Introduction
The number of patients with colorectal cancer (CRC)
worldwide is increasing annually, with an incidence rate of 6.1% in
2018 (1). Chronic inflammation is
the leading cause of immune cell infiltration and proliferation,
and it has been suggested to be a high-risk factor for
colitis-associated CRC (CAC) (2).
Inflammatory bowel disease (IBD), which encompasses both ulcerative
colitis (UC) and Crohn's disease, was established as an important
precursor to CRC (3,4). For example, the incidence of
IBD-associated CRC in patients with UC was reported to have a
cumulative risk rate of 2% at 10 years, 8% at 20 years and 18% at
30 years of disease duration (3).
Therefore, further in vivo studies are required for
researchers to gain an improved understanding of the molecular
mechanisms of CAC, which may provide more exact molecular targets
for the diagnosis and treatment of CAC during the early stages.
Toll-like receptor (TLR)9, a member of the TLR
family, is located in the cytoplasm and intracellular endosomes,
and can be activated by unmethylated bacterial CpG DNA (5). The activation of the TLR9 signaling
pathway induces a Type 1 T helper cell immune response and
stimulates the proliferation of B cells, thus protecting the host
from external microbial invasion (6–9).
Multiple studies have revealed that abnormal TLR9 expression levels
were involved in the pathogenesis and progression of UC (10,11). In
addition, abnormal expression levels of TLR9 were also identified
during the tumorigenesis and development of CRC (12–15).
NF-κB is an important transcription factor involved
in various biological processes, including inflammatory reactions,
immune responses, apoptosis and proliferation (16). In fact, NF-κB is regarded as a
molecular hub that links inflammation and cancer (17). It was previously suggested that NF-κB
may serve an important role in colorectal carcinogenesis by
regulating matrix metalloproteinase-9 expression (18–20).
Previous studies have revealed that TLR9 was related to the
biological characteristics of various types of cancer, including
bladder, lung and prostate cancer, such as cell proliferation,
invasion, tumor growth and progression (21–24). In
fact, one previous study reported that TLR9 regulated the
expression levels of interleukin (IL)-6 through the myeloid
differentiation primary response protein MyD88 (MyD88)/NF-κB
signaling pathway in myeloid cells to promote tumor recurrence
after irradiation, including in melanoma, bladder carcinoma and
colorectal carcinoma (25).
The current study aimed to investigate the effect of
TLR9 on the development of CAC through its regulation of the NF-κB
signaling pathway. Owing to the synergistic effects of azoxymethane
(AOM), a tumor-inducing agent, and dextran sodium sulfate (DSS), a
tumor-promoting agent (26), the
present study established CAC model mice by co-administering AOM
and DSS to analyze the expression levels of TLR9, NF-κB and Ki67 in
CAC tissues.
Materials and methods
Reagents and antibodies
AOM (cat. no. A5486) and chloroquine (TLR9
inhibitor; cat. no. C6628-25G) were obtained from Sigma-Aldrich;
Merck KGaA. DSS (cat. no. 0216011080-100G) was purchased from MP
Biomedicals, LLC. Anti-TLR9 (cat. no. ab134368) and anti-MyD88
(cat. no. ab135693) primary antibodies were obtained from Abcam.
Anti-NF-κB (NF-κB p65; cat. no. 8242S), anti-Bcl-xl (cat. no. 2764)
and anti-Ki67 (cat. no. 9449S) primary antibodies were obtained
from Cell Signaling Technology, Inc. The anti-proliferating cell
nuclear antigen (PCNA; cat. no. sc-56) primary antibody was
obtained from Santa Cruz Biotechnology, Inc. and the anti-GAPDH
(cat. no. TA309157) primary antibody was obtained from OriGene
Technologies, Inc. Horseradish peroxidase secondary goat
anti-rabbit (cat. no. ZB-2301) and goat anti-mouse (cat. no.
ZB-2305) antibodies, used for western blotting, and goat
anti-mouse/rabbit antibodies (cat. no. TA130001/TA130015) used for
immunohistochemistry (IHC) were obtained from OriGene Technologies,
Inc.
Cell lines and culture
The human CRC cell line HT29 was obtained from the
American Type Culture Collection and cultured in McCoy's 5A
(Modified) medium (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
50 mg/ml penicillin and 50 mg/ml streptomycin, maintained in a
humidified atmosphere of 5% CO2 at 37°C.
Western blotting
Cells were lysed in RIPA lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd.) at 4°C for 30 min. The
protein concentration was measured following centrifugation at
12,000 × g for 15 min at 4°C and quantified using a bicinchoninic
acid protein assay kit (Beijing Solarbio Science & Technology
Co., Ltd.). An equal amount of protein (50 µg/lane) was separated
via 10% SDS-PAGE and electrophoretically transferred onto
polyvinylidene difluoride membranes (EMD Millipore). The membranes
were blocked with 5% skimmed milk for 2 h at room temperature and
were subsequently incubated overnight at 4°C with the following
primary antibodies: Anti-TLR9 (1:1,000), anti-NF-κB (1:1,000),
anti-Bcl-xl (1:1,000), anti-PCNA (1:1,000), anti-MyD88 (1:500) and
anti-GAPDH (1:1,000). Following the primary antibody incubation,
the membranes were washed 3 times with PBS for 10 min each and
incubated with the corresponding goat anti-rabbit or goat
anti-mouse secondary antibodies at 4°C for 4 h. Protein bands were
visualized using an ECL reagent (Thermo Fisher Scientific, Inc.) on
a Gel Doc XR+ system (Bio-Rad Laboratories, Inc.) and analyzed
using Image Lab version 2.0 software (Bio-Rad Laboratories,
Inc.).
Wound healing assay
A wound healing assay was performed to analyze the
cell migratory ability. Briefly, HT29 cells (3×105/well)
were seeded into six-well plates and cultured to 90–100%
confluence. Subsequently, a 200-µl pipette tip was used to scratch
a wound in the cell monolayer. Fresh serum-free McCoy's 5A
(Modified) medium containing different concentrations of
chloroquine (0, 15 or 25 µg/ml) was added to each well and cultured
for 48 h in a humidified atmosphere of 5% CO2 at 37°C.
Images of each well were captured at 0 and 48 h using a light phase
contrast microscope (Olympus, ZKX-41; Olympus Corporation) with a
magnification of ×100. The wound-healing areas were assessed using
ImageJ 1.52a software (National Institutes of Health). The
migratory rate of cells = (wound area at 0 h-wound area at 48
h)/area at 0 h.
Colony formation assay
HT29 cells (1.2×105/well) were cultured
in 6-well plates for 48 h in medium containing different
concentrations of chloroquine (0, 15 or 25 µg/ml) at 37°C, and then
seeded into 6-cm cell culture dishes (500 cells/dish) and incubated
in complete medium at 37°C. After 14 days, the cells were fixed
with 4% paraformaldehyde for 15 min and stained with 0.1% crystal
violet for 30 min, both at room temperature. The number of
colonies, defined as >50 cells/colony, was counted manually
using a light microscope with a magnification of ×100. Relative
colony number = number of colonies in observed group/number of
colonies in control group. All of the experiments were repeated ≥3
times.
