Cancer is one of the leading causes of mortality
worldwide, and has been the second highest cause of mortality in
the USA in recent years (1,2). Cancer is a group of diseases that is
characterised by aberrant and uncontrolled growth of tissues or
cells. The development of cancer is attributed to dysregulation of
cell proliferation, apoptosis, differentiation, migration and
autophagy (3). Transcription factors
and their co-factors are the basic machinery controlling cell
processes. Activity of several transcription factors is altered in
a number of types of cancer through numerous and varying
mechanisms, including chromosomal translocations, gene
amplification or deletion, point mutations and dysregulated
expression, and indirectly through non-coding DNA mutations that
affect transcription factor binding (4,5). These
transcription factors are also considered candidate oncogenic
genes. Furthermore, transcription co-factors alter the activity of
transcription factors by interacting with them, serving critical
roles in cancer (5).
Cbp/P300 interacting transactivator with
Glu/Asp-rich carboxy-terminal domain 2 (CITED2) is a protein
encoded by the Cited2 gene and is a transcription co-factor.
The Cited2 gene was cloned around two decades ago (6,7), and was
reported to promote the development of cancer when overexpressed in
cells in vitro (7). CITED2 is
essential for mouse embryonic development, as deletion of CITED2
results in embryonic lethality around embryonic day 10.5 (8). CITED2 is also essential for the
development of the liver, lungs, heart, neural tube, left-right
patterning and eye development (8–13).
CITED2 modulates gene transcription by directly or indirectly
interacting with transcription factors or co-factors without a DNA
binding motif (14–22). Cited2-null mouse embryonic
fibroblasts exhibited premature arrest of proliferation
(senescence) (23), which suggests
that CITED2 is essential for cell proliferation. Taken together,
these findings demonstrated that CITED2 serves a critical role in
several fundamental cellular processes.
In addition to the critical role of CITED2 in
several fundamental cell processes, CITED2 has been reported to
serve roles in numerous different types of cancer. For example,
lung cancer (21), gastric cancer
(24) and breast cancer (19,25,26).
Cancer stem cells (CSCs) are hypothesized to be a population of
cells with multipotent stem cell-like properties, and can cause
cancer relapse, metastasis, multidrug resistance and radiation
resistance through their ability to arrest in the G0 phase, giving
rise to new tumours when they finally leave cell-cycle arrest
(27–29). CSCs exhibit strong self-renewal
capacity, in a similar way to normal stem cells (30). As the function of CITED2 in CSCs has
not been extensively studied, reviewing the function of CITED2 in
stem cells may provide directions for future studies. In the
present review, the molecular mechanisms of CITED2 function, and
the role of CITED2 in stem cells and different types of cancer are
discussed.
CITED2 is a transcriptional co-regulator without a
DNA binding domain, that can directly interact with a host of
transcription factors and co-factors, including LIM homeobox 2,
transcription factor AP-2 (TFAP2), SMAD2/3, peroxisome
proliferator-activated receptor (PPAR)-γ, oestrogen receptor, MYC,
Nucleolin and p300/CBP, thus regulating the ability of these
binding partners to activate or inactivate gene transcription
(14–19,21,22). The
molecular mechanism of CITED2 function varies based on the type of
tissue and the binding partner. In this section, the molecular
function of CITED2 in general is briefly summarized. The
transcription factors interacting with CITED2 or regulated by
CITED2 are listed in Table I. CITED2
was originally found to displace p300/CBP from hypoxia-inducible
factor (HIF)-1α, thereby negatively regulating HIF-1α function
(14). HIF-1α functions as a master
regulator of cellular and systemic homeostatic response to hypoxia
by activating transcription of a number of genes, including those
involved in energy metabolism, angiogenesis, apoptosis, and other
genes whose protein products increase oxygen delivery or facilitate
metabolic adaptation to hypoxia (31). HIF-1α also serves an important role
in stem cells and cancer (32–34). The
molecular mechanisms through which CITED2 interacts with p300/CBP
to inhibit HIF-1α function has been intensively studied (35–39).
CITED2 acts as a bridge, directly interacting with and
co-activating TFAP2 and the p300/CBP transcriptional co-activator
complex to stimulate TFAP2-mediated transcriptional activation
(8,12,16,40,41).
CITED2 positively regulates TGF-β signalling through its
association with the SMAD2/3-mediated transcriptional co-activator
complex, and upregulates the expression of downstream targets,
including matrix metalloproteinase (MMP)-9 and vascular endothelial
growth factor (VEGF) (18,42). CITED2 co-activates PPAR-α and PPAR-γ
transcriptional activities (17,43–45).
CITED2 also functions as a transcriptional co-activator of the
oestrogen receptor in breast cancer cells (19). Notably, CITED2 participates in sex
determination and early gonad development through its combined
action with WT1 and SF1, and regulates transcription activation of
the genes located in the sex-determining region of the Y chromosome
(46,47). By regulating the Nodal-Pitx2c
pathway, CITED2 serves a role in controlling left-right gene
transcription in the left lateral plate mesoderm (12,48–50).
CITED2 is also reported to serve an essential role in the
differentiation of the adrenal cortex from the adrenogonadal
primordium, which stimulates WT1-mediated transcription activation,
thereby increasing the promoter activity of the nuclear hormone
receptor NR5A1 (47,51). CITED2 functions as a transcriptional
co-repressor by interfering with the binding of HIF1-α or STAT2
with their transcription co-activator, p300/CBP (9,35,36,52–54).
By displacing p300 from binding with ETS-1, CITED2 was shown to
co-repress expression of MMPs, including MMP-1 and MMP-13 (55). Through downregulation of MMP
expression, CITED2 was revealed to exhibit a chondroprotective role
and is considered a potential drug target for treatment of
osteoarthritis (56,57). A previous study demonstrated that by
physically interacting with the DNA-binding transcription activator
ISL1, CITED2 enhanced embryonic stem cell (ESC) cardiac
differentiation (58). ISL1 has been
shown to serve a role in several different types of cancer,
including gastric cancer (59) and
breast cancer (60,61). Therefore, whether CITED2 serves a
role in cancer through its interaction with ISL1 will require
further investigation in future studies.
