Introduction
Oral cancer is mainly represented by
epithelial-derived oral squamous cell carcinoma (OSCC) (1). Over the past two decades, the incidence
of OSCC has increased to ~40%, particularly in women aged 15–49
years (2). The increasing incidence
and younger onset of OSCC have become global healthcare issues
(3). Furthermore, the 5-year
survival rate of patients with OSCC remains at <60%, without
major improvements over the last three decades (4,5). The
high prevalence of death is mainly due to late diagnoses, poor
prognosis and absence of effective treatments (6). Therefore, the study of the biochemical
pathways and the potential molecules involved in OSCC could provide
valuable evidence for the development of further preventive
strategies and treatments.
Placenta-specific 8 (PLAC8) is a 12.4-kDa protein
that was first identified in human dendritic cells (7). PLAC8 has been demonstrated to
participate in immunity, adipocyte differentiation and embryo
development (8–10). Previous studies have revealed that
PLAC8 is closely associated with the proliferation, apoptosis and
autophagy of tumor cells (11–14).
Some studies have reported that high PLAC8 expression is positively
associated with progression of malignancy and that it promotes the
proliferation of cancer cells in the colon and lungs, as well as in
renal cell carcinoma (12,15,16).
Others studies have demonstrated that downregulation of PLAC8
expression may promote the growth and viability of hepatocellular
cancer cells (17). Therefore, PLAC8
may be considered as a novel biomarker of cancer. However, to the
best of our knowledge, the expression and function of PLAC8 in OSCC
remain unknown.
The present study aimed to reveal the role of PLAC8
in OSCC progression and investigated the function and the potential
mechanism of action of PLAC8 by overexpressing and silencing it in
OSCC cell lines.
Materials and methods
Cell lines and culture
Human oral epithelial cells (HOECs) were purchased
from the Cell Bank of Suzhou University (Suzhou, China). Three OSCC
cell lines (HN4, HN30 and HN6) were provided by the Shanghai Key
Laboratory of Stomatology (School of Medicine, Ninth People's
Hospital, Shanghai Jiaotong University, Shanghai, China). Cells
were seeded in Dulbecco's modified Eagle's medium (cat. no.
21013024; Gibco; Thermo Fisher Scientific, Inc.) containing 10%
fetal bovine serum (cat. no. 35-010-CV; Corning, Inc.) at 37°C in
5% CO2. XAV939 (100 µM; cat. no. X3004; Sigma-Aldrich;
Merck KGaA), a potent small-molecule inhibitor of β-catenin
(18), was used to treat HN4 cells
for 72 h.
Western blotting
Western blotting was performed as previously
described (19). Total protein was
analyzed by 10% SDS-PAGE and blotted on a nitrocellulose filter
membrane (cat. no. 10401196; Whatman plc; GE Healthcare Life
Sciences). The primary antibodies (all used at 1:1,000) were all
from Cell Signaling Technology, Inc., and were as follows: PLAC8
(cat. no. 13885), proliferating cell nuclear antigen (PCNA; cat.
no. 13110), cyclin D1 (cat. no. 2978), vimentin (cat. no. 5741),
c-Myc (cat. no. 5605), E-cadherin (cat. no. 14472), total-β-catenin
(cat. no. 9587), glycogen synthase kinase 3β (GSK3β; cat. no.
12456), non-phospho (Active) β-catenin (Ser33/37/Thr41; cat. no.
8814S), phosphorylated-GSK3β (cat. no. 5558), Akt (cat. no. 4685)
and phosphorylated-Akt (cat. no. 4060). β-actin (1:2,000; cat. no.
3700) was used as the control standard. Horseradish
peroxidase-conjugated rabbit anti-mouse (1:2,000; cat. no. p0161;
Dako; Agilent Technologies, Inc.) or goat anti-rabbit (1:5,000;
cat. no. sc2357; Santa Cruz Biotechnology, Inc.) were used as
secondary antibodies. The blots were probed with primary antibodies
overnight at 4°C and then incubated with the secondary antibody for
1 h at room temperature. An electrochemiluminescence
western-blotting system (cat. no. 7003; Cell Signaling Technology,
Inc.) was used to detect protein bands (Tanon 4600SF; Tanon Science
and Techonlogy Co., Ltd.).
RNA extraction and reverse
transcription-quantitative PCR
Total RNA was extracted from HOEC, HN4, HN30 or HN6
cells using TRIzol® reagent (cat. no. 15596026;
Invitrogen; Thermo Fisher Scientific, Inc.). Complementary DNA was
synthesized using the RNA reverse transcriptase kit (cat. no.
RR036A; Takara Bio, Inc.) according to the manufacturer's protocol.
The temperature protocol was as follows: 37°C for 15 min and 85°C
for 5 sec. Quantitative PCR was performed using SYBR™ Premix Ex
Taq™ II (cat. no. RR820Q; Takara Bio, Inc.) in an ABI 7300 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 94°C for 5 min; 40 cycles
at 94°C for 30 sec, 58°C for 45 sec and 70°C for 50 sec. The
expression of mRNA was assessed by evaluating the threshold cycle
(CT) values, and GAPDH was used as the internal reference gene.
Relative expression was calculated using the 2−ΔΔCq
method (20). The following primers
were used: GAPDH forward, 5′-AGGTCGGAGTCAACGGATTTGGT-3′ and
reverse, 5′-GTGCAGGAGGCATTGCTGATGAT-3′; and PLAC8 forward,
5′-CTGTCTGTGTGGAACAAGC-3′ and reverse,
5′-GAGGACAGCAAAGAGTTGCC-3′.
Cell transfection
Transfection experiments were performed using
Lipofectamine® 2000 (cat. no. 11668030; Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Briefly, HN4 and HN30 cells were transfected with PLAC8
plasmid (Sangon Biotech, Co., Ltd.) and control vector (cat. no.
631070; Takara Bio Inc.). HN6 cells were transfected with PLAC8
small interfering (si)RNA and negative control (Hippobiotec, Inc.,
http://www.hippobiotec.com). The OSCC
cell lines were seeded into 6-well plates (2.5×105
cells/well) and treated with 10 ug plasmid or 20 µM siRNA after 24
h of culture. Cells were harvested after 72 h of culture for
western blot analysis. Cells were regularly passaged for the Cell
Counting Kit-8 (CCK-8) assay. Targeted sequences of PLAC8 siRNAs
were: siRNA#1 sense, 5′-CUUUGCCAAAUCAAGAGAGAUdTdT-3′ and antisense,
5′-AUCUCUCUUGAUUUGGCAAAGdTdT-3′; siRNA#2 sense,
5′-GCUGAUAUGAAUGAAUGCUGUdTdT-3′ and antisense,
5′-ACAGCAUUCAUUCAUAUCAGCdTdT-3′; and negative control siRNA (siNC)
sense, 5′-UUCUCCGAACGUGUCACGUTT-3′. Altered expression of PLAC8 was
verified by western blotting.