Cell viability assay
The viability of HT29 cells was analyzed using an
MTT assay. Briefly, HT29 cells were seeded at a density of 5,000
cells/well in a 96-well plate and treated for 24, 48 or 72 h with
chloroquine (0, 100, 125 or 150 µg/ml) at 37°C and then analyzed
using an MTT assay as previously described (27).
Animal studies
All animal experiments were approved by the ethics
committee of The First Affiliated Hospital of Nanchang University
(Nanchang, China). A total of 144 female BALB/c mice (weight, 20–24
g; age, 8–10 weeks) were obtained from Beijing Vital River
Laboratory Animal Technology Co. Ltd. (Laboratory Animal License
no. SCXK 2017-0012). The animals were maintained with a normal diet
and tap water ad libitum at a temperature of 23±2°C and a relative
humidity of 40–60%, and artificially illuminated on an approximate
12 h light/dark cycle at the Animal Care Facility in the Medical
College of Nanchang University. All of the mice experiments were
approved by the Animal Care and Use Committee of Nanchang
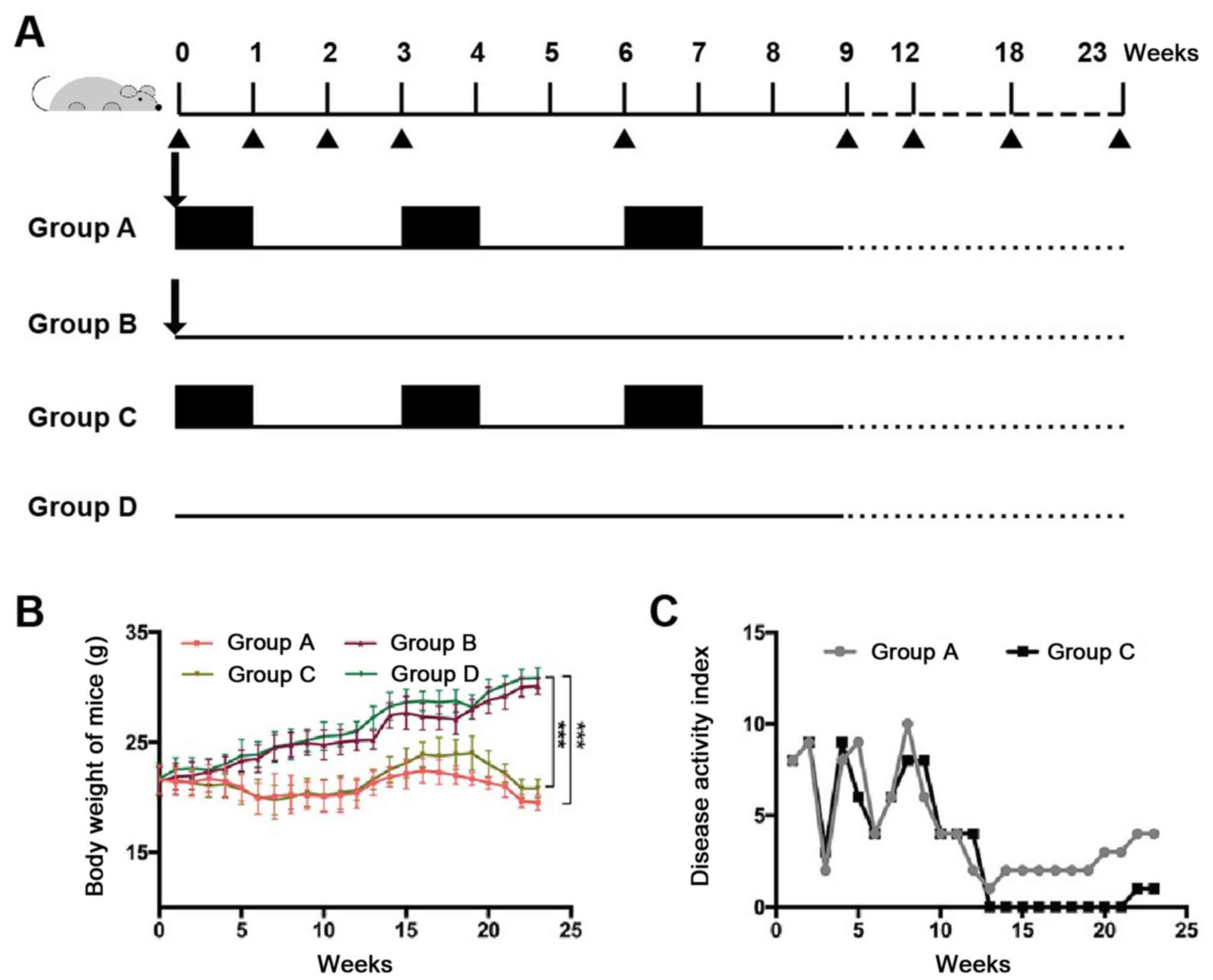
University. The total experiment lasted for 23 weeks. The mice were
divided into four groups (36 mice/group): i) Group A (AOM + DSS),
which was intraperitoneally injected without anesthesia with 10
mg/kg AOM once on the first day, followed by 3% DSS given in the
drinking water for 1 week and then 2 weeks of distilled water (one
cycle), which was repeated for two additional cycles; ii) Group B
(AOM), which was intraperitoneally injected with 10 mg/kg AOM once
on the first day and provided with distilled drinking water during
weeks 0–9; iii) Group C (DSS), which was treated with DSS as
described for group A but without the AOM treatment; and iv) Group
D (blank control), which received neither DSS nor AOM treatment and
was provided with distilled drinking water for the first 9 weeks.
All the mice were provided with a normal diet and tap water during
weeks 10–23 (Fig. 1A).
The disease activity index (DAI) was evaluated at
the end of the experiment using the numerical system described by
Tian et al (28). The DAI
parameters included total body weight loss (0, none; 1, 1–5%; 2,
5–10%; 3, 10–20%; 4, >20%), stool consistency (0, well-formed
pellets; 2, loose stool; 4, diarrhoea) and the presence of fecal
occult blood (0, negative; 2, positive; 4, gross bleeding).
Several mice were randomly sacrificed at certain
times points following AOM injection (the 1st,
2nd, 3rd, 6th, 9th,
12th, 18th and 23rd weeks). Six mice were
sacrificed at weeks 1, 2, 3 and 6, one mouse was sacrificed at week
9 and four mice were sacrificed at weeks 12 and 18 in each group.
Additionally, six mice in group D were randomly sacrificed at week
0 as control. All remaining mice were sacrificed at week 23. After
the large bowels were resected and washed with PBS, they were
carefully examined, photographed and fixed in 10% formalin at room
temperature for 24 h. Further histological examinations were
subsequently performed.
