Introduction
Oral squamous cell carcinoma (OSCC) affects
>600,000 individuals worldwide annually (1). Presently, the clinical treatment of
OSCC is primarily surgery, radiotherapy or chemotherapy (2,3). Over
the past decades, the overall survival (OS) rate of patients with
OSCC has not significantly improved, with a 5-year survival rate of
29–45% (4). Insufficient sensitive
and specific biomarkers may lead to the diagnosis of OSCC at
advanced stages (5). Therefore, it
is necessary to identify novel biomarkers for the early diagnosis
and treatment of OSCC.
Recently, with the continuous development of
sequencing technology, researchers can efficiently distinguish
differentially expressed genes (DEGs) by transcriptome sequencing,
which allows screening of potential tumor markers or therapeutic
drug targets (6). For example, a
number of new potential tumor markers have been found in human
malignancies, such as breast cancer (7), epithelial ovarian cancer (8) and glioma (9).
Minichromosome maintenance protein 5 (MCM5), a
member of mini-chromosome maintenance family of proteins, plays an
important role in cell proliferation and DNA replication (10,11).
Some studies have confirmed that MCM5 is highly expressed in
numerous human malignancies, such as renal cell carcinoma (12), pancreatic ductal adenocarcinoma
(13), cervical cancer (14) and skin cancer (15). Further studies have found that high
expression of MCM5 is closely associated with the
clinicopathological features of specific cancer types. For example,
overexpression of MCM5 is significantly associated with overall
survival rate (OS) in hepatocellular carcinoma (16). Moreover, increased expression of MCM5
is positively correlated with larger tumor size, positive lymph
node metastasis, more advanced clinical stage, higher histological
grade, deeper invasion depth and perineural invasion of OSCC
(17). However, thus far, the
expression, function and potential mechanisms of MCM5 in OSCC are
still unclear. Therefore, the present study aimed to analyze the
DEGs in OSCC using a microarray, screen for MCM5 and further
evaluate the possible functions of MCM5 in OSCC. The present
results may provide evidence to support the value of MCM5 as a
biomarker or a therapeutic target of OSCC.
Materials and methods
Tissue sampling
Pairs of OSCC tissues and adjacent normal tissues
were obtained from 18 patients undergoing resection operations at
the China-Japan Union Hospital of Jilin University (Changchun,
China). Clinicopathological data were also collected. No patient
received preoperative treatment, including radiotherapy or
chemotherapy. No other inclusion/exclusion criteria were used.
Matched normal OSCC tissues were obtained from a segment of the
resected specimens >5-cm away from the tumor. Pathological
analysis was used to identify surgically resected specimens.
Pathological analysis was performed by our group with no specific
diagnostic guidelines. Three paired samples were obtained for
transcriptome sequencing. Then, to confirm the reliability of
sequencing data, the samples size was increased using the remaining
15 paired tissues and analyzed using quantitative (q)PCR. All
comparisons between OSCC tissues and adjacent normal tissues were
performed simultaneously. The Kaplan-Meier analysis of OS and
survival curves were from the Cancer Genome Atlas database (TCGA,
http://www.cancer.gov/).
The study was approved by the Ethics Committee of
China-Japan Union Hospital of Jilin University. Written informed
consent was obtained from all patients who participated in this
study.
Transcriptome sequencing and
functional annotation analysis
Total RNA extraction, RNA library construction and
transcriptome sequencing were performed at Sangon Biotech Co., Ltd.
The biological relevance of unique genes in expression profiles of
DEGs were screened according to the threshold values of
log2|fold-change|≥1 and P<0.05. Then the
differentially expressed mRNAs were analyzed by Gene Ontology (GO)
whose annotations were downloaded from Gene Ontology (http://geneontology.org/), UniProt (https://sparql.uniprot.org/) and NCBI (https://www.ncbi.nlm.nih.gov/). Significant GO
categories were identified using Fisher's exact test with a
P<0.05, which indicated that significantly upregulated genes in
the set of DEGs were assigned to a specific functional category
more often than expected by chance. Significant pathways of the
DEGs were then analyzed and identified according to the Kyoto
Encyclopedia of Genes and Genomes (KEGG) database (https://www.kegg.jp/).
Cell lines
The human tongue squamous cell carcinoma SCC-15 and
CAL-27 were obtained from the American Type Culture Collection.
CAL-27 cells were cultured in DMEM medium with 10% fetal bovine
serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. SCC-15 cells were cultured in MEM
medium with 10% FBS, 1% NEAA, 100 U/ml penicillin and streptomycin
at 37°C in a humidified atmosphere containing 5%
CO2.
MCM5-specific siRNA and
transfection
Three MCM5 siRNA sequences were synthesized by
Suzhou GenePharma Co., Ltd. The sequences were as follows (5′-3′):
siRNA-1, Forward: CCGACUACUUGUACAAGCATT and reverse:
UGCUUGUACAAGUAGUCGGTT; siRNA-2, forward: CCAAAUGUCUAUGAGGUCATT and
reverse: UGACCUCAUAGACAUUUGGTT, siRNA-3, forward:
GUCGUCUGUAUUGACGAGUTT and reverse: ACUCGUCAAUACAGACGACTT and
scrambled, forward: UUCUCCGAACGUGUCACGUTT and reverse:
ACGUGACACGUUCGGAGAATT. The mock was an untransfected empty vector,
serving as the control.
SCC-15 cells (4.5×104 cells/well) were
cultured in 6-well plates overnight at 37°C. Then, cells were
transfected with 50 nM negative control siRNA or MCM5 siRNA using
Lipofectamine® 2000 Transfection Reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. After 48 h transfection, cells were collected, and then
RNA was extracted by TRIzol® regent (Invitrogen; Thermo
Fisher Scientific, Inc.) for further experiments as indicated.
Reverse transcription (RT)qPCR
RNA extracted from tissues samples were reverse
transcribed into cDNA using a GoScript Reverse Transcription System
kit (Monad; http://www.monadbiotech.com/) according to the
manufacturer's instructions. Relative mRNA expressions were
quantified by qPCR using the QuantiTect SYBR Green PCR kit (Roche
Diagnostics) and normalized to GAPDH using primers listed in
Table I. The cycling parameters were
40 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C for 30 sec.
Relative mRNA levels were assessed by the comparative
2−ΔΔCq method (18). All
analyses of the samples were conducted in triplicate.
 | Table I.Primers used for reverse
transcription quantitative-PCR. |
Table I.
Primers used for reverse
transcription quantitative-PCR.
| mRNA | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| MCM5 |
GATCCTGGCATTTTCTACAG |
CCCTGTATTTGAAGGTGAAG |
| P21 |
GGAGACTCTCAGGGTCGAAA |
GGATTAGGGCTTCCTCTTGG |
| CyclinE |
TTCTTGAGCAACACCCTCTTCTGCAGCC |
TCGCCATATACCGGTCAAAGAAATCTTGTGCC |
| CDK2 |
GCTAGCAGACTTTGGACTAGCCAG |
AGCTCGGTACCACAGGGTCA |
| GAPDH |
AGAAGGCTGGGGCTCATTTG |
AGGGGCCATCCACAGTCTTC |
For association of MCM5 expression levels with
clinicopathologic features of OSCC, the relative expression levels
of MCM5 were evaluated using qPCR as aforementioned. Relative mRNA
levels of paired samples (adjacent vs. cancer tissues) were
assessed by the comparative 2−ΔΔCq method. A ratio >1
was considered to have high MCM5 expression, whereas a ratio ≤1 was
considered to have low MCM5 expression.
