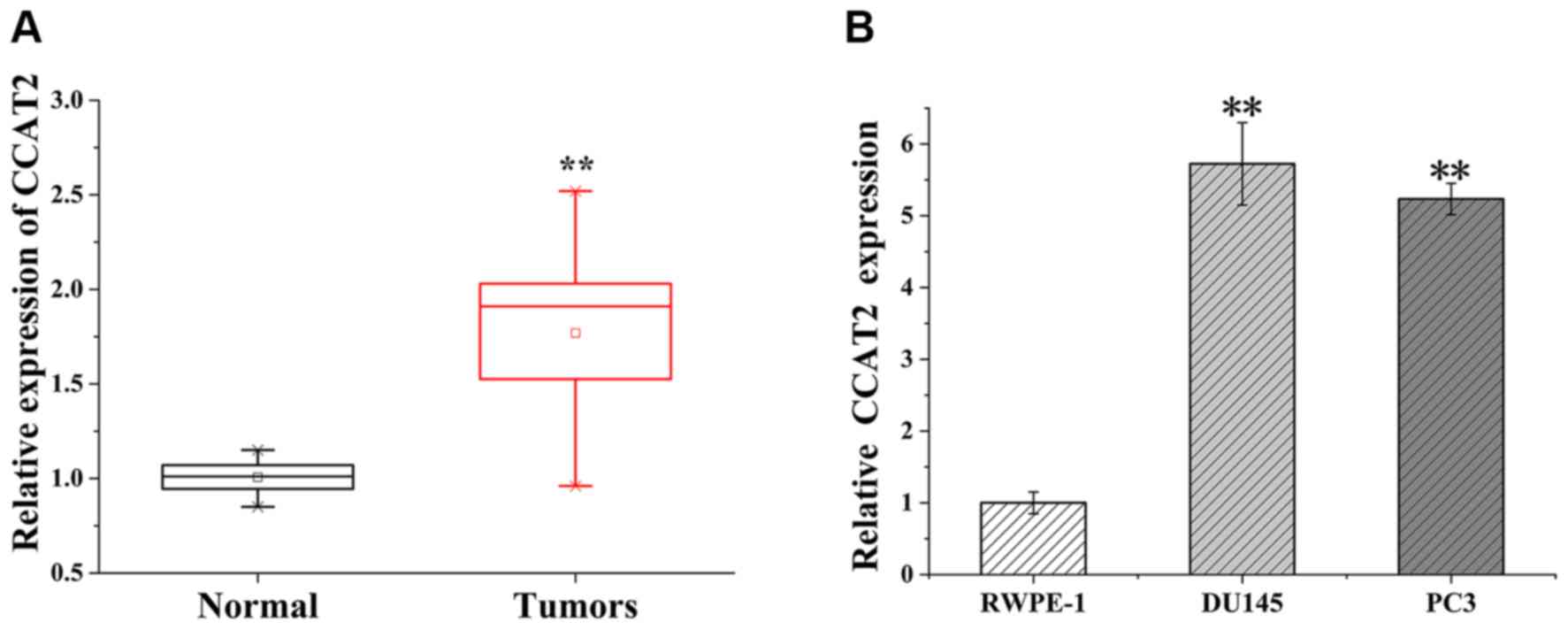
CCAT2 is upregulated in PCa tissues
and cell lines
RT-qPCR was performed to detect the expression of
lncRNA CCAT2 in paired tissue samples obtained from 18 patients
with PCa. CCAT2 expression levels were significantly higher in PCa
tissues compared with adjacent non-tumor tissues (P<0.01;
Fig. 1A). Subsequently, RT-qPCR was
performed to detect the expression levels of CCAT2 in the human
prostate epithelial RWPE-1 cell line and the human PCa DU145 and
PC3 cell lines. The results revealed that CCAT2 expression levels
were significantly higher in DU145 and PC3 cells compared with
RWPE-1 cells (P<0.01; Fig.1B).
The results indicated that abnormal CCAT2 expression may be
associated with the pathogenesis of PCa and may serve a crucial
role during the progression of the disease.
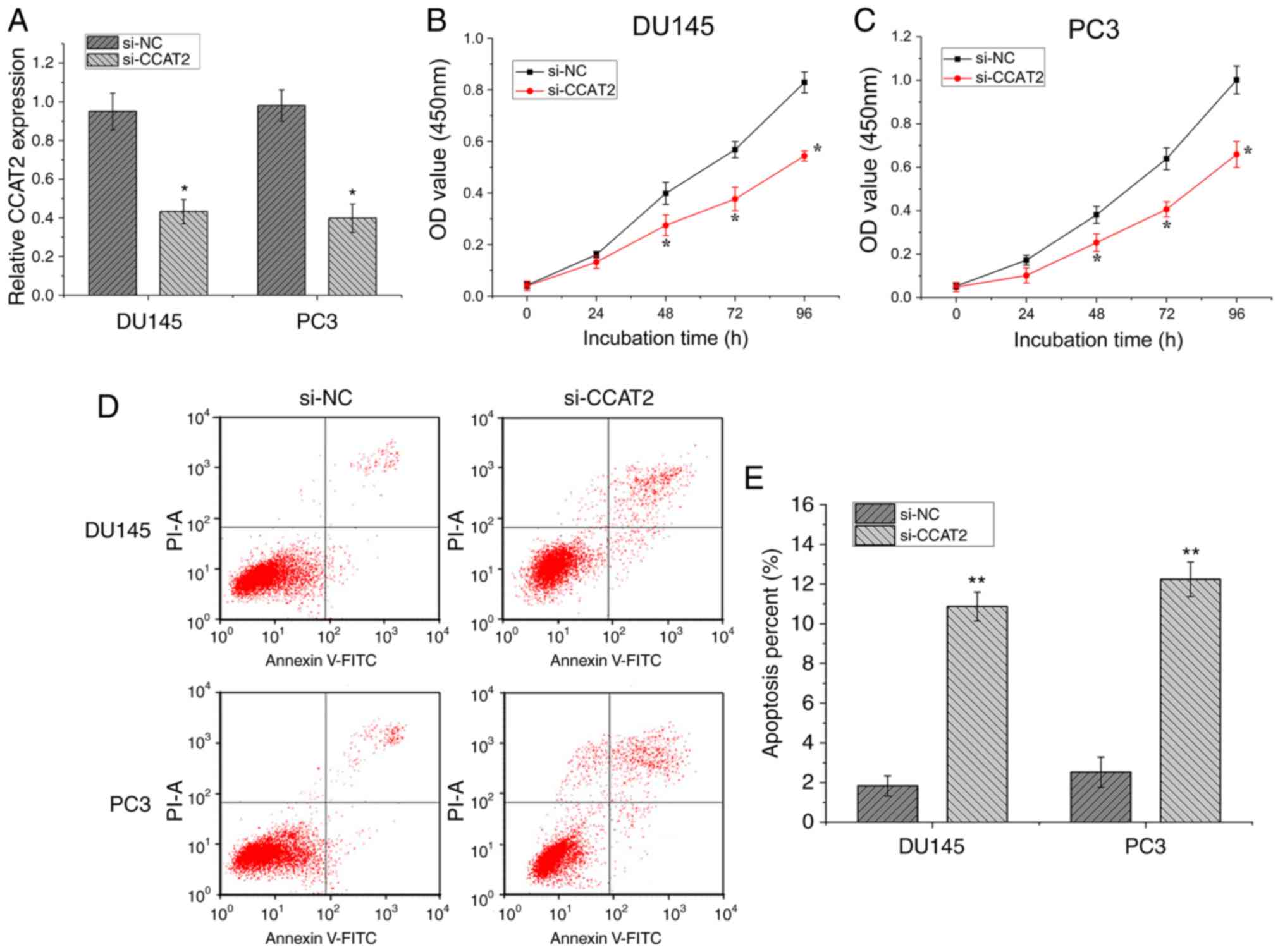
CCAT2 regulates cell proliferation and
cell apoptosis in PCa
To further investigate the role of CCAT2 during the
progression of PCa, CCAT2 expression was knocked down in DU145 and
PC3 cells using a specific siRNA to determine its effect on the
biological function of PCa cells. The expression level of CCAT2
mRNA in transfected si-CCAT2 cells was significantly lower compared
with cells transfected with si-NC control siRNA (Fig. 2A; P<0.05). The CCK-8 assay
indicated that DU145 (Fig. 2B) and
PC3 (Fig. 2C) cells exhibited
significantly reduced cell proliferation at 48, 72 and 96 h in the
