Introduction
Lung cancer is the most common cause of
cancer-associated mortality worldwide (1). In 2012, there were 1.82 million new
lung cancer cases (accounting for 12.9% of the total newly
diagnosed cancers) and 1.59 million lung cancer deaths (accounting
for 19.4% of the total cancer deaths) globally (1). Non-small cell lung cancer (NSCLC)
accounts for ~85% of lung cancer cases (1,2). Lung
adenocarcinoma represents the most common histological subtype of
NSCLC (2). The number of diagnosed
small-sized lung adenocarcinomas has now increased due to the
improvement of imaging techniques (3). However, the clinical outcome remains
unsatisfactory even after curative surgery.
In 2011, a novel lung adenocarcinoma classification
was advocated by the International Association for the Study of
Lung Cancer, the American Thoracic Society and the European
Respiratory Society. Adenocarcinoma in situ (AIS) is defined
as a ≤3 cm tumor with a pure lepidic pattern without invasion;
minimally invasive adenocarcinoma (MIA) is defined as a ≤3 cm tumor
with a lepidic-predominant pattern with ≤0.5 cm invasion; and
invasive adenocarcinoma (IAC) is further classified according to
the predominant subtype: Lepidic, papillary, acinar, solid or
micropapillary-predominant adenocarcinoma (4). The progression of lung adenocarcinoma
is thought to develop in a stepwise manner (5), but the underlying mechanism is not well
elucidated.
Patients with AIS/MIA have an ~100% disease-free
survival rate when the tumors are radically resected (4). However, a considerable proportion of
patients with stage I IAC ultimately exhibit recurrence (6). Therefore, improving the knowledge of
the mechanisms underlying the development and progression of lung
adenocarcinoma is essential, which may lead to novel risk
classifications for lung adenocarcinoma and to the development of
more effective treatment strategies.
The tumor tissue is composed of cancer cells, as
well as various types of stromal cells, including endothelial
cells, fibroblasts and immune cells, in addition to the
extracellular matrix (ECM) they produce, blood vessels and
lymphatics, which together create a specific tumor microenvironment
critical for tumor progression (7,8).
Cancer-associated fibroblasts (CAFs) are representative cells of
the tumor microenvironment (9).
Extensive clinical and experimental evidence has suggested that
CAFs may promote tumor progression in various ways, including
immune response regulation (10),
angiogenesis (11) and ECM
remodeling (12). Studies have
demonstrated that CAFs are associated with a poor prognosis in lung
adenocarcinoma (13,14).
An important characteristic of the aggressiveness of
tumor cells is their ability to remodel and degrade the ECM using
specific enzymes (15). Matrix
metalloproteinase-9 (MMP-9) is a member of the zinc-containing
endopeptidase family and the most important component in tumor
tissue remodeling (16). MMP-9 can
degrade types IV, V, VII, IX and X collagen, elastin, fibrin,
fibrinogen and plasminogen, thus enabling tumor cell invasion and
metastasis (16). Furthermore, MMP-9
can influence the vascular endothelial growth factor
(VEGF)-mediated development of an angiogenic vasculature (17,18).
High MMP-9 expression in NSCLC has an independent prognostic value
for the diagnosis of distant metastasis or local recurrence
(19).
In addition to the invasive ability of tumor cells,
lymphangiogenesis, referring to the growth of lymphatic vessels, is
considered to be the initial step and basic requirement for
lymphatic metastasis (20).
Lymphatic vessel density (LVD) is the parameter most frequently
used to quantify tumor lymphangiogenesis (20). Studies have demonstrated that LVD is
an independent prognostic factor in NSCLC (21,22).
However, previous studies have focused mainly on the
advanced stages of NSCLC, and only a few AIS and MIA cases were
examined in these studies (13,14,19,21,22).
Therefore, the role of CAFs, MMP-9 and LVD, and their association
in the progression from AIS to IAC remains mostly unknown. In the
present study, immunohistochemical (IHC) analysis was used to
evaluate the importance of CAFs, MMP-9 and LVD in both non-invasive
(lepidic) and invasive (other) tumor components in the progression
from AIS to IAC. Additionally, the potential correlations among
these parameters and their prognostic values were investigated.