Histopathological analysis and
immunohistochemistry (IHC)
Paraffin-embedded colorectal sections (5-µm-thick)
were stained with hematoxylin for 2 min and eosin for 5 min at room
temperature to analyze the degree of inflammation using a light
microscope with magnifications of ×40 and ×100. Briefly, the
severity of inflammation, the thickness of inflammation the
severity of epithelial damage and the extent of the lesions were
each graded from 0 to 3 by two investigators who were blinded to
the treatment groups, as previously described (29,30). The
severity of inflammation was adapted from the grading system
developed by Truelove and Richards (31,32) as
follows: i) Grade 0, no neutrophil infiltration in the lamina
propria; ii) Grade I, infiltration of a small number of neutrophils
[<10 cells/high power field (HPF)] in the lamina propria,
involving a few crypts; iii) Grade II, obvious neutrophil
infiltration in the lamina propria (10–50 cells/HPF), involving
>50% of the crypts; iv) Grade III, infiltration of neutrophils
(>50 cells/HPF) in the lamina propria with crypt abscess; and v)
Grade IV, obvious acute inflammation in the lamina propria with
ulcer formation. Grade I was classified as mild, grade II was
classified as moderate, and grades III and IV were classified as
severe. The severity of inflammation ranged from 0 to 3 (0, no
inflammation; 1, mild; 2, moderate; 3, severe), the thickness of
inflammation ranged from 0 to 3 (0, no inflammation; 1, mucosa; 2,
mucosa plus submucosa; 3, transmural), the severity of epithelial
damage ranged from 0 to 3 (0, intact epithelium; 1, disruption of
architectural structure; 2, erosion; 3, ulceration) and the extent
of lesions ranged from 0 to 3 (0, no lesions; 1, punctuate; 2,
multifocal; 3, diffuse).
IHC was performed as described in our
previous study (33)
Colorectal tissues were fixed in 10% formalin for 24
h at room temperature. Formalin-fixed and paraffin-embedded tissue
blocks were cut into 5-µm-thick sections and mounted on glass
slides. Slides were heated in an oven at 70°C for 90 min and
deparaffined in xylene twice for 10 min each at room temperature,
rehydrated in a descending ethanol series (100, 95 and 85% ethanol
for 5 min each at room temperature) and incubated in 3%
H2O2 for 8 min at room temperature to block
endogenous peroxidase. Antigen retrieval was performed by heating
in a microwave at 100°C in sodium citrate buffer (3 mM, pH 6.0) for
15 min. Slides were blocked with 5% bovine serum albumin (Beijing
Solarbio Science & Technology Co., Ltd.) for 1 h at room
temperature to block non-specific antibody binding and incubated
overnight at 4°C with the following primary antibodies: Anti-TLR9
(1:400), anti-NF-κB (1:200) and anti-Ki67 (1:100). Following the
primary antibody incubation, the sections were washed three times
with PBS and incubated with horseradish peroxidase secondary goat
anti-mouse/rabbit antibodies (ready to use) at 37°C for 30 min. The
sections were stained with 3,3′-diaminobenzidine at room
temperature; the duration of staining was based on the staining
observed under a light microscope with a magnification of ×100, and
the reaction was terminated when the staining was yellowish-brown.
The sections were then counterstained with hematoxylin for 1 min at
room temperature. The slides were observed under a light microscope
with a magnification of ×100. The widely accepted German
semi-quantitative scoring system (34,35) was
used to determine the staining intensity and area of staining,
according to the recommendations of Remmele and Stegner (36). Each specimen was assigned a score
according to the intensity of the nucleic, cytoplasmic and/or
membrane staining (no staining, not detected=0; weak staining,
light yellow=1; moderate staining, yellowish brown=2; strong
staining, brown=3) and the extent of stained cells (no staining, 0;
1–24%, 1; 25–49%, 2; 50–74%, 3; 75–100%, 4). The final
immunoreactive score was determined by multiplying the intensity
score with the extent of stained cells score, ranging from 0 (the
minimum) to 12 (the maximum).
Co-immunoprecipitation assay
HT29 cells were lysed in RIPA lysis buffer (Beijin
Solarbio Science & Technology Co., Ltd.) at 4°C for 30 min.
Whole-cell lysates were pelleted via centrifugation at 10,000 × g
for 10 min at 4°C. The supernatant was incubated with an anti-TLR9
antibody (1:200) or goat anti-mouse IgG (1:200; cat. no. ZB-2305;
OriGene Technologies, Inc.), together with protein A/G PLUS-Agarose
beads (Santa Cruz Biotechnology) at 4°C overnight. The beads were
washed three times with the non-lubrol lysis buffer at 2,500 × g
centrifugation for 5 min at 4°C, then subjected to SDS-PAGE and
subsequent western blotting analysis as aforementioned. Whole cell
lysate was used as a control.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (IBM Corp.). The data are presented as the mean ± SD. All
experiments were performed at least in triplicate. A one-way ANOVA
followed by a Tukey's post hoc test was used to determine the
statistical differences between >2 groups, whereas an unpaired
Student's t-test were used to determine the statistical differences
between 2 groups. A Spearman's rank correlation test was used to
determine the correlation between the expression levels of TLR9,
NF-κB and Ki67 in CRC tissues. P<0.05 was considered to indicate
a statistically significant difference.
Results
Construction of the CAC model
mice
In groups A (AOM + DSS) and C (DSS), the body
weights of the mice decreased with time compared with group D
(blank control group; Fig. 1B). The
average weight of the mice in group C declined from 21.6±1.3 g at
the beginning of the experiment to 20.8±0.8 g by week 23, while the
average weight of the mice in group A decreased from 21.6±1.23 g at
the beginning of the experiment to 19.5±0.7 g by week 23; the
differences between groups A and D, and groups C and D were
statistically significant at week 23 (P<0.05). The DAI, which
reflects the severity of colitis, was also markedly increased in
groups A and C after DSS administration (on the 1st,
3rd and the 6th week; Fig.
1C). No weight loss or any signs of inflammation, such as loose
stool or diarrhea, positive fecal occult blood or gross bleeding,
were observed in groups B (AOM) and D (blank control). The DAI of
groups B and D was zero throughout the modeling process and is
therefore not shown in Fig. 1C. The
lengths of the large bowels in groups A and C were decreased
compared with groups B and D (Fig.
2A). Notably, the lengths of the large bowels were
significantly decreased in groups A and C from week 3 compared with
the control group (mice sacrificed in group D at week 0) or with
group D(P<0.05; Fig. 2B).
However, the severity of inflammation was significantly increased
in group A compared with in group C at week 23, and the extent of
inflammation was significantly increased in group A compared with
in group C after week 18 (P<0.05), while there was no
significant differences in the thickness of inflammation and the
severity of epithelial damage between groups A and C (Fig. 2C).