Cell proliferation assay
To analyze cell proliferation, a Cell Counting Kit
(CCK)-8 (Dojindo Molecular Technologies, Inc.) was used according
to the manufacturer's instructions. In total, 5,000 cells were
cultured to each well of 96-well plates. After 24 h post siRNA
transfection, cells were incubated for 24, 48 and 72 h. CCK-8
reagent was added 2 h prior to detection. The OD was measured at
450 nm using a microplate reader (Bio-Tek). The experiment was
performed three times.
Colony formation assay
This assay was performed according to a previous
study (19). Briefly, cells were
cultured in 6-well plates at 1,000 cells/well, followed by culture
in complete medium (DMEM supplemented with 10% FBS, 100 U/ml
penicillin and streptomycin) for 2 weeks. The colonies were fixed
with methanol for 15 min at room temperature and washed with PBS
and stained with 0.1% crystal violet at room temperature (Beyotime
Institute of Biotechnology) solution for 20 min. A cell colony was
defied as a group formation of at least 50 cells. Finally, formed
colonies were observed and images were captured under a light
microscope at magnification, ×200.
Cell migration analysis using
scratching assays
SCC-15 cells were cultured in a 6-well plate at
5×105 cells/well overnight at 37°C. Then the cells were
scratched and scraped with fresh DMEM. Cells were observed and
images were captured under a light microscope (magnification, ×200)
at 0 h. The width of the scratch was measured and referred to as
Wbefore. Then, the cells were starved with no FBS and
returned to the incubator for 6 h at 37°C. The width of the same
scratch was measured and referred to as Wafter.
Migrating distance was calculated by subtracting Wafter
from Wbefore. The migration of the control was set as
100.
Western blot analysis
The protein extractions from the cells were isolated
using RIPA Lysis Buffer (P0013B; Beyotime Institute of
Biotechnology). Then, 50–100 µg protein was loaded per lane on a
12% gel, resolved using SDS-PAGE and electroblotted onto PVDF
membranes (Roche Diagnostics). After blocking at 37°C for 1 h (5%
non-fat milk in PBS plus 0.1% Tween 20), the blots were incubated
with primary antibodies against anti-MCM5 (D220960-0025; 1:1,000;
BBI Life Sciences), anti-p21 (0053657; 1:1,000; Proteintech),
anti-cyclin E (0047453; 1:1,000; ProteinTech Group, Inc.) and
anti-β-actin (D16H11; 1:1,000; CST Biological Reagents Co., Ltd.)
at 37°C for 1 h. Western blots were probed with secondary
antibodies and detected using the Odyssey infrared imaging system
(LI-COR Biosciences).
Cell cycle analysis
SCC-15 cells were harvested and fixed with 70%
ethanol on ice for 30 min, and then washed with PBS to decant the
ethanol solution. Then the cells were suspended and stained by PI
and RNase A treatment. Cell cycle analysis was performed using a
flow cytometer (FACSARIAII; BD Biosciences). The data was performed
using CXP Analysis software (Beckman Coulter, Inc).
Statistical analysis
All the data analysis was performed using SPSS
version 18.0 (SPSS, Inc.). The results are presented as mean + SD.
Associations between MCM5 mRNA expression and
clinicopathological factors were analyzed using the Pearson's
χ2 test or Fisher's exact test. The differences in
MCM5 mRNA expression between carcinoma and adjacent normal
tissues were evaluated by a paired t-test. One-way ANOVA followed
by Tukey's post hoc test was used to determine the differences
between groups, and unpaired t-tests for the rest of the data. The
survival rate was calculated by the Kaplan-Meier method and
compared using the log-rank test. P<0.05 was considered to
indicate a statistically significant difference. All experiments
were performed in triplicate.
Results
RNA sequencing and functional
annotation analysis
To explore novel biomarkers for OSCC, the RNAs
derived from tissue samples by sequencing were detected. Three
matched primary OSCC tissues and adjacent normal tissues were
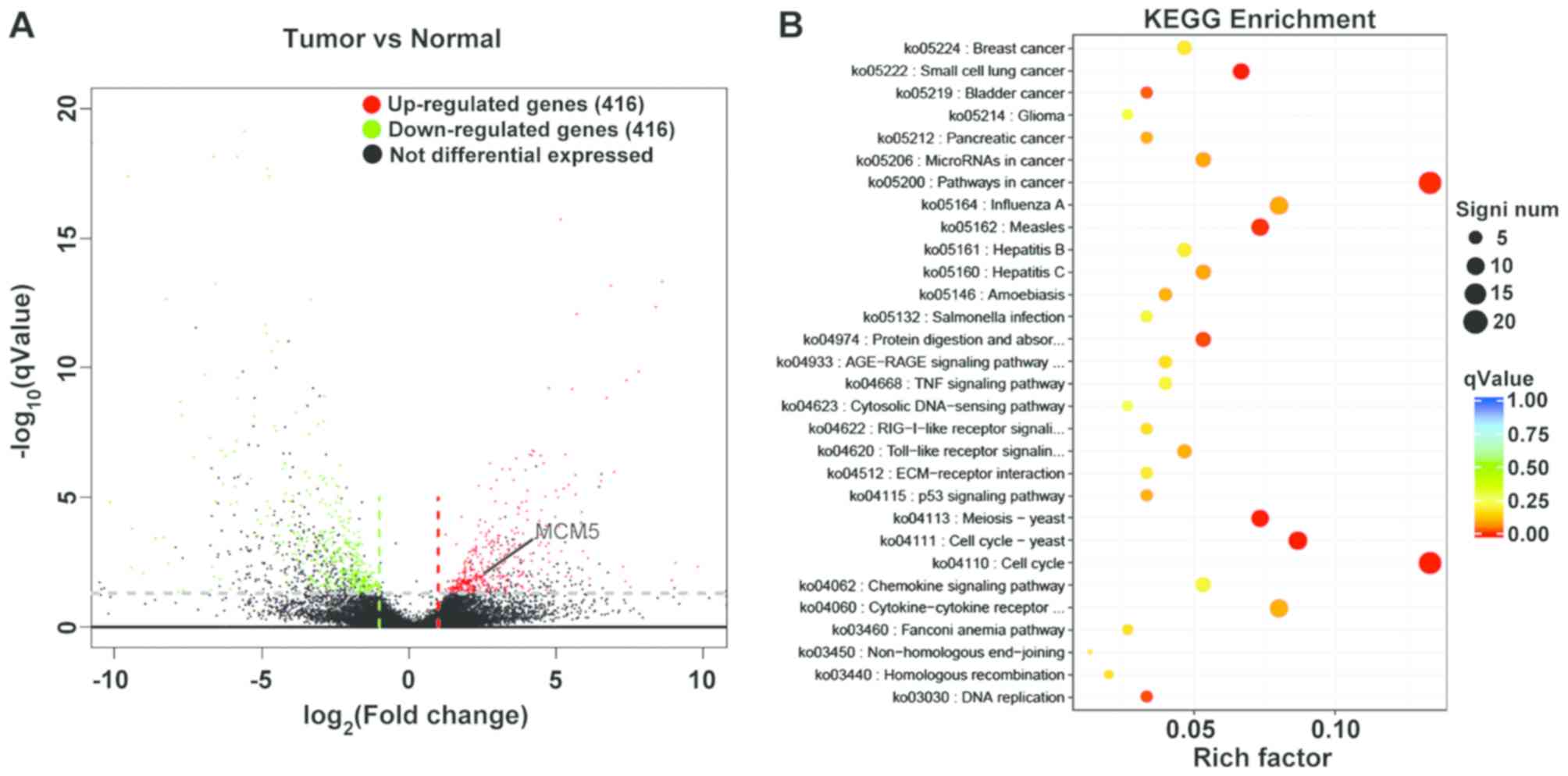
randomly selected. As shown in Fig.