Materials and methods
Patients
A total of 77 patients with stage 0-IA lung
adenocarcinoma who underwent complete resection at the Department
of Thoracic Surgery of Zhoushan Hospital (Zhoushan, China) between
January 2013 and December 2013 were included in the present
retrospective study. Mean age at the time of surgery was 62 years
(age range: 34–83 years) and the mean follow-up period was 57.3
months (range: 13–60 months). The clinicopathological
characteristics of patients are summarized in Table I. The adenocarcinoma subtypes were
classified according to the World Health Organization
classification (fourth edition) (23). Each component (lepidic, papillary,
acinar, solid and micropapillary) presented in MIA or ≥5% in IAC
was recorded and assessed in individual cases. Breast cancer tissue
from a 55-year-old female patient who underwent radical mastectomy
at Zhoushan Hospital (Zhoushan, China) in 2013 was used as the
positive control for the present study. The present study was
approved by the Institutional Review Board of Zhoushan Hospital.
The informed consent from patients was waived by the Institutional
Review Board of Zhoushan Hospital due to the retrospective nature
of the study.
 | Table I.Summary of clinicopathological
characteristics. |
Table I.
Summary of clinicopathological
characteristics.
| Variables | AIS (n=9) | MIA (n=24) | IAC (n=44) |
P-valuea |
P-valueb |
|---|
| Age, years |
|
|
| 0.38c | 0.67 |
|
≤65 | 8 | 16 | 27 |
|
|
|
>65 | 1 | 8 | 17 |
|
|
| Sex |
|
|
|
>0.999c | 0.13 |
|
Male | 4 | 9 | 25 |
|
|
|
Female | 5 | 15 | 19 |
|
|
| Smoking |
|
|
|
>0.999c | 0.06c |
| No | 7 | 20 | 26 |
|
|
|
Yes | 2 | 4 | 18 |
|
|
| Carcinoembryonic
antigen, ng/ml |
|
|
|
|
|
| ≤5 | 9 | 22 | 39 |
>0.999c |
>0.999c |
|
>5 | 0 | 2 | 5 |
|
|
| Tumor size, cm |
|
|
|
>0.999c |
<0.01c,d |
| ≤2 | 9 | 23 | 28 |
|
|
| >2
and ≤3 | 0 | 1 | 16 |
|
|
| Location |
|
|
| 0.03c | 0.37c |
| Right
upper lobe | 4 | 9 | 20 |
|
|
| Right
middle lobe | 0 | 2 | 3 |
|
|
| Right
lower lobe | 3 | 4 | 6 |
|
|
| Left
upper lobe | 0 | 9 | 10 |
|
|
| Left
lower lobe | 2 | 0 | 5 |
|
|
| Vascular
invasion |
|
|
| – | 0.54c |
|
Absent | 9 | 24 | 42 |
|
|
|
Present | 0 | 0 | 2 |
|
|
IHC analysis
The resected tissue specimens were fixed in 10%
formalin for 24 h embedded in paraffin, stored at room temperature
and cut into 4-µm-thick serial sections. The sections were
deparaffinized in xylene and rehydrated through graded alcohol
(100% alcohol, 5 min; 95% alcohol, 5 min and 75% alcohol, 5 min)
and deionized water, and then incubated with 3%
H2O2 at room temperature for 10 min to
inhibit endogenous peroxidase activity. After extensive washing
with PBS, the sections were further treated with 2% normal goat
serum (OriGene Technologies, Inc.) in PBS for 30 min at room
temperature to block non-specific binding. Next, the sections were
rinsed with PBS three times and then incubated with primary
antibodies against MMP-9 (1:300; cat. no. 13667S; Abcam), D2-40
(1:200; cat. no. ab77854; Abcam) and α-smooth muscle actin (α-SMA;
1:600; cat. no. 19245S, Cell Signaling Technology, Inc.) overnight
at 4°C. The sections were then incubated with the HRP labeled
polymer conjugated with secondary antibody (1:200; cat. no.