CITED2 is essential for differentiation of
hematopoietic stem cells (HSC) in the foetal liver and adult bone
marrow, as Cited2-null mice exhibit impaired HSC function in
the foetal liver and adult bone marrow (62–64). In
the foetal liver, expression of self-renewal and
survival-associated genes in HSCs (including Bmi-1, Wnt5a,
LEF-1, Notch-1 and GATA2) were revealed to be
significantly downregulated in Cited2-null HSCs (62). Studies have further revealed that
PU.1 co-operates with CITED2 to maintain HSC (65,66). The
role of CITED2 in hematopoietic stem cells has been previously
reviewed (64). Thus, in the present
review, a focus is placed on adult tissue stem cells, ESCs and
induced pluripotent stem cells (iPSC).
Adult tissue stem cells: Tendon-derived
stem/progenitor cells (TSPC) are the adult stem cells resident in
the tendon tissue and are responsible for regeneration of tenocytes
and healing of injuries to the tendons (67). A tendon's healing capacity is
gradually reduced with age, which may be due to decreased CITED2
expression in aged TSPCs, which results in defective self-renewal
and altered differentiation fates (68). The proliferation rate is decreased,
cell cycle progression is delayed and cell fate patterns are also
altered in aged TSPCs (68). In
particular, expression of tendon lineage marker genes is decreased,
while adipocytic differentiation is increased in aged TSPCs
(68). Another study suggested that
CITED2 prevented TGF-β2-induced TSPC senescence through
downregulation of SP1 and P21, and upregulation of Myc (69). Whether increasing CITED2 expression
can restore proliferation, cell cycle progression and determination
of differentiation fate in aged TSPC, or even enhance tendon
healing in vivo remains to be determined and should be the
focus of future studies.
ESCs: Interactions between transcription factors and
transcription co-factors determine the fate of ESCs (71). Chromatin immunoprecipitation-seq
analysis revealed that p300, a transcription co-factor, was mapped
in the circuit of ESC stemness via its co-occupancy with stem cell
marker transcription factor OCT4 on target genes (71). p300 is directly involved in
regulating Nanog expression in mouse ESCs (72), whereas CITED2 directly interacts with
p300. By performing genome-wide screening of CITED2-overexpressing
mouse ESCs, CITED2 was shown to be able to rescue the ESC phenotype
following removal of leukaemia inhibitory factor (LIF) from the
growth media (73). Loss-of-function
of Cited2 in mouse ESC does not affect ESC proliferation and
does not alter the undifferentiated state of ESCs in the presence
of LIF (74). However, knockout of
Cited2 delayed ESC differentiation due to delayed silencing
of the genes involved in the maintenance of pluripotency and
self-renewal of stem cells (including Oct4, Klf4, Sox2 and
c-Myc) (74). A recent study
revealed that Cited2-depleted stem cells retain higher
expression levels of pluripotency-related transcription factors,
including Nanog and Klf4, and that loss of Cited2 in
differentiating ESCs delayed differentiation (75). However, Kranc et al (76) demonstrated that CITED2 is required
for ESC proliferation, survival and self-renewal, by directly
regulating the transcriptional expression of Nanog, Klf4 and Tbx3.
Spontaneous differentiation of Cited2-knockdown ESCs
occurred through downregulation of stem cell markers (including
Nanog, Oct4, Sox2 and Tbx3), and upregulation of mesoderm gene
markers (including Brachyury and Cdx2) and the ectoderm gene marker
(Foxa2) (76). A possible
explanation for the discrepancy between these previous studies is a
result of the different methods of deleting the Cited2 gene
used in the ESCs. Li et al (74) used a sequential targeting method;
deleting the floxed Cited2 allele first by using Cag-cre,
and then using a knockout targeting vector to delete the other
allele. It is possible that the selected ESC clones were adapted to
loss of Cited2 and thus survived. In fact, Kranc et
al (76) observed a small
portion of ESCs (~3%) that were able to self-renew without CITED2.
Perhaps this small portion of self-renewing ESCs may be used to
elucidate the mechanisms of how ESCs survive and self-renew.
iPSCs: Generation of human iPSCs from somatic cells
have increased the potential prospects of personalized medicine
(77,78). Four key transcription factors (Oct4,
Sox2, Klf4 and c-Myc) comprise the key regulatory network of ESCs,
and overexpression of these transcription factors in somatic cells
give rise to pluripotent stem cells, or iPSCs (79,80). As
described earlier, CITED2 regulates expression of stem cell marker
transcription factors. Charneca et al (81), assessed whether overexpression of
Cited2 alone was sufficient for generation of iPSCs.
Notably, overexpression of Cited2 in mouse embryonic
fibroblasts did activate expression of certain stem cell marker
transcription factors, including Nanog, Sox2 and Rex1, but this was
not sufficient for generation of iPSCs (81). Furthermore, overexpression of CITED2
in the pre-senescent fibroblasts significantly increased the
efficiency of iPSC generation using the combination of the four
transcription factors that Yamanaka established (79–81). It
has been reported that aged cells are more difficult to transform
into iPSCs compared with younger cells (82,83), and
improving our understanding of the differences between aged and
younger cells may highlight a possible solution for the generation
of iPSCs from aged cells.
Breast cancer accounts for 30% of all cancer cases
in females in the USA and is the leading cause of cancer-associated
mortality worldwide (2).
Cited2 expression was shown to be upregulated in primary
breast cancer specimens and bone metastatic tumours compared with
the normal mammary epithelium (25,85).
Notably, cell lines with bone metastatic capacity in animal models
exhibit the highest expression levels of Cited2 compared
with less metastatic cell lines (25). A recent study also confirmed that
human metastatic tumours express higher mRNA levels of
Cited2 compared with primary tumours and normal epithelium
(26). Additionally, expression
levels of Cited2 are negatively associated with survival
(19,25). Cited2 expression levels are
associated with disease-free survival in patients with breast
cancer, and has been proposed to serve as potential prognostic
marker (85,86). However, van Agthoven et al
(87) reported that Cited2
mRNA expression levels were significantly increased in human breast
cancer cell lines, and an analysis of data obtained from the
Genomic Spatial Event database showed that Cited2 levels are
lower in breast cancer tissue compared with normal tissues, albeit
not statistically significant (22).