1A, the aberrant expression of genes was detected in tissue
samples. To screen the candidate biomarkers for diagnosing OSCC,
the DEGs were selected when changes in RNA expression were >2
fold-changes. As shown in Fig. 1A,
416 aberrant RNAs were significantly downregulated, while 416 RNAs
were upregulated. In order to represent all differentially
expressed genes, twenty randomly selected dysregulated genes
between OSCC and adjacent tissue samples are summarized in Table II. The P-value and log2
fold-changes of all aberrant expression genes are shown in Table SI. The DEGs were selected randomly
instead of listing based on rank or fold-change in expression.
 | Table II.Twenty randomly selected
differentially expressed genes between oral squamous cell carcinoma
and adjacent tissue samples. The genes selected randomly instead of
listing based on rank or fold-change in expression. |
Table II.
Twenty randomly selected
differentially expressed genes between oral squamous cell carcinoma
and adjacent tissue samples. The genes selected randomly instead of
listing based on rank or fold-change in expression.
| Gene ID | Log2
fold-change | P-value | Result | Gene name |
|---|
|
ENSG00000160182 | −18.41080047 |
8.81×10−6 | Downregulation | TFF1 |
|
ENSG00000205592 | −15.71681384 |
4.68×10−5 | Downregulation | MUC19 |
|
ENSG00000171195 | −9.405322765 |
1.44×10−6 | Downregulation | MUC7 |
|
ENSG00000126549 | −8.237011727 |
1.40×10−16 | Downregulation | STATH |
|
ENSG00000090382 | −6.918550063 |
2.09×10−13 | Downregulation | LYZ |
|
ENSG00000161798 | −6.421368217 |
5.28×10−4 | Downregulation | AQP5 |
|
ENSG00000161055 | −5.852302695 |
9.59×10−8 | Downregulation | SCGB3A1 |
|
ENSG00000107562 | −4.504588372 |
6.04×10−11 | Downregulation | CXCL12 |
|
ENSG00000214711 | −3.313192374 |
6.01×10−4 | Downregulation | CAPN14 |
|
ENSG00000106066 | −2.169225527 |
8.46×10−6 | Downregulation | CPVL |
|
ENSG00000184330 | 16.33797846 |
2.34×10−14 | Upregulation | S100A7A |
|
ENSG00000137745 | 9.059136388 |
7.53×10−5 | Upregulation | MMP13 |
|
ENSG00000243207 | 8.610243304 |
2.22×10−17 | Upregulation |
PPAN-P2RY11 |
|
ENSG00000107159 | 7.805639054 |
1.61×10−13 | Upregulation | CA9 |
|
ENSG00000183072 | 6.976257283 |
3.80×10−9 | Upregulation | NKX2-5 |
|
ENSG00000196611 | 5.435774905 |
1.54×10−8 | Upregulation | MMP1 |
|
ENSG00000171217 | 4.773764562 |
7.05×10−5 | Upregulation | CLDN20 |
|
ENSG00000178445 | 4.489639485 |
7.51×10−6 | Upregulation | GLDC |
|
ENSG00000100297 | 2.043710595 |
5.65×10−4 | Upregulation | MCM5 |
|
ENSG00000127564 | 2.036248873 |
2.40×10−5 | Upregulation | PKMYT1 |
To explore the role of differentially expressed RNAs
in OSCC, KEGG pathway analysis was performed. Depending on the
P-value and enrichment, 261 signal pathways associated with OSCC
were identified (Table SII). The
top 30 KEGG enrichment terms of DEGs are shown in Fig. 1B, including ‘cell cycle’, ‘pathways
in cancer’, ‘cell cycle-yeast’, ‘meiosis-yeast’ and
‘cytokine-cytokine receptor interaction’. Among these, it was
reported that some genes, such as MCM5, cell division cycle
7-related protein kinase and Cyclin-dependent kinase 4 homolog,
were primarily enriched in the ‘cell cycle’ pathway. Some genes,
such as lysozyme C, statherin and aquaporin-5, were enriched in the
‘saliva secretion’ pathway. MCM5, which participated in cell cycle
regulation and had high expression in OSCC, was selected for
further study and it was hypothesized that MCM5 might be a
candidate tumor marker for OSCC.