PV-6000; Universal immunohistochemical kit, OriGene Technologies,
Inc.) at room temperature for 20 min. For color development, the
sections were incubated with 2% DAB solution (DAB detection kit,
OriGene Technologies, Inc.) for 5 min at room temperature. Each
section was counterstained with hematoxylin for 2 min at room
temperature, dehydrated by sequential immersions in 70% ethanol (1
min) and 100% ethanol (twice for 1 min) and finally mounted.
Positive controls were breast carcinoma tissues, which are known to
express MMP-9, D2-40 and α-SMA. For negative controls, the primary
antibody was replaced with PBS.
IHC staining was assessed independently by two
pathologists with >10 years of experience using a light
microscope, who were blinded to all clinicopathological features.
When the evaluation results differed, a final consensus was reached
by revaluation and discussion. The non-invasive and invasive
components were determined based on hematoxylin and eosin staining
according to the international multidisciplinary classification of
lung adenocarcinoma (6). The
staining score of each component was evaluated for every sample.
The most densely populated staining areas (hot spot) were first
identified under a magnification, ×40 and then magnification, ×400
was used to calculate staining scores. The mean value of three hot
spots was considered the representative of one component. For
comparing the non-invasive and invasive components, the highest
score of one invasive component was considered the representative
of the case. The area fraction of CAFs was defined as the ratio of
the α-SMA+ area to the total area of the microscopic field using
ImageJ software version 1.48 (National Institutes of Health)
(24,25). The expression levels of MMP-9 were
evaluated based on the staining intensity and the percentage of
positive cells. The scores for the percentage of positive cells
were as follows: 0, 0–5; 1, 6–25; 2, 26–50; 3, 51–75; and 4,
76–100% (26). The staining
intensity scoring was as follows: 0, absent=no staining; 1,
weak=faint yellow; 2, moderate=brown; and 3, strong=dark brown. The
final staining scores were calculated by multiplying the scores for
the percentage of positive cells and staining intensity, with the
final scores ranging from 0 to 12. D2-40 positive single
endothelial cell or cell cluster, separated from adjacent lymphatic
vessels, tumor cells and connective tissue elements, regardless of
the presence of lumen was counted as one lymphatic vessel.
Statistical analysis
Statistical analysis was performed using SPSS
version 18.0 (SPSS, Inc.). The data are expressed as the mean ±
standard deviation. Categorical variables were compared using the
χ2 test or Fisher's exact test, as appropriate.
Continuous variables were compared using the independent-samples
Student's t-test (for differences between 2 groups) or a one-way
ANOVA followed by Fisher's least significant difference (LSD) post
hoc test (for comparisons among >2 groups). Variables in
different components of one tumor were compared using the
paired-samples Student's t-test. The correlation between continuous
variables was analyzed using Pearson's correlation coefficient.
Overall survival (OS) was defined as the time between surgery and
mortality or last observation. OS was analyzed using the
Kaplan-Meier method and the log-rank test. P<0.05 (two-sided)
was considered to indicate a statistically significant difference.
Multiple comparisons were made using the Bonferroni correction as
appropriate (P<0.05/n).
Results
Clinicopathological characteristics of
patients with AIS, MIA and IAC
The clinicopathological characteristics for all 77
patients are summarized in Table I.
No significant differences in age, sex, smoking, carcinoembryonic
antigen level, tumor location and vascular invasion were observed
among the three groups. However, patients with IAC had a
significantly larger tumor size compared with patients with MIA
(P<0.01).