si-CCAT2 group compared with the si-NC group (P<0.05).
Subsequently, flow cytometry was performed to examine the effect of
CCAT2 on the PCa cell apoptosis (Fig.
2D). si-CCAT2 significantly promoted DU145 and PC3 cell
apoptosis compared with si-NC (P<0.01; Fig. 2E). Collectively, the aforementioned
results indicated that CCAT2-knockdown inhibited PCa cell
proliferation and promoted PCa cell apoptosis.
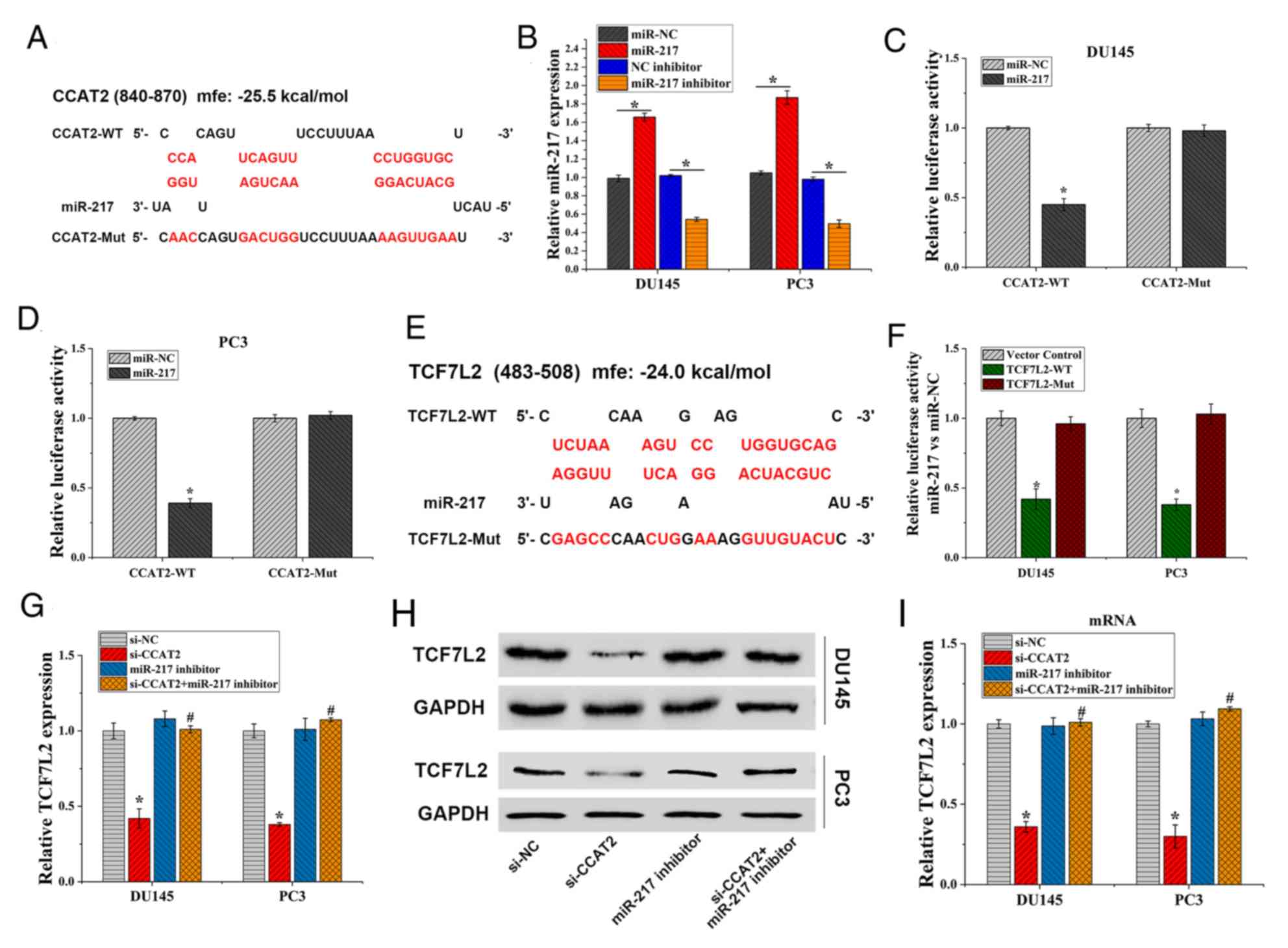
CCAT2 regulates TCF7L2 expression by
binding to miR-217
To further investigate the mechanism of action
underlying CCAT2 during the development of PCa, RNA Hybrid was used
to predict potential target miRs of CCAT2. The results indicated
that CCAT2 bound to miR-217 with −25.5 kcal/mol binding energy
(Fig. 3A). As shown in Fig. 3B, after PCa cells were transfected
with miR-217 mimic and miR-217 inhibitor, the mRNA expression of
miR-217 was significantly different from that of the NC, indicating
successful transfection. To investigate the binding function of the
genes, a dual-luciferase gene reporter assay was performed. In
DU145 and PC3 cells, luciferase activities of CCAT2-WT were
significantly reduced by miR-217 compared with miR-NC (P<0.05),
but not that of the CCAT2-MUT (Fig. 3C
and D). Moreover, the RNA Hybrid prediction analysis identified
binding sites between miR-217 and TAF7L2, which displayed −24.0
kcal/mol binding energy (Fig. 3E).