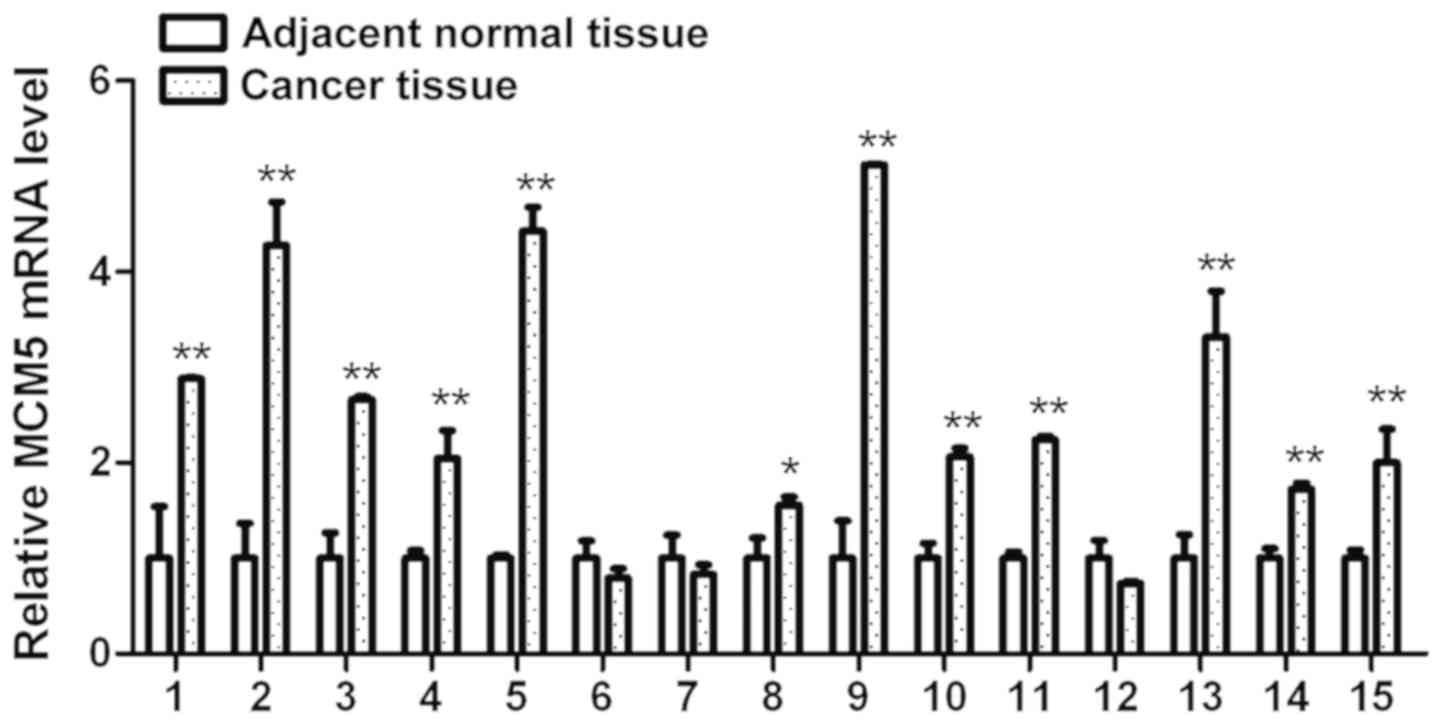
Validation of MCM5 using RT-qPCR
To further verify the aforementioned expression
profile data, MCM5 expression levels were investigated using
RT-qPCR in 15 tumor and adjacent normal tissues. As shown in
Fig. 2, MCM5 mRNA was
significantly upregulated in 80.0% (12/15) of tumor tissues
compared with that in matched normal tissues. These results showed
that MCM5 was highly expressed in OSCC tissues, which was in line
with the sequencing data.
Association of MCM5 expression levels
with clinicopathologic features of OSCC and survival analysis
The results of the potential association between
MCM5 expression and clinicopathological features in 15 patients
with OSCC are presented in Table
III. No significant association with MCM5 expression was found
for age, sex, histological differentiation, metastasis/recurrence
and survival status (P>0.5).
 | Table III.Association between expression of
MCM5 and clinicopathologic features of 15 patients with oral
squamous cell carcinoma. |
Table III.
Association between expression of
MCM5 and clinicopathologic features of 15 patients with oral
squamous cell carcinoma.
|
|
| MCM5
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Value, n | High, n=12 | Low, n=3 | P-value |
|---|
| Age, years |
|
|
| 0.792 |
|
<60 | 6 | 5 | 1 |
|
|
≥60 | 9 | 7 | 2 |
|
| Sex |
|
|
| 0.519 |
|
Male | 12 | 10 | 2 |
|
|
Female | 3 | 2 | 1 |
|
| Histological
differentiation |
|
|
| 0.438 |
| Well
and Moderate | 8 | 7 | 1 |
|
|
Poor | 7 | 5 | 2 |
|
|
Metastasis/Recurrence |
|
|
| 0.605 |
|
Yes | 8 | 6 | 2 |
|
| No | 7 | 6 | 1 |
|
| Survival
status |
|
|
| 0.438 |
|
Death | 7 | 5 | 2 |
|
|
Survival | 8 | 7 | 1 |
|
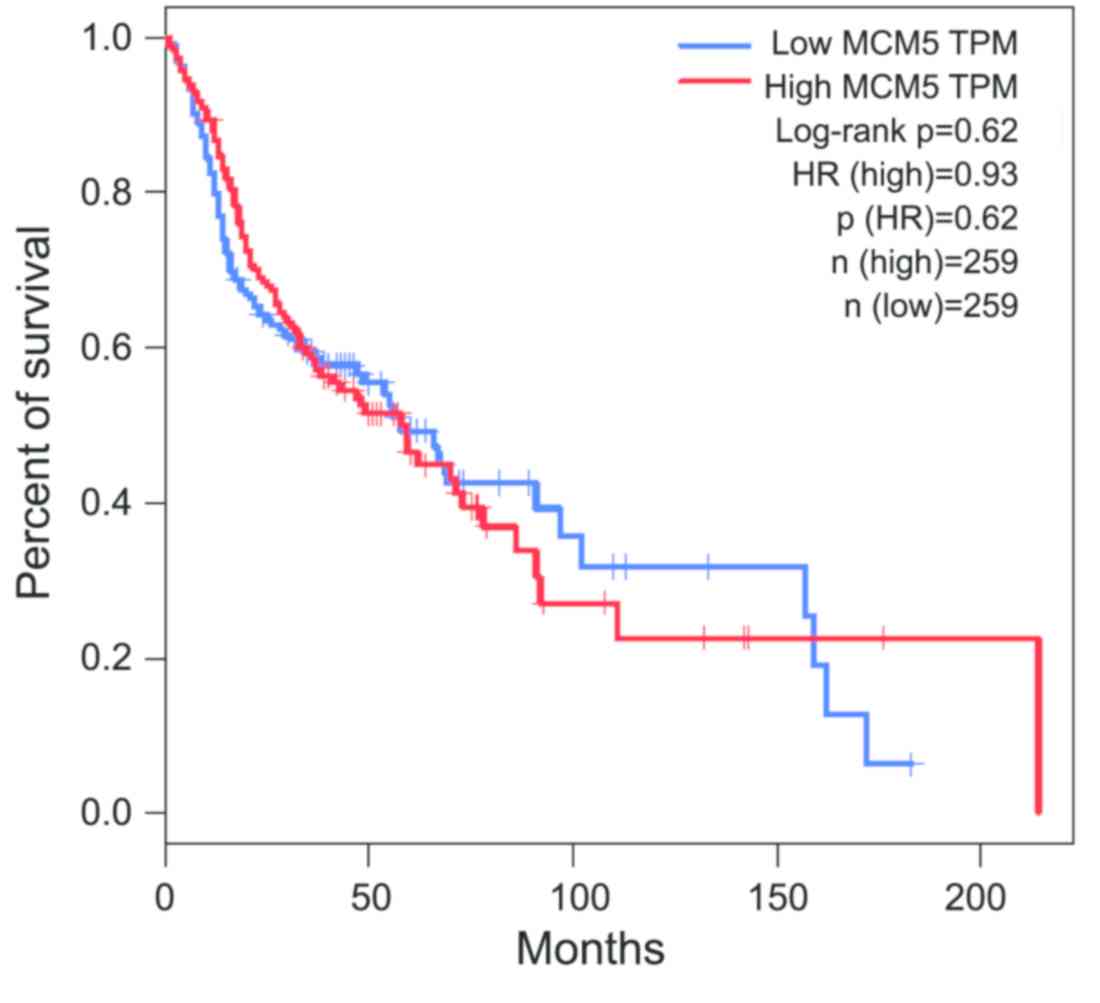
A Kaplan-Meier analysis of OS is shown in Fig. 3. Analysis of clinical data from TCGA
showed that high MCM5 expression was no associated with a shorter
OS in patients with OSCC (log-rank P=0.62). These results suggested
that MCM5 might not be a prognostic biomarker for OSCC.