Despite the discrepancy between studies, CITED2 is able to modulate
oestrogen receptor transcriptional activity in breast cancer cells,
and thus, elevated Cited2 expression may lead to
oestrogen-independent oestrogen receptor activation, resulting in a
reduction in oestrogen dependence and thus a reduced response to
anti-oestrogen therapy (19). CITED2
may also modulate the capability of breast cancer metastases by
positively regulating IKKα (26).
Using breast cancer cell lines, it was demonstrated that knockdown
of Cited2 expression resulted in reduced expression of the
NF-κB signalling pathway regulator IKKα, and of the NF-κB
signalling pathway downstream targets, OPN, MMP9, µPA, SPARC, IL-11
and IL-1β, which are known to serve roles in metastasis (26). Furthermore, knockdown of
Cited2 expression in breast cancer cell lines attenuates
breast tumour growth in mice (42),
further highlighting the role of CITED2 in breast cancer
progression. Therefore, it was proposed that CITED2 regulated
vascularization of breast tumours through TGF-β dependent VEGF
expression (42). Tumour-associated
macrophages are important immune cells that serve a role in
promoting primary tumour growth, metastatic progression, poor
overall survival and therapeutic resistance (88–91).
Notably, silencing Cited2 expression in breast cancer cell
lines decreased the expression of the macrophage chemoattractant,
CCL20, and thus attenuated macrophage recruitment (92). In fast growing tumours, cells usually
encounter hypoxic stress, which results in induction of HIF
expression, and apoptosis can be induced by HIF (93). Bakker et al (94) reported that in MCF7 breast cancer
cells, HIF-1α induced FOXO3a expression and upregulated the
downstream CITED2 expression. Increased expression of CITED2
inhibits HIF-1α-induced cell apoptosis (94). Although the mechanism of CITED2
function in breast cancer is not fully understood, the results of
the aforementioned studies have demonstrated that CITED2 may be a
potential therapeutic target for treatment of breast cancer.
Prostate cancer is the most frequently diagnosed
type of cancer in males, and the second leading cause of
cancer-associated mortality among males (2). Despite the huge advances in prostate
cancer therapy in recent years, the prognosis of patients with
advanced prostate cancer remains generally poor due to metastasis
(101), as the molecular and
cellular mechanisms of prostate metastasis are not well understood.
A recent study revealed that CITED2 is highly expressed in prostate
cancer tissue from patients compared with normal tissues. Notably,
metastatic prostate tumours express even higher CITED2 mRNA levels
than non-metastatic prostate tumours (22). This study found that high CITED2
expression is significantly correlated with the overall survival of
patients with prostate cancer. The authors further elucidated the
mechanisms of this and proposed that CITED2 recruits Protein
Arginine Methyltransferase 5 and p300 to nucleolin, which promotes
epithelial-mesenchymal transition and prostate cancer metastasis
(22). Therefore, CITED2 may be a
potential therapeutic target for treatment of metastatic prostate
cancer (22).
Colon cancer or colorectal cancer is the third most
frequently diagnosed cancer type, in males and females (2). The function of CITED2 in colon cancer
has not yet been extensively studied. One study using a colon
cancer cell line reported that knockdown of Cited2 increased
cancer cell invasiveness in vitro by upregulating MMP-13
expression (102). The histone
deacetylase (HDAC) inhibitor, butyrate, resulted in upregulated
expression of Cited2 in colon cancer cells and consequently
downregulated MMP-13 expression. Ectopic expression of
Cited2 induced colon cancer cell growth arrest (102). RNA-seq analysis showed that
Cited2 expression was correlated with resistance to the
chemotherapeutic reagent, irinotecan (103). The role of CITED2 in irinotecan
resistance and the underlying mechanisms remain to be
determined.
Gastric cancer (colloquially referred to as stomach
cancer) is a major category of cancer and is a leading cause of
mortality in cancer-associated diseases (2). A few recent studies investigated the
possible roles of CITED2 in gastric cancer. Data mining using
existing gene expression data revealed that Cited2 was a
signature prognostic gene among 16 genes (104). A Human Protein Atlas program used
systems biology analysis and revealed that CITED2 is a prognostic
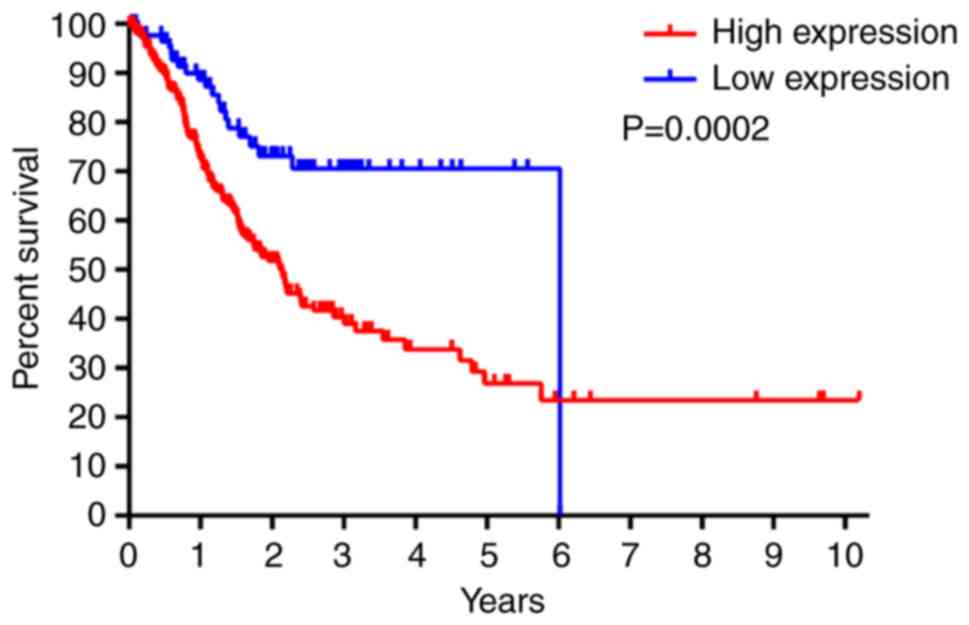
predictor of stomach cancer (proteinatlas.org) (84). High expression of Cited2 is
associated with a worse prognosis in patients with stomach cancer
(Fig. 1; patient information in
Table II; data derived proteinatlas.org) (84). Notably, low expression of
Cited2 in gastric cancer cells is associated with
chemoresistance (24). By
overexpressing Cited2 or inducing Cited2 expression
using an HDAC inhibitor in gastric cell lines, the cells were
sensitized to the chemotherapeutic reagent, anthracycline, both
in vitro and in vivo (24). There was also a small subset of
patients with higher Cited2 expression levels in gastric
cancer with a more complete response to chemotherapy, including
epirubicin, although the number of patients was too small to draw
any conclusions from (24). One
possible explanation for this is that usually, high expression
levels of Cited2 results in increased rates of cell
proliferation and DNA synthesis, which in turn results in increased
sensitivity to chemotherapy or radiotherapy. Mycophenolic acid
(MPA), a metabolized product and active element of mycophenolate
mofetil was revealed to inhibit gastric cancer cell invasion and
migration (105). Based on
microarray analysis, MPA may have downregulated the expression of a
large number of pro-migratory genes and upregulated the expression
of a number of anti-migratory genes, including Cited2
(105). An in vitro study by
Tang et al (106) reported
that knockdown of Cited2 expression in gastric cells
resulted in decreased cell proliferation, mitochondrial membrane
potential and colony formation. Our understanding of the role of
CITED2 in gastric cancer is limited and requires further study.