Furthermore, the dual-luciferase gene reporter assay indicated that
miR-217 mimic inhibited luciferase activities in the TCF7L2-WT
group, but exhibited no effect in the TCF7L2-MUT group (Fig. 3F). The results confirmed that TCF7L2
was a target gene of miR-217. In addition, CCAT2-knockdown
significantly reduced the mRNA (Fig.
3G) and protein (Fig. 3H and I)
expression levels of TCF7L2. However, miR-217 inhibitor reversed
the inhibitory effect of CCAT2-knockdown on TCF7L2 expression. The
results suggested that CCAT2 regulated TCF7L2 expression by binding
to miR-217.
CCAT2-knockdown inhibits the
Wnt/β-catenin signaling pathway by downregulating TCF7L2
Wnt/β-catenin signaling can regulate a variety of
developmental events, including proliferation and migration
(40). In addition, it has been
reported that lncRNA CCAT2 affects glioma, breast cancer and other
tumors by regulating the Wnt/β-catenin pathway (35–37).
TCF7L2 is an effector of the Wnt/E-catenin signaling pathway
(41). Previous studies have
indicated that TCF7L2 is involved in regulating the Wnt/β-catenin
signaling pathway in a number of different types of cancer
(42,43). Therefore, whether CCAT2 regulated PCa
by increasing miR-217 expression and consequently inhibiting the
TCF7L2-mediated Wnt/β-catenin signaling pathway was investigated.
The TOPflash assay was performed to evaluate the activity of the
Wnt/β-catenin signaling pathway in PCa cells. CCAT2-knockdown
inhibited the Wnt/β-catenin signaling pathway and TCF7L2
overexpression increased the activity of the Wnt/β-catenin
signaling pathway (Fig. 4A). In
addition, TCF7L2 overexpression partially reversed the inhibitory
effect of CCAT2 on the Wnt/β-catenin signaling pathway. Western
blotting was performed to measure the protein expression levels of
TCF7L2 and Wnt/β-catenin signaling pathway-related proteins
β-catenin, c-Myc and Cyclin D1 following si-CCAT2 and TCF7L2
transfection in PCa cells. The western blotting results were
consistent with the TOPflash assay results (Fig. 4B-E). Collectively, the results
suggested that CCAT2 regulated the progression of PCa by targeting
miR-217 to inhibit TCF7L2 expression and the Wnt/β-catenin
signaling pathway; therefore, this signaling pathway may serve as
an intrinsic molecular mechanism by which CCAT2 regulates PCa.
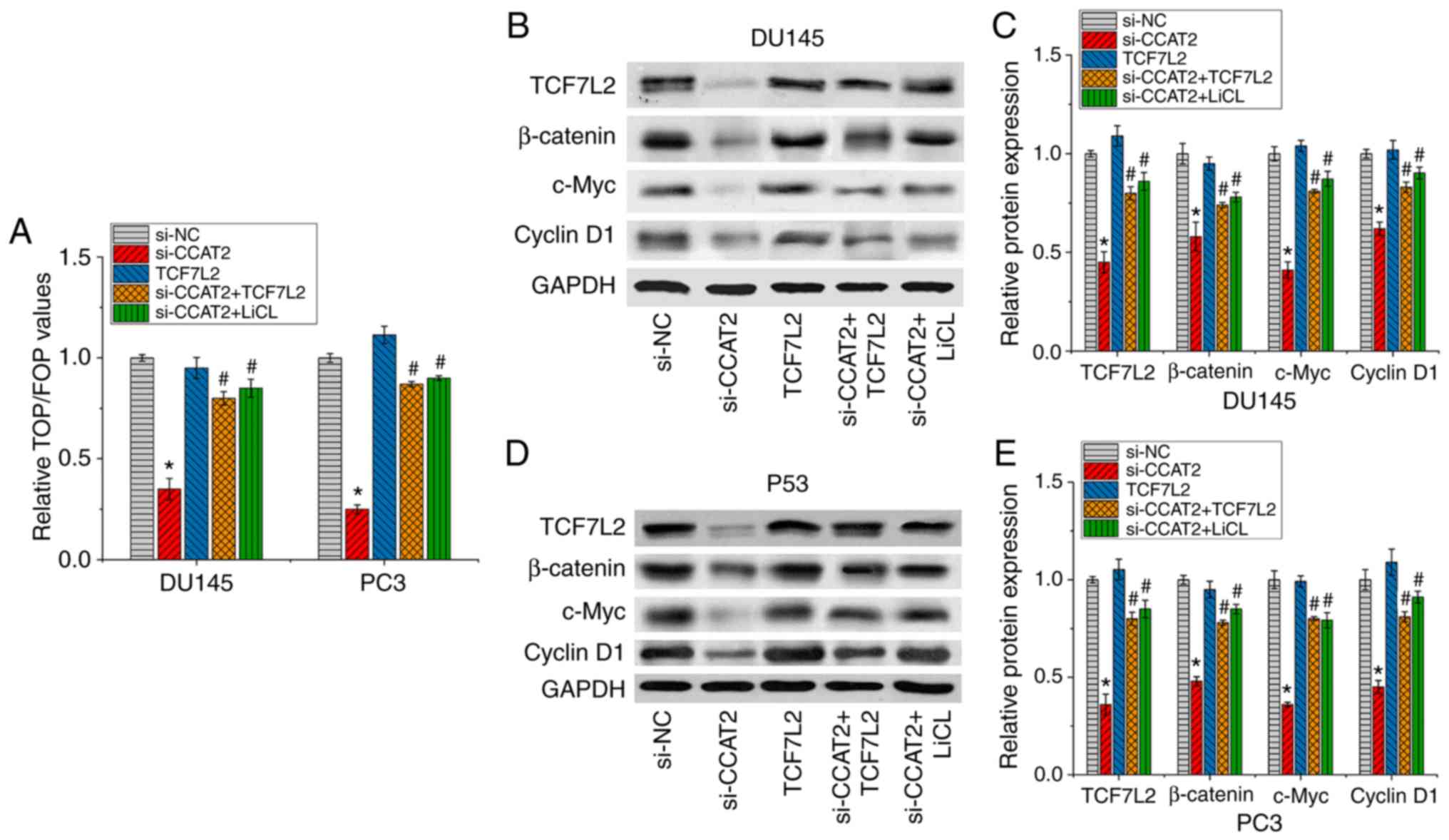 | Figure 4.CCAT2-knockdown inhibits TCF7L2 via
miR-217, thereby inhibiting the Wnt/β-catenin signaling pathway.