Inhibitory effect of MCM5 in OSCC cell
lines
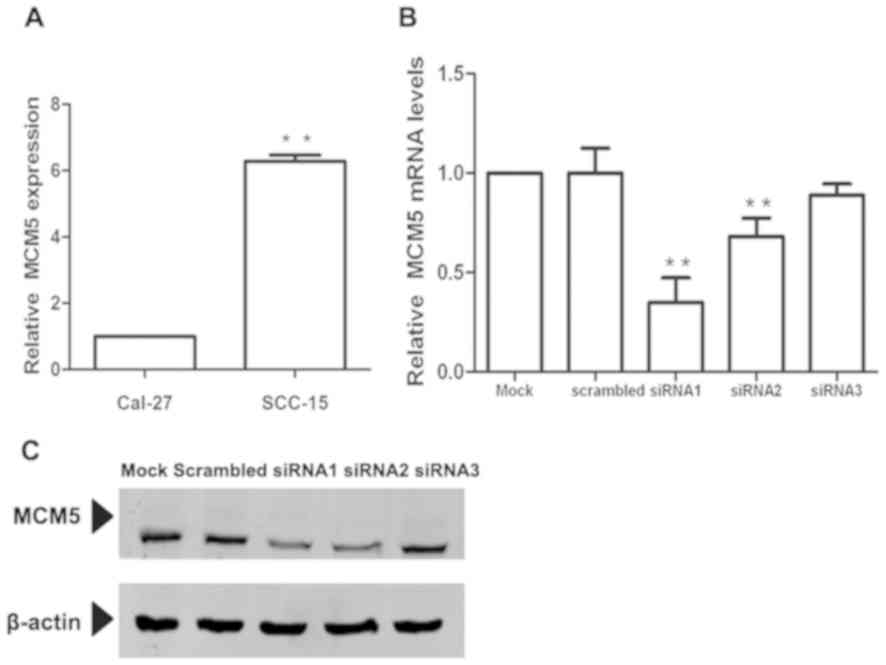
To determine the functional role of MCM5, first,
MCM5 expression was analyzed using RT-qPCR in two OSCC cell lines.
Notably, SCC-15 cells expressed significantly higher levels of MCM5
compared with Cal-27 cells (P<0.01; Fig. 4A). Considering that knockdown of MCM5
in the cell line with high MCM5 expression may bring about more
significant changes, the SCC-15 cell line was selected for further
investigation of the functional role of MCM5. Three specific siRNA
sequences were designed to inhibit MCM5 expression and transfected
in SCC-15 cells, and the impact on MCM5 expression was determined
using RT-qPCR. As shown in Fig. 4B,
siRNA1, siRNA2 and siRNA3 transfection decreased MCM5 expression by
65.1(P<0.01), 31.7 (P<0.01) and 11.0 (P<0.01),
respectively, compared with the negative control. Then, the
efficiencies were confirmed using western blotting (Fig. 4C). The inhibitory effect of siRNA1
and siRNA2 was significant, but not found in siRNA3. The results
were consistent with those of RNA expression. siRNA1 transfection
reduced MCM5 expression significantly in SCC-15 cells. Therefore,
siRNA1 was used for subsequent experiments.
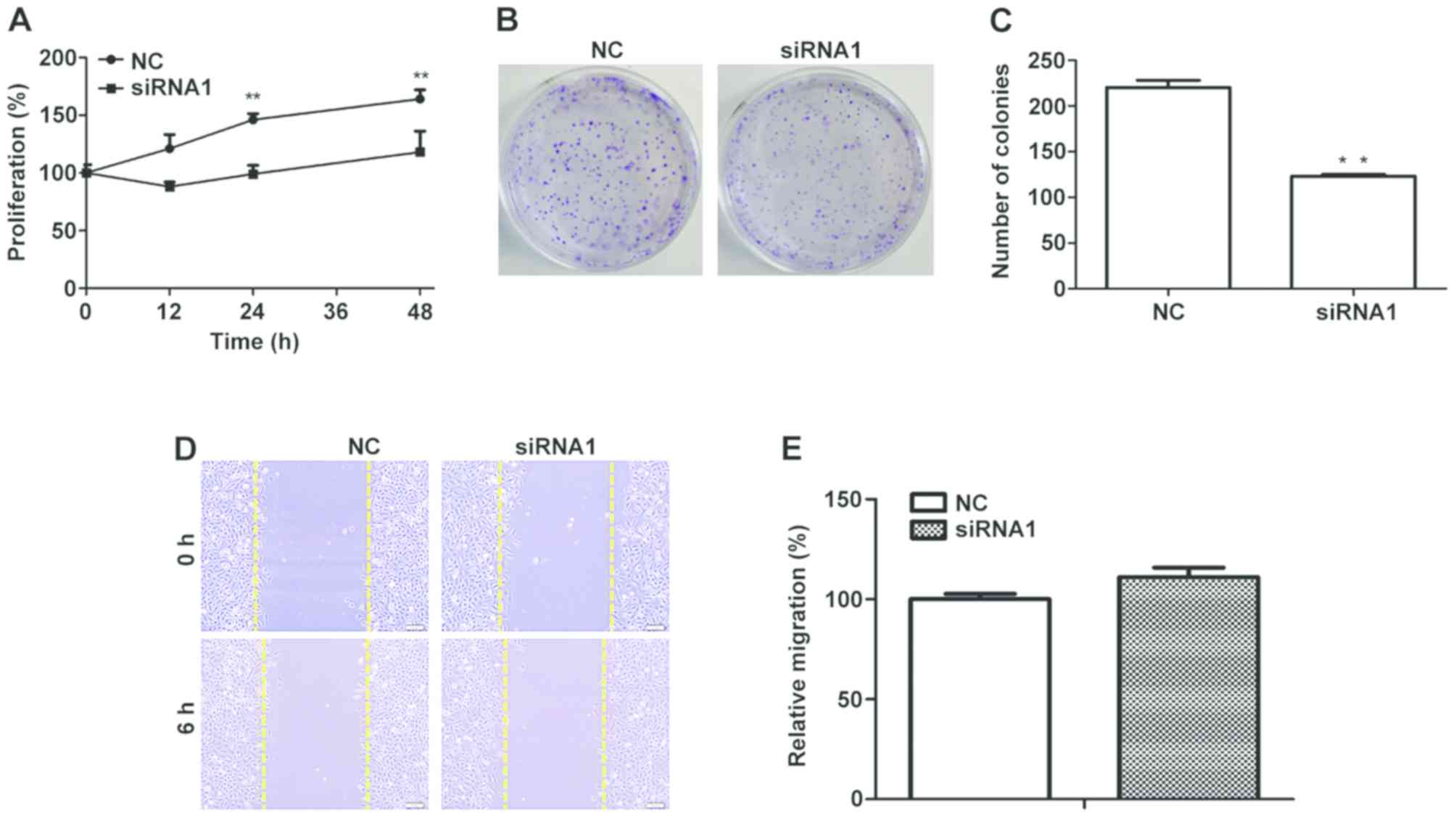
Effect of MCM5 inhibition on
proliferation, colony formation and migration
To determine whether MCM5 regulates cell cycle and
modulates cell proliferation in OSCC, the effect of inhibiting MCM5
expression on SCC-15 cell proliferation was investigated. As shown
in Fig. 5A, the results showed that
downregulation of MCM5 had significant anti-proliferative effect
compared with the negative control (P<0.01). Colony formation
assays were performed, and the results revealed that, compared with
the number of colonies in the control group, downregulation of MCM5
significantly reduced colony formation (P<0.01; Fig. 5B and C). To estimate the impact of
MCM5 on OSCC migration, scratching assays were conducted, and
inhibition of MCM5 showed no significant impact on the migration of
SCC-15 cells (P>0.05; Fig. 5D).