As CITED2 serves critical roles in numerous
fundamental cell processes, it may be a suitable target for
treatment of several types of cancer. Cited2-null normal
tissue cells, including mouse embryonic fibroblast cells, HSCs,
foetal liver cells, midbrain cells and neuroepithelium cells,
exhibit premature senescence or an increase in the levels of
apoptosis (8,10,23,107,108).
Although resistance to apoptosis is a hallmark of cancer cells,
induction of senescence or apoptosis, is a hypothesized means of
controlling cancer growth. Notably, several studies have reported
that CITED2 inhibits P53 activation in cancer cells (97,99,109,110),
and upregulated expression of Cited2 in cancer cells
inhibits P53 activation and apoptosis. Therefore, targeting CITED2
by silencing Cited2 gene expression and increasing cancer
cell apoptosis (99,106) may be a possible treatment for
cancer. Knocking down CITED2 expression in lung cancer cells
resulted in a shrinkage of tumour size in nude mice and increased
host mouse survival rates (21).
Other methods, including inducing expression of microRNAs targeting
and downregulating Cited2 expression, also results in
apoptosis of cancer cells (111).
Relapse and metastasis are the major hurdles of cancer therapy,
and, whether CITED2 serves a role in cancer relapse or metastasis
will be a topic of interest. As mentioned earlier, CITED2 directly
or indirectly regulates expression of key stem cell transcription
factors, OCT4, Nanog, Klf4 and Tbx3. These transcription factors
are also known to be key players in cancer stem-like cells
(112–115). Therefore, it is possible that
CITED2 serves a key role in cancer stem cell function. Indirectly
downregulating Cited2 expression in chronic myeloid
leukaemia using PPAR-γ agonists resulted in an erosion of the
leukaemia stem cell pool (116),
which suggests that targeting CITED2 expression in cancer stem cell
in general may be a therapeutic method for treatment of cancer, and
preventing relapse. In conclusion, CITED2 is an essential
transcription co-factor and may serve as a therapeutic target for
the treatment of cancer.
Not applicable.
No funding was received.
All datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
YG conceived the presented idea and supervised the
project. BA and XJ collected all the references and data. BA wrote
the manuscript. All authors discussed, contributed toward and
approved the final manuscript.
This study does not contain any studies with human
participants or animals performed by any of the authors.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lampe JW: Dairy products and cancer. J Am
Coll Nutr. 30 (5 Suppl 1):464S–470S. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lambert M, Jambon S, Depauw S and
David-Cordonnier MH: Targeting transcription factors for cancer
treatment. Molecules. 23:14792018. View Article : Google Scholar
|
|
5
|
Bushweller JH: Targeting transcription
factors in cancer-from undruggable to reality. Nat Rev Cancer.
19:611–624. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shioda T, Fenner MH and Isselbacher KJ:
MSG1 and its related protein MRG1 share a transcription activating
domain. Gene. 204:235–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun HB, Zhu YX, Yin T, Sledge G and Yang
YC: MRG1, the product of a melanocyte-specific gene related gene,
is a cytokine-inducible transcription factor with transformation
activity. Proc Natl Acad Sci USA. 95:13555–13560. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bamforth SD, Braganca J, Eloranta JJ,
Murdoch JN, Marques FI, Kranc KR, Farza H, Henderson DJ, Hurst HC
and Bhattacharya S: Cardiac malformations, adrenal agenesis, neural
crest defects and exencephaly in mice lacking Cited2, a new Tfap2
co-activator. Nat Genet. 29:469–474. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin Z, Haynie J, Yang X, Han B,
Kiatchoosakun S, Restivo J, Yuan S, Prabhakar NR, Herrup K, Conlon
RA, et al: The essential role of Cited2, a negative regulator for
HIF-1alpha, in heart development and neurulation. Proc Natl Acad
Sci USA. 99:10488–10493. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qu X, Lam E, Doughman YQ, Chen Y, Chou YT,
Lam M, Turakhia M, Dunwoodie SL, Watanabe M, Xu B, et al: Cited2, a
coactivator of HNF4alpha, is essential for liver development. EMBO
J. 26:4445–4456. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu B, Qu X, Gu S, Doughman YQ, Watanabe M,
Dunwoodie SL and Yang YC: Cited2 is required for fetal lung
maturation. Dev Biol. 317:95–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bamforth SD, Braganca J, Farthing CR,
Schneider JE, Broadbent C, Michell AC, Clarke K, Neubauer S, Norris
D, Brown NA, et al: Cited2 controls left-right patterning and heart
development through a Nodal-Pitx2c pathway. Nat Genet.