PCa DU145 and PC3 cells were treated with si-NC, si-CCAT2, TCFL2,
si-CCAT2+ TCFL2 or si-CCAT2 + LiCl. (A) The TOPflash assay was used
to measure Wnt/β-catenin signaling activity. Western blotting was
performed to detect the expression levels of TCF7L2, β-catenin,
c-Myc and CyclinD1 in PCa (B and C) DU145 and (D and E) PC3 cells.
Data are presented as the mean ± SD (n=3). *P<0.05 vs. si-NC;
#P<0.05 vs. si-CCAT2. CCAT2, colon cancer associated
transcript 2; TCF7L2, transcription factor 7 like 2; miR, microRNA;
si, small interfering RNA; NC, negative control; LiCl, lithium
chloride; PCa, prostate cancer. |
CCAT2-knockdown arrests PCa cell cycle
and impairs cell migration and invasion in vitro
Compared with si-NC cells, the percentage of cells
in the G0/G1 phase was significantly
increased in si-CCAT2-transfected DU145 and PC3 cells (Fig. 5C and C). By contrast, the percentage
of cells in the S phase was significantly decreased in
si-CCAT2-transfected DU145 and PC3 cells compared with si-NC cells
(Fig. 5C and D). Therefore, the
results suggested that CCAT2-knockdown shifted the cell cycle from
S phase to G0/G1 phase. Transwell assays were
performed to assess the role of CCAT2 in DU145 and PC3 cell
migration and invasion. Compared with si-NC cells, the number of
migratory cells was significantly reduced in the si-CCAT2 group,
which indicated that CCAT2-knockdown reduced the migratory ability
of PCa cells (P<0.05; Fig. 5G and
H). CCAT2-knockdown also significantly inhibited the invasion
of PCa cells compared with the si-NC group (P<0.05; Fig. 5I and J). Furthermore,
epithelial-mesenchymal transition (EMT) is an important factor
during cell migration and invasion. The western blotting results
indicated that the expression levels of N-cadherin and vimentin
were decreased, while E-cadherin expression levels were increased
following CCAT2-knockdown in DU145 cells and PC3 cells (Fig. 5E and F). In summary, the results
suggested that CCAT2-knockdown arrested PCa cell cycle, and
inhibited PCa cell migration and invasion.
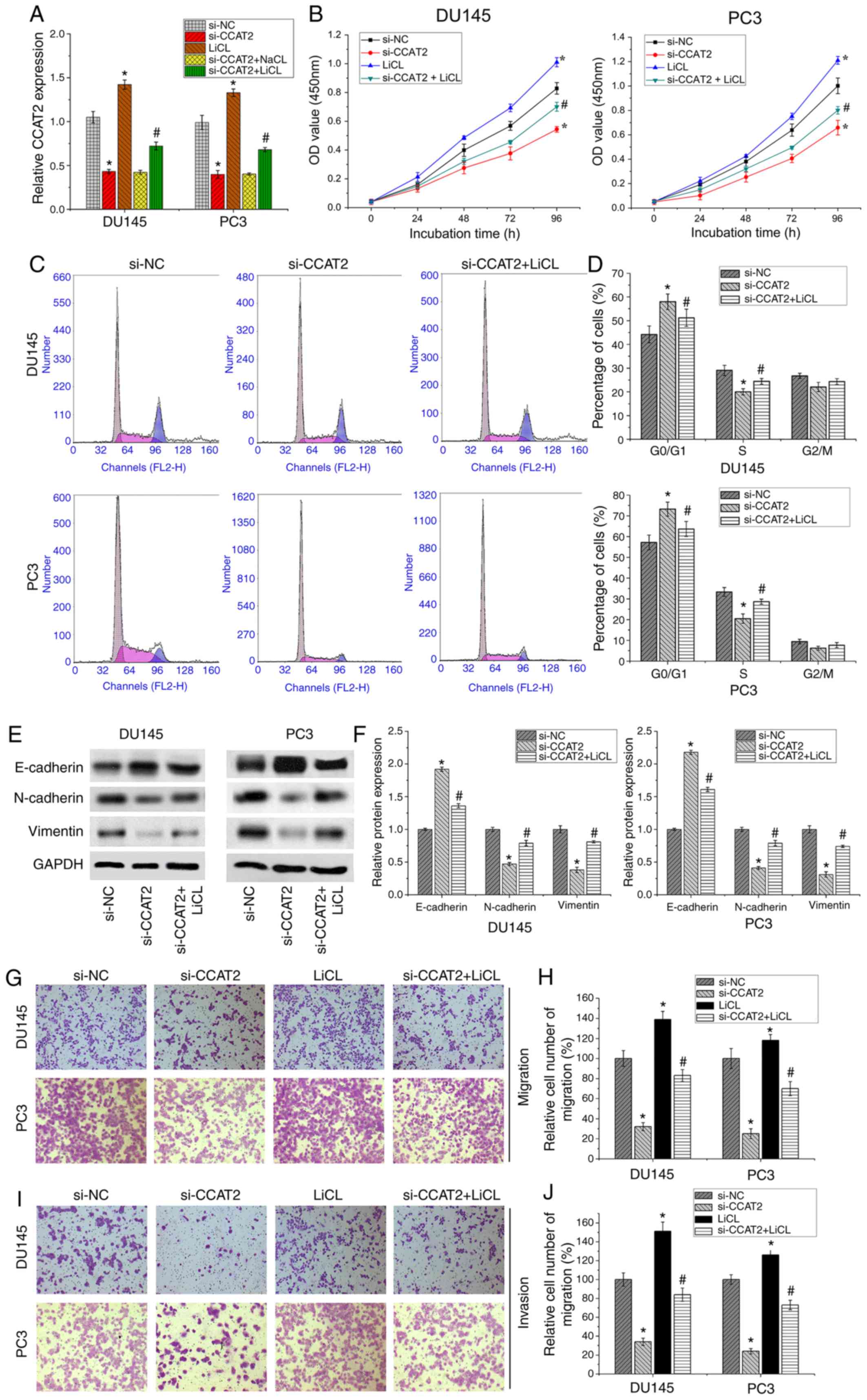 | Figure 5.CCAT2 and Wnt signaling agonist LiCl
affect PCa cell proliferation, cell cycle, migration and invasion.