These results suggested that inhibiting MCM5 expression inhibited
cell proliferation and colony formation, but had no effect on
migration in SCC-15 cells.
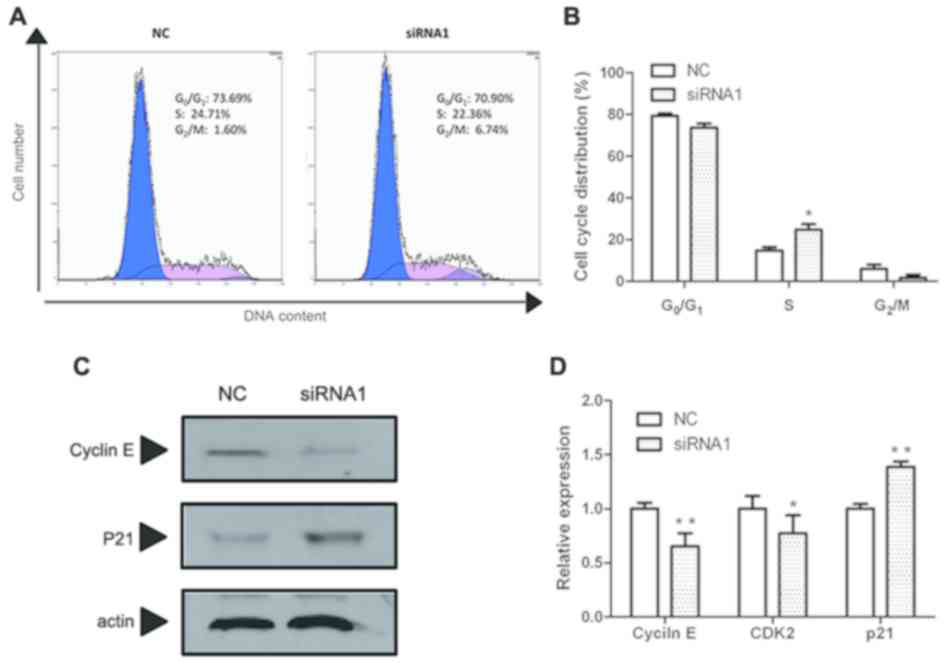
Effect of MCM5 inhibition on cell
cycle
To determine the potential mechanism for the
observed proliferation inhibition of SCC-15 cells by MCM5
inhibition, cell cycle analysis was performed using flow cytometry.
As shown in Fig. 6A and B, after
MCM5 inhibition, the number of cells were decreased in the
G0/G1 phase and the S phase but significantly
increased in the G2/M phase compared with the negative
control. These results indicated that MCM5 inhibition significantly
induced G2/M phase arrest compared with that in the
control group.
To elucidate the mechanism underlying this effect,
the expressions levels of cyclin E, cyclin-dependent kinase 2
(CDK2) and p21, related to cell cycle arrest (20), were determined using RT-qPCR. As
shown in Fig. 6D, MCM5 inhibition
decreased both cyclin E and CDK2 mRNA levels but increased
the mRNA expression of p21 significantly. Then, cyclin E and p21
were selected to detect the protein levels using western blotting.
As shown in Fig. 6C, cyclin E levels
decreased, while p21 levels increased in MCM5-downregulated SCC-15
cells, which was consistent with the RT-qPCR results.
Discussion
Despite notable progress in cancer research and
treatment, the survival rate of patients with OSCC has not
significantly improved in the past few decades (2). To date, there are no effective
tumor-specific biomarkers for the early detection and prognosis
prediction of OSCC (2). Several
studies have shown that DEGs serve an important role in the
development of tumors in different cancer types (7–9).
However, few studies have reported differentially expressed genes
in OSCC. The present screened genes that regulate the progression
of oral cancer by gene expression profiling and found that 832
genes were dysregulated, of which 416 DEGs were upregulated and 416
were downregulated. DEGs significantly affected 1,490 GO terms and
29 KEGG pathways. MCM5 did not have one of the highest
log2 (fold-change) values and log10
(qValues); however, MCM5 is one of the differentially expressed
genes in cell cycle signaling pathway, which was the most
significant enrichment of differentially expressed genes.
Therefore, MCM5, which regulates the cell cycle (10), was selected for further
investigation. However, previously published studies on biomarkers
in OSCC mainly focused on pathological studies (2,3,17). The present study not only verified
the overexpression of MCM5 in OSCC, but also confirmed, using cell
experiments, that MCM5 affects cell proliferation by regulating the
cell cycle.
MCM5 is a member of the MCM family of proteins and
is a component of the starting complex for DNA synthesis (21). MCM5 has been identified as a cell
cycle biomarker of aberrant proliferation, which is associated with
the progression of various cancer types (22,23).
Previously, MCM5 has been found to be overexpressed in numerous
human malignancies, such as esophageal (21), thyroid (24) and ovarian cancer (25). For example, increased MCM5 levels in
urine sediment cells predicts the presence of bladder cancer
(26,27). Inhibition of transcription factor
SOX-10 can inhibit the proliferation of skin melanocytes, and MCM5
expression is significantly decreased following downregulation of
SOX-10 (11). Moreover, high
expression of MCM5 is associated with poor prognosis and poor
malignant status in patients with cervical adenocarcinoma (14,28).
It is well known that immunohistochemistry and
western blotting are necessary methods to evaluate protein
expression (29). However, due to a
limited number of tissue samples, the present study did not have
enough samples for simultaneous qPCR, western blotting and
immunohistochemistry detection. Therefore, this is a limitation of
the present study. However, the study did perform qPCR to evaluate
the expression of MCM5 at the mRNA level. The results
demonstrated that 80.0% of patients with OSCC have high MCM5
expression, which is consistent with the results of the
aforementioned studies, indicating that high MCM5 expression may
play an important role in the pathogenesis of OSCC. In addition,
other members of the MCM family of proteins have also served as
biological markers of dysplasia and malignancy, such as glioma,
cervical, colorectal, breast, prostate and lung cancer (30–35).
Therefore, some researchers even suggested that changes in MCM5
expression may be a sign of cell cycle disorders (36,37).
