Introduction
Glioma is a type of brain tumor with very high
recurrence and mortality rates (1).
According to the American Cancer Society, 77% of patients with
glioblastoma die within 1 year of diagnosis (2). Gliomas are mainly caused by mutations
in normal interstitial cells. The recurrence rate of glioma is
positively associated with the degree of malignancy (3). With the continuous development of
medical technology, the treatment of glioma is improving. Surgical
resection continues to be the principal treatment for glioma, but
is only applicable in patients with early-stage glioma (4,5). The
tumors of patients with early-stage gliomas are small, well-defined
and easily removed surgically (4,5).
However, surgical resection is not an ideal treatment option for
patients with malignant glioma because malignant gliomas are very
large and present invasive growth (6). In recent years, the use of microscopy
during surgery has enabled invasive gliomas to be resected more
effectively (7). Radiation therapy
is considered an important treatment option for various types of
tumors, such as glioma, renal cell carcinoma and bladder cancer
(8–10). A series of clinical studies have
confirmed that radiotherapy can improve the survival rate of
patients with glioma; however, radiotherapy is associated with
numerous adverse reactions and side effects (11–13).
Therefore, it remains important to identify potential drugs for the
effective treatment of glioma.
15,16-Dihydrotanshinone I (DHI) is extracted from
tanshen (Salvia miltiorrhiza bunge). Previous studies have
shown that tanshen has various biological uses, which include the
treatment of cardiovascular diseases, especially angina pectoris
and myocardial infarction (14,15). Lee
and Lee (16) reported that DHI is
able to inhibit the proliferation and induce the apoptosis of K562
leukemia cells. DHI has also been shown to inhibit breast and colon
cancer through the mitochondrial apoptosis pathway (17,18).
Furthermore, DHI inhibits the proliferation of gastric cancer cells
through the c-Jun N-terminal kinase/P38 signaling pathway (19). However, the effect of DHI on glioma
cells and the underlying mechanisms have not yet been elucidated.
The present study explored the antitumor effect and mechanism of
DHI in gliomas.
Ferroptosis is a type of programmed cell death
(20). The main morphological
features of ferroptosis are shrinkage of the mitochondria and a
reduction in the number of mitochondrial ridges (20). Ferroptosis of tumor cells, such as
pancreatic and liver cancer cells, and normal tissue cells,
including renal tubular cells and fibroblasts, can be induced by
some small molecules and common clinical drugs, including
vincristine, sorafenib and artemisinin (21–23).
Ferroptosis is predominantly caused by the accumulation of
intracellular lipid peroxides and the release of reactive oxygen
species (ROS), which are closely associated with the accumulation
of large amounts of iron ions in the cell. The iron chelator
deferoxamine and the ferroptosis-specific inhibitors ferrostatin-1
or ferrostatin-1 are able to inhibit the ferroptosis process
(24). The metabolism of iron ions
and lipid peroxides is considered to be critical in the process of
ferroptosis and to regulate its occurrence (25). Ferroptosis plays an important role in
the development of tumors. The activation of ferroptosis has great
therapeutic potential for tumors. Although the main regulatory
network of ferroptosis has been established (26,27),
drugs that have been reported to regulate ferroptosis are limited
in number and their exact regulatory mechanisms are unclear
(28–30). Therefore, the identification of new
drugs for ferroptosis regulation would be of great value.
A previous study indicated that tanshen induced
ferroptosis in breast cancer cells, and significantly reduced the
final tumor volume in a xenograft nude mouse model without adverse
effects (31). Therefore, the
present study aimed to evaluate the effects of DHI on the
proliferation of glioma cells and investigate the contribution of
ferroptosis to the underlying mechanism.
Materials and methods
Chemicals and treatment groups
DHI was obtained from ChemFaces Natural Products
Co., Ltd. (cat. no. CFN-90162; purity, 98%; solubility in DMSO,
>5 mg/ml; PubChem CID: 11425923). HEB, U87 and U251 cells were
treated with the DHI and/or the ferroptosis inhibitor
ferrostatin-1, or with an equivalent volume of DMSO for 72 h at
37°C with 5% CO2. The cells were divided into four
groups as follows: i) Control group, cells treated with DMSO; ii)
DHI group, cells treated with DHI (1, 10, 100 or 1,000 µM); iii)
ferroptosis inhibitor-control group, cells treated with
ferrostatin-1 (1 µM); iv) DHI + ferroptosis inhibitor group, cells
treated with DHI (100 µM) and ferrostatin-1 (1 µM). Ferrostatin-1
was purchased from Santa Cruz Biotechnology, Inc.
Cell culture
The HEB human glial cell line was purchased from
Shanghai Bioleaf Biotech Co., Ltd. The U251 human glioblastoma cell
line was purchased from the European Collection of Authenticated
Cell Cultures (cat. no. 09063001) and the U87 human glioblastoma
cell line of unknown origin was purchased from American Type
Culture Collection (ATCC; catalogue number: HTB-14). All the cell
lines were identified by STR. All cells were cultured in DMEM
(HyClone; GE Healthcare Lifesciences) supplemented with 10% fetal
calf serum (Invitrogen; Thermo Fisher Scientific, Inc.) and
penicillin-streptomycin combination (100 mg/ml; Thermo Fisher
Scientific, Inc.), and maintained at 37°C in a 5%
CO2-humidified incubator.
Cell proliferation analysis
Cell proliferation was detected by Cell Counting
Kit-8 (CCK-8) assay (cat. no. C0037; Beyotime Institute of
Biotechnology). The HEB, U251 and U87 cells were inoculated on a
96-well plate at a density of 5,000 cells/well and treated as
described above. Cell proliferation was detected at specified time
points (0, 24, 48 and 72 h) using a CellTiter 96®
AQueous One Solution Cell Proliferation Assay kit (Promega
Corporation). The absorbance was measured at 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
Western blot analysis
Total protein was extracted from HEB, U251 and U87
cells using the M-PER™ Mammalian Protein Extraction Reagent
(Pierce; Thermo Fisher Scientific, Inc.). The protein concentration
of the extract was determined using the BCA method. Equal amounts
of protein extract and 2X SDS loading buffer were mixed and boiled
for 5 min. Proteins (30 µg/lane) were separated via SDS-PAGE on a
10% gel and then transferred to polyvinylidene difluoride membranes
(Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.). The membranes
were blocked with 5% skimmed milk at room temperature for 2 h and
then incubated with primary antibodies against glutathione
peroxidase 4 (GPX4; 1:3,000; cat. no. sc-166570; Santa Cruz
Biotechnology, Inc.), long-chain-fatty-acid-CoA ligase 4 (ACSL-4;
1:2,000; cat. no. sc-365230; Santa Cruz, USA) and anti-β-actin
(1:500; cat. no. SA00001-9; ProteinTech Group Inc.) overnight at
4°C. Following primary antibody incubation, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:1,000; cat. no. SA00001-9; ProteinTech Group, Inc.)
at room temperature for 3 h. Protein bands were observed using
Amersham ECL Prime Western Blotting Detection Reagent (GE
Healthcare Life Sciences) and scanned using Amersham Imager 600 (GE
Healthcare).