(A) CCAT2 expression was detected by reverse
transcription-quantitative PCR. LiCl treatment enhanced CCAT2
expression. (B) The Cell Counting Kit-8 assay detected PCa cell
proliferation following transfection with si-NC, si-CCAT2, LiCl or
si-CCAT2 + LiCl. (C and D) Flow cytometry analysis of the cell
cycle distribution of PCa cells following transfection with si-NC,
si-CCAT2 or si-CCAT2 + LiCl. (E and F) Western blotting was
performed to detect the expression of epithelial-mesenchymal
transition-related proteins, including E-cadherin, N-cadherin and
Vimentin. (G and H) Transwell migration assay in PCa cells
transfected with si-NC, si-CCAT2, LiCl and si-CCAT2 + LiCl.
Magnification, ×200. (I and J) Transwell invasion assay in PCa
cells transfected with si-NC, si-CCAT2, LiCl or si-CCAT2 + LiCl.
Magnification, ×200. Data are presented as the mean ± SD (n=3).
*P<0.05 vs. si-NC; #P<0.05 vs. si-CCAT2. CCAT2,
colon cancer associated transcript 2; LiCl, lithium chloride; PCa,
prostate cancer; si, small interfering RNA; NC, negative control;
OD, optical density. |
Wnt/β-catenin signaling activation
partly restores CCAT2-mediated effects on PCa cells
To further verify whether CCAT2 controlled PCa cell
proliferation, migration and invasion by regulating Wnt/β-catenin
signaling, 20 mmol/l LiCl was used to activate Wnt/β-catenin
signaling. PCa DU145 and PC3 cell lines were divided into the
following groups: i) si-NC control group; ii) si-CCAT2 group; iii)
Wnt/β-catenin signal activator LiCl group; and iv) si-CCAT2
combined with LiCl. LiCl not only activated the Wnt/β-catenin
signaling pathway, increased the recruitment of β-catenin, and
promoted the expression of the classical downstream genes of the
Wnt/β-catenin signaling pathway, Cyclin D1 and c-Myc (Fig. 4), but also promoted cell
proliferation (Fig. 5B), migration
(Fig. 5G and H) and invasion
(Fig. 5I and J).
The western blotting results indicated that the
combined treatment of si-CCAT2 and LiCl partially increased the
transcriptional activity of the Wnt/β-catenin signaling pathway,
increased the recruitment of β-catenin, and increased the
expression of downstream genes CyclinD1 and c-Myc compared with
si-CCAT2. However, si-CCAT2 and LiCl did not restore the activation
level of the Wnt/β-catenin signaling pathway to level of the LiCl
group (Fig. 4).
In addition, the RT-qPCR results indicated that LiCl
treatment significantly enhanced CCAT2 expression (Fig. 5A). An in vitro cell assay
further suggested that DU145 and PC3 cells treated with si-CCAT2
and LiCl displayed significantly increased cell proliferation,
migration and invasion compared with si-CCAT2 (Fig. 5B and G-J). Regarding the cell cycle,
LiCl partially reversed si-CCAT2-mediated S phase arrest in PCa
cells. Furthermore, following treatment with LiCl,
CCAT2-knockdown-mediated effects on EMT-related protein expression
were partially reversed (Fig. 5E and
F). The results indicated that activation of the Wnt/β-catenin
signaling pathway partially restored CCAT2-mediated malignant
biological behavior in PCa cells. Furthermore, the results
suggested that CCAT2 controlled the progression of PCa via the
Wnt/β-catenin signaling pathway.
Discussion
Increasing evidence has suggested that lncRNAs serve
an important role in various malignancies (44,45).
Previous studies have suggested that lncRNAs may serve as novel
tumor biomarkers, providing a new approach to the early diagnosis
and treatment of cancer (46). As a
novel lncRNA, CCAT2 was first reported to be highly overexpressed
in colorectal cancer, which affects Wnt signaling activity, and
promotes tumor metastasis and chromosomal instability (47). The carcinogenic effect of CCAT2 in
human cancer has been confirmed in multiple previous studies
(48–50). It has been reported that CCAT2 is
upregulated in ovarian, lung and cervical cancer, as well as in
esophageal squamous cell carcinoma, and high expression of CCAT2 is
often associated with tumor progression and poor clinical outcomes
(20,49,50). It
has been reported that CCAT2 is also upregulated in PCa (24); however, to the best of our knowledge,
the molecular regulation mechanism underlying lncRNA CCAT2 during
PCa has not been previously reported.
In the present study, RT-qPCR detection was
performed on 18 paired PCa and paracancerous tissues, as well as on
PCa cell lines (DU145 and PC3). Consistent with previous studies,
the expression level of CCAT2 in PCa tissues and cell lines was
significantly higher compared with proximal prostate tissue and
normal human prostate epithelial RWPE-1 cells. To further
investigate the clinical role of CCAT2 during PCa, the functions of
CCAT2 in PCa were investigated using CCK-8, cell cycle, cell
apoptosis and Transwell assays. The present experiments
demonstrated that inhibition of CCAT2 expression arrests the cell
cycle and induces cell apoptosis to inhibit PCa cell proliferation,
migration and invasion, which indicated that CCAT2 displayed an
oncogene role during PCa.