CCK-8 assay
Cells were seeded at 5×103 cells/well in
a 96-well plate for 1–5 days. Cell proliferation was measured using
CCK-8 (CK04; Dojindo Molecular Technologies, Inc.) according to the
manufacturer's protocol. The absorbance was measured at a
wavelength of 450 nm using a spectrophotometer (Omega Bio-Tek,
Inc.).
Transwell invasion assay
Invasion of OSCC cells was measured using
Matrigel®-coated Transwell chambers (cat. no. 354480;
Corning Inc.). Briefly, Matrigel-coated Transwell chambers were
rehydrated at 37°C in 5% CO2 for 2 h. Subsequently,
2.5×104 cells were seeded in the Matrigel-coated
Transwell chambers and culture medium without serum was placed in
the lower compartment and incubated for 24 h at 37°C in 5%
CO2. Non-invasive cells in the upper membrane were
removed using a cotton swab, whilst the invasive cells were stained
at room temperature for 2 min using the Differential Quik Stain kit
(cat. no. B4132-1A; Allegiance Chemicals LLC), and manually counted
in five pre-determined fields under a light microscope
(magnifications, ×10 and ×40; BX51, Olympus Corporation).
Immunofluorescence staining
Immunofluorescence staining was performed as
previously described (21). Briefly,
adherent cells grown on coverslips were washed thrice with PBS and
incubated in PBS containing 0.2–0.3% Triton X-100. Subsequently,
E-cadherin (cat. no. 14472) and vimentin (cat. no. 5741) antibodies
(both Cell Signaling Technology, Inc.) were diluted to 1:100 and
incubated overnight at 4°C with cells. Next, Alexa Fluor 555
anti-mouse IgG (H+L) (cat. no. 4409) and Alexa Fluor 488
anti-rabbit IgG (H+L) (cat. no. 4412) (both Cell Signaling
Technology, Inc.) were incubated for 1 h at room temperature using
a 1:200 dilution. Subsequently, DAPI (cat. no. H-1200; Vector
Laboratories, Inc.) was used for nuclear staining for 1 min at room
temperature. Images were captured using a fluorescence microscope
(BX51; Olympus Corporation; magnification, ×200).
Statistical analysis
Statistical analyses were conducted using GraphPad
Prism 5.0 (GraphPad Software, Inc.). Paired Student's t-test and
one-way ANOVA followed by Tukey's post hoc test were used for
comparisons of two or multiple groups, respectively. All
experiments were performed in triplicate and data are presented as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
PLAC8 expression is downregulated in
OSCC cells
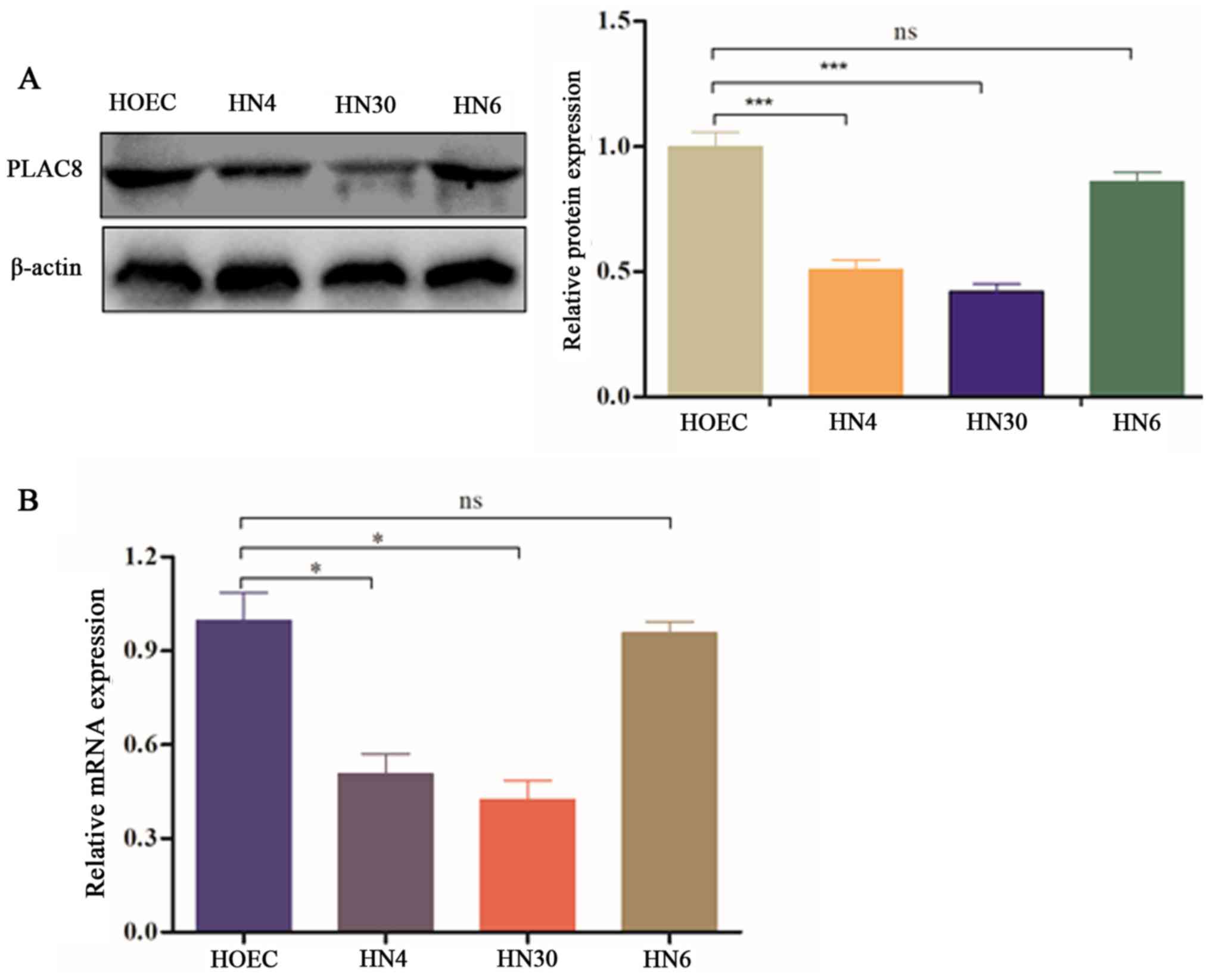
To assess whether PLAC8 is expressed in OSCC, three
OSCC cell lines and primary HOECs were selected to measure PLAC8
expression. Relative expression levels of the PLAC8 protein were
decreased in HN4, HN30 and HN6 cells compared with in HOECs, with
statistically significant differences in HN4 and HN30 cells
(P<0.001; Fig. 1A). Additionally,
PLAC8 mRNA expression was significantly downregulated in HN4 and
HN30 cells compared with that in HOECs (P<0.05); however, HN6
cells exhibited no significant difference in PLAC8 mRNA expression
compared with HOECs (Fig. 1B).