5-Hydroxyeicosatetraenoic acid (HETE)
assay
5-HETE, a ferroptotic marker, was assessed using a
5-HETE ELISA kit (cat. no. CED739Ge; Wuhan USCN Business Co.,
Ltd.), according to the manufacturer's protocol.
12/15-HETE assay
The levels of 12- and 15-HETE, which are two
ferroptotic markers, were determined using 12/15-HETE ELISA kits
(cat. nos. ab133034 and ab133035; Abcam), according to the
manufacturer's protocols.
Lactate dehydrogenase (LDH) assay
LDH is a cytoplasmic enzyme that is released from
cells when they are damaged (32).
LDH levels were measured using a colorimetric CytoTox 96™
Cytotoxicity kit (cat. no. G1780; Promega Corporation) according to
the manufacturer's protocol. Cells were treated with 10× lysis
reagent to determine the maximum LDH release level. A 96-well plate
reader (Molecular Devices, LLC) was used to measure the absorbance
at 490 nm. According to the manufacturer's instructions (33), the ratio of LDH release was
calculated as the ratio of experimental LDH release to maximum LDH
release.
Determination of the reduced
glutathione (GSH)/oxidized glutathione (GSSG) ratio and
malondialdehyde (MDA) levels
The levels of GSH and MDA in the cell extracts were
investigated as described previously (34). The cellular extracts of the human
glioma cell lines treated with DMSO, DHI (100 µM) and/or
ferrostatin-1 (1 µM) at 37°C for 24 h were prepared according to
the manufacturer's instructions. MDA levels were detected using a
Lipid Peroxidation (MDA) assay kit (cat. no. K739-100; BioVision,
Inc.) according to the manufacturer's instructions. The GSH/GSSG
ratio was detected using a Glutathione assay kit (cat. no.
K264-100; BioVision, Inc.) according to the manufacturer's
instructions.
Mitochondrial membrane potential (MMP)
assay
The MMP of the cells was assayed using
tetramethylrhodamine methyl ester (TMRM) according to the
manufacturer's instructions (AAT Bioquest, Inc.). TMRM is readily
sequestered by healthy mitochondria, but its fluorescence is
rapidly lost when the MMP dissipates. In total, 1×106
cells were seeded in confocal dishes before treatment with DHI (100
µM) with or without ferrostatin-1 (1 µM) at 37°C for 24 h. The
cells were washed twice with PBS, incubated for 20 min at 37°C with
100 nM TMRM to stain the mitochondria and then carefully washed
three times with PBS. Then, they were washed with PBS before
nuclear staining with 4,6-diamidino-2-phenylindole (DAPI) (cat. no.
28718-90-3; Beyotime Institute of Biotechnology) for 15 min at room
temperature. Finally, the cells were analyzed by confocal
fluorescence microscopy (Nikon N-SIM; Nikon Corporation) to assess
the intensity of red fluorescence (excitation at 549 nm; emission
at 573 nm).
Statistical analysis
All data are presented as means ± standard
deviation. Each experiment was repeated three times. SPSS 22.0
software (IBM Corp.) was used for the statistical analysis.
Two-tailed unpaired t-tests were used to compare two groups, while
one- or two-way analysis of variance (ANOVA) was used to compare
three or more groups. Pairwise group comparisons were conducted
using Tukey's test as a post hoc test following ANOVA. P<0.05
was considered to indicate a significant result.
Results
Ferroptosis levels are reduced in
human glioma cells
Ferroptosis has been shown to be involved in the
pathogenesis of gliomas (35).
However, the relevant research is very limited. Therefore, in the
present study, ferroptosis in human glioma cells was assessed by
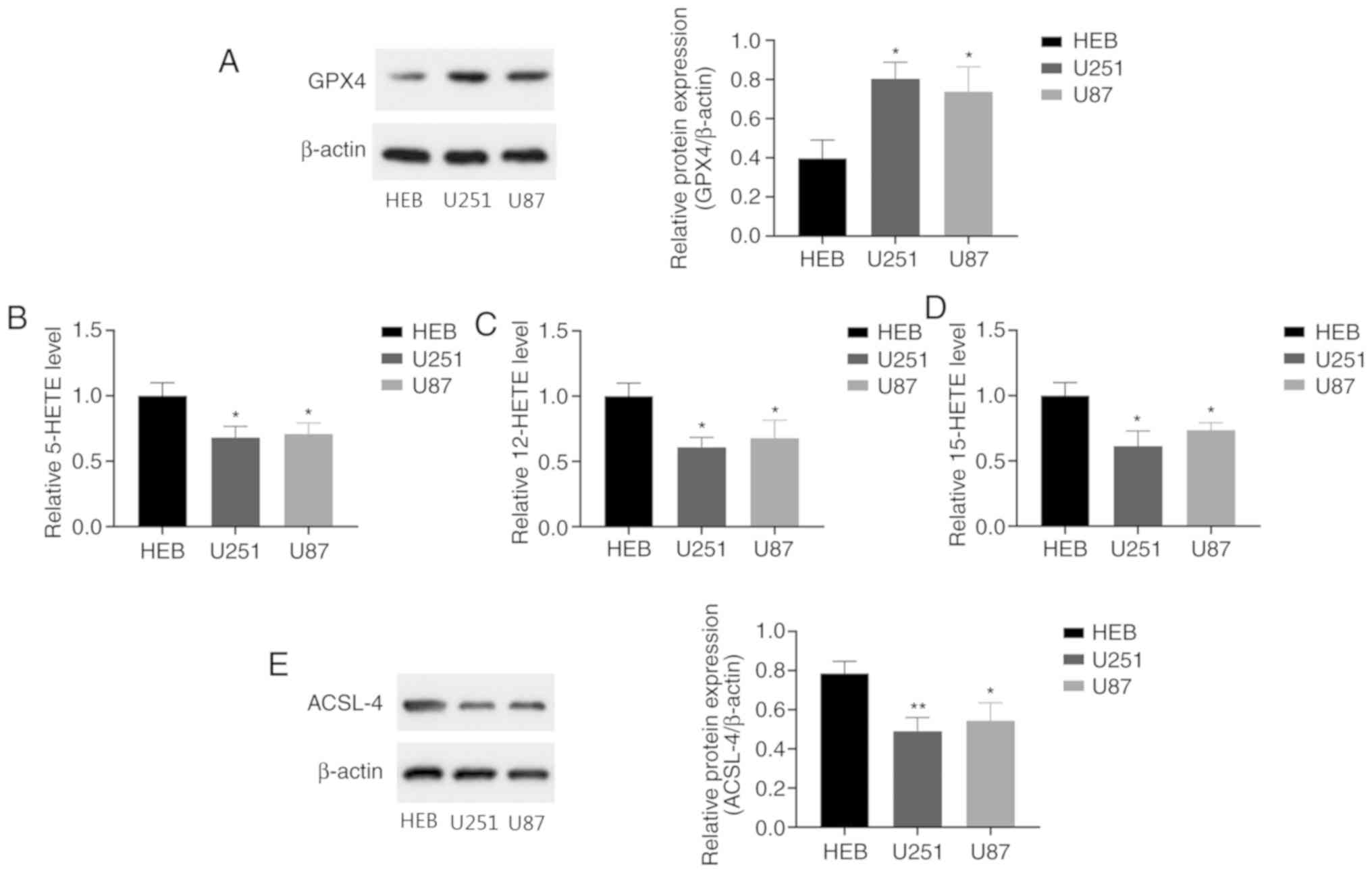
measuring the levels of GPX4, 5-, 12- and 15-HETE. GPX4 is an
important membrane lipid peroxidase reductase, and the
downregulation of GPX4 causes the accumulation of reactive oxygen
species (ROS) on membrane lipids, which leads to ferroptosis
(36). The latter three are lipid
peroxidation products that are associated with the deposition of
ferritin in ferroptosis, and recognized as indicators of
ferroptosis levels (20,26). Western blotting was performed to
detect GPX4 expression in human glioma cells. The results revealed
that GPX4 levels were significantly higher in human glioma cells
compared with normal human glial cells (Fig. 1A). ELISAs were performed to evaluate
the levels of 5-, 12- and 15-HETE in the cells. The 5-, 12- and
15-HETE levels were significantly decreased in the glioma cells
compared with the control glial cells (Fig. 1B-D). These results demonstrate that
U251 and U87 human glioma cells have higher levels of GPX4 and
lower 5-, 12- and 15-HETE levels than normal human glial cells.