36:1189–1196. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang TQ, Wang Y, Ebrahem Q, Chen Y, Cheng
C, Doughman YQ, Watanabe M, Dunwoodie SL and Yang YC: Deletion of
HIF-1α partially rescues the abnormal hyaloid vascular system in
Cited2 conditional knockout mouse eyes. Mol Vis. 18:1260–1270.
2012.PubMed/NCBI
|
|
14
|
Bhattacharya S, Michels CL, Leung MK,
Arany ZP, Kung AL and Livingston DM: Functional role of p35srj, a
novel p300/CBP binding protein, during transactivation by HIF-1.
Genes Dev. 13:64–75. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glenn DJ and Maurer RA: MRG1 binds to the
LIM domain of Lhx2 and may function as a coactivator to stimulate
glycoprotein hormone alpha-subunit gene expression. J Biol Chem.
274:36159–36167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bragança J, Eloranta JJ, Bamforth SD,
Ibbitt JC, Hurst HC and Bhattacharya S: Physical and functional
interactions among AP-2 transcription factors, p300/CREB-binding
protein, and CITED2. J Biol Chem. 278:16021–16029. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tien ES, Davis JW and Vanden Heuvel JP:
Identification of the CREB-binding protein/p300-interacting protein
CITED2 as a peroxisome proliferator-activated receptor alpha
coregulator. J Biol Chem. 279:24053–24063. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chou YT, Wang H, Chen Y, Danielpour D and
Yang YC: Cited2 modulates TGF-beta-mediated upregulation of MMP9.
Oncogene. 25:5547–5560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lau WM, Doucet M, Huang D, Weber KL and
Kominsky SL: CITED2 modulates estrogen receptor transcriptional
activity in breast cancer cells. Biochem Biophys Res Commun.
437:261–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujita M, Takasaki T, Nakajima N, Kawano
T, Shimura Y and Sakamoto H: MRG-1, a mortality factor-related
chromodomain protein, is required maternally for primordial germ
cells to initiate mitotic proliferation in C. Elegans. Mech Dev.
114:61–69. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou YT, Hsieh CH, Chiou SH, Hsu CF, Kao
YR, Lee CC, Chung CH, Wang YH, Hsu HS, Pang ST, et al: CITED2
functions as a molecular switch of cytokine-induced proliferation
and quiescence. Cell Death Differ. 19:2015–2028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin SH, Lee GY, Lee M, Kang J, Shin HW,
Chun YS and Park JW: Aberrant expression of CITED2 promotes
prostate cancer metastasis by activating the nucleolin-AKT pathway.
Nat Commun. 9:41132018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kranc KR, Bamforth SD, Braganca J, Norbury
C, van Lohuizen M and Bhattacharya S: Transcriptional coactivator
Cited2 induces Bmi1 and Mel18 and controls fibroblast proliferation
via Ink4a/ARF. Mol Cell Biol. 23:7658–7666. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Regel I, Merkl L, Friedrich T,
Burgermeister E, Zimmermann W, Einwächter H, Herrmann K, Langer R,
Röcken C, Hofheinz R, et al: Pan-histone deacetylase inhibitor
panobinostat sensitizes gastric cancer cells to anthracyclines via
induction of CITED2. Gastroenterology. 143:99–109.e10. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lau WM, Weber KL, Doucet M, Chou YT, Brady
K, Kowalski J, Tsai HL, Yang J and Kominsky SL: Identification of
prospective factors promoting osteotropism in breast cancer: A
potential role for CITED2. Int J Cancer. 126:876–884.
2010.PubMed/NCBI
|
|
26
|
Jayaraman S, Doucet M, Lau WM and Kominsky
SL: CITED2 modulates breast cancer metastatic ability through
effects on IKKα. Mol Cancer Res. 14:730–739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen W, Dong J, Haiech J, Kilhoffer MC and
Zeniou M: Cancer stem cell quiescence and plasticity as major
challenges in cancer therapy. Stem Cells Int. 2016:17409362016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang
J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem
cell pathways for cancer therapy. Signal Transduct Target Ther.
5:82020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bjerkvig R, Tysnes BB, Aboody KS, Najbauer
J and Terzis AJ: Opinion: The origin of the cancer stem cell:
Current controversies and new insights. Nat Rev Cancer. 5:899–904.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pugh CW: Modulation of the hypoxic
response. Adv Exp Med Biol. 903:259–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gezer D, Vukovic M, Soga T, Pollard PJ and
Kranc KR: Concise review: genetic dissection of hypoxia signaling
pathways in normal and leukemic stem cells. Stem Cells.
32:1390–1397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Du J and Yang YC: HIF-1 and its antagonist
Cited2: Regulators of HSC quiescence. Cell Cycle. 11:2413–2414.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Henze AT and Acker T: Feedback regulators
of hypoxia-inducible factors and their role in cancer biology. Cell
Cycle. 9:2749–2763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Guzman RN, Martinez-Yamout MA, Dyson HJ
and Wright PE: Interaction of the TAZ1 domain of the CREB-binding
protein with the activation domain of CITED2: Regulation by
competition between intrinsically unstructured ligands for
non-identical binding sites. J Biol Chem. 279:3042–3049. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matt T, Martinez-Yamout MA, Dyson HJ and
Wright PE: The CBP/p300 TAZ1 domain in its native state is not a
binding partner of MDM2. Biochem J. 381:685–691. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoon H, Lim JH, Cho CH, Huang LE and Park
JW: CITED2 controls the hypoxic signaling by snatching p300 from
the two distinct activation domains of HIF-1α. Biochim Biophys
Acta. 1813:2008–2016. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berlow RB, Martinez-Yamout MA, Dyson HJ
and Wright PE: Role of backbone dynamics in modulating the
interactions of disordered ligands with the TAZ1 domain of the
CREB-binding protein. Biochemistry. 58:1354–1362. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ruiz-Ortiz I and De Sancho D: Competitive
binding of HIF-1α and CITED2 to the TAZ1 domain of CBP from
molecular simulations. Phys Chem Chem Phys. 22:8118–8127. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen CM, Bentham J, Cosgrove C, Braganca
J, Cuenda A, Bamforth SD, Schneider JE, Watkins H, Keavney B,
Davies B and Bhattacharya S: Functional significance of SRJ domain
mutations in CITED2. PLoS One. 7:e462562012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
MacDonald ST, Bamforth SD, Braganca J,
Chen CM, Broadbent C, Schneider JE, Schwartz RJ and Bhattacharya S:
A cell-autonomous role of Cited2 in controlling myocardial and
coronary vascular development. Eur Heart J. 34:2557–2565. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jayaraman S, Doucet M and Kominsky SL:
Down-regulation of CITED2 attenuates breast tumor growth, vessel
formation and TGF-β-induced expression of VEGFA. Oncotarget.