Overall, the present findings suggested that OSCC cells had a low
PLAC8 expression.
PLAC8 overexpression inhibits the
proliferation of OSCC cells
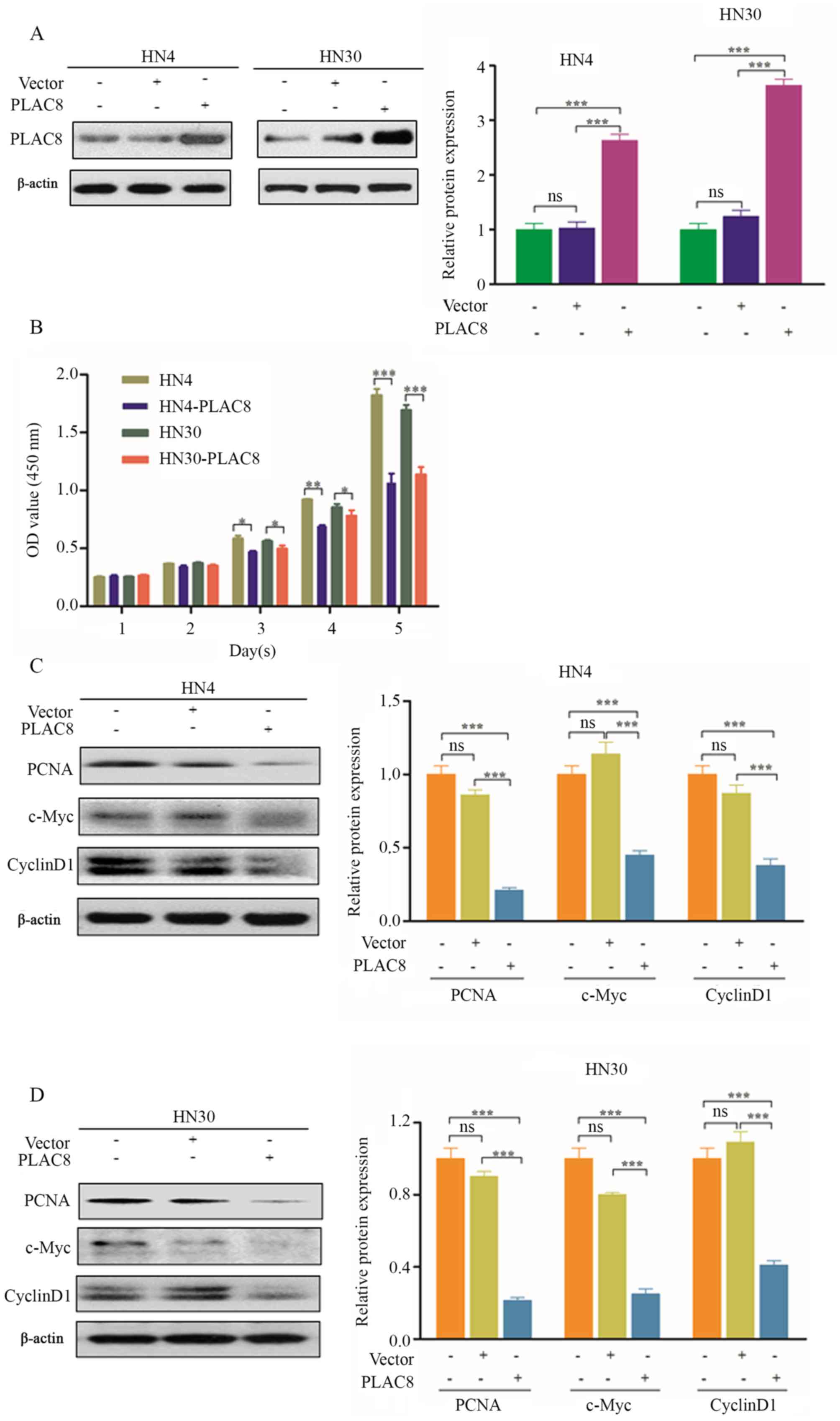
In order to understand the function of PLAC8 in
OSCC, HN4 and HN30 cells were selected for the overexpression of
PLAC8, since PLAC8 expression was lower in these two cell lines
compared with that in HN6 cells (Fig.
1). Western blot analysis was performed to confirm that PLAC8
was significantly increased following transfection with PLAC8
plasmid compared with non-transfected cells and cells transfected
with empty vector (Fig. 2A). PLAC8
overexpression significantly suppressed cells proliferation from
day 3 according to the CCK-8 assay, compared with non-transfected
HN4 and HN30 cells (P<0.05; Fig.
2B. In additional experiments, PLAC8 overexpression
significantly decreased the expression levels of PCNA, c-Myc and
cyclin D1 in HN4 and HN30 cells (P<0.001; Fig. 2C and D, respectively). The present
results suggested that PLAC8 overexpression suppressed the
proliferation of OSCC cells.
PLAC8-silencing promotes the
proliferation of OSCC cells
Two siRNAs were used to silence PLAC8 expression for
further evaluation of the function of PLAC8 in OSCC. Expression
levels of the PLAC8 protein were significantly decreased by
transfection with siRNA#1 (si-#1) and siRNA#2 (si-#2) compared with
those transfected with the negative control, therefore, si-#1 was
randomly selected for further experimentation (P<0.001; Fig. 3A). The proliferation of HN6 cells was
significantly increased from day 2 after silencing of PLAC8
expression (P<0.05; Fig. 3B).