ACSL-4 is considered to be a key regulatory gene for
ferroptosis. Previous studies have shown that ACSL-4 overexpression
upregulates ferroptosis levels in gliomas (37). Compared with normal human glial
cells, the human glioma cells exhibited significantly lower ACSL-4
levels (Fig. 1E). The reduction in
ACSL-4 expression may be associated with dysfunctional ferroptosis
in glioma. These results indicate that ferroptosis levels are
reduced in glioma cells.
DHI inhibits the proliferation of
human glioma cells
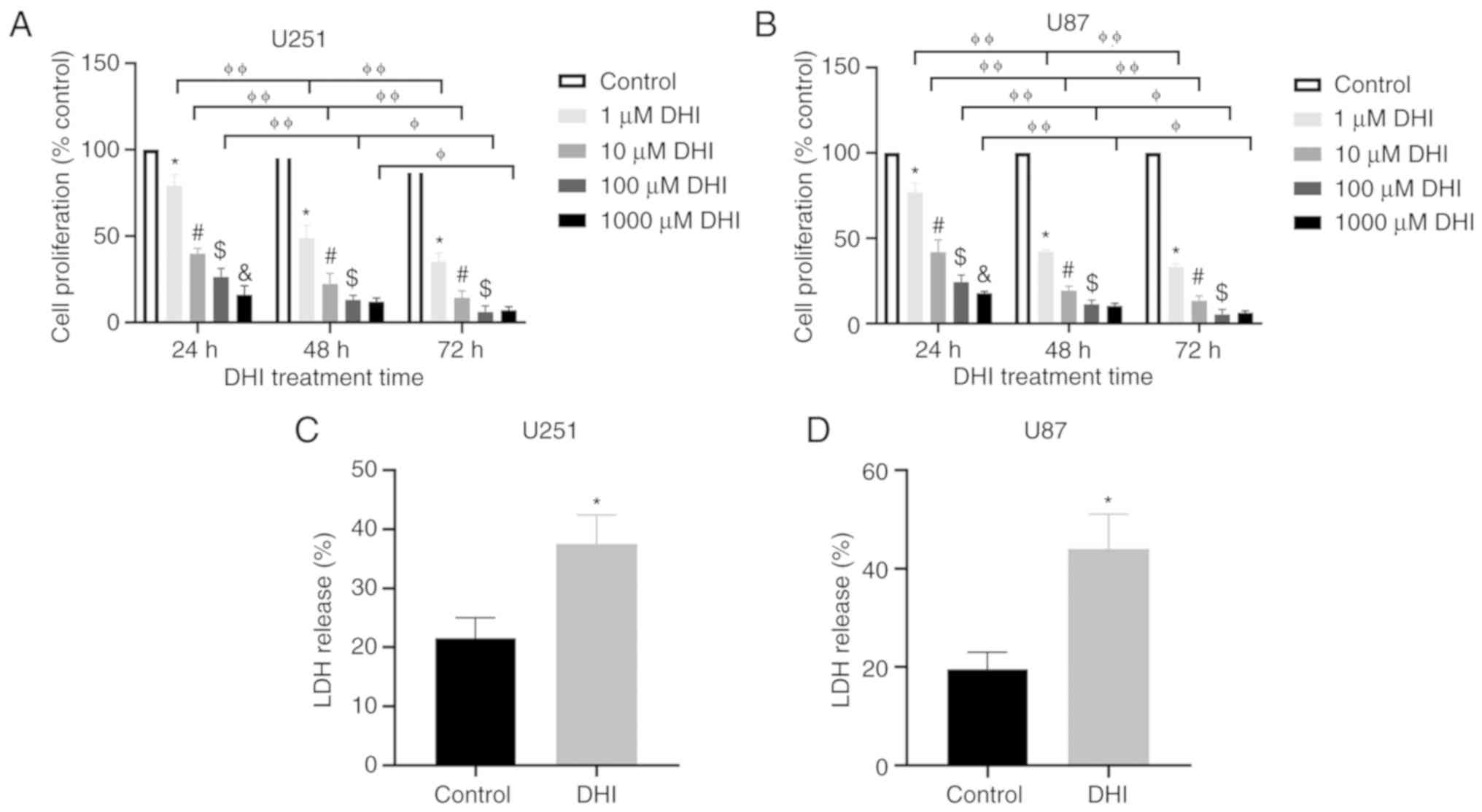
To investigate the effect of DHI on glioma cells,
two glioma cell lines were treated with different concentrations of
DHI for 24, 48 and 72 h. The CCK8 assay was used to measure cell
proliferation. The cell viability of the U251 and U87 glioma cell
lines was significantly reduced after treatment with DHI (Fig. 2A and B). Fig. 2A shows that DHI had significant time-
and dose-dependent effects on U251 glioma cells. The inhibitory
effect on cell growth increased as the treatment time increased. At
each time point, the inhibition rates of higher concentrations of
DHI were higher than those of lower concentrations of DHI in the
majority of cases. However, no significant difference was observed
between the antiproliferative effects of 100 and 1,000 µM DHI
following treatment for either 48 or 72 h. Therefore, DHI at a
concentration of 100 µM was used for subsequent experiments. When
the experiment was repeated using U87 cells, similar results to
those in U251 cells were obtained (Fig.
2B).
To test whether DHI affects the cytotoxicity of
human glioma cells, the levels of LDH in the cells were examined.
The more LDH is released, the more severe the cell damage, which
results in a reduction in cell proliferation. Following DHI
treatment, the release of LDH increased significantly (Fig. 2C and D). These data indicate that DHI
reduces the viability of human glioma cells.