Differences in CAFs, MMP-9 and LVD in
the non-invasive and invasive components of tumors of patients with
AIS, MIA and IAC
The representative samples of IHC staining are shown
in Fig. 1. Within the non-invasive
component, the mean area percentage for CAFs was 6.04±3.00,
9.70±3.28 and 12.10±4.44 in patients with AIS, MIA and IAC,
respectively. The mean staining score for MMP-9 was 1.56±1.74,
4.00±2.09 and 5.23±1.98 in patients with AIS, MIA and IAC,
respectively. The mean LVD count was 4.33±1.66, 3.33±1.58 and
3.95±1.68 in patients with AIS, MIA and IAC, respectively (Table II). One-way ANOVA followed by the
LSD test revealed that the proportion of CAFs and the expression
levels of MMP-9 significantly increased from AIS to IAC (CAFs,
F=8.497 and P=0.001; AIS vs. MIA, P=0.016; AIS vs. IAC, P<0.001;
MIA vs. IAC, P=0.035; MMP-9, F=10.908 and P<0.001; AIS vs. MIA,
P=0.003; AIS vs. IAC, P<0.001; MIA vs. IAC, P=0.042; Tables II and III). The LVD was not significantly
different while progressing from AIS to IAC (F=1.532 and P=0.226;
AIS vs. MIA, P=0.123; AIS vs. IAC, P=0.56; MIA vs. IAC, P=0.203;
Tables II and III). Within the invasive component, the
mean area percentage for CAFs was 11.04±4.33 and 13.43±4.15 in MIA
and IAC, respectively. The mean staining score for MMP-9 was
4.58±2.26 and 5.68±1.86 in MIA and IAC, respectively. The mean LVD
was 4.83±2.41 and 5.45±2.87 in MIA and IAC, respectively. CAFs
(P=0.001), MMP-9 (P=0.004) and LVD (P=0.013) all significantly
increased from MIA to IAC (Table
II).
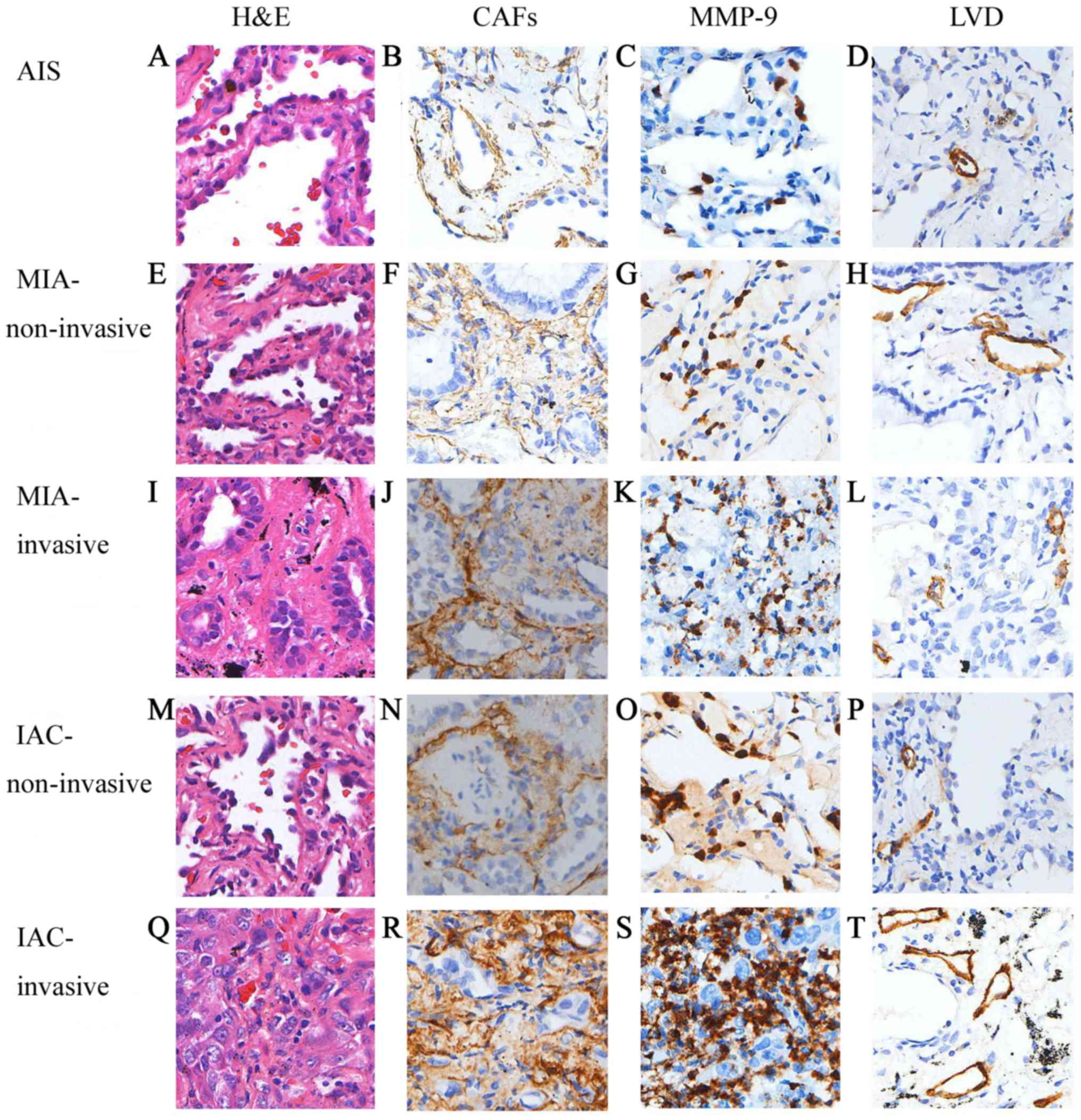 | Figure 1.Representative immunostaining results
for H&E, CAFs, MMP-9 and LVD. Staining results in (A-D) AIS,
(E-H) non-invasive component of MIA, (I-L) invasive component of
MIA, (M-P) non-invasive component of IAC and (Q-T) invasive
component of IAC. Magnification, ×200. H&E, hematoxylin and
eosin; CAFs, cancer-associated fibroblasts; MMP-9, matrix
metalloproteinase-9; LVD, lymphatic vessel density; AIS,