Western blotting revealed that protein levels of PCNA, c-Myc and
cyclin D1 were significantly increased following PLAC8-knockdown
(P<0.001; Fig. 3C). The present
findings further indicated that PLAC8 repressed the proliferation
of OSCC cells.
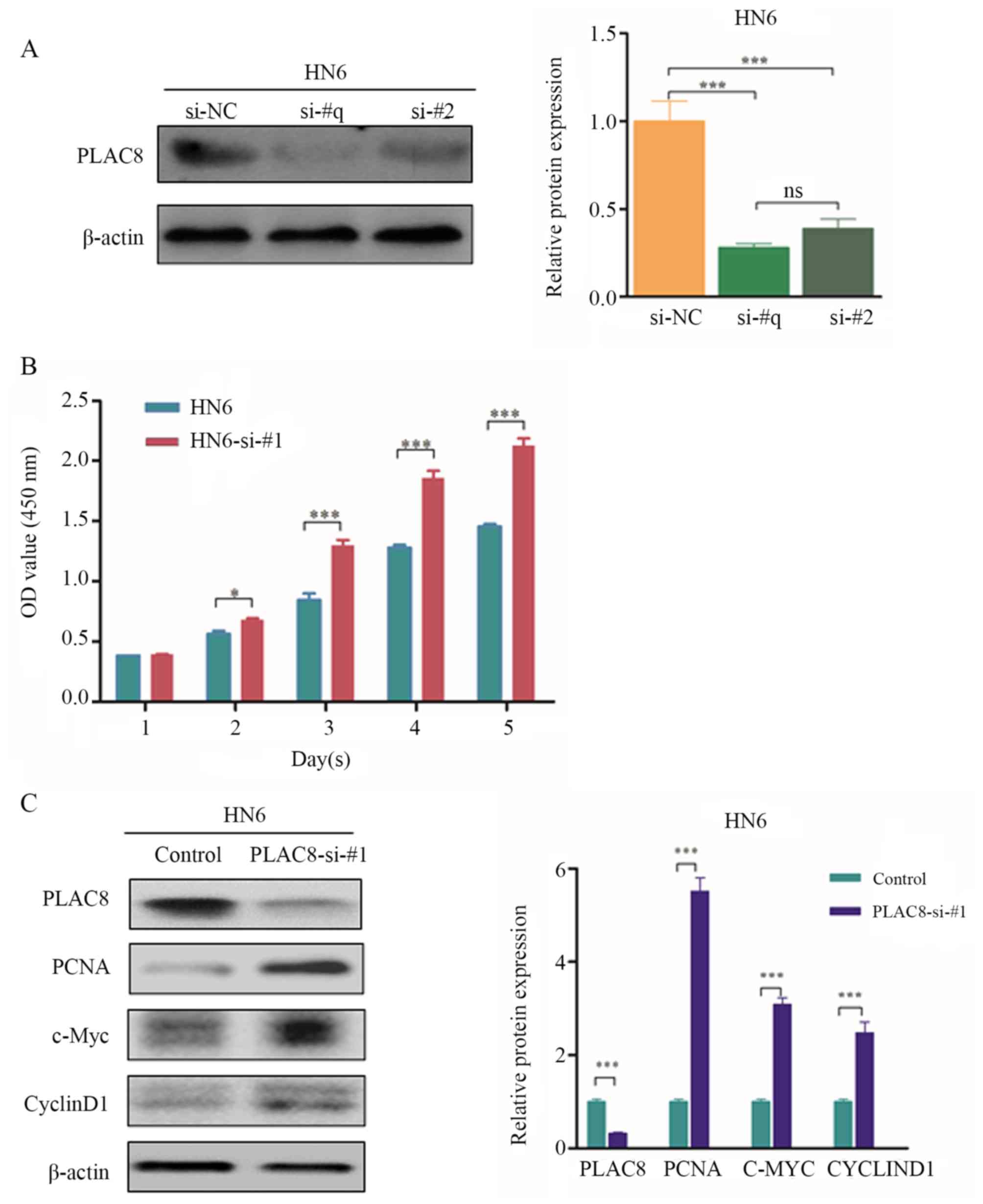 | Figure 3.Effect of PLAC8 silencing on the
proliferation of oral squamous cell carcinoma cells. (A) Western
blot analysis of PLAC8 expression in HN6 cells after transfection
with two PLAC8 siRNAs. Data are presented as the mean ± SD and
analyzed by one-way ANOVA with Tukey's post hoc test. (B)
Proliferation of control and PLAC8 siRNA#1-transfected cells at 1,
2, 3, 4 and 5 days measured via the Cell Counting Kit-8 assay. Data
are presented as the mean ± SD. (C) Western blot analysis of PCNA,
c-Myc and cyclin D1 expression in PLAC8-knockdown HN6 cells. Data
are presented as the mean ± SD. *P<0.05, ***P<0.001. PLAC8,
placenta-specific 8; OD, optical density; PCNA, proliferating cell
nuclear antigen; si, small interfering; NC, negative control; ns,
not significant. |
PLAC8 inhibits the invasion and
epithelial-mesenchymal transition (EMT) of OSCC cells
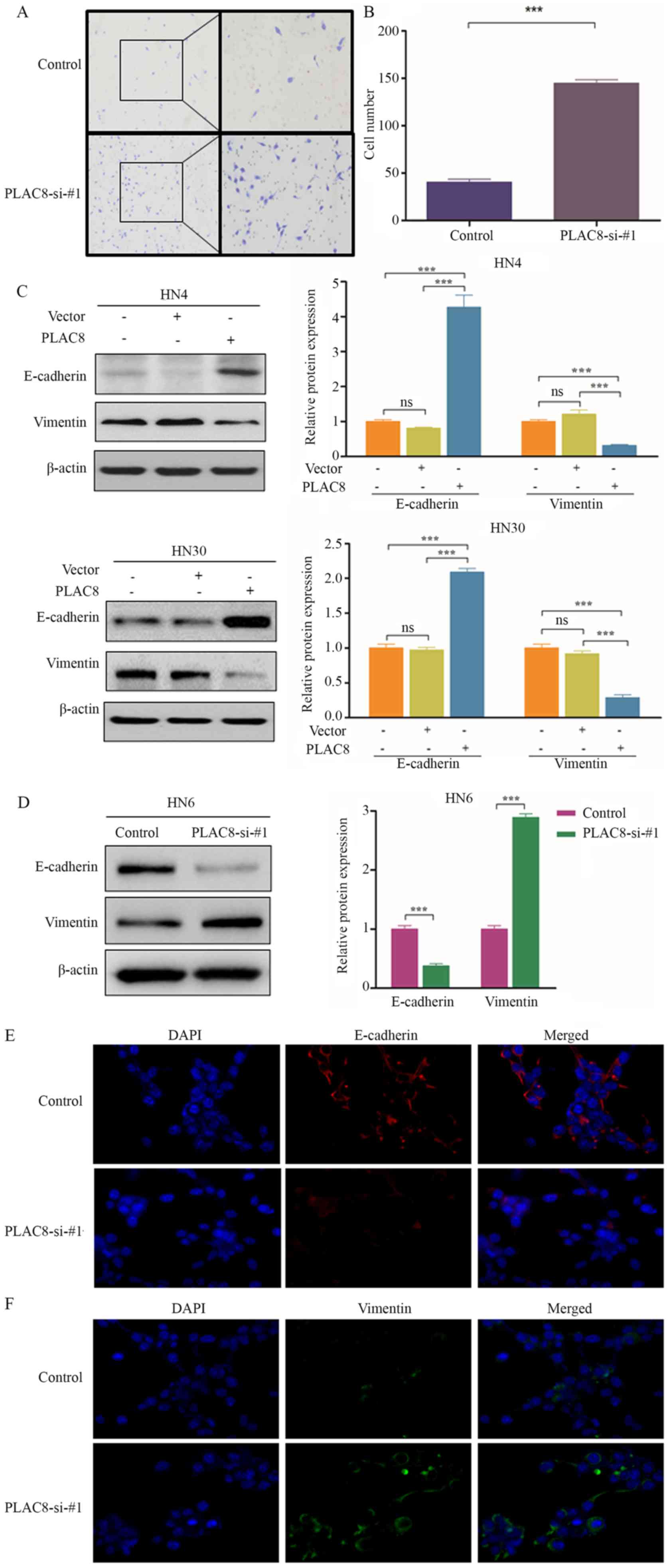
Transwell assays were performed to assess the role
of PLAC8 on the invasive ability of OSCC cells. The invasive
ability of HN6 cells was significantly increased after PLAC8
expression was silenced (P<0.001; Fig. 4A and B), which suggested that PLAC8
inhibited the invasion of OSCC cells.
To ascertain if EMT is involved in how PLAC8
influences the invasion of cells, western blotting was used to
detect EMT-associated markers. E-cadherin expression was
significantly increased, whereas vimentin expression was
significantly decreased following PLAC8-overexpression in HN4 and
HN30 cells (P<0.001; Fig. 4C).
Knockdown of PLAC8 expression resulted in a significant
downregulation of E-cadherin expression and significant
upregulation of vimentin expression in HN6 cells (P<0.001;
Fig. 4D). Immunofluorescence
staining indicated higher EMT activity in HN6 cells in the
silenced-PLAC8 group compared with in the control group (Fig. 4E and F). Overall, the present data
suggested that PLAC8 may inhibit the invasive ability of OSCC cells
via EMT suppression.
β-catenin helps to repress PLAC8
expression in OSCC cells
Overexpression or knockdown of PLAC8 affected c-Myc
and cyclin D1 expression (Figs. 2D
and 3D), which are target genes of
the Wnt signaling pathway. β-catenin is a pivotal player of Wnt
signaling and promotes EMT in cancer (22). Therefore, the role of PLAC8 in
regulating β-catenin expression was analyzed. PLAC8-overexpression