DHI induces ferroptosis in human
glioma cells by downregulating GPX4 and upregulating ACSL-4
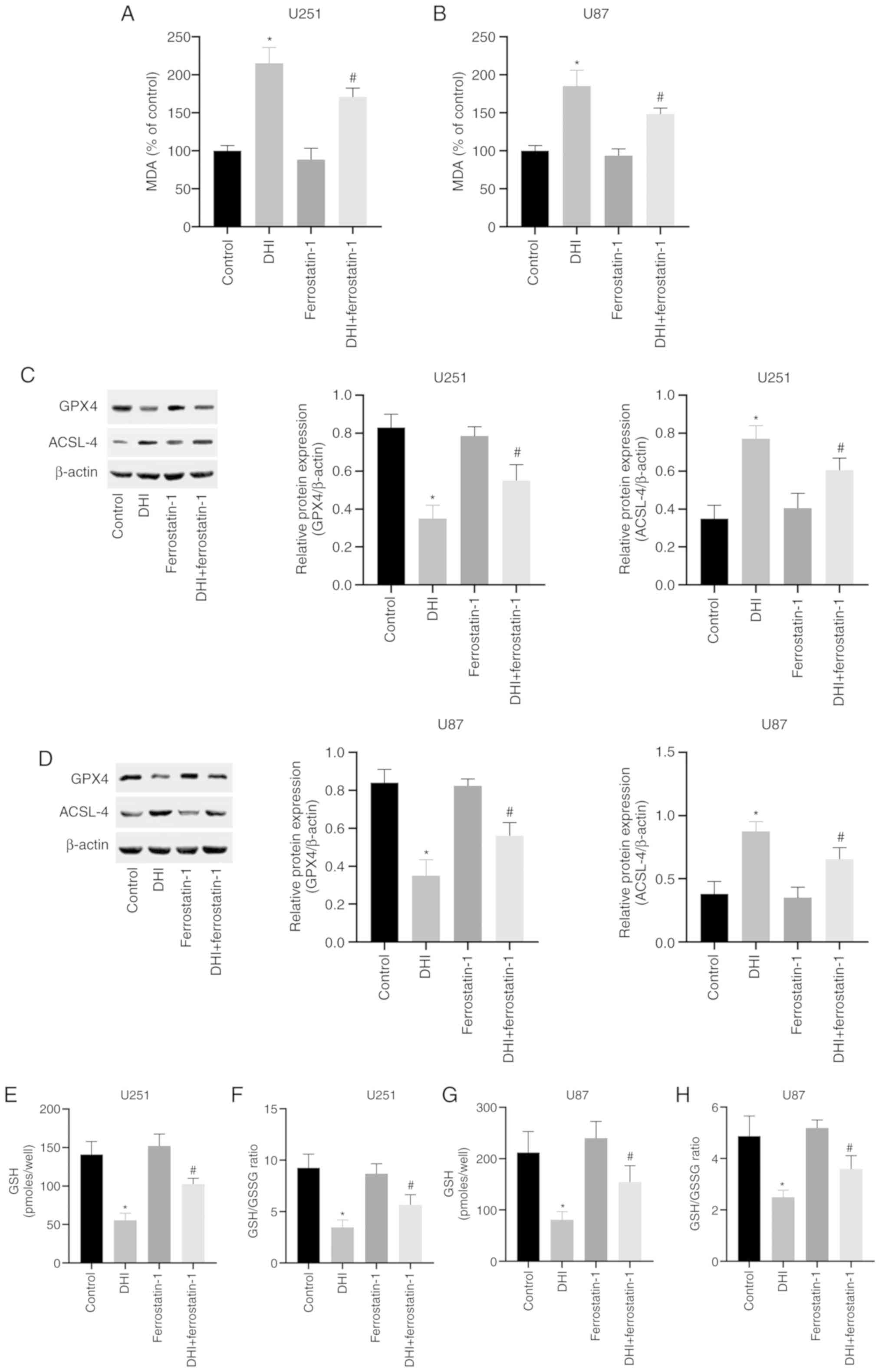
To evaluate whether the inhibition of cell
proliferation by DHI is associated with ferroptosis, the mechanism
of action of DHI in human glioma cells was investigated. Lipid
peroxidation is a key process during ferroptosis (38). MDA is a natural product of lipid
peroxidation that is often used as a marker for lipid peroxidation.
The results of the present study indicate that following treatment
with 100 µM DHI for 24 h, the MDA levels of the U251 and U87 cells
were significantly increased (Fig. 3A
and B).
GSH and GSSG constitute an important cellular
antioxidant system that provides a reducing environment for the
reduction of oxidative substances. GPX4 is an important regulator
of ferroptosis, the absence of which causes a sharp increase in
GSSG, leading to a reduction in the GSH/GSSG ratio (39) GSH and GSSG constitute a critical
cellular antioxidant system and provide a reducing environment to
reduce oxidative species. The loss of GPX4 activity can cause a
drastic increase in GSSG, leading to a decrease in the GSH/GSSG
ratio and ultimately ferroptosis (30). In the present study, it was found
that after 24 h of DHI treatment, intracellular GPX4 expression in
human glioma cells was significantly inhibited (Fig. 3C and D). Notably, DHI also caused
significant reductions in GSH levels and the GSH/GSSG ratio
(Fig. 3E-H). In addition, DHI
significantly upregulated ACSL-4 expression in human glioma cells
(Fig. 3C and D). These results
indicate that ferroptosis is one of the mechanisms by which DHI
induces cell death in human glioma cells.
DHI induces a reduction in the MMP of
human glioma cells
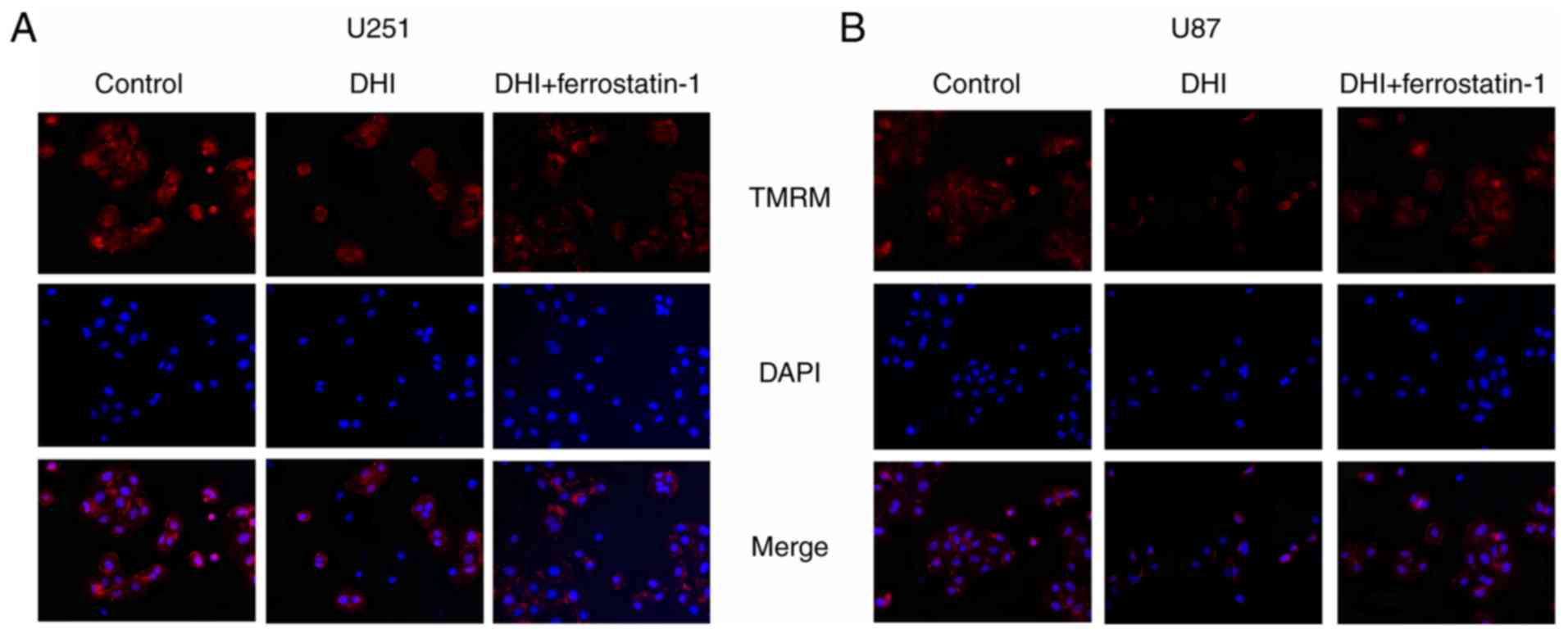
The probe TMRM was used to detect the MMP of the
glioma cells. TMRM accumulates in the mitochondria of living cells
and produces bright red fluorescence. DAPI makes cells produce blue
fluorescence in order to locate cells. Confocal microscopy of
TMRM-stained cells revealed that glioma cells treated with DHI
showed reduced red fluorescence compared with that of untreated
cells. These results indicate that DHI induces a reduction in the
MMP of glioma cells (Fig. 4).