It is worth noting that some researchers reported
that the high expression of MCM family members may be closely
related to tumorigenesis and prognosis. For example, MCM2, MCM4,
and MCM6 are overexpressed in breast cancer of high histological
grades (33). MCM7 expression is a
potent prognostic marker in non-small cell lung cancer (38), while MCM5 may be an independent
adverse prognostic marker in lung squamous cell carcinoma (34). It is well known that the cell cycle
is related to cell proliferation signaling pathways (10). In most tumors, an increase in the
expression level of genes encoding proteins that regulate cell
proliferation is observed. The abnormal expression of
cell-cycle-related genes is associated with infinite proliferation
of tumors and poor prognosis (10,11,20).
Thus far, only Yu et al (17)
reported the relationship between MCM5 and OSCC. The study reported
that overexpression of MCM5 in patients with OSCC was significantly
associated with tumor site, tumor size, positive lymph node
metastasis, later clinical stage, higher histological grade, deeper
infiltration depth and peripheral nerve infiltration. However, in
the present study, association between high expression of MCM5 with
survival, metastasis and poor histologic differentiation was not
observed. A Kaplan-Meier analysis of the overall survival rate was
not significantly changed in patients with high MCM5 expression
compared with patients with low MCM5 expression. It was speculated
that due to the small number of samples that there were large
differences between individuals. Therefore, in future research, a
larger sample size should be used to clarify the relationship
between high expression of MCM5 and prognosis of OSCC.
Little is known about the role and potential
function of MCM5 in OSCC. In the present study, a loss-of-function
analysis was conducted and it was demonstrated that MCM5
participated in regulating cell cycle and cell proliferation in
OSCC cells. In fact, inhibiting the expression of MCM5 in SCC-15
cells resulted in the downregulation of cyclin E and CDK expression
and upregulation of p21 expression, which ultimately led to
G2/M phase arrest in oral cancer cells. These results
further verified that MCM5 is highly expressed in patients with
OSCC, which promotes the proliferation of OSCC cells and regulates
cell cycle. In addition, it was observed that MCM5 was not only
expressed in SCC-15 cells, but also expressed in CAL-27 cells
(Fig. 4A). Notably, according to the
ATCC, the MCM5 gene had no mutations in either of the two
cell lines, indicating that the two cell lines selected in this
study have similar genetic backgrounds, and could be used for the
study of MCM5 cell functions.
Considering MCM5-knockdown experiments in SCC-15
cells with high MCM5 expression received more significant results,
SCC-15 cells were selected for follow-up studies. However,
analyzing the functional effects of MCM5-knockdown in CAL27 cell
lines may provide more information. In addition, both SCC-15 and
CAL-27 cells are transformed cell lines. In future investigations,
untransformed cell lines for multiple comparisons should be used to
clarify the role of MCM5 in OSCC.
Surgical resection is currently the main method to
treat OSCC. However, considering the particularity of the oral
structure, surgical resection will lead to a huge impact on
patients' quality of life (2,3).
Therefore, it is important to find effective diagnostic biomarkers
for early detection or to develop targeted drugs for OSCC. In the
present study, it was reported that MCM5 is overexpressed in OSCC
and that MCM5 can affect cell proliferation by regulating cell
cycle. Therefore, the results suggested that MCM5 might be one of
the important pathogenic factors of OSCC and is expected to be used
as a potential tumor marker for OSCC target drugs. The specific
mechanism of action of MCM5 is still worthy of further
investigation.
Overall, the present study evaluated 832
differentially expressed genes using sequencing patterns in OSCC
tumor tissues, and further validated MCM5 upregulated expression in
OSCC tissues. By knocking down MCM5 expression in SCC-15 cells, it
was revealed that cell proliferation and colony formation was
significantly inhibited by inducing G2/M phase arrest.
The results also suggested that during this process, cyclin E and
cell cycle-related gene expression levels were decreased, while p21
was significantly upregulated. Therefore, MCM5 may modulate OSCC
cell proliferation by regulating the cell cycle. MCM5 is an
important pathogenic factor and might have important role as a
potential diagnostic marker or drug target for OSCC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant no. 81903881), the Bethune
Project of Jilin University of China (grant no. 2018B02), the
Education Department of Jilin Province (grant no. JJKH20190074KJ),
and Department of Science and Technology of Jilin Province (grant
no. 20190103086JH and 20200201398JC).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request. The other datasets generated and/or analyzed during the
current study are available in The Cancer Genome Atlas (https://www.cancer.gov/), Gene Ontology (http://geneontology.org/), UniProt (https://sparql.uniprot.org/), NCBI (https://www.ncbi.nlm.nih.gov/) and Kyoto Encyclopedia
of Genes and Genomes (https://www.kegg.jp/) databases.
Authors' contributions
HW, CZ and CL performed the experiments. HW, MH and
XW analyzed the data. MH and HW wrote the manuscript. MH and XW
designed and supervised the study.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
China-Japan Union Hospital of Jilin University (Changchun, China).
Written informed consent was obtained from all patients who
participated in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ruicci KM, Meens J, Sun RX, Rizzo G, Pinto
N, Yoo J, Fung K, MacNeil D, Mymryk JS, Barrett JW, et al: A
controlled trial of HNSCC patient-derived xenografts reveals broad
efficacy of PI3Kα inhibition in controlling tumor growth. Int J
Cancer. 145:2100–2106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khurshid Z, Zafar MS, Khan RS, Najeeb S,
Slowey PD and Rehman IU: Role of salivary biomarkers in oral cancer
detection. Adv Clin Chem. 86:23–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thomson PJ: Perspectives on oral squamous
cell carcinoma prevention-proliferation, position, progression and
prediction. J Oral Pathol Med. 47:803–807. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sim F, Leidner R and Bell RB:
Immunotherapy for Head and Neck cancer. Oral Maxillofac Surg Clin
North Am. 31:85–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao Y, Chen X, Lu S, Zhou C, Xu G, Yan Z,
Yang J, Yu T, Chen W, Qian Y, et al: Circulating long noncoding
rnas as biomarkers for predicting head and neck squamous cell
carcinoma. Cell Physiol Biochem. 50:1429–1440. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Long T, Liu Z, Zhou X, Yu S, Tian H and
Bao Y: Identification of differentially expressed genes and
enriched pathways in lung cancer using bioinformatics analysis. Mol
Med Rep. 19:2020–2040. 2019.
|
|
7
|
Lei B, Zhang XY, Zhou JP, Mu GN, Li YW,
Zhang YX and Pang D: Transcriptome sequencing of HER2-positive
breast cancer stem cells identifies potential prognostic marker.
Tumour Biol. 37:14757–14764. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu L, Guo Q, Jin S, Feng H, Zhuang H, Liu
C, Tan M, Liu J, Li X and Lin B: Analysis of the gene expression
profile in response to human epididymis protein 4 in epithelial
ovarian cancer cells. Oncol Rep. 36:1592–1604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie C, Xu M, Lu D, Zhang W, Wang L, Wang
H, Li J, Ren F and Wang C: Candidate genes and microRNAs for glioma
pathogenesis and prognosis based on gene expression profiles. Mol
Med Rep. 18:2715–2723. 2018.PubMed/NCBI
|
|
10
|
Chen Y, Hennessy KM, Botstein D and Tye
BK: CDC46/MCM5, a yeast protein whose subcellular localization is
cell cycle-regulated, is involved in DNA replication at
autonomously replicating sequences. Proc Natl Acad Sci USA.