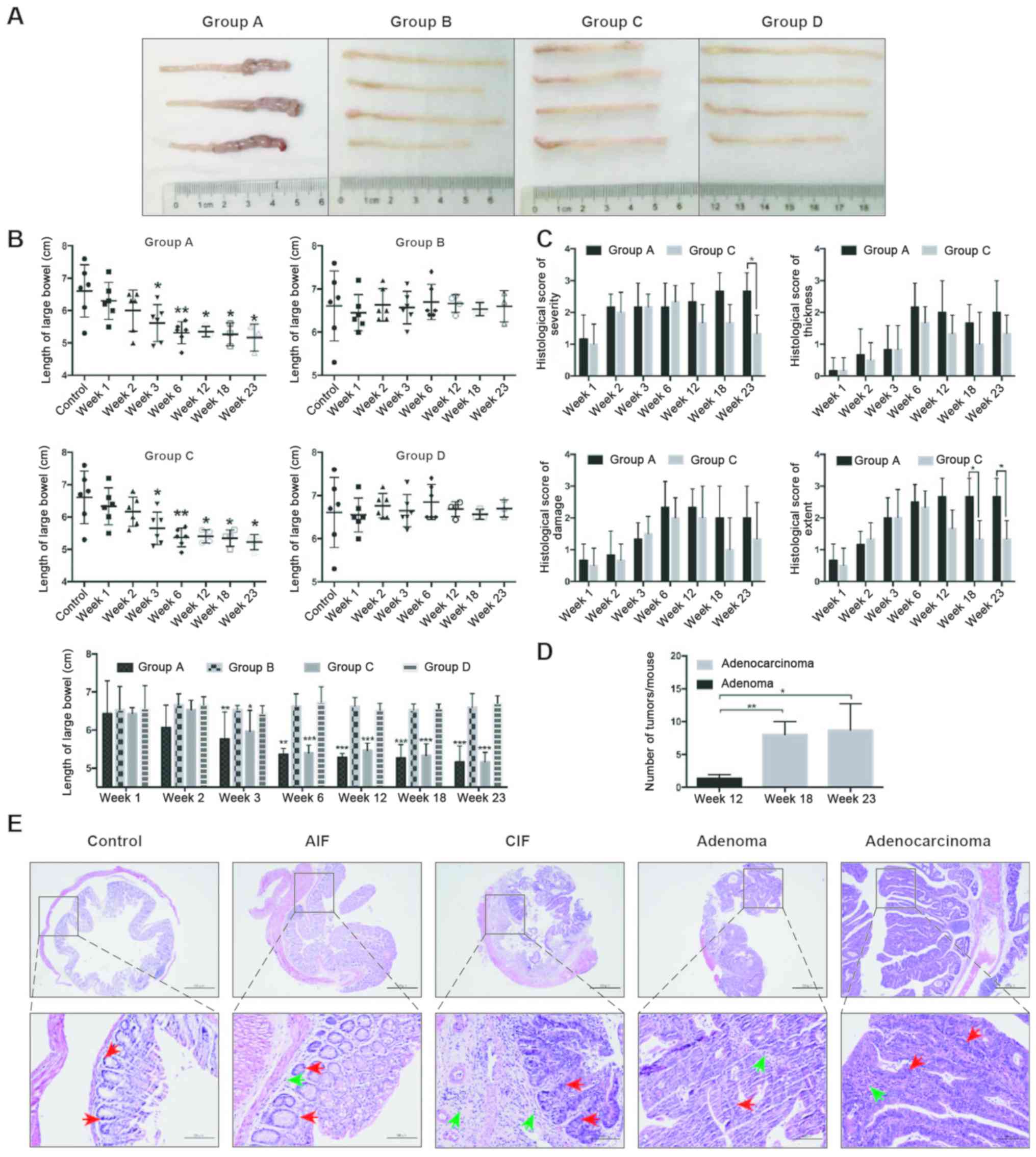 | Figure 2.Colorectal pathological results in
mice. (A) Length of the large bowels of mice in each group at week
18. Representative bowels from 3–4 independent animals are shown.
(B) Mean length of large bowel of mice in each group. *P<0.05,
**P<0.01, ***P<0.001 vs. control (mice sacrificed in group D
at week 0) or group D (bottom graph). (C) Histological score of
severity, thickness, damage and extent of lesions of the
colorectums from mice in groups A and C. *P<0.05. (D) Large
bowels were retrieved at week 12, 18 and 23 from mice in group A to
determine the numbers of tumors formed. *P<0.05, **P<0.01.
(E) Histological analysis of various pathological changes in the
colons of mice in group A were determined using hematoxylin and
eosin staining. The control groups represents tissues taken at week
0 from group D. Scale bars, 500-µm (upper) and 100-µm (lower).
Inflammatory cells (green arrows) and intestinal epithelial cells
(red arrows) are indicated. Group A, 10 mg/kg azoxymethane and 3%
dextran sodium sulfate water; group C, 3% dextran sodium sulfate
water; AIF, acute inflammation; CIF, chronic inflammation. |
By the 12th week, colorectal tumors were only
observed in the mice of group A (Fig.
2D). Pathological results further revealed that the mice in
group A presented with acute inflammation (AIF), chronic
inflammation (CIF), adenoma and adenocarcinoma of the colorectum at
weeks 3, 6, 12 and 18, respectively (Fig. 2D and E).
Expression levels of TLR9 and NF-κB
are simultaneously upregulated during the development of chronic
colitis in CRC
IHC staining revealed that TLR9 was located in the
cytoplasm of the intestinal epithelial cells (IECs) and
inflammatory cells in the lamina propria (Fig. 3A). TLR9 expression levels were
significantly upregulated in AIF, CIF, adenoma and adenocarcinoma
tissues compared with the corresponding tissues from control mice
(group D; P=0.0334, P=0.0379, P=0.0437 and P=0.0008, respectively;
Fig. 3B). Furthermore, the protein
expression levels of TLR9 was significantly upregulated in the
adenocarcinoma tissue compared with the AIF (P=0.0077), CIF
(P=0.0278) and adenoma (P=0.0273) tissue (Fig. 3B).