Anticancer effect of DHI on human
glioma cells can be blocked by ferroptosis inhibitors
Ferrostatin-1 is a ferroptosis inhibitor that
reduces the accumulation of intracellular ROS and cell death. A
previous study demonstrated that ferrostatin-1 is able to inhibit
the induction of ferroptosis in vitro (40). The potent inhibition of ferroptosis
by ferrostatin-1 has been ascribed to its ability to slow the
accumulation of lipid hydroperoxides (41). Ferrostatin-1 was used to determine
whether DHI can induce ferroptosis in human glioma cells in the
present study. The western blotting results indicate that
ferrostatin-1 inhibited the DHI-induced reductions in GPX4
expression levels and increases in ACSL-4 expression levels in U251
and U87 cells (Fig. 3C and D).
Furthermore, ferrostatin-1 attenuated the increases in MDA levels
(Fig. 3A and B) and reductions in
GSH levels and GSH/GSSG ratio that were induced by DHI (Fig. 3E-H). Also, confocal microscopy of
TMRM-stained cells revealed that glioma cells treated with DHI and
ferrostatin-1 exhibited increased red fluorescence compared with
that of glioma cells treated with DHI alone.
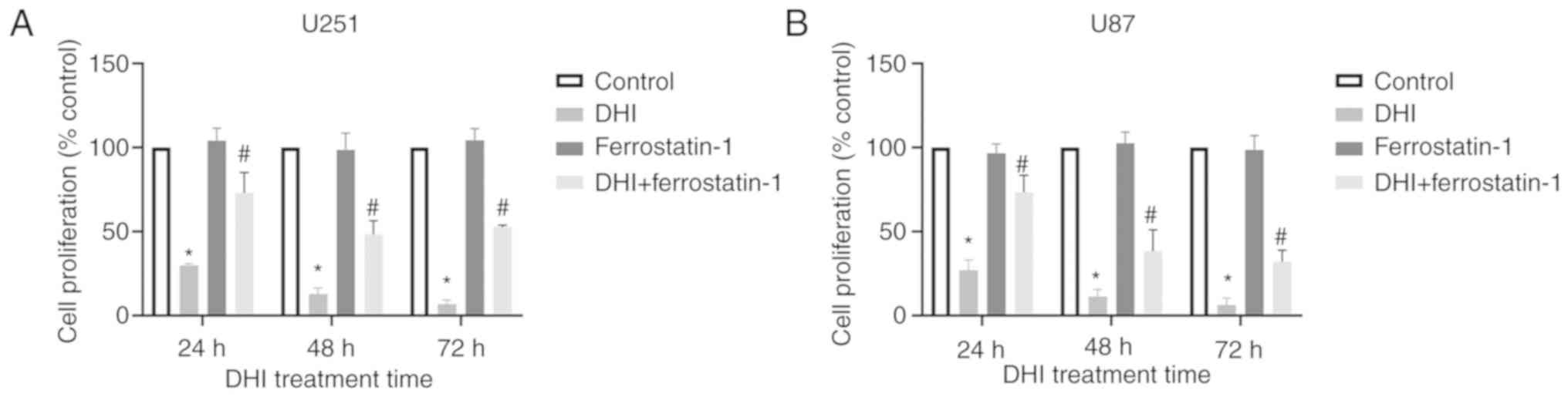
The effect of ferrostatin-1 on the proliferation of
human glioma cells was evaluated using the CCK-8 assay. The results
revealed that ferrostatin-1 increased the proliferative activity of
DHI-treated human glioma cells compared with that of cells treated
with DHI alone (Fig. 5). Therefore,
it may be concluded that ferrostatin-1 blocks the inhibitory effect
of DHI on human glioma cell proliferation to a certain extent.
Discussion
Increasing attention is being paid to the antitumor
activity of natural substances, a number of which are being
developed into antitumor drugs. Tanshen is one of the most commonly
used natural medicines in clinical practice. It has been reported
to have cardiovascular effects, including increased coronary blood
flow, the expansion of coronary arteries, and prevention of
myocardial infarction and myocardial ischemia, as well as being
used to treat gastric cancer, liver cancer, leukemia and cervical
cancer (42,43). Tanshinones are the main components of
tanshen, and tanshinone IIA is one of the most abundant
tanshinones. Therefore, previous research has focused on the
antitumor activity of tanshinone IIA (44,45).
However, there are few studies on other single agents that may be
isolated from tanshen, including DHI, tanshinone I and
cryptotanshinone (CPT). Liu et al (46) explored the inhibitory effect of
tanshinones on lung cancer cells, and Mosaddik (47) investigated the inhibitory effects of
tanshinones on leukemia cells. These studies demonstrated that the
inhibitory effect of DHI on tumor cells was stronger than that of
tanshinone IIA, tanshinone I and CPT. DHI is easy to extract, has
low toxicity against normal cells and is a promising natural
antitumor drug (48). A number of
studies have reported the antitumor mechanism of DHI (49–51). A
previous study revealed that DHI exerts antitumor effects by
inhibiting the proliferation of cancer cells, inducing cancer cell
apoptosis and promoting differentiation (52). However, there are relatively few
studies on the effect of DHI on glioma.
Gliomas are the malignant tumors with the highest
incidence in the central nervous system in adults (53). Due to substantial heterogeneity and
invasive growth characteristics, glioma is very difficult to cure
completely (54). As research has
advanced, new therapies such as immunotherapy and gene therapy have
been developed but the survival time of glioma patients has not
been significantly prolonged (55,56).