8:6169–6178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gonzalez YR, Zhang Y, Behzadpoor D, Cregan
S, Bamforth S, Slack RS and Park DS: CITED2 signals through
peroxisome proliferator-activated receptor-gamma to regulate death
of cortical neurons after DNA damage. J Neurosci. 28:5559–5569.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim GD, Das R, Rao X, Zhong J, Deiuliis
JA, Ramirez-Bergeron DL, Rajagopalan S and Mahabeleshwar GH: CITED2
restrains proinflammatory macrophage activation and response. Mol
Cell Biol. 38:e00452–17. 2018.PubMed/NCBI
|
|
45
|
Liu Z, Wang Y, Dou C, Sun L, Li Q, Wang L,
Xu Q, Yang W, Liu Q and Tu K: MicroRNA-1468 promotes tumor
progression by activating PPAR-γ-mediated AKT signaling in human
hepatocellular carcinoma. J Exp Clin Cancer Res. 37:492018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Buaas FW, Val P and Swain A: The
transcription co-factor CITED2 functions during sex determination
and early gonad development. Hum Mol Genet. 18:2989–3001. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Val P, Martinez-Barbera JP and Swain A:
Adrenal development is initiated by Cited2 and Wt1 through
modulation of Sf-1 dosage. Development. 134:2349–2358. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Weninger WJ, Lopes Floro K, Bennett MB,
Withington SL, Preis JI, Barbera JP, Mohun TJ and Dunwoodie SL:
Cited2 is required both for heart morphogenesis and establishment
of the left-right axis in mouse development. Development.
132:1337–1348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bentham J, Michell AC, Lockstone H, Andrew
D, Schneider JE, Brown NA and Bhattacharya S: Maternal high-fat
diet interacts with embryonic Cited2 genotype to reduce Pitx2c
expression and enhance penetrance of left-right patterning defects.
Hum Mol Genet. 19:3394–3401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lopes Floro K, Artap ST, Preis JI, Fatkin
D, Chapman G, Furtado MB, Harvey RP, Hamada H, Sparrow DB and
Dunwoodie SL: Loss of Cited2 causes congenital heart disease by
perturbing left-right patterning of the body axis. Hum Mol Genet.
20:1097–1110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Combes AN, Spiller CM, Harley VR, Sinclair
AH, Dunwoodie SL, Wilhelm D and Koopman P: Gonadal defects in
Cited2-mutant mice indicate a role for SF1 in both testis and ovary
differentiation. Int J Dev Biol. 54:683–689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Berlow RB, Dyson HJ and Wright PE:
Hypersensitive termination of the hypoxic response by a disordered
protein switch. Nature. 543:447–451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shin DH, Li SH, Chun YS, Huang LE, Kim MS
and Park JW: CITED2 mediates the paradoxical responses of
HIF-1alpha to proteasome inhibition. Oncogene. 27:1939–1944. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Freedman SJ, Sun ZY, Kung AL, France DS,
Wagner G and Eck MJ: Structural basis for negative regulation of
hypoxia-inducible factor-1alpha by CITED2. Nat Struct Biol.
10:504–512. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yokota H, Goldring MB and Sun HB:
CITED2-mediated regulation of MMP-1 and MMP-13 in human
chondrocytes under flow shear. J Biol Chem. 278:47275–47280. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
He Z, Leong DJ, Zhuo Z, Majeska RJ,
Cardoso L, Spray DC, Goldring MB, Cobelli NJ and Sun HB:
Strain-induced mechanotransduction through primary cilia,
extracellular ATP, purinergic calcium signaling, and ERK1/2
transactivates CITED2 and downregulates MMP-1 and MMP-13 gene
expression in chondrocytes. Osteoarthritis Cartilage. 24:892–901.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
He Z, Leong DJ, Xu L, Hardin JA, Majeska
RJ, Schaffler MB, Thi MM, Yang L, Goldring MB, Cobelli NJ and Sun
HB: CITED2 mediates the cross-talk between mechanical loading and
IL-4 to promote chondroprotection. Ann N Y Acad Sci. 1442:128–137.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pacheco-Leyva I, Matias AC, Oliveira DV,
Santos JM, Nascimento R, Guerreiro E, Michell AC, van De Vrugt AM,
Machado-Oliveira G, Ferreira G, et al: CITED2 cooperates with ISL1
and promotes cardiac differentiation of mouse embryonic stem cells.
Stem Cell Reports. 7:1037–1049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guo T, Wen XZ, Li ZY, Han HB, Zhang CG,
Bai YH, Xing XF, Cheng XJ, Du H, Hu Y, et al: ISL1 predicts poor
outcomes for patients with gastric cancer and drives tumor
progression through binding to the ZEB1 promoter together with
SETD7. Cell Death Dis. 10:332019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang Y, Wang L, Gao P, Sun Z, Li N, Lu Y,
Shen J, Sun J, Yang Y, Dai H and Cai H: ISL1 promotes cancer
progression and inhibits cisplatin sensitivity in triple-negative
breast cancer cells. Int J Mol Med. 42:2343–2352. 2018.PubMed/NCBI
|
|
61
|
Li L, Sun F, Chen X and Zhang M: ISL1 is
upregulated in breast cancer and promotes cell proliferation,
invasion, and angiogenesis. Onco Targets Ther. 11:781–789. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen Y, Haviernik P, Bunting KD and Yang
YC: Cited2 is required for normal hematopoiesis in the murine fetal
liver. Blood. 110:2889–2898. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kranc KR, Schepers H, Rodrigues NP,
Bamforth S, Villadsen E, Ferry H, Bouriez-Jones T, Sigvardsson M,
Bhattacharya S, Jacobsen SE and Enver T: Cited2 is an essential
regulator of adult hematopoietic stem cells. Cell Stem Cell.