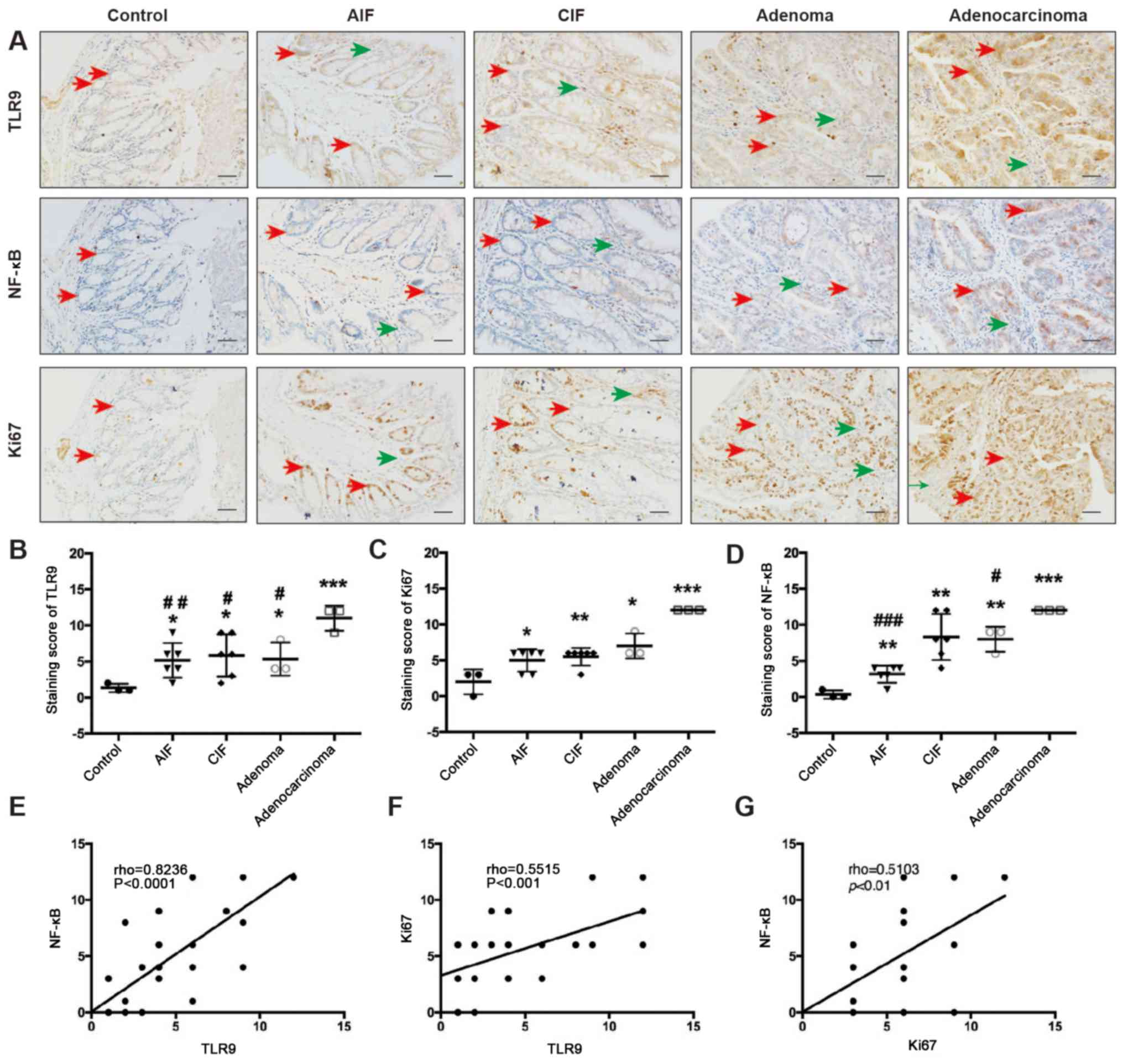 | Figure 3.TLR9 and NF-κB are simultaneously
upregulated as CAC develops. (A) IHC was used to analyze the
expression levels and localization of TLR9, NF-κB and Ki67 in
colorectal sections obtained from mice in group A. Inflammatory
cells (green arrows) and intestinal epithelial cells (red arrows)
are indicated. IHC staining scores of (B) TLR9, (C) Ki67 and (D)
NF-κB in sections of normal control (group D), AIF, CIF, adenoma
and adenocarcinoma tissues, which were obtained as described in the
methods section. Scale bar, 50-µm. *P<0.05, **P<0.01 and
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs.
adenocarcinoma. Correlation analysis of (E) TLR9 and NF-κB
expression levels, (F) Ki67 and TLR9 expression levels and (G)
NF-κB and Ki67 expression levels was conducted using Spearman's
rank correlation. Group A, 10 mg/kg azoxymethane and 3% dextran
sodium sulfate water; AIF, acute inflammation; CIF, chronic
inflammation; TLR9, Toll-like receptor 9; IHC,
immunohistochemistry. |
The positive NF-κB region was mainly confined to the
cytoplasm of the IECs and inflammatory cells (Fig. 3A). The IHC results revealed that the
expression levels of NF-κB were significantly upregulated in the
AIF, CIF, adenoma and adenocarcinoma tissues compared with the
corresponding tissues from control mice (P=0.0061, P=0.0043,
P=0.0019 and P<0.0001, respectively; Fig. 3D). Moreover, NF-κB expression levels
were significantly upregulated in the adenocarcinoma tissue
compared with the AIF (P<0.0001) and adenoma (P=0.0161) tissues
(Fig. 3D).
Ki67 expression levels were observed in the nuclei
of IECs and inflammatory cells (Fig.
3A). The IHC results revealed that the Ki67 expression levels
were gradually upregulated across the intestinal lesions, including
in the AIF, CIF, adenoma and adenocarcinoma tissues (P=0.0331,
P=0.0092, P=0.0241 and P=0.0006, respectively) compared with the
expression levels in the corresponding tissues from the control
mice (Fig. 3C). Interestingly, the
expression levels of TLR9 and NF-κB were discovered to be
significantly positively correlated with other (rho=0.8236;
P<0.0001; Fig. 3E). In addition,
a significant positive correlation was also identified between TLR9
and Ki67 expression levels (rho=0.5515; P<0.001; Fig. 3F) and between NF-κB and Ki67
expression levels (rho=0.5103; P<0.01; Fig. 3G).
Downregulated TLR9 expression levels
reduces the migration, viability and colony formation of HT29
cells
To further investigate the role of TLR9 in CRC, the
human CRC cell line HT29 was treated with chloroquine (an inhibitor
of TLR9) in vitro. The results revealed that suppressing
TLR9 with chloroquine (at both doses) inhibited the migration,
viability and colony formation ability of HT29 cells in a
dose-dependent manner (Fig. 4A-E).
Additionally, lysates were collected from HT29 cells treated with
either different concentrations of chloroquine for 72 h or 25 µg/ml
chloroquine for different time periods (Fig. 4F-I) and western blotting was
performed. The analysis revealed that the expression levels of
TLR9, NF-κB, PCNA, MyD88 and Bcl-xl were gradually downregulated in
HT29 cells treated with increasing doses of chloroquine compared
with the control group (Fig. 4F and
G). A similar trend was observed in the expression levels of
these proteins as the duration of chloroquine treatment increased
(Fig. 4H and I). Thus, these results
indicated that the expression levels of TLR9, NF-κB, PCNA, MyD88
and Bcl-xl in HT29 cells treated with chloroquine may be
downregulated in both a dose- and time-dependent manner (Fig. 4F-I). To verify whether TLR9 affected
the colorectal carcinogenesis by interacting with NF-κB,
co-immunoprecipitation assay was used to detect the interaction
between TLR9 and NF-κB in HT-29 cells. The results revealed that
there was an interaction between TLR9 and NF-κB (Fig. 4J)
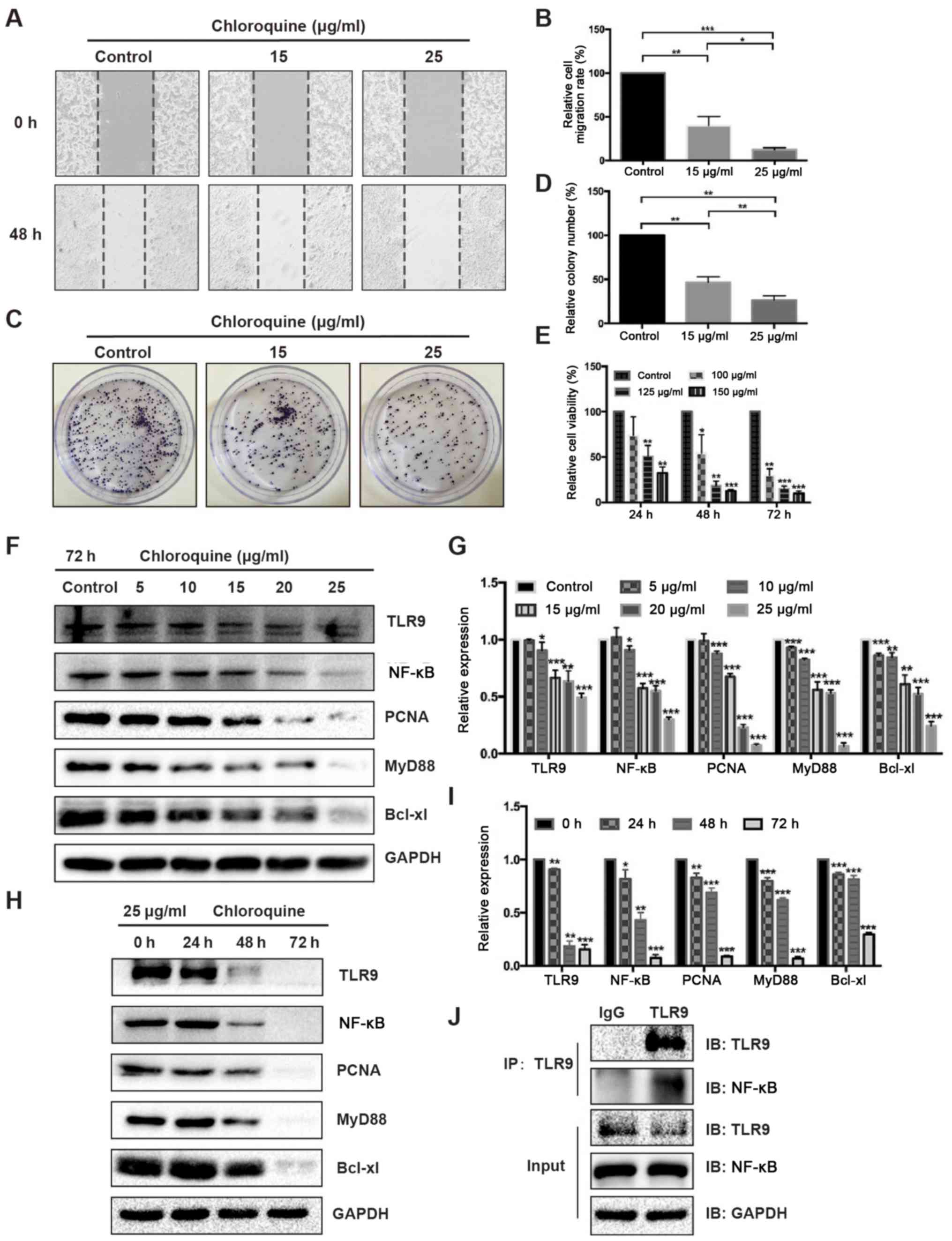 | Figure 4.Chloroquine inhibits the migration,
viability and colony formation of HT29 cells by inhibiting TLR9.
Chloroquine (15 and 25 µg/ml) inhibited the migration of HT29 cells
in a dose-dependent manner at 48 h compared with the control group,
which was determined using a wound healing assay. (A)
Representative images were photographed (magnification ×100) and
(B) migration rates were calculated. Proliferation rate of HT29
cells was reduced by chloroquine treatment (15 and 25 µg/ml), which
was determined using a colony formation assay. (C) Representative
images were photographed and (D) the relative colony number was
analyzed. *P<0.05, **P<0.01, ***P<0.001. (E) Viability of