significantly decreased the protein levels of non-phospho β-catenin
(Ser33/37/Thr41) compared with HN4 cells transfected with empty
vector, whereas PLAC8 silencing performed after overexpression of
PLAC8 reverted non-phospho β-catenin (Ser33/37/Thr41) expression in
HN4 cells (P<0.001). Furthermore, PLAC8-overexpression slightly
increased the protein levels of total β-catenin compared with HN4
cells transfected with empty vector, whereas PLAC8 silencing
performed after overexpression of PLAC8 significantly decreased
total β-catenin expression in HN4 cells (P<0.05; Fig. 5A).
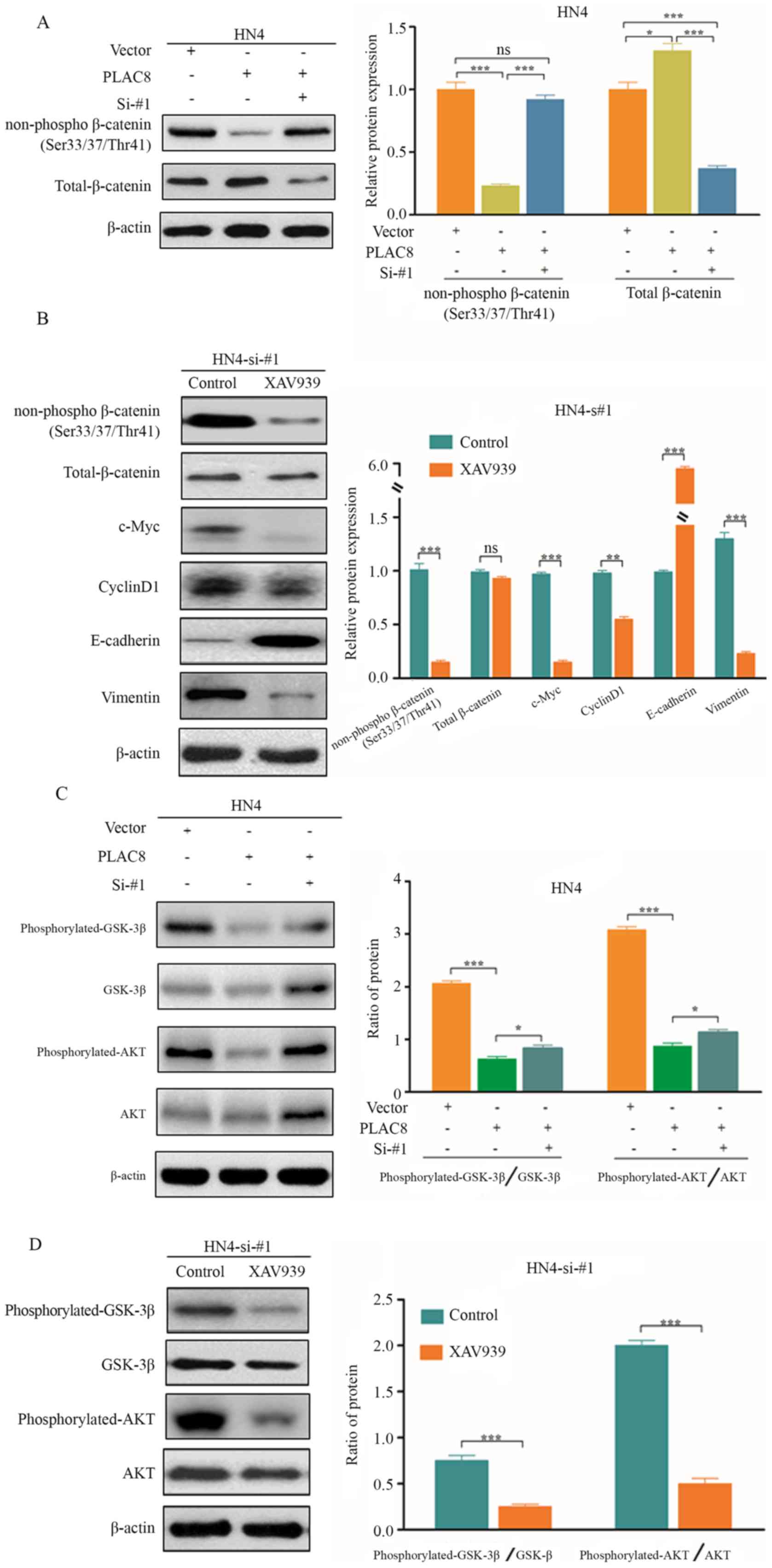 | Figure 5.β-catenin contributes to the
inhibitory effect of PLAC8 in HN4 cells. (A) Protein levels of
non-phospho β-catenin (Ser33/37/Thr41) and total β-catenin in HN4
cells treated with PLAC8-overexpressed and/or PLAC8-siRNA. (B)
Protein levels of non-phospho β-catenin (Ser33/37/Thr41), total
β-catenin, c-Myc, cyclin D1, E-cadherin and vimentin in
PLAC8-knockdown HN4 cells with or without XAV939 treatment. (C)
Protein levels of phosphorylated-GSK3β, total GSK3β,
phosphorylated-Akt and total Akt in HN4 cells treated with
PLAC8-overexpressed and/or PLAC8-siRNA. (D) Protein levels of
phosphorylated-GSK3β, total GSK3β, phosphorylated-Akt and total Akt
in PLAC8-knockdown HN4 cells with or without XAV939 treatment. Data
are presented as the mean ± SD. *P<0.05, **P<0.01,
***P<0.001 according to one-way ANOVA with Tukey's post hoc
test. PLAC8, placenta-specific 8; si, small interfering; GSK3β,
glycogen synthase kinase 3β; ns, not significant. |
Whether inhibition of β-catenin expression affected
PLAC8 function was also evaluated. Expression levels of non-phospho
β-catenin (Ser33/37/Thr41) were significantly decreased when XAV939
(a β-catenin inhibitor) was used to treat HN4 cells in which PLAC8
expression had been knocked down (Fig.
5B). Furthermore, protein levels of c-Myc, cyclin D1 and
vimentin were significantly downregulated, while those of
E-cadherin were significantly upregulated after XAV939 treatment
(P<0.001; Fig. 5B). The present
findings indicated that PLAC8 may inhibit the proliferation and EMT
pathway of OSCC cells by inactivating the Wnt/β-catenin
pathway.
Akt phosphorylation of GSK3β may lead to
stabilization of β-catenin and accumulation of nuclear β-catenin
(22). Numerous studies have
suggested that Wnt signaling cross-talks with the PI3K/Akt
signaling pathway (17,23). Therefore, the present study
investigated whether PLAC8 could also contribute to the
PI3K/Akt/GSK3β signaling pathway in OSCC. PLAC8 overexpression
significantly decreased the ratio of phosphorylated/total protein
of GSK3β and Akt (P<0.001), while silencing overexpressed-PLAC8