5:659–665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Du J and Yang YC: Cited2 in hematopoietic
stem cell function. Curr Opin Hematol. 20:301–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Korthuis PM, Berger G, Bakker B,
Rozenveld-Geugien M, Jaques J, de Haan G, Schuringa JJ, Vellenga E
and Schepers H: CITED2-mediated human hematopoietic stem cell
maintenance is critical for acute myeloid leukemia. Leukemia.
29:625–635. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mattes K, Geugien M, Korthuis PM,
Brouwers-Vos AZ, Fehrmann RSN, Todorova TI, Steidl U, Vellenga E
and Schepers H: Transcriptional regulators CITED2 and PU.1
cooperate in maintaining hematopoietic stem cells. Exp Hematol.
73:38–49.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bi Y, Ehirchiou D, Kilts TM, Inkson CA,
Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, et al:
Identification of tendon stem/progenitor cells and the role of the
extracellular matrix in their niche. Nat Med. 13:1219–1227. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhou Z, Akinbiyi T, Xu L, Ramcharan M,
Leong DJ, Ros SJ, Colvin AC, Schaffler MB, Majeska RJ, Flatow EL
and Sun HB: Tendon-derived stem/progenitor cell aging: Defective
self-renewal and altered fate. Aging Cell. 9:911–915. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hu C, Zhang Y, Tang K, Luo Y, Liu Y and
Chen W: Downregulation of CITED2 contributes to TGFβ-mediated
senescence of tendon-derived stem cells. Cell Tissue Res.
368:93–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
AlAbdi L, He M, Yang Q, Norvil AB and
Gowher H: The transcription factor Vezf1 represses the expression
of the antiangiogenic factor Cited2 in endothelial cells. J Biol
Chem. 293:11109–11118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen X, Xu H, Yuan P, Fang F, Huss M, Vega
VB, Wong E, Orlov YL, Zhang W, Jiang J, et al: Integration of
external signaling pathways with the core transcriptional network
in embryonic stem cells. Cell. 133:1106–1117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhong X and Jin Y: Critical roles of
coactivator p300 in mouse embryonic stem cell differentiation and
Nanog expression. J Biol Chem. 284:9168–9175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pritsker M, Ford NR, Jenq HT and Lemischka
IR: Genomewide gain-of-function genetic screen identifies
functionally active genes in mouse embryonic stem cells. Proc Natl
Acad Sci USA. 103:6946–6951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li Q, Ramirez-Bergeron DL, Dunwoodie SL
and Yang YC: Cited2 gene controls pluripotency and cardiomyocyte
differentiation of murine embryonic stem cells through Oct4 gene. J
Biol Chem. 287:29088–29100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Santos JMA, Mendes-Silva L, Afonso V,
Martins G, Machado RSR, Lopes JA, Cancela L, Futschik ME,
Sachinidis A, Gavaia P and Bragança J: Exogenous WNT5A and WNT11
proteins rescue CITED2 dysfunction in mouse embryonic stem cells
and zebrafish morphants. Cell Death Dis. 10:5822019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kranc KR, Oliveira DV, Armesilla-Diaz A,
Pacheco-Leyva I, Catarina Matias A, Luisa Escapa A, Subramani C,
Wheadon H, Trindade M, Nichols J, et al: Acute loss of Cited2
impairs Nanog expression and decreases self-renewal of mouse
embryonic stem cells. Stem Cells. 33:699–712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Fakunle ES: iPSCs for personalized
medicine: What will it take for Africa? Trends Mol Med. 18:695–699.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gurwitz D: Human iPSC-derived neurons and
lymphoblastoid cells for personalized medicine research in
neuropsychiatric disorders. Dialogues Clin Neurosci. 18:267–276.
2016.PubMed/NCBI
|
|
79
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Takahashi K, Okita K, Nakagawa M and
Yamanaka S: Induction of pluripotent stem cells from fibroblast
cultures. Nat Protoc. 2:3081–3089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Charneca J, Matias AC, Escapa AL,
Fernandes C, Alves A, Santos JMA, Nascimento R and Bragança J:
Ectopic expression of CITED2 prior to reprogramming, promotes and
homogenises the conversion of somatic cells into induced
pluripotent stem cells. Exp Cell Res. 358:290–300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li H, Collado M, Villasante A, Strati K,
Ortega S, Cañamero M, Blasco MA and Serrano M: The Ink4/Arf locus
is a barrier for iPS cell reprogramming. Nature. 460:1136–1139.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mahmoudi S and Brunet A: Aging and
reprogramming: A two-way street. Curr Opin Cell Biol. 24:744–756.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357:eaan25072017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Minemura H, Takagi K, Sato A, Takahashi H,
Miki Y, Shibahara Y, Watanabe M, Ishida T, Sasano H and Suzuki T:
CITED2 in breast carcinoma as a potent prognostic predictor
associated with proliferation, migration and chemoresistance.
Cancer Sci. 107:1898–1908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yang X, Vasudevan P, Parekh V, Penev A and
Cunningham JM: Bridging cancer biology with the clinic: Relative
expression of a GRHL2-mediated gene-set pair predicts breast cancer
metastasis. PLoS One. 8:e561952013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
van Agthoven T, Sieuwerts AM, Veldscholte
J, Meijer-van Gelder ME, Smid M, Brinkman A, den Dekker AT, Leroy
IM, van Ijcken WF, Sleijfer S, et al: CITED2 and NCOR2 in
anti-oestrogen resistance and progression of breast cancer. Br J
Cancer. 101:1824–1832. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
De Palma M and Lewis CE: Cancer:
Macrophages limit chemotherapy. Nature. 472:303–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Nielsen SR and Schmid MC: Macrophages as
key drivers of cancer progression and metastasis. Mediators
Inflamm. 2017:96247602017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ruffell B and Coussens LM: Macrophages and
therapeutic resistance in cancer. Cancer Cell. 27:462–472. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jayaraman S, Doucet M and Kominsky SL:
CITED2 attenuates macrophage recruitment concordant with the
downregulation of CCL20 in breast cancer cells. Oncol Lett.