Therefore, it is necessary to find new and effective drugs for the
targeted treatment of gliomas. The main aim of the present study
was to explore the mechanism by which DHI inhibits human glioma
cell proliferation and promotes human glioma cell death.
In the present study, the human glioma cell lines
U251 and U87 were used to investigate the effect of DHI on the
proliferation of human glioma cells and its mechanism. The results
of the CCK8 assay showed that the proliferation of human glioma
cells was significantly inhibited following treatment with DHI for
24, 48 and 78 h; the antiproliferative effect was time- and
concentration-dependent
Ferroptosis is a mode of iron-dependent
cell-regulated death. It is characterized by the abnormal
accumulation of iron-dependent lipid peroxides (lipid ROS) in
cells, which disrupts the redox balance and ultimately leads to
cell death (57). These processes
are caused by the loss of intracellular GPX4 activity. The cell
membrane-localized system Xc- is composed of SLC3A2 and SLC7A11
(58). SLC3A2 is a binding protein
of SLC7A11, which takes up cystine and excretes glutamate (58). In cell metabolism, GSH is synthesized
from cystine and is an important antioxidant in the cell (59). It scavenges free radicals and
maintains the redox balance inside and outside the cell (59). SLC7A11 is highly expressed in a
variety of tumors; by increasing cystine uptake it leads to
increased intracellular GPX4 synthesis, reduced intracellular
oxidative stress and the suppression of ferroptosis, thereby
promoting tumor growth (60,61). GPX4 is a GSH-dependent enzyme, and
selenocysteine, which is one of the amino acids in the active
center of GPX4, can be inserted into GPX4 through selenocysteine
transfer RNA (tRNA) (62,63). In addition, the maturation of
selenocysteine tRNA may also be regulated by the mevalonate pathway
to act on GPX4 (62,63). GSH and selenocysteine both regulate
the occurrence of ferroptosis in cells by affecting GPX4. GPX4 has
been recognized as a key target in the induction of ferroptosis by
various agents, including erastin and RSL3 (64). Erastin consumes GSH to inhibit the
activity of GPX4, while RSL3 directly inhibits the activity of GPX4
(64). Therefore, the reduction of
GPX4 activity appears to be a necessary condition for ferroptosis.
The role of ferroptosis in tumorigenesis is becoming a prominent
topic of research, and a number of studies have shown that multiple
drugs are able to induce ferroptosis in tumor cells and affect the
expression of ferroptosis-associated genes (65–67).
Previous studies have found that cisplatin induces ferroptosis in
A549 and HCT116 cells and cause changes in GPX4 expression
(68). Similarly, sorafenib targets
the Xc-system and causes ferroptosis in various cancer cells such
as liver cancer cells (69,70). In addition, sulfasalazine induces
ferroptosis in breast cancer cells, and the mechanism may be
associated with the increased expression of DMT1 Mrna (71). Furthermore, a number of studies have
shown that fatty acid metabolism is essential for maintaining the
microenvironment of malignant tumors and participating in the
occurrence and development of tumors (72,73).
ACSL4 serves a key role in fatty acid metabolism. Previous studies
have shown that ACSL4 activates long-chain unsaturated fatty acids
to participate in the synthesis of membrane phospholipids and
trigger cell ferroptosis (74).
Ferroptosis is associated with tumor suppression and is a potential
therapeutic mechanism for the treatment of tumors. ACSL4 may be
used as an indicator to predict whether cells have the potential to
undergo ferroptosis (37). The
present study confirmed that DHI affects the expression of
ferroptosis-related genes in human glioma cells. After DHI
treatment, the expression of GPX4 in human glioma cells decreased,
while the expression of ACSL-4 increased. However, following
treatment with the ferroptosis inhibitor ferrostatin-1, the
expression of GPX4 in human glioma cells increased, the expression
of ACSL-4 decreased, and the cell survival rate increased
significantly. These results indicate that the effect of DHI on
human glioma cells was mediated by ferroptosis to a certain
extent.
In summary, the results of the present study confirm
that DHI attenuates the proliferation of human glioma cells by
inducing ferroptosis. They also provide a greater understanding of
the mechanism of DHI that may be of benefit in the development of
methods using DHI for the treatment of glioma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LM conceived and designed the study. ST performed
the experiments. ST and XH analyzed the data and wrote the
manuscript. All authors read and approved the final manuscript and
agreed to be accountable for all aspects of the research.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ohtaki S, Wanibuchi M, Kataoka-Sasaki Y,
Sasaki M, Oka S, Noshiro S, Akiyama Y, Mikami T, Mikuni N, Kocsis
JD and Honmou O: ACTC1 as an invasion and prognosis marker in
glioma. J Neurosurg. 126:467–475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohgaki H and Kleihues P: Population-based
studies on incidence, survival rates, and genetic alterations in
astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol.
64:479–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hadjipanayis CG and Van Meir EG: Tumor
initiating cells in malignant gliomas: Biology and implications for
therapy. J Mol Med (Berl). 87:363–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Becker D, Scherer M, Neher P, Jungk C,
Jesser J, Pflüger I, Brinster R, Bendszus M, Bruckner T, Maier-Hein
K and Unterberg A: Going beyond Diffusion Tensor Imaging
tractography in eloquent glioma surgery-high resolution fiber
tractography: Q-ball or constrained spherical deconvolution? World
Neurosurg. 134:e596–e609. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cajigas I, Mahavadi AK, Shah AH, Borowy V,
Abitbol N, Ivan ME, Komotar RJ and Epstein RH: Analysis of
intra-operative variables as predictors of 30-day readmission in
patients undergoing glioma surgery at a single center. J
Neurooncol. 145:509–518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slof J, Diez Valle R and Galvan J:
Cost-effectiveness of 5-aminolevulinic acid-induced fluorescence in
malignant glioma surgery. Neurologia. 30:163–168. 2015.(In En,
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brokinkel B, Yavuz M, Warneke N, Brentrup
A, Hess K, Bleimüller C, Wölfer J and Stummer W: Endoscopic
management of a low-grade thalamic glioma: A safe alternative to
open microsurgery? Acta Neurochir (Wien). 159:1237–1240. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maruyama A, Yasuoka S, Katoh N and Asai J:
Radiation-induced osteosarcoma of the skull mimicking cutaneous
tumor after treatment for frontal glioma. J Dermatol. 47:69–71.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siva S, Kothari G, Muacevic A, Louie AV,
Slotman BJ, The BS and Lo SS: Radiotherapy for renal cell
carcinoma: Renaissance of an overlooked approach. Nat Rev Urol.
14:549–563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milosevic M, Gospodarowicz M, Zietman A,
Abbas F, Haustermans K, Moonen L, Rödel C, Schoenberg M and Shipley
W: Radiotherapy for bladder cancer. Urology. 69 (1 Suppl):S80–S92.