Previous studies have reported that lncRNAs may
affect the biological behavior of malignant tumors via various
signaling pathways. For example, lncRNA HOXA distal transcript
antisense RNA regulates osteosarcoma by activating the
Wnt/β-catenin signaling pathway (51). Furthermore, lncRNA small nucleolar
RNA host gene 20 promotes glioblastoma and tumor progression by
activating the PI3K/Akt/mTOR signaling pathway (52) and lncRNA colorectal neoplasia
differentially expressed promotes liver cancer by enhancing the
Wnt/β-catenin signaling pathway (53). Several studies have demonstrated that
the typical Wnt/β-catenin signaling pathways are involved in a
variety of cellular processes, including cell proliferation,
autophagy, cell cycle, cell differentiation and tumorigenesis
(54–57). When Wnt/β-catenin is activated,
cytoplasmic β-catenin protein is upregulated, leading to
accumulation and nuclear transfer, which can result in activation
of the transcription of downstream target genes, including
CyclinD1, c-Myc and matrix metallopeptidases (58,59).
CCAT2 is a downstream target of the Wnt signaling
pathway (46). A number of studies
have reported that CCAT2 affects breast and non-small cell lung
cancer, as well as glioma, renal cell carcinoma and other tumors by
activating the Wnt/β-catenin signaling pathway (35–37). To
further study the regulatory mechanism underlying CCAT2 during PCa,
luciferase reporter assays were performed to verify the interaction
between CCAT2 and miR-217, as well as the interaction between
miR-217 and TCF7L2. TCF7L2 is a key member of the Wnt/β-catenin
signaling pathway, representing a central factor in cell
proliferation, invasion and death (41,60). The
results of the TOPflash assay and western blotting indicated that
CCAT2-knockdown inhibited the expression of TCF7L2, which led to a
decrease in β-catenin activity in the Wnt/β-catenin signaling
pathways in both PCa cell lines. In addition, the downstream
targeting genes Cyclin D1 and c-Myc were also downregulated
following CCAT2-knockdown. Therefore, the results indicated that
si-CCAT2 may regulate PCa by inhibiting the Wnt/β-catenin signaling
pathway via TCF7L2.
To further identify the potential molecular
mechanisms underlying CCAT2 during PCa, cells were treated with
si-CCAT2 and LiCl, a Wnt/β-catenin signaling pathway activator
(34,61). LiCl treatment not only reversed
CCAT2-knockdown-mediated effects on the expression of β-catenin,
CyclinD1 and c-Myc in the Wnt/β-catenin signaling pathway, but also
reversed the inhibitory effects of CCAT2-knockdown on PCa cell
proliferation, cell cycle, migration and invasion. It was further
suggested that CCAT2 inhibited the expression of N-cadherin and
vimentin, and promoted the expression of E-cadherin by inhibiting
the Wnt/β-catenin signaling pathway, thereby inhibiting cell EMT.
Therefore, the results indicated that CCAT2 regulated PCa via the
Wnt/β-catenin signaling pathway.
In conclusion, the present study extended the
existing knowledge of the molecular mechanisms underlying
CCAT2-mediated regulation of PCa. The results indicated that CCAT2
regulated the expression of TCF7L2 by targeting miR-217, which
consequently regulated Wnt/β-catenin signaling to alter the
biological behavior of PCa cells. Therefore, CCAT2 may serve as a
novel molecular therapeutic target for PCa; however, further
studies with larger sample sizes are required to verify the results
of the present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PH performed the experiments and wrote the
manuscript. GX and GJ collected the samples and acquired the data.
GX and WG analyzed the data. YL assisted in the experiments and
data processing. HL conceived the study and revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Evaluation
Committee of The Fifth Hospital of Wuhan (approval no. 2018-k-003;
Wuhan, China). Written informed consent was obtained from each
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Logothetis C, Morris MJ, Den R and Coleman
RE: Current perspectives on bone metastases in castrate-resistant
prostate cancer. Cancer Metastasis Rev. 37:189–196. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frieling JS, Basanta D and Lynch CC:
Current and emerging therapies for bone metastatic
castration-resistant prostate cancer. Cancer Contr. 22:109–120.
2015. View Article : Google Scholar
|
|
6
|
Zhu Z, Zhang H, Lang F, Liu G, Gao D, Li B
and Liu Y: Pin1 promotes prostate cancer cell proliferation and
migration through activation of Wnt/β-catenin signaling. Clin
Transl Oncol. 18:792–797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu LF, Song LD, Xu Q and Zhan JF: Highly
expressed long non-coding RNA FEZF1-AS1 promotes cells
proliferation and metastasis through Notch signaling in prostate
cancer. Eur Rev Med Pharmacol Sci. 23:5122–5132. 2019.PubMed/NCBI
|
|
8
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Crea F, Clermont PL, Parolia A, Wang Y and
Helgason CD: The non-coding transcriptome as a dynamic regulator of
cancer metastasis. Cancer Metastasis Rev. 33:1–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Viereck J and Thum T: Circulating
noncoding RNAs as biomarkers of cardiovascular disease and injury.
Circ Res. 120:381–399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meseure D, Drak Alsibai K, Nicolas A,
Bieche I and Morillon A: Long noncoding RNAs as new architects in
cancer epigenetics, prognostic biomarkers, and potential
therapeutic targets. BioMed Res Int. 2015:3202142015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Lu X, Chen J, Wu D, Qiu F, Xiong
H, Pan Z, Yang L, Yang B, Xie C, et al: Association of nsv823469
copy number loss with decreased risk of chronic obstructive
pulmonary disease and pulmonary function in Chinese. Sci Rep.
7:400602017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang CY, Hua L, Yao KH, Chen JT, Zhang JJ
and Hu JH: Long non-coding RNA CCAT2 is up-regulated in gastric
cancer and associated with poor prognosis. Int J Clin Exp Pathol.