15:871–878. 2018.PubMed/NCBI
|
|
93
|
Greijer AE and van der Wall E: The role of
hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J
Clin Pathol. 57:1009–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bakker WJ, Harris IS and Mak TW: FOXO3a is
activated in response to hypoxic stress and inhibits HIF1-induced
apoptosis via regulation of CITED2. Mol Cell. 28:941–953. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Du J, Li Q, Tang F, Puchowitz MA, Fujioka
H, Dunwoodie SL, Danielpour D and Yang YC: Cited2 is required for
the maintenance of glycolytic metabolism in adult hematopoietic
stem cells. Stem Cells Dev. 23:83–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Deschler B and Lübbert M: Acute myeloid
leukemia: Epidemiology and etiology. Cancer. 107:2099–2107. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Mattes K, Berger G, Geugien M, Vellenga E
and Schepers H: CITED2 affects leukemic cell survival by
interfering with p53 activation. Cell Death Dis. 8:e31322017.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Jang SM, An JH, Kim CH, Kim JW and Choi
KH: Transcription factor FOXA2-centered transcriptional regulation
network in non-small cell lung cancer. Biochem Biophys Res Commun.
463:961–967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wu ZZ, Sun NK and Chao CC: Knockdown of
CITED2 using short-hairpin RNA sensitizes cancer cells to cisplatin
through stabilization of p53 and enhancement of p53-dependent
apoptosis. J Cell Physiol. 226:2415–2428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yao Y, Zhang T, Qi L, Liu R, Liu G, Wang
X, Li J, Li J and Sun C: Competitive endogenous RNA network
construction and comparison of lung squamous cell carcinoma in
smokers and nonsmokers. Dis Markers. 2019:52927872019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Attard G and Antonarakis ES: Prostate
cancer: AR aberrations and resistance to abiraterone or
enzalutamide. Nat Rev Urol. 13:697–698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bai L and Merchant JL: A role for CITED2,
a CBP/p300 interacting protein, in colon cancer cell invasion. FEBS
Lett. 581:5904–5910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Li XX, Zheng HT, Peng JJ, Huang LY, Shi
DB, Liang L and Cai SJ: RNA-seq reveals determinants for irinotecan
sensitivity/resistance in colorectal cancer cell lines. Int J Clin
Exp Pathol. 7:2729–2736. 2014.PubMed/NCBI
|
|
104
|
Zhao X, Cai H, Wang X and Ma L: Discovery
of signature genes in gastric cancer associated with prognosis.
Neoplasma. 63:239–245. 2016.PubMed/NCBI
|
|
105
|
Dun B, Sharma A, Teng Y, Liu H, Purohit S,
Xu H, Zeng L and She JX: Mycophenolic acid inhibits migration and
invasion of gastric cancer cells via multiple molecular pathways.
PLoS One. 8:e817022013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tang Z, He G, Xu J and Zhongfu L:
Knockdown of Cbp/P300-interacting transactivator with Glu/Asp-rich
carboxy-terminal domain 2 inhibits cell division and increases
apoptosis in gastric cancer. J Surg Res. 211:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Barbera JP, Rodriguez TA, Greene ND,
Weninger WJ, Simeone A, Copp AJ, Beddington RS and Dunwoodie S:
Folic acid prevents exencephaly in Cited2 deficient mice. Hum Mol
Genet. 11:283–293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Du J, Chen Y, Li Q, Han X, Cheng C, Wang
Z, Danielpour D, Dunwoodie SL, Bunting KD and Yang YC: HIF-1α
deletion partially rescues defects of hematopoietic stem cell
quiescence caused by Cited2 deficiency. Blood. 119:2789–2798. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu YC, Chang PY and Chao CC: CITED2
silencing sensitizes cancer cells to cisplatin by inhibiting p53
trans-activation and chromatin relaxation on the ERCC1 DNA repair
gene. Nucleic Acids Res. 43:10760–10781. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yoshida T, Sekine T, Aisaki K, Mikami T,
Kanno J and Okayasu I: CITED2 is activated in ulcerative colitis
and induces p53-dependent apoptosis in response to butyric acid. J
Gastroenterol. 46:339–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Jiang Y, Zhou Z, Fei R, Zhou X, Wang J,
Tao Y, Li J and Chen T: Role of miR-182-5p overexpression in
trichloroethylene-induced abnormal cell cycle functions in human
HepG2 cells. J Toxicol Environ Health A. 82:920–927. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Rasti A, Mehrazma M, Madjd Z, Abolhasani
M, Saeednejad Zanjani L and Asgari M: Co-expression of cancer stem
cell markers OCT4 and NANOG predicts poor prognosis in renal cell
carcinomas. Sci Rep. 8:117392018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jeter CR, Yang T, Wang J, Chao HP and Tang
DG: Concise review: NANOG in cancer stem cells and tumor
development: An update and outstanding questions. Stem Cells.
33:2381–2390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yu F, Li J, Chen H, Fu J, Ray S, Huang S,
Zheng H and Ai W: Kruppel-like factor 4 (KLF4) is required for
maintenance of breast cancer stem cells and for cell migration and
invasion. Oncogene. 30:2161–2172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Prost S, Relouzat F, Spentchian M,
Ouzegdouh Y, Saliba J, Massonnet G, Beressi JP, Verhoeyen E,
Raggueneau V, Maneglier B, et al: Erosion of the chronic myeloid
leukaemia stem cell pool by PPARγ agonists. Nature. 525:380–383.
2015. View Article : Google Scholar : PubMed/NCBI
|