2007. View Article : Google Scholar
|
|
11
|
Bitterman DS, MacDonald SM, Yock TI,
Tarbell NJ, Wright KD, Chi SN, Marcus KJ and Haas-Kogan DA:
Revisiting the role of radiation therapy for pediatric low-grade
glioma. J Clin Oncol. 37:3335–3339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu VM, Welby JP, Laack NN, Mahajan A and
Daniels DJ: Pseudoprogression after radiation therapies for low
grade glioma in children and adults: A systematic review and
meta-analysis. Radiother Oncol. 142:36–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kieran MW, Goumnerova L, Manley P, Chi SN,
Marcus KJ, Manzanera AG, Silva Polanco ML, Guzik BW,
Aguilar-Cordova E, Diaz-Montero CM, et al: Phase I study of
gene-mediated cytotoxic immunotherapy with AdV-tk as adjuvant to
surgery and radiation for pediatric malignant glioma and recurrent
ependymoma. Neuro Oncol. 21:537–546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Morris-Natschke SL and Lee KH: New
developments in the chemistry and biology of the bioactive
constituents of Tanshen. Med Res Rev. 27:133–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tzen JT, Jinn TR, Chen YC, Li FY, Cheng
FC, Shi LS, She HK, Chen BC, Hsieh V and Tu ML: Magnesium
lithospermate B possesses inhibitory activity on Na+,K+-ATPase and
neuroprotective effects against ischemic stroke. Acta Pharmacol
Sin. 28:609–615. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee DS and Lee SH: Biological activity of
dihydrotanshinone I: Effect on apoptosis. J Biosci Bioeng.
89:292–293. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai SL, Suk FM, Wang CI, Liu DZ, Hou WC,
Lin PJ, Hung LF and Liang YC: Anti-tumor potential of
15,16-dihydrotanshinone I against breast adenocarcinoma through
inducing G1 arrest and apoptosis. Biochem Pharmacol. 74:1575–1586.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Hu T, Shen J, Zhang L, Chan RLY,
Lu L, Li M, Cho CH and Wu WKK: Dihydrotanshinone I induced
apoptosis and autophagy through caspase dependent pathway in colon
cancer. Phytomedicine. 22:1079–1087. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng R, Chen J, Wang Y, Ge Y, Huang Z and
Zhang G: Dihydrotanshinone induces apoptosis of SGC7901 and MGC803
cells via activation of JNK and p38 signalling pathways. Pharm
Biol. 54:3019–3025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng J, Fan YQ, Liu BH, Zhou H, Wang JM
and Chen QX: ACSL4 suppresses glioma cells proliferation via
activating ferroptosis. Oncol Rep. 43:147–158. 2020.PubMed/NCBI
|
|
21
|
Houessinon A, Francois C, Sauzay C,
Louandre C, Mongelard G, Godin C, Bodeau S, Takahashi S, Saidak Z,
Gutierrez L, et al: Metallothionein-1 as a biomarker of altered
redox metabolism in hepatocellular carcinoma cells exposed to
sorafenib. Mol Cancer. 15:382016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ooko E, Saeed ME, Kadioglu O, Sarvi S,
Colak M, Elmasaoudi K, Janah R, Greten HJ and Efferth T:
Artemisinin derivatives induce iron-dependent cell death
(ferroptosis) in tumor cells. Phytomedicine. 22:1045–1054. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dixon SJ and Stockwell BR: The role of
iron and reactive oxygen species in cell death. Nat Chem Biol.
10:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu B, Chen XB, Ying MD, He QJ, Cao J and
Yang B: The role of ferroptosis in cancer development and treatment
response. Front Pharmacol. 8:9922018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wenzel SE, Tyurina YY, Zhao J, St Croix
CM, Dar HH, Mao G, Tyurin VA, Anthonymuthu TS, Kapralov AA,
Amoscato AA, et al: PEBP1 wardens ferroptosis by enabling
lipoxygenase generation of lipid death signals. Cell. 171:628–641.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin YS, Shen YC, Wu CY, Tsai YY, Yang YH,
Lin YY, Kuan FC, Lu CN, Chang GH, Tsai MS, et al: Danshen improves
survival of patients with breast cancer and dihydroisotanshinone I
induces ferroptosis and apoptosis of breast cancer cells. Front
Pharmacol. 10:12262019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parhamifar L, Andersen H and Moghimi SM:
Lactate dehydrogenase assay for assessment of polycation
cytotoxicity. Methods Mol Biol. 1943:291–299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shan Y, Liu B, Li L, Chang N, Li L, Wang
H, Wang D, Feng H, Cheung C, Liao M, et al: Regulation of PINK1 by
NR2B-containing NMDA receptors in ischemic neuronal injury. J
Neurochem. 111:1149–1160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baysal M, Ilgin S, Kilic G, Kilic V,
Ucarcan S and Atli O: Reproductive toxicity after levetiracetam
administration in male rats: Evidence for role of hormonal status
and oxidative stress. PLoS One. 12:e1759902017. View Article : Google Scholar
|
|
35
|
Imai H, Matsuoka M, Kumagai T, Sakamoto T
and Koumura T: Lipid peroxidation-dependent cell death regulated by
GPx4 and ferroptosis. Curr Top Microbiol Immunol. 403:143–170.
2017.PubMed/NCBI
|
|
36
|
Li C, Deng X, Zhang W, Xie X, Conrad M,
Liu Y, Angeli JPF and Lai L: Novel allosteric activators for
ferroptosis regulator glutathione peroxidase 4. J Med Chem.
62:266–275. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan H, Li X, Zhang X, Kang R and Tang D:
Identification of ACSL4 as a biomarker and contributor of
ferroptosis. Biochem Biophys Res Commun. 478:1338–1343. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang WS and Stockwell BR: Ferroptosis:
Death by lipid peroxidation. Trends Cell Biol. 26:165–176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Y: The protective effects of
cryptochlorogenic acid on β-cells function in diabetes in vivo and
vitro via inhibition of ferroptosis. Diabetes Metab Syndr Obes.
13:1921–1931. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Friedmann Angeli AJ, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View Article : Google Scholar
|
|
41
|
Zilka O, Shah R, Li B, Friedmann Angeli
JP, Griesser M, Conrad M and Pratt DA: On the mechanism of
cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of
lipid peroxidation in ferroptotic cell death. ACS Cent Sci.
3:232–243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Ma R, Liu C, Liu H, Zhu R, Guo S,
Tang M, Li Y, Niu J, Fu M, et al: Salvia miltiorrhiza: A potential
red light to the development of cardiovascular diseases. Curr Pharm
Des. 23:1077–1097. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu Y, Chen T, Li X, Qu YK, An JN, Zheng
HX, Zhang ZJ and Lin N: Salvia miltiorrhiza bunge increases
estrogen level without side effects on reproductive tissues in
immature/ovariectomized mice. Aging (Albany NY). 9:156–172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang PR and Lü Q: A study on anticancer
activity of tanshinone II A against human breast cancer. Sichuan Da
Xue Xue Bao Yi Xue Ban. 40:245–249. 2009.(In Chinese). PubMed/NCBI
|
|
45
|
Zhang HS, Zhang FJ, Li H, Liu Y, Du GY and
Huang YH: Tanshinone A inhibits human esophageal cancer cell growth
through miR-122-mediated PKM2 down-regulation. Arch Biochem
Biophys. 598:50–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu D, Yuan R, Yang F and Zhang D: Effects
of tanshinones mediated by forkhead box O3a transcription factor on
the proliferation and apoptosis of lung cancer cells. Oncol Lett.