8:779–785. 2015.PubMed/NCBI
|
|
14
|
Zhao Z, Wang J, Wang S, Chang H, Zhang T
and Qu J: LncRNA CCAT2 promotes tumorigenesis by over-expressed
Pokemon in non-small cell lung cancer. Biomed Pharmacother.
87:692–697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao C, Qiao C, Zong L and Chen Y: Long
non-coding RNA-CCAT2 promotes the occurrence of non-small cell lung
cancer by regulating the Wnt/β-catenin signaling pathway. Oncol
Lett. 16:4600–4606. 2018.PubMed/NCBI
|
|
16
|
Wang J, Qiu M, Xu Y, Li M, Dong G, Mao Q,
Yin R and Xu L: Long noncoding RNA CCAT2 correlates with smoking in
esophageal squamous cell carcinoma. Tumour Biol. 36:5523–5528.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X and Wang X: Long non-coding RNA
colon cancer-associated transcript 2 may promote esophageal cancer
growth and metastasis by regulating the Wnt signaling pathway.
Oncol Lett. 18:1745–1754. 2019.PubMed/NCBI
|
|
18
|
Wu ER, Hsieh MJ, Chiang WL, Hsueh KC, Yang
SF and Su SC: Association of lncRNA CCAT2 and CASC8 gene
polymorphisms with hepatocellular carcinoma. Int J Environ Res
Public Health. 16:162019.
|
|
19
|
Liu Y, Wang D, Li Y, Yan S, Dang H, Yue H,
Ling J, Chen F, Zhao Y, Gou L, et al: Long noncoding RNA CCAT2
promotes hepatocellular carcinoma proliferation and metastasis
through up-regulation of NDRG1. Exp Cell Res. 379:19–29. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu L, Jin L, Zhang W and Zhang L: Roles of
long non-coding RNA CCAT2 in cervical cancer cell growth and
apoptosis. Med Sci Monit. 22:875–879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Zhuang C, Liu Y, Chen M, Zhou Q,
Chen Z, He A, Zhao G, Guo Y, et al: shRNA targeting long non-coding
RNA CCAT2 controlled by tetracycline-inducible system inhibits
progression of bladder cancer cells. Oncotarget. 7:28989–28997.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Kong D, Sun D and Li J: Long
non-coding RNA CCAT2 acts as an oncogene in osteosarcoma through
regulation of miR-200b/VEGF. Artif Cells Nanomed Biotechnol.
47:2994–3003. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ling H, Spizzo R, Atlasi Y, Nicoloso M,
Shimizu M, Redis RS, Nishida N, Gafà R, Song J, Guo Z, et al:
CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic
progression and chromosomal instability in colon cancer. Genome
Res. 23:1446–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng J, Zhao S, He X, Zheng Z, Bai W,
Duan Y, Cheng S, Wang J, Liu X and Zhang G: The up-regulation of
long non-coding RNA CCAT2 indicates a poor prognosis for prostate
cancer and promotes metastasis by affecting epithelial-mesenchymal
transition. Biochem Biophys Res Commun. 480:508–514. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren X, Zhu R, Liu G, Xue F, Wang Y, Xu J,
Zhang W, Yu W and Li R: Effect of sitagliptin on tubulointerstitial
Wnt/β-catenin signalling in diabetic nephropathy. Nephrology
(Carlton). 24:1189–1197. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian FM, Meng FQ and Wang XB:
Overexpression of long-noncoding RNA ZFAS1 decreases survival in
human NSCLC patients. Eur Rev Med Pharmacol Sci. 20:5126–5131.
2016.PubMed/NCBI
|
|
27
|
Kasagi Y, Oki E, Ando K, Ito S, Iguchi T,
Sugiyama M, Nakashima Y, Ohgaki K, Saeki H, Mimori K, et al: The
expression of CCAT2, a novel long noncoding RNA transcript, and
rs6983267 single-nucleotide polymorphism genotypes in colorectal
cancers. Oncology. 92:48–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lyu X, Li J, Yun X, Huang R, Deng X, Wang
Y, Chen Y and Xiao G: miR-181a-5p, an inducer of Wnt-signaling,
facilitates cell proliferation in acute lymphoblastic leukemia.
Oncol Rep. 37:1469–1476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao L, Chen Y, Yuan Y, Xu B, Gao Q, Chen
P, Zhang T and Guan T: PC-1 NF suppresses high glucose-stimulated
inflammation and extracellular matrix accumulation in glomerular
mesangial cells via the Wnt/β-catenin signaling. Exp Ther Med.
18:2029–2036. 2019.PubMed/NCBI
|
|
30
|
Chen JJ, Xiao ZJ, Meng X, Wang Y, Yu MK,
Huang WQ, Sun X, Chen H, Duan YG, Jiang X, et al: MRP4 sustains
Wnt/β-catenin signaling for pregnancy, endometriosis and
endometrial cancer. Theranostics. 9:5049–5064. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rothe M, Kanwal N, Dietmann P, Seigfried
FA, Hempel A, Schütz D, Reim D, Engels R, Linnemann A, Schmeisser
MJ, et al: An Epha4/Sipa1l3/Wnt pathway regulates eye development
and lens maturation. Development. 144:321–333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chopra S, Al-Sammarraie N, Lai Y and Azhar
M: Increased canonical WNT/β-catenin signalling and myxomatous
valve disease. Cardiovasc Res. 113:6–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma Y, Hu X, Shang C, Zhong M and Guo Y:
Silencing of long non-coding RNA CCAT2 depressed malignancy of oral
squamous cell carcinoma via Wnt/β-catenin pathway. Tumour Biol.
39:10104283177176702017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang B, Liu M, Zhuang R, Jiang J, Gao J,
Wang H, Chen H, Zhang Z, Kuang Y and Li P: Long non-coding RNA
CCAT2 promotes epithelial-mesenchymal transition involving
Wnt/β-catenin pathway in epithelial ovarian carcinoma cells. Oncol
Lett. 15:3369–3375. 2018.PubMed/NCBI
|
|
35
|
Guo H, Hu G, Yang Q, Zhang P, Kuang W, Zhu
X and Wu L: Knockdown of long non-coding RNA CCAT2 suppressed
proliferation and migration of glioma cells. Oncotarget.