slightly increased the ratio of phosphorylated/total protein of
GSK3β and Akt in HN4 cells (P<0.005; Fig. 5C). XAV939 treatment resulted in a
significant decrease in the protein levels of phosphorylated-GSK3β
and phosphorylated-Akt in PLAC8-silenced HN4 cells, which suggested
that β-catenin contributed to the inhibitory effect of PLAC8 in the
PI3K/Akt signaling pathway (Fig.
5D). Overall, these results suggested that PLAC8 may regulate
the PI3K/Akt and Wnt/β-catenin signaling pathways via suppression
of β-catenin expression in OSCC cell lines.
Discussion
Oral cancer has become a global health problem due
to its high incidence, high mortality and early onset. According to
the GLOBOCAN, oral cancer may have caused 350,000 deaths with
710,000 new cases worldwide in 2018 (24,25).
OSCC accounts for >90% of oral malignant tumors (26). In the present study, PLAC8 expression
in OSCC cells. PLAC8 expression was lower in OSCC cell lines
compared with primary HOECs. In addition, PLAC8 appeared to repress
cell invasion and EMT features via downregulation of the expression
levels of non-phospho β-catenin (Ser33/37/Thr41) in OSCC cells.
Furthermore, protein levels of Wnt/β-catenin target genes and
phosphorylated-Akt/GSK3β were inhibited in PLAC8-knockdown cells
after treatment with a β-catenin inhibitor. The present results
indicated that PLAC8 may inhibit the proliferation and EMT features
of OSCC cells by repressing β-catenin expression, which regulates
the Wnt/β-catenin and PI3K/Akt/GSK3β signaling pathways.
PLAC8 has been observed in several cell types,
including dendritic, myeloid, lymphoid and epithelial cells, and
its functions vary depending on cellular processes and human
diseases (9,7). PLAC8 has been described as a tumor
suppressor and a tumor promoter in different types of cancer. For
example, PLAC8 overexpression promotes the tumorigenic
transformation in clear-cell renal-cell carcinoma, lung cancer,
colon cancer and breast cancer, but it also suppresses the
proliferation of hepatocellular carcinoma cells (12–17). In
accordance with the latter finding, the present study identified
that PLAC8 expression was significantly lower in OSCC cell lines
compared with human oral epithelial cells. Furthermore, PLAC8
expression inhibited the proliferation of OSCC cells, and
downregulated the protein levels of PCNA, c-Myc and cyclin D1,
which are transcriptional markers of tumor cell proliferation
(21,27). The present results suggested that
downregulated expression of PLAC8 may be an indicator of oral tumor
progression, and may serve an important part in the inhibition of
this malignancy.