89:10459–10463. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su Z, Zheng X, Zhang X, Wang Y, Zhu S, Lu
F, Qu J and Hou L: Sox10 regulates skin melanocyte proliferation by
activating the DNA replication licensing factor MCM5. J Dermatol
Sci. 85:216–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong H, Chen B, Neves H, Xing J, Ye Y,
Lin Y, Zhuang G, Zhang SD, Huang J and Kwok HF: Expression of
minichromosome maintenance genes in renal cell carcinoma. Cancer
Manag Res. 9:637–647. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liao X, Han C, Wang X, Huang K, Yu T, Yang
C, Huang R, Liu Z, Han Q and Peng T: Prognostic value of
minichromosome maintenance mRNA expression in early-stage
pancreatic ductal adenocarcinoma patients after
pancreaticoduodenectomy. Cancer Manag Res. 10:3255–3271. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang D, Li Q, Li Y and Wang H: The role of
MCM5 expression in cervical cancer: Correlation with progression
and prognosis. Biomed Pharmacother. 98:165–172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Takeuchi S, Moroi Y, Lin N, Urabe
K, Kokuba H, Imafuku S, Dainichi T, Uchi H, Furue M and Tu Y:
Expression of minichromosome maintenance 5 protein in proliferative
and malignant skin diseases. Int J Dermatol. 46:1171–1176. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liao X, Liu X, Yang C, Wang X, Yu T, Han
C, Huang K, Zhu G, Su H, Qin W, et al: Distinct diagnostic and
prognostic values of minichromosome maintenance gene expression in
patients with hepatocellular carcinoma. J Cancer. 9:2357–2373.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu SY, Wang YP, Chang JY, Shen WR, Chen HM
and Chiang CP: Increased expression of MCM5 is significantly
associated with aggressive progression and poor prognosis of oral
squamous cell carcinoma. J Oral Pathol Med. 43:344–349. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jia CY, Li HH, Zhu XC, Dong YW, Fu D, Zhao
QL, Wu W and Wu XZ: MiR-223 suppresses cell proliferation by
targeting IGF-1R. PLoS One. 6:e270082011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saleem M, Asif J, Asif M and Saleem U:
Amygdalin from apricot kernels induces apoptosis and causes cell
cycle arrest in cancer cells: An updated review. Anticancer Agents
Med Chem. 18:1650–1655. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seo YS and Kang YH: The Human replicative
helicase, the CMG Complex, as a target for anti-cancer therapy.
Front Mol Biosci. 5:262018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Everson M, Magee C, Alzoubaidi D, Brogden
S, Graham D, Lovat LB, Novelli M and Haidry R: Minichromosomal
maintenance component complex 5 (MCM5) as a marker of barrett's
esophagus-related neoplasia: A feasibility study. Dig Dis Sci.
64:2815–2822. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Margalit DN and Lin A: Two sides of the
same coin: Head and neck cancer treatment De-Intensification and
intensification with induction chemotherapy. Int J Radiat Oncol
Biol Phys. 102:1–4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mio C, Lavarone E, Conzatti K, Baldan F,
Toffoletto B, Puppin C, Filetti S, Durante C, Russo D, Orlacchio A,
et al: MCM5 as a target of BET inhibitors in thyroid cancer cells.
Endocr Relat Cancer. 23:335–347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gakiopoulou H, Korkolopoulou P, Levidou G,
Thymara I, Saetta A, Piperi C, Givalos N, Vassilopoulos I, Ventouri
K, Tsenga A, et al: Minichromosome maintenance proteins 2 and 5 in
non-benign epithelial ovarian tumours: Relationship with cell cycle
regulators and prognostic implications. Br J Cancer. 97:1124–1134.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kelly JD, Dudderidge TJ, Wollenschlaeger
A, Okoturo O, Burling K, Tulloch F, Halsall I, Prevost T, Prevost
AT, Vasconcelos JC, et al: Bladder cancer diagnosis and
identification of clinically significant disease by combined
urinary detection of Mcm5 and nuclear matrix protein 22. PLoS One.
7:e403052012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen X, Scapa JE, Liu DX and Godbey WT:
Cancer-specific promoters for expression-targeted gene therapy:
Ran, brms1 and mcm5. J Gene Med. 18:89–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang D, Wang H, Li Y and Li Q: MiR-362-3p
functions as a tumor suppressor through targeting MCM5 in cervical
adenocarcinoma. Biosci Rep. 38:BSR201806682018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Janardhan K, Jensen H, Clayton NP and
Herbert RA: Immunohistochemistry in investigative and toxicologic
pathology. Toxicol Pathol. 46:488–510. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hua C, Zhao G, Li Y and Bie L:
Minichromosome maintenance (MCM) Family as potential diagnostic and
prognostic tumor markers for human gliomas. BMC Cancer. 14:5262014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Majid S, Dar AA, Saini S, Chen Y,
Shahryari V, Liu J, Zaman MS, Hirata H, Yamamura S, Ueno K, et al:
Regulation of minichromosome maintenance gene family by
microRNA-1296 and genistein in prostate cancer. Cancer Res.
70:2809–2818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Das M, Prasad SB, Yadav SS, Govardhan HB,
Pandey LK, Singh S, Pradhan S and Narayan G: Over expression of
minichromosome maintenance genes is clinically correlated to
cervical carcinogenesis. PLoS One. 8:e696072013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Issac MSM, Yousef E, Tahir MR and Gaboury
LA: MCM2, MCM4, and MCM6 in breast cancer: Clinical utility in
diagnosis and prognosis. Neoplasia. 21:1015–1035. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giaginis C, Georgiadou M, Dimakopoulou K,
Tsourouflis G, Gatzidou E, Kouraklis G and Theocharis S: Clinical
significance of MCM-2 and MCM-5 expression in colon cancer:
Association with clinicopathological parameters and tumor
proliferative capacity. Dig Dis Sci. 54:282–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu YZ, Wang BS, Jiang YY, Cao J, Hao JJ,
Zhang Y, Xu X, Cai Y and Wang MR: MCMs expression in lung cancer:
Implication of prognostic significance. J Cancer. 8:3641–3647.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Korkolopoulou P, Givalos N, Saetta A,
Goudopoulou A, Gakiopoulou H, Thymara I, Thomas-Tsagli E and
Patsouris E: Minichromosome maintenance proteins 2 and 5 expression
in muscle-invasive urothelial cancer: A multivariate survival study
including proliferation markers and cell cycle regulators. Hum
Pathol. 36:899–907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Passerini V, Ozeri-Galai E, de Pagter MS,
Donnelly N, Schmalbrock S, Kloosterman WP, Kerem B and Storchová Z:
The presence of extra chromosomes leads to genomic instability. Nat
Commun. 7:107542016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aparicio T, Guillou E, Coloma J, Montoya G
and Méndez J: The human GINS complex associates with Cdc45 and MCM
and is essential for DNA replication. Nucleic Acids Res.
37:2087–2095. 2009. View Article : Google Scholar : PubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBI
|