17:450–455. 2019.PubMed/NCBI
|
|
47
|
Mosaddik MA: In vitro cytotoxicity of
tanshinones isolated from Salvia miltiorrhiza Bunge against P388
lymphocytic leukemia cells. Phytomedicine. 10:682–685. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Park JW, Lee SH, Yang MK, Lee JJ, Song MJ,
Ryu SY, Chung HJ, Won HS, Lee CS, Kwon SH, et al:
15,16-dihydrotanshinone I, a major component from Salvia
miltiorrhiza Bunge (Dansham), inhibits rabbit platelet aggregation
by suppressing intracellular calcium mobilization. Arch Pharm Res.
31:47–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Suk FM, Jou WJ, Lin RJ, Lin SY, Tzeng FY
and Liang YC: 15,16-Dihydrotanshinone I-induced apoptosis in human
colorectal cancer cells: Involvement of ATF3. Anticancer Res.
33:3225–3231. 2013.PubMed/NCBI
|
|
50
|
Wu CY, Yang YH, Lin YY, Kuan FC, Lin YS,
Lin WY, Tsai MY, Yang JJ, Cheng YC, Shu LH, et al: Anti-cancer
effect of danshen and dihydroisotanshinone I on prostate cancer:
Targeting the crosstalk between macrophages and cancer cells via
inhibition of the STAT3/CCL2 signaling pathway. Oncotarget.
8:40246–40263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin YY, Lee IY, Huang WS, Lin YS, Kuan FC,
Shu LH, Cheng YC, Yang YH and Wu CY: Corrigendum to ‘Danshen
improves survival of patients with colon cancer and
dihydroisotanshinone I inhibit the proliferation of colon cancer
cells via apoptosis and skp2 signaling pathway’. J Ethnopharmacol.
213:4462018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ju LX, Chen Z and Ren RZ: Progress in
research on the treatment of primary liver cancer with traditional
Chinese medicine for activating blood to resolve stasis. Zhong Xi
Yi Jie He Xue Bao. 3:491–494. 2005.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aquilanti E, Miller J, Santagata S, Cahill
DP and Brastianos PK: Updates in prognostic markers for gliomas.
Neuro Oncol. 20 (Suppl 7):vii17–vii26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mair DB, Ames HM and Li R: Mechanisms of
invasion and motility of high-grade gliomas in the brain. Mol Biol
Cell. 29:2509–2515. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hanaei S, Afshari K, Hirbod-Mobarakeh A,
Mohajer B, Amir DD and Rezaei N: Therapeutic efficacy of specific
immunotherapy for glioma: A systematic review and meta-analysis.
Rev Neurosci. 29:443–461. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Natsume A and Yoshida J: Gene therapy for
high-grade glioma: Current approaches and future directions. Cell
Adh Migr. 2:186–191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Latunde-Dada GO: Ferroptosis: Role of
lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta
Gen Subj. 1861:1893–1900. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang L, Liu W, Liu F, Wang Q, Song M, Yu
Q, Tang K, Teng T, Wu D, Wang X, et al: IMCA Induces ferroptosis
mediated by SLC7A11 through the AMPK/mTOR pathway in colorectal
cancer. Oxid Med Cell Longev. 2020:16756132020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Homma T and Fujii J: Application of
glutathione as anti-oxidative and anti-aging drugs. Curr Drug
Metab. 16:560–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang Y, Zhao Y, Wang H, Zhang C, Wang M,
Yang Y, Xu X and Hu Z: Histone demethylase KDM3B protects against
ferroptosis by upregulating SLC7A11. FEBS Open Bio. 10:637–643.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim DH, Kim WD, Kim SK, Moon DH and Lee
SJ: TGF-β1-mediated repression of SLC7A11 drives vulnerability to
GPX4 inhibition in hepatocellular carcinoma cells. Cell Death Dis.
11:4062020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Friedmann AJ and Conrad M: Selenium and
GPX4, a vital symbiosis. Free Radic Biol Med. 127:153–159. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ingold I, Berndt C, Schmitt S, Doll S,
Poschmann G, Buday K, Roveri A, Peng X, Freitas FP, Seibt T, et al:
Selenium utilization by GPX4 is required to prevent
hydroperoxide-induced ferroptosis. Cell. 172:409–422.e21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sui X, Zhang R, Liu S, Duan T, Zhai L,
Zhang M, Han X, Xiang Y, Huang X, Lin H and Xie T: RSL3 drives
ferroptosis through GPX4 Inactivation and ROS production in
colorectal cancer. Front Pharmacol. 9:13712018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Belavgeni A, Bornstein SR and Linkermann
A: Prominin-2 suppresses ferroptosis sensitivity. Dev Cell.
51:548–549. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li W, Li W, Leng Y, Xiong Y and Xia Z:
Ferroptosis is involved in diabetes myocardial ischemia/reperfusion
injury through endoplasmic reticulum stress. DNA Cell Biol.
39:210–225. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu N, Lin X and Huang C: Activation of
the reverse transsulfuration pathway through NRF2/CBS confers
erastin-induced ferroptosis resistance. Br J Cancer. 122:279–292.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C,
Dai X, Li Z and Wu G: Ferroptosis: A novel anti-tumor action for
cisplatin. Cancer Res Treat. 50:445–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Louandre C, Marcq I, Bouhlal H, Lachaier
E, Godin C, Saidak Z, François C, Chatelain D, Debuysscher V,
Barbare JC, et al: The retinoblastoma (Rb) protein regulates
ferroptosis induced by sorafenib in human hepatocellular carcinoma
cells. Cancer Lett. 356:(2 Pt B). 971–977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Louandre C, Ezzoukhry Z, Godin C, Barbare
JC, Mazière JC, Chauffert B and Galmiche A: Iron-dependent cell
death of hepatocellular carcinoma cells exposed to sorafenib. Int J
Cancer. 133:1732–1742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yu H, Yang C, Jian L, Guo S, Chen R, Li K,
Qu F, Tao K, Fu Y, Luo F and Liu S: Sulfasalazineinduced
ferroptosis in breast cancer cells is reduced by the inhibitory
effect of estrogen receptor on the transferrin receptor. Oncol Rep.
42:826–838. 2019.PubMed/NCBI
|
|
72
|
Bach DH, Luu TT, Kim D, An YJ, Park S,
Park HJ and Lee SK: BMP4 upregulation is associated with acquired
drug resistance and fatty acid metabolism in EGFR-mutant
non-small-cell lung cancer cells. Mol Ther Nucleic Acids.
12:817–828. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang C, Liao Y, Liu P, Du Q, Liang Y, Ooi
S, Qin S, He S, Yao S and Wang W: FABP5 promotes lymph node
metastasis in cervical cancer by reprogramming fatty acid
metabolism. Theranostics. 10:6561–6580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Radif Y, Ndiaye H, Kalantzi V, Jacobs R,
Hall A, Minogue S and Waugh MG: The endogenous subcellular
localisations of the long chain fatty acid-activating enzymes ACSL3
and ACSL4 in sarcoma and breast cancer cells. Mol Cell Biochem.
448:275–286. 2018. View Article : Google Scholar : PubMed/NCBI
|