OSCC cells often display EMT features in invasion
and metastasis (28). In the present
study, PLAC8-knockdown increased the invasion of OSCC cells,
suggesting that PLAC8 may be involved in EMT regulation. EMT is
characterized by epithelial cells acquiring a mesenchymal behavior,
caused by loss of intercellular adhesions and apicobasal polarity,
and gain of mesenchymal proteins, which promotes the proliferation
of cancer cells (29,30). Data from western blotting and
immunofluorescence experiments in the present study revealed that
E-cadherin expression was increased, while vimentin expression was
decreased after PLAC silencing. Conversely, PLAC8 overexpression
upregulated E-cadherin expression and downregulated vimentin
expression. The present results suggested that PLAC8 inhibited the
invasion of OSCC cells accompanied by EMT progression.
The functions of β-catenin include the regulation of
gene transcription and cell-cell adhesions (31). Nuclear expression of β-catenin
inhibits the transcription of E-cadherin and induces EMT by binding
to members of the T-cell factor/lymphoid enhancer factor family
(32). The lower expression of
E-cadherin causes β-catenin accumulation (33). Additionally, β-catenin, as the main
component of the Wnt/β-catenin signaling pathway, induces the
transcription of c-Myc and cyclin D1 that stimulates cancer cell
proliferation (22). It has been
previously demonstrated that the Wnt/β-catenin signaling pathway is
important in cathelicidin-promoted growth of lung and colon cancer
cells (19,23,34). In
the present study, PLAC8 overexpression downregulated c-Myc and
cyclin D1 expression. Overall, the present data indicated that
β-catenin may be involved in the inhibitory effect of PLAC8 on OSCC
cells. Therefore, β-catenin expression was measured in OSCC cells:
PLAC8 overexpression inhibited the expression of non-phospho
β-catenin (Ser33/37/Thr41), whereas PLAC8 knockdown promoted the
expression of non-phospho β-catenin (Ser33/37/Thr41). Additionally,
the expression levels of c-Myc and cyclin D1 were significantly
decreased, and EMT reversion was induced after XAV939 (a β-catenin
inhibitor) treatment in HN4 cells in which PLAC8 expression had
been knocked down. The present findings indicated that PLAC8 may
attenuate EMT in OSCC cells by suppressing β-catenin expression and
inactivating the Wnt/β-catenin signaling pathway.
GSK3b is a downstream target of Akt
phosphorylation/activation (35).
GSK3β is also a major component of the Wnt/β-catenin signaling
pathway. PLAC8 has been reported to suppress cell growth by
inhibiting phosphorylation of Akt and GSK3β in hepatocellular
carcinoma (17). Similarly, the
present study revealed a significant decrease in
phosphorylated-GSK3β and phosphorylated-Akt expression in
PLAC8-silenced HN4 cells after treatment with a β-catenin
inhibitor. Therefore, it was suggested that β-catenin may serve an
important role in inhibiting PLAC8 expression by suppressing the
PI3K/Akt/GSK3β signaling pathway in OSCC cell lines.
The limitation of the present study is that only
in vitro experiments were performed. Further studies with
animal experiments and clinical samples are required to confirm the
preliminary results, and thus better understand the clinical
value.
In conclusion, PLAC8 may inhibit carcinogenesis and
EMT via downregulation of β-catenin expression in the
PI3K/Akt/GSK3β and Wnt/β-catenin signaling pathways in OSCC. The
present results suggested that PLAC8 may serve a novel role in the
inhibition of OSCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81873975, 81802084,
81974314 and 81902984), the Excellent Academic Leader Training
Program of Shanghai Health System (grant no. 2018BR31), the Medical
Guidance Science and Technology Support Project of Shanghai (grant
no. 19411964800), the Natural Science Foundation of Shanghai (grant
no. 19ZR1448800), the Project of Shanghai Health and Family
Planning Commission (grant no. 20164Y0071) and the Project
supported by Clinical Research Project of Tongji Hospital of Tongji
University [grant nos. ITJ(ZD)1803, ITJ(ZD)1905 and
ITJ(QN)1905].
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LW, XTW, AQS, GV, ZJS, PJ, DYY, AMW YWY and DL
conceived and designed the present study. JLW, XTW, AQS, PJ, DYY
and AMW performed the experiments. ZJS provided the cell lines. YWY
and JLW drafted the initial manuscript. YWY, GV, JLW and DL
reviewed and edited the initial manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kerawala C, Roques T, Jeannon JP and
Bisase B: Oral cavity and lip cancer: United kingdom national
multidisciplinary guidelines. J Laryngol Otol. 130((S2)): S83–S89.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Du M, Nair R, Jamieson L, Liu Z and Bi P:
Incidence trends of lip, oral cavity, and pharyngeal cancers:
Global burden of disease 1990–2017. J Dent Res. 99:143–151. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farquhar DR, Tanner AM, Masood MM, PATEl
SR, Hackman TG, Olshan AF, Mazul AL and Zevallos JP: Oral tongue
carcinoma among young patients: An analysis of risk factors and
survival. Oral Oncol. 84:7–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jou A and Hess J: Epidemiology and
molecular biology of head and neck cancer. Oncol Res Treat.
40:328–332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Z, Hu S, Luo Q, Ye D, Hu D and Chen F:
Overexpression of SENP3 in oral squamous cell carcinoma and its
association with differentiation. Oncol Rep. 29:1701–1706. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Z, Luo Q, Ye D, Chen W and Chen F:
Role of toll-like receptor 4 on the immune escape of human oral
squamous cell carcinoma and resistance of cisplatin-induced
apoptosis. Mol Cancer. 11:332012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rissoan MC, Duhen T, Bridon JM,
Bendriss-Vermare N, Péronne C, de Saint Vis B, Brière F and Bates
EE: Subtractive hybridization reveals the expression of
immunoglobulin-like transcript 7, Eph-B1, granzyme B, and 3 novel
transcripts in human plasmacytoid dendritic cells. Blood.
100:3295–3303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jimenez-Preitner M, Berney X, Uldry M,
Vitali A, Cinti S, Ledford JG and Thorens B: Plac8 is an inducer of
C/EBPβ required for brown fat differentiation, thermoregulation,
and control of body weight. Cell Metab. 14:658–670. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ledford JG, Kovarova M and Koller BH:
Impaired host defense in mice lacking ONZIN. J Immunol.