HT29 cells was decreased by chloroquine (100, 125 and 150 µg/ml) at
24, 48 and 72 h compared with the control cells, as determined
using an MTT assay. (F) Expression levels of TLR9, NF-κB, PCNA,
MyD88 and Bcl-xl in lysates obtained from HT29 cells treated with
numerous concentrations of chloroquine (0, 5, 10, 15, 20 or 25
µg/ml) for 72 h were analyzed using western blotting. (G)
Semi-quantification of the expression levels of the proteins
presented in part (F) was performed using ImageJ software. GAPDH
was used as the loading control and for normalization. (H)
Expression levels of TLR9, NF-κB, PCNA, MyD88 and Bcl-xl in lysates
obtained from HT29 cells treated with 25 µg/ml chloroquine for
numerous durations (0, 24, 48 or 72 h) were analyzed using western
blotting. (I) Semi-quantification of the expression levels of the
proteins presented in part (H) was performed using ImageJ software.
GAPDH was used as the loading control and for normalization. (J)
TLR9 interacted with the NF-κB protein in HT29 cells, which was
determined using a co-immunoprecipitation assay. Goat anti-mouse
IgG antibody was used as the negative control. All data are
expressed as the mean ± SD of three independent experiments.
*P<0.05, **P<0.01, ***P<0.001 vs. control/0 h. TLR9,
Toll-like receptor 9; PCNA, proliferating cell nuclear antigen;
MyD88, myeloid differentiation primary response protein MyD88; IP,
immunoprecipitated; IB, immunoblotting. |
Discussion
Colorectal carcinogenesis is a multi-step process,
starting from normal crypts to aberrant crypt foci, then to polyps,
adenoma and eventually adenocarcinoma (37,38). It
has been reported that individuals with IBD may have an increased
risk of developing CRC, which is directly proportional to the
extent and duration of their disease (39,40).
However, the exact mechanism and duration required for chronic
colitis to develop into adenoma and then adenocarcinoma remains
unclear (40). It has been suggested
that patients with IBD have an increased risk of CRC following the
inflammation-dysplasia-carcinoma model (41), including dysplasia and CRC as primary
consequences of chronic inflammation. However, there are currently
still no defined molecular biomarkers or existing monitoring
protocols for detecting the occurrence of a malignant tumor, except
for frequent colonoscopy examinations.
In the present study, an acute colitis-chronic
colitis-adenoma-adenocarcinoma model was successfully constructed
via AOM/DSS induction. Using this model, TLR9 expression levels
were discovered to be upregulated as the severity of the colorectal
lesions increased, which indicated that TLR9 protein expression
levels may be continuously activated during colitis-CRC
development. TLR9 is a critical protein associated with innate and
acquired immunity (42), and it has
been demonstrated to serve a significant role in the development of
colitis (11,43) and sporadic CRC (12,13).
However, the mechanism by which TLR9 regulates the development of
CRC remains to be elucidated.
Interestingly, IHC analysis revealed that the
expression levels of NF-κB and Ki67 were simultaneously upregulated
alongside TLR9 expression levels. Notably, inhibiting TLR9
decreased the migration, proliferation and viability of HT29 cells
in vitro, and TLR9 expression levels in vivo were
identified to be significantly positively correlated with the
expression levels of NF-κB and Ki67 (a cell proliferation marker)
during the transition from colitis to CRC. The present study
further revealed that the inhibition of TLR9 in vitro
significantly downregulated the expression levels of NF-κB, MyD88,
PCNA and the anti-apoptotic protein Bcl-xl in a dose- and
time-dependent manner. Notably, a previous study reported that TLR9
promoted the tumor-propagating potential of prostate cancer cells
via NF-κB signaling (22). Thus, the
findings of the present study indicated that TLR9 may promote CAC
through NF-κB signaling. However, these findings may be
controversial because other previous studies have revealed that
TLR9 agonists exerted an antitumor effect in CRC (14,15,44,45). The
majority of these studies primarily focused on the role of TLR9 in
colitis or sporadic CRC, whereas the current study focused on the
role of TLR9 in the pathogenesis of CAC to provide novel targets
for the treatment of CAC. For example, alterations in microtubule
end-binding protein 1 were identified as a characteristic of
sporadic, but not UC-associated CRC (46), and in another previous study, the
immune profiling patterns of patients with CAC were significantly
different compared with the patients with sporadic CRC (47).