adenocarcinoma in situ; MIA, minimally invasive
adenocarcinoma; IAC, invasive adenocarcinoma. |
 | Table II.Differences in CAFs, MMP-9 and LVD in
the non-invasive and invasive components of patients with AIS
(n=9), MIA (n=24) and IAC (n=22). |
Table II.
Differences in CAFs, MMP-9 and LVD in
the non-invasive and invasive components of patients with AIS
(n=9), MIA (n=24) and IAC (n=22).
|
| Non-invasive
component | Invasive
component |
|---|
|
|
|
|
|---|
| Variable | AIS | MIA | IAC | F ratio |
P-valuea | MIA | IAC |
P-valueb |
|---|
| CAFs | 6.04±3.00 | 9.70±3.28 | 12.10±4.44 | 8.497 | 0.001 | 11.04±4.33 | 13.43±4.15 | 0.001 |
| MMP-9 | 1.56±1.74 | 4.00±2.09 | 5.23±1.98 | 10.908 | <0.001 | 4.58±2.26 | 5.68±1.86 | 0.004 |
| LVD | 4.33±1.66 | 3.33±1.58 | 3.95±1.68 | 1.532 | 0.226 | 4.83±2.41 | 5.45±2.87 | 0.013 |
 | Table III.LSD post hoc test for CAFs, MMP-9 and
LVD between groups of non-invasive components in patients with AIS,
MIA and IAC. |
Table III.
LSD post hoc test for CAFs, MMP-9 and
LVD between groups of non-invasive components in patients with AIS,
MIA and IAC.
|
| AIS | MIA | IAC |
|---|
| CAFs |
|
|
|
| AIS | – | P=0.016 | P<0.001 |
| MIA | – | – | P=0.035 |
| IAC | – | – | – |
|
| AIS | MIA | IAC |
| MMP-9 |
|
|
|
| AIS | – | P=0.003 | P<0.001 |
| MIA | – | – | P=0.042 |
| IAC | – | – | – |
|
| AIS | MIA | IAC |
| LVD |
|
|
|
| AIS | – | P=0.123 | P=0.56 |
| MIA | – | – | P=0.203 |
| IAC | – | – |
|
Differences in CAFs, MMP-9 and LVD
between the non-invasive and invasive components of tumors of
patients with MIA and IAC
Of the 44 IAC cases, 22 also had a non-invasive
component (lepidic) and thus were included in this comparison. The
scores of CAFs, MMP-9 and LVD were all significantly higher in the
invasive component compared with in the non-invasive component in
both MIA and IAC (P<0.05; Table
IV).
 | Table IV.Differences in CAFs, MMP-9 and LVD
between the non-invasive and invasive components in patient with
MIA (n=24) and IAC (n=22). |
Table IV.