178:5132–5143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Sayed A, Hoelker M, Rings F, Jennen DS,
Tholen E, Sirard MA, Schellander K and Tesfaye D: Large-scale
transcriptional analysis of bovine embryo biopsies in relation to
pregnancy success after transfer to recipients. Physiol Genomics.
28:84–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kinsey C, Balakrishnan V, O'Dell MR, Huang
JL, Newman L, Whitney-Miller CL, Hezel AF and Land H: Plac8 links
oncogenic mutations to regulation of autophagy and is critical to
pancreatic cancer progression. Cell Rep. 7:1143–1155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li C, Ma H, Wang Y, Cao V, Graves-Deal R,
Powell AE, Starchenko A, Ayers GD, Washington MK, Kamath V, et al:
Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon
cancer. J Clin Invest. 124:2172–2187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mao M, Chen Y, Jia Y, Yang J, Wei Q, Li Z,
Chen L, Chen L and Wang L: PLCA8 suppresses breast cancer apoptosis
by activating the PI3k/AKT/NF-κB pathway. J Cell Mol Med.
23:6930–6941. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kolluru V, Pal D, AMS PJ, Ankem MK,
Freedman JH and Damodaran C: Induction of Plac8 promotes
pro-survival function of autophagy in cadmium-induced prostate
carcinogenesis. Cancer Lett. 408:121–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia Y, Ying X, Zhou J, Chen Y, Luo X, Xie
S, Wang QC, Hu W and Wang L: The novel KLF4/PLAC8 signaling pathway
regulates lung cancer growth. Cell Death Dis. 9:6032018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi L, Xiao L, Heng B, Mo S, Chen W and Su
Z: Overexpression of placenta specific 8 is associated with
malignant progression and poor prognosis of clear cell renal cell
carcinoma. Int Urol Nephrol. 49:1165–1176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou L, Chai J, Gao Y, Guan J, Liu Q and Du
JJ: Down-regulated PLAC8 promotes hepatocellular carcinoma cell
proliferation by enhancing PI3K/Akt/GSK3β/Wnt/β-catenin signaling.
Biomed Pharmacother. 84:139–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Togashi Y, Hayashi H, Terashima M, de
Velasco MA, Sakai K, Fujita Y, Tomida S, Nakagawa K and Nishio K:
Inhibition of β-Catenin enhances the anticancer effect of
irreversible EGFR-TKI in EGFR-mutated non-small-cell lung cancer
with a T790M mutation. J Thorac Oncol. 10:93–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao Y, Wu J, Zhou H, Firrman J, Xiao W,
Sun Z and Li D: A deficiency in cathelicidin reduces lung tumor
growth in NNK/NTHi-induced A/J mice. Am J Cancer Res. 8:1190–1199.
2018.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li D, Sun J, Liu W, Wang X, Bals R, Wu J,
Quan W, Yao Y, Zhang Y, Zhou H and Wu K: Rig-G is a growth
inhibitory factor of lung cancer cells that suppresses STAT3 and
NF-κB. Oncotarget. 7:66032–66050. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji P, Zhou Y, Yang Y, Wu J, Zhou H, Quan
W, Sun J, Yao Y, Shang A, Gu C, et al: Myeloid cell-derived LL-37
promotes lung cancer growth by activating Wnt/β-catenin signaling.
Theranostics. 9:2209–2223. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Z, Liu Q, Ye D, Ye K, Yang Z and Li D:
Role of c-Met in the progression of human oral squamous cell
carcinoma and its potential as a therapeutic target. Oncol Rep.
39:209–216. 2018.PubMed/NCBI
|
|
26
|
Feng Z, Xu QS, Wang C, Li B, Li JZ, Mao
MH, Li H, Qin Z and Han Z: Clinicopathological features, management
and outcome of patients with poorly-differentiated oral and
oropharyngeal squamous cell carcinoma. J Craniomaxillofac Surg.
45:1478–1485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li D, Beisswenger C, Herr C, Hellberg J,
Han G, Zakharkina T, Voss M, Wiewrodt R, Bohle RM, Menger MD, et
al: Myeloid cell RelA/p65 promotes lung cancer proliferation
through Wnt/β-catenin signaling in murine and human tumor cells.
Oncogene. 33:1239–1248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kansy BA, Dißmann PA, Hemeda H, Bruderek
K, Westerkamp AM, Jagalski V, Schuler P, Kansy K, Lang S, Dumitru
CA and Brandau S: The bidirectional tumor-mesenchymal stromal cell
interaction promotes the progression of head and neck cancer. Stem
Cell Res Ther. 5:952014. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Böhrnsen F, Fricke M, Sander C, Leha A,
Schliephake H and Kramer FJ: Interactions of human MSC with head
and neck squamous cell carcinoma cell line PCI-13 reduce markers of
epithelia-mesenchymal transition. Clin Oral Investig. 19:1121–1128.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu S, Shi L, Wang Y, Ye D, Ju H, Ma H,
Yang W, Wang Y, Hu J, Deng J and Zhang Z: Stabilization of slug by
NF-κB is essential for TNF-α -induced migration and
epithelial-mesenchymal transition in head and neck squamous cell
carcinoma cells. Cell Physiol Biochem. 47:567–578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brembeck FH, Rosário M and Birchmeier W:
Balancing cell adhesion and Wnt signaling, the key role of
beta-catenin. Curr Opin Genet Dev. 16:51–59. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Orsulic S, Huber O, Aberle H, Arnold S and
Kemler R: E-cadherin binding prevents beta-catenin nuclear
localization and beta-catenin/LEF-1-mediated transactivation. J
Cell Sci. 112:1237–1245. 1999.PubMed/NCBI
|
|
34
|
Li D, Liu W, Wang X, Quan W, Yao Y, Bals
R, Ji S, Wu K, Guo J and Wan H: Cathelicidin, an antimicrobial
peptide produced by macrophages, promotes colon cancer by
activating the Wnt/β-catenin pathway. Oncotarget. 6:2939–2950.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xue Q, Yu C, Wang Y, Liu L, Zhang K, Fang
C, Liu F, Bian G, Song B, Yang A, et al: miR-9 and miR-124
synergistically affect regulation of dendritic branching via the
AKT/GSK3β pathway by targeting Rap2a. Sci Rep. 6:267812016.
View Article : Google Scholar : PubMed/NCBI
|