Chloroquine, a non-specific inhibitor of TLR9, is an
old antimalarial drug (48), which
has recently attracted significant interest for its potential
antitumor properties; for example, numerous studies have reported
that chloroquine directly regulated cancer cells by inducing
apoptosis, inhibiting autophagy, interacting with nucleotides,
eliminating cancer stem cells and enhancing the growth of cancer
cells (49–51). Chloroquine also inhibited the
expression levels of TLR9 by preventing the acidification and
maturation of the endosomes, and the trafficking of TLRs (52). Due to the multiple effects of
chloroquine on tumor cells, different concentrations of chloroquine
were selected for use in the present study based on the lowest dose
according to previous studies (49,53), in
order to obtain the best possible results with low toxicity to the
cells. In the future, investigations using small interfering RNA
targeting TLR9 should be performed to determine the effect on the
biological processes of CRC cell lines to further verify the
findings of the present study. Thus, our future studies will focus
on investigating the precise molecular mechanism by which TLR9
participates in the early occurrence of colorectal
carcinogenesis.
In conclusion, the present study developed a CAC
animal model. The findings indicated that TLR9 may be closely
associated with the process of inflammation-dysplasia-carcinoma and
may impact carcinogenesis by regulating the NF-κB signaling
pathway. These results may provide promising potential to be
developed into an early detection protocol or therapeutic molecular
target for CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81660404
and 81560398), the Foundation of Jiangxi Educational Committee
(grant no. GJJ170016) and the Graduate Student Innovation Funding
Program of Nanchang University (grant no. CX2019119).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article. The original data are available
from the corresponding author on reasonable request.
Authors' contributions
CZ and QL designed the study and drafted the
manuscript. QL and LZ performed the experiments. QL, CT and ZZ
analyzed the data. QL, CZ and YC revised the manuscript for
important intellectual content. CZ and YC made substantial
contributions to conception, design and coordination of the study
and gave final approval of the version to be published. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of The First Affiliated Hospital of Nanchang University
(Nanchang, China; approval no. 2015- 045).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AIF
|
acute inflammation
|
|
AOM
|
azoxymethane
|
|
CAC
|
colitis-associated colorectal
cancer
|
|
CIF
|
chronic inflammation
|
|
CRC
|
colorectal cancer
|
|
DAI
|
disease activity index
|
|
DSS
|
dextran sodium sulfate
|
|
IBD
|
inflammatory bowel disease
|
|
IECs
|
intestinal epithelial cells
|
|
IHC
|
immunohistochemistry
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
TLR9
|
Toll-like receptor 9
|
|
UC
|
ulcerative colitis
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eaden JA, Abrams KR and Mayberry JF: The
risk of colorectal cancer in ulcerative colitis: A meta-analysis.
Gut. 48:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moldoveanu AC, Diculescu M and Braticevici
CF: Cytokines in inflammatory bowel disease. Rom J Intern Med.
53:118–127. 2015.PubMed/NCBI
|
|
5
|
Hemmi H, Takeuchi O, Kawai T, Kaisho T,
Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K and
Akira S: A toll-like receptor recognizes bacterial DNA. Nature.
408:740–745. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krieg AM, Yi AK, Matson S, Waldschmidt TJ,
Bishop GA, Teasdale R, Koretzky GA and Klinman DM: CpG motifs in
bacterial DNA trigger direct B-cell activation. Nature.
374:546–549. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kolumam GA, Thomas S, Thompson LJ, Sprent
J and Murali-Krishna K: Type I interferons act directly on CD8 T
cells to allow clonal expansion and memory formation in response to
viral infection. J Exp Med. 202:637–650. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Havenar-Daughton C, Kolumam GA and
Murali-Krishna K: Cutting Edge: The direct action of type I IFN on
CD4 T cells is critical for sustaining clonal expansion in response
to a viral but not a bacterial infection. J Immunol. 176:3315–3319.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akira S and Takeda K: Toll-Like receptor
signaling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanchez-Munoz F, Fonseca-Camarillo G,
Villeda-Ramirez MA, Miranda-Pérez E, Mendivil EJ, Barreto-Zúñiga R,
Uribe M, Bojalil R, Domínguez-López A and Yamamoto-Furusho JK:
Transcript levels of toll-like receptors 5, 8 and 9 correlate with
inflammatory activity in ulcerative colitis. BMC Gastroenterol.
11:1382011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan Y and Liu B: Expression of Toll-Like
receptors in the mucosa of patients with ulcerative colitis. Exp
Ther Med. 9:1455–1459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eiro N, Gonzalez L, Gonzalez LO,
Andicoechea A, Fernández-Díaz M, Altadill A and Vizoso FJ: Study of
the expression of toll-like receptors in different histological
types of colorectal polyps and their relationship with colorectal
cancer. J Clin Immunol. 32:848–854. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao C, Qiao T, Zhang B, Yuan S, Zhuang X
and Luo Y: TLR9 signaling activation at different stages in
colorectal cancer and NF-kappaB expression. Onco Targets Ther.
11:5963–5971. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nojiri K, Sugimoto K, Shiraki K, Tameda M,
Inagaki Y, Kusagawa S, Ogura S, Tanaka J, Yoneda M, Yamamoto N, et
al: The expression and function of Toll-like receptors 3 and 9 in
human colon carcinoma. Oncol Rep. 29:1737–1743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shahriari S, Rezaeifard S, Moghimi HR,
Khorramizadeh MR and Faghih Z: Cell membrane and intracellular
expression of toll-like receptor 9 (TLR9) in colorectal cancer and
breast cancer cell-lines. Cancer Biomark. 18:375–380. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Capece D, Verzella D, Tessitore A, Alesse
E, Capalbo C and Zazzeroni F: Cancer secretome and inflammation:
The bright and the dark sides of NF-kappaB. Semin Cell Dev Biol.
78:51–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Said AH, Raufman JP and Xie G: The role of
matrix metalloproteinases in colorectal cancer. Cancers. 6:366–375.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue Q, Cao L, Chen XY, Zhao J, Gao L, Li
SZ and Fei Z: High expression of MMP9 in glioma affects cell
proliferation and is associated with patient survival rates. Oncol
Lett. 13:1325–1330. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ha SH, Kwon KM, Park JY, Abekura F, Lee
YC, Chung TW, Ha KT, Chang HW, Cho SH, Kim JK and Kim CH:
Esculentoside H inhibits colon cancer cell migration and growth
through suppression of MMP-9 gene expression via NF-kB signaling
pathway. J Cell Biochem. 120:9810–9819. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu L, Wang C, Wen Z, Yao X, Liu Z, Li Q,
Wu Z, Xu Z, Liang Y and Ren T: Selective up-regulation of CDK2 is
critical for TLR9 signaling stimulated proliferation of human lung
cancer cell. Immunol Lett. 127:93–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moreira D, Zhang Q, Hossain DM, Nechaev S,
Li H, Kowolik CM, D'Apuzzo M, Forman S, Jones J, Pal SK and
Kortylewski M: TLR9 signaling through NF-kappaB/RELA and STAT3
promotes tumor-propagating potential of prostate cancer cells.