7:81806–81814. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sarrafzadeh S, Geranpayeh L, Tasharrofi B,
Soudyab M, Nikpayam E, Iranpour M, Mirfakhraie R, Gharesouran J
Ghafouri-Fard S and Ghafouri-Fard S: Expression study and clinical
correlations of MYC and CCAT2 in breast cancer patients. Iran
Biomed J. 21:303–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang JL, Liao Y, Qiu MX, Li J and An Y:
Long non-coding RNA CCAT2 promotes cell proliferation and invasion
through regulating Wnt/β-catenin signaling pathway in clear cell
renal cell carcinoma. Tumour Biol. 39:1010428317711312017.
View Article : Google Scholar
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rehmsmeier M, Steffen P, Hochsmann M and
Giegerich R: Fast and effective prediction of microRNA/target
duplexes. RNA. 10:1507–1517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Teo JL and Kahn M: The Wnt signaling
pathway in cellular proliferation and differentiation: A tale of
two coactivators. Adv Drug Deliv Rev. 62:1149–1155. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hrckulak D, Kolar M, Strnad H and Korinek
V: TCF/LEF transcription factors: An update from the internet
resources. Cancers (Basel). 8:182016. View Article : Google Scholar
|
|
42
|
Liu Z and Habener JF: Stromal cell-derived
factor-1 promotes survival of pancreatic beta cells by the
stabilisation of beta-catenin and activation of transcription
factor 7-like 2 (TCF7L2). Diabetologia. 52:1589–1598. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng A, Song X, Zhang L, Zhao L, Mao X,
Wei M and Jin F: Long non-coding RNA LUCAT1/miR-5582-3p/TCF7L2 axis
regulates breast cancer stemness via Wnt/β-catenin pathway. J Exp
Clin Cancer Res. 38:3052019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cai Q, Wang Z, Wang S, Weng M, Zhou D, Li
C, Wang J, Chen E and Quan Z: Long non-coding RNA LINC00152
promotes gallbladder cancer metastasis and epithelial-mesenchymal
transition by regulating HIF-1α via miR-138. Open Biol. 7:72017.
View Article : Google Scholar
|
|
45
|
Ji J, Tang J, Deng L, Xie Y, et al:
LINC00152 promotes proliferation in hepatocellular carcinoma by
targeting EpCAM via the mTOR signaling pathway. Oncotarget.
6:42813–42824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Meryet-Figuière M, Lambert B, Gauduchon P,
et al: An overview of long non-coding RNAs in ovarian cancers.
7:44719–44734. 2016.PubMed/NCBI
|
|
47
|
Ling H, Spizzo R, Atlasi Y, et al: CCAT2,
a novel noncoding RNA mapping to 8q24, underlies metastatic
progression and chromosomal instability in colon cancer. Genome
Res. 23:1446–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xin Y, Li Z, Zheng H, Chan MTV and Ka Kei
Wu W: CCAT2: A novel oncogenic long non-coding RNA in human
cancers. Cell Prolif. 50:e123422017. View Article : Google Scholar
|
|
49
|
Wu SW, Hao YP, Qui JH, Zhang DB, Yu CG and
Li WH: High expression of long noncoding RNA CCAT2 indicates poor
prognosis of gastric cancer and promotes cell proliferation and
invasion. Minerva Med. 108:317–323. 2017.PubMed/NCBI
|
|
50
|
Cai Y, He J and Zhang D: Long noncoding
RNA CCAT2 promotes breast tumor growth by regulating the Wnt
signaling pathway. Onco Targets Ther. 8:2657–2664. 2015.PubMed/NCBI
|
|
51
|
Li Z, Liang Z and Wang Q: Overexpression
of long non-coding RNA HOTTIP increases chemoresistance of
osteosarcoma cell by activating the Wnt/β-catenin pathway. Am J
Transl Res. 8:2385–2393. 2016.PubMed/NCBI
|
|
52
|
Gao XF, He HQ, Zhu XB, Xie SL and Cao Y:
LncRNA SNHG20 promotes tumorigenesis and cancer stemness in
glioblastoma via activating PI3K/Akt/mTOR signaling pathway.
Neoplasma. 66:532–542. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhu L, Yang N, Du G, Li C, Liu G, Liu S,
Xu Y, Di Y, Pan W and Li X: LncRNA CRNDE promotes the
epithelial-mesenchymal transition of hepatocellular carcinoma cells
via enhancing the Wnt/β-catenin signaling pathway. J Cell Biochem.
120:1156–1164. 2018. View Article : Google Scholar
|
|
54
|
Dong ZCZD, Zhang D, Wang SB and Lin ZQ:
Target inhibition on GSK-3β by miR-9 to modulate proliferation and
apoptosis of bladder cancer cells. Eur Rev Med Pharmacol Sci.
22:3018–3026. 2018.PubMed/NCBI
|
|
55
|
Yoshida T, Sopko NA, Kates M, Liu X, Joice
G, McConkey DJ and Bivalacqua TJ: Three-dimensional organoid
culture reveals involvement of Wnt/β-catenin pathway in
proliferation of bladder cancer cells. Oncotarget. 9:11060–11070.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Qi X, Mirja K and Anatoly S; Cancers VSJ,
: Wnt Signal Ren Cell Carcinoma. 8:572016.
|
|
57
|
Nwabo Kamdje AH, Seke Etet PF, Vecchio L,
Muller JM, Krampera M and Lukong KE: Signaling pathways in breast
cancer: Therapeutic targeting of the microenvironment. Cell Signal.
26:2843–2856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ying Y and Tao Q: Epigenetic disruption of
the WNT/β-catenin signaling pathway in human cancers. Epigenetics.
4:307–312. 2009. View Article : Google Scholar
|
|
59
|
Sebio A, Kahn M and Lenz H-J: The
potential of targeting Wnt/β-catenin in colon cancer. Expert Opin
Ther Targets. 18:611–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao C, Deng Y, Liu L, Yu K, Zhang L, Wang
H, He X, Wang J, Lu C, Wu LN, et al: Dual regulatory switch through
interactions of Tcf7l2/Tcf4 with stage-specific partners propels
oligodendroglial maturation. Nat Commun. 7:108832016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li Z, Chen S, Chen S, Huang D, Ma K and
Shao Z: Moderate activation of Wnt/β-catenin signaling promotes the
survival of rat nucleus pulposus cells via regulating apoptosis,
autophagy, and senescence. Journal of Cellular Biochemistry.
120:12519–12533. 2019. View Article : Google Scholar : PubMed/NCBI
|