Differences in CAFs, MMP-9 and LVD
between the non-invasive and invasive components in patient with
MIA (n=24) and IAC (n=22).
|
| MIA | IAC |
|---|
|
|
|
|
|---|
| Variable | Non-invasive | Invasive | P-value | Non-invasive | Invasive | P-value |
|---|
| CAFs | 9.70±3.28 | 11.04±4.33 | 0.019 | 12.10±4.44 | 13.43±4.15 | 0.017 |
| MMP-9 | 4.00±2.09 | 4.58±2.26 | 0.013 | 5.23±1.98 | 5.68±1.86 | 0.021 |
| LVD | 3.33±1.58 | 4.83±2.41 | <0.001 | 3.95±1.68 | 5.45±2.87 | 0.027 |
Differences in CAFs, MMP9 and LVD in
the invasive subtypes of IAC samples
The scores of CAFs, MMP-9 and LVD in the subtypes of
IAC in 44 cases (since most cases of lung adenocarcinoma exhibited
mixed subtypes, 32 papillary, 23 acinar, 11 solid and 10
micropapillary) were compared to further explore the differences in
CAFs, MMP-9 and LVD in the invasive subtypes of IAC samples. The
cases with solid and micropapillary patterns were fewer, and hence
included in the solid + micropapillary group, while the other two
patterns were included in the acinar + papillary group. As shown in
Table V, the scores of CAFs, MMP-9
and LVD were all significantly higher in the solid + micropapillary
group compared with in the acinar + papillary group (P=0.011,
P=0.045 and P<0.001, respectively).
 | Table V.Differences in CAFs, MMP9 and LVD in
the invasive subtypes of patients with invasive adenocarcinoma. |
Table V.
Differences in CAFs, MMP9 and LVD in
the invasive subtypes of patients with invasive adenocarcinoma.
| Variable | Acinar + papillary
(n=55) | Solid +
micropapillary (n=21) | P-value |
|---|
| CAFs | 12.88±3.76 | 16.36±5.34 | 0.011 |
| MMP-9 | 5.27±2.00 | 6.33±2.11 | 0.045 |
| LVD | 5.38±2.22 | 8.38±3.01 | <0.001 |
Correlations between CAFs, MMP-9 and
LVD in the non-invasive and invasive components
Considering all of the samples as a whole, CAFs were
positively correlated with MMP-9 in the non-invasive component
(r=0.398; P=0.003; Fig. 2A). No
statistically significant correlation was observed between CAFs and