Oncotarget. 6:17302–17313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai Y, Huang J, Xing H, Li B, Li L, Wang
X, Peng D and Chen J: Contribution of FPR and TLR9 to
hypoxia-induced chemoresistance of ovarian cancer cells. Onco
Targets Ther. 12:291–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Olbert PJ, Kesch C, Henrici M, Subtil FS,
Honacker A, Hegele A, Hofmann R and Hänze J: TLR4- and
TLR9-dependent effects on cytokines, cell viability, and invasion
in human bladder cancer cells. Urol Oncol. 33:e119–e127. 2015.
View Article : Google Scholar
|
|
25
|
Gao C, Kozlowska A, Nechaev S, Li H, Zhang
Q, Hossain DM, Kowolik CM, Chu P, Swiderski P, Diamond DJ, et al:
TLR9 signaling in the tumor microenvironment initiates cancer
recurrence after radiotherapy. Cancer Res. 73:7211–7221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanaka T: Colorectal carcinogenesis:
Review of human and experimental animal studies. J Carcinog.
8:52009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng CY, Zhan YS, Huang J and Chen YX:
MicroRNA7 suppresses human colon cancer invasion and proliferation
by targeting the expression of focal adhesion kinase. Mol Med Rep.
13:1297–1303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tian Y, Xu Q, Sun L, Ye Y and Ji G:
Short-Chain fatty acids administration is protective in
colitis-associated colorectal cancer development. J Nutr Biochem.
57:103–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tian Y, Wang K, Wang Z, Li N and Ji G:
Chemopreventive effect of dietary glutamine on colitis-associated
colon tumorigenesis in mice. Carcinogenesis. 34:1593–1600. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hudert CA, Weylandt KH, Lu Y, Wang J, Hong
S, Dignass A, Serhan CN and Kang JX: Transgenic mice rich in
endogenous omega-3 fatty acids are protected from colitis. Proc
Natl Acad Sci USA. 103:11276–11281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Truelove SC and Richards WC: Biopsy
studies in ulcerative colitis. Br Med J. 9:1315–1318. 1956.
View Article : Google Scholar
|
|
32
|
Sangfelt P, Carlson M, Thörn M, Lööf L and
Raab Y: Neutrophil and eosinophil granule proteins as markers of
response to local prednisolone treatment in distal ulcerative
colitis and proctitis. Am J Gastroenterol. 96:1085–1090. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeng C, Wang Y, Lu Q, Chen J, Zhang J, Liu
T, Lv N and Luo S: SPOP suppresses tumorigenesis by regulating
Hedgehog/Gli2 signaling pathway in gastric cancer. J Exp Clin
Cancer Res. 33:752014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scharl A, Vierbuchen M, Conradt B, Moll W,
Würz H and Bolte A: Immunohistochemical detection of progesterone
receptor in formalin-fixed and paraffin-embedded breast cancer
tissue using a monoclonal antibody. Arch Gynecol Obstet. 247:63–71.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan X, Zhou T, Tai YH, Wang C, Zhao J, Cao
Y, Chen Y, Zhang PJ, Yu M, Zhen C, et al: Elevated expression of
CUEDC2 protein confers endocrine resistance in breast cancer. Nat
Med. 17:708–714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.PubMed/NCBI
|
|
37
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Beaugerie L and Itzkowitz SH: Cancers
complicating inflammatory bowel disease. N Engl J Med.
372:1441–1452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Terzić J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology. 138:2101–2114.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Itzkowitz SH and Yio X: Inflammation and
cancer IV. Colorectal cancer in inflammatory bowel disease: The
role of inflammation. Am J Physiol Gastrointest Liver Physiol.
287:G7–G17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Akira S, Takeda K and Kaisho T: Toll-Like
receptors: Critical proteins linking innate and acquired immunity.
Nat Immunol. 2:675–680. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Atreya R, Bloom S, Scaldaferri F, Gerardi
V, Admyre C, Karlsson Å, Knittel T, Kowalski J, Lukas M, Löfberg R,
et al: Clinical effects of a topically applied toll-like receptor 9
agonist in active moderate-to-severe ulcerative colitis. J Crohn's
Colitis. 10:1294–1302. 2016. View Article : Google Scholar
|
|
44
|
Schmoll HJ, Wittig B, Arnold D,
Riera-Knorrenschild J, Nitsche D, Kroening H, Mayer F, Andel J,
Ziebermayr R and Scheithauer W: Maintenance treatment with the
immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in
patients with metastatic colorectal carcinoma and disease control
after chemotherapy: A randomised, double-blind, placebo-controlled
trial. J Cancer Res Clin Oncol. 140:1615–1624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dong T, Yi T, Yang M, Lin S, Li W, Xu X,
Hu J, Jia L, Hong X and Niu W: Co-Operation of
alpha-galactosylceramide-loaded tumour cells and TLR9 agonists
induce potent anti-tumour responses in a murine colon cancer model.
Biochem J. 473:7–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gemoll T, Kollbeck SL, Karstens KF, Hò GG,
Hartwig S, Strohkamp S, Schillo K, Thorns C, Oberländer M, Kalies
K, et al: EB1 protein alteration characterizes sporadic but not
ulcerative colitis associated colorectal cancer. Oncotarget.
8:54939–54950. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Soh JS, Jo SI, Lee H, Do EJ, Hwang SW,
Park SH, Ye BD, Byeon JS, Yang SK, Kim JH, et al: Immunoprofiling
of colitis-associated and sporadic colorectal cancer and its
clinical significance. Sci Rep. 9:68332019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kuznik A, Bencina M, Svajger U, Jeras M,
Rozman B and Jerala R: Mechanism of endosomal TLR inhibition by
antimalarial drugs and imidazoquinolines. J Immunol. 186:4794–4804.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pascolo S: Time to use a dose of
chloroquine as an adjuvant to anti-cancer chemotherapies. Eur J
Pharmacol. 771:139–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Maes H, Kuchnio A, Carmeliet P and
Agostinis P: Chloroquine anticancer activity is mediated by
autophagy-independent effects on the tumor vasculature. Mol Cell
Oncol. 3:e9700972016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin YC, Lin JF, Wen SI, Yang SC, Tsai TF,
Chen HE, Chou KY and Hwang TI: Chloroquine and hydroxychloroquine
inhibit bladder cancer cell growth by targeting basal autophagy and
enhancing apoptosis. Kaohsiung J Med Sci. 33:215–223. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mohamed FE, Al-Jehani RM, Minogue SS,
Andreola F, Winstanley A, Damink SW, Habtesion A, Malagó M, Davies
N, Luong TV, et al: Effect of toll-like receptor 7 and 9 targeted
therapy to prevent the development of hepatocellular carcinoma.
Liver Int. 35:1063–1076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang Y, Li Y, Li Y, Ma Y, Wang H and Wang
Y: Chloroquine inhibits MGC803 gastric cancer cell migration via
the Toll-like receptor 9/nuclear factor kappa B signaling pathway.
Mol Med Rep. 11:1366–1371. 2015. View Article : Google Scholar : PubMed/NCBI
|