LVD (r=0.183; P=0.181; Fig. 2B), and
between MMP-9 and LVD (r=0.238; P=0.08; Fig. 2C). In the invasive component, CAFs
were positively correlated with MMP-9 (r=0.395; P=0.001; Fig. 2D) and LVD (r=0.408; P=0.001; Fig. 2E). Additionally, MMP-9 was positively
correlated with LVD (r=0.427; P<0.001; Fig. 2F).
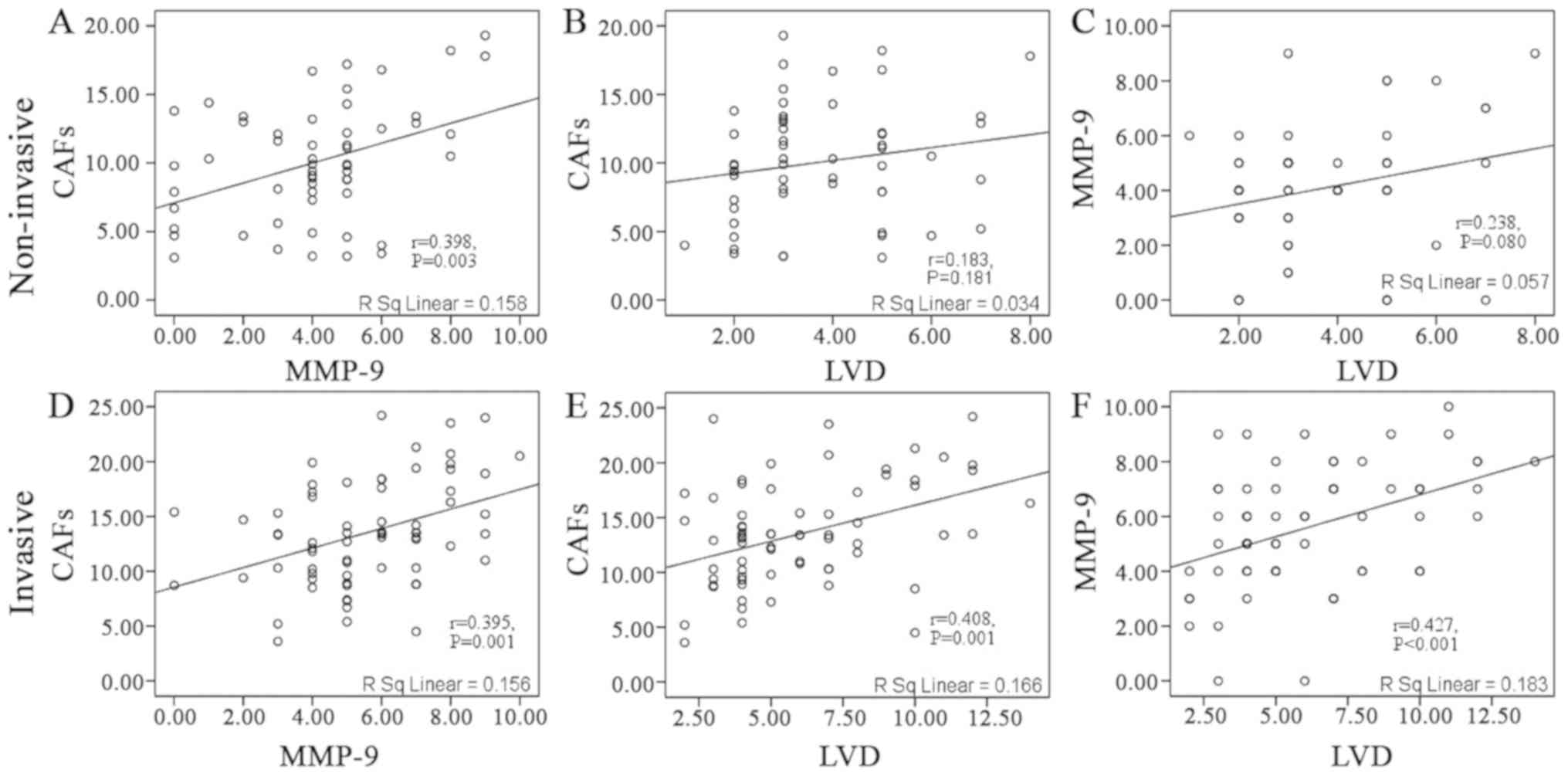 | Figure 2.Correlation between CAFs, MMP-9 and
LVD in the non-invasive and invasive components. In the
non-invasive component, a significant correlation was observed
between (A) CAFs and MMP-9, but not between (B) CAFs and LVD, and
(C) MMP-9 and LVD. In the invasive component, significant
correlations were observed between (D) CAFs and MMP-9, (E) CAFs and
LVD, and (F) MMP-9 and LVD. CAFs, cancer-associated fibroblasts;
MMP-9, matrix metalloproteinase-9; LVD, lymphatic vessel
density. |
Associations of invasive subtypes,
CAFs, MMP-9 and LVD with OS in patients with IAC
All patients with AIS and MIA achieved a 100%
disease-free survival rate in the present study (data not shown).
Therefore, the prognostic value of clinicopathological variables,
invasive subtypes, CAFs, MMP-9 and LVD in the 44 patients with IAC
was investigated. The highest scores for CAFs, MMP-9 and LVD in the
invasive component were used as the respective scores for the
survival analysis. Patients were classified into high or low groups
based on the median scores of CAFs, MMP-9 and LVD (6, 13.5 and 5,
respectively), and positive or negative invasive subtypes, an
invasive subtype with a proportion ≥5% was defined as positive. A
significant association was observed between patients with OS and
LVD, patients with high LVD had a poorer OS rate compared with
those with low LVD (P=0.005; Fig.
3E), but not with MMP-9 and CAFs (P=0.530 and P=0.468,
respectively; Fig. 3F and G). The
micropapillary pattern was associated with poor OS (P=0.031;
Fig. 3A), while other patterns had
no significant association with OS (solid, P=0.336; papillary,
P=0.090; acinar, P=0.533; Fig.
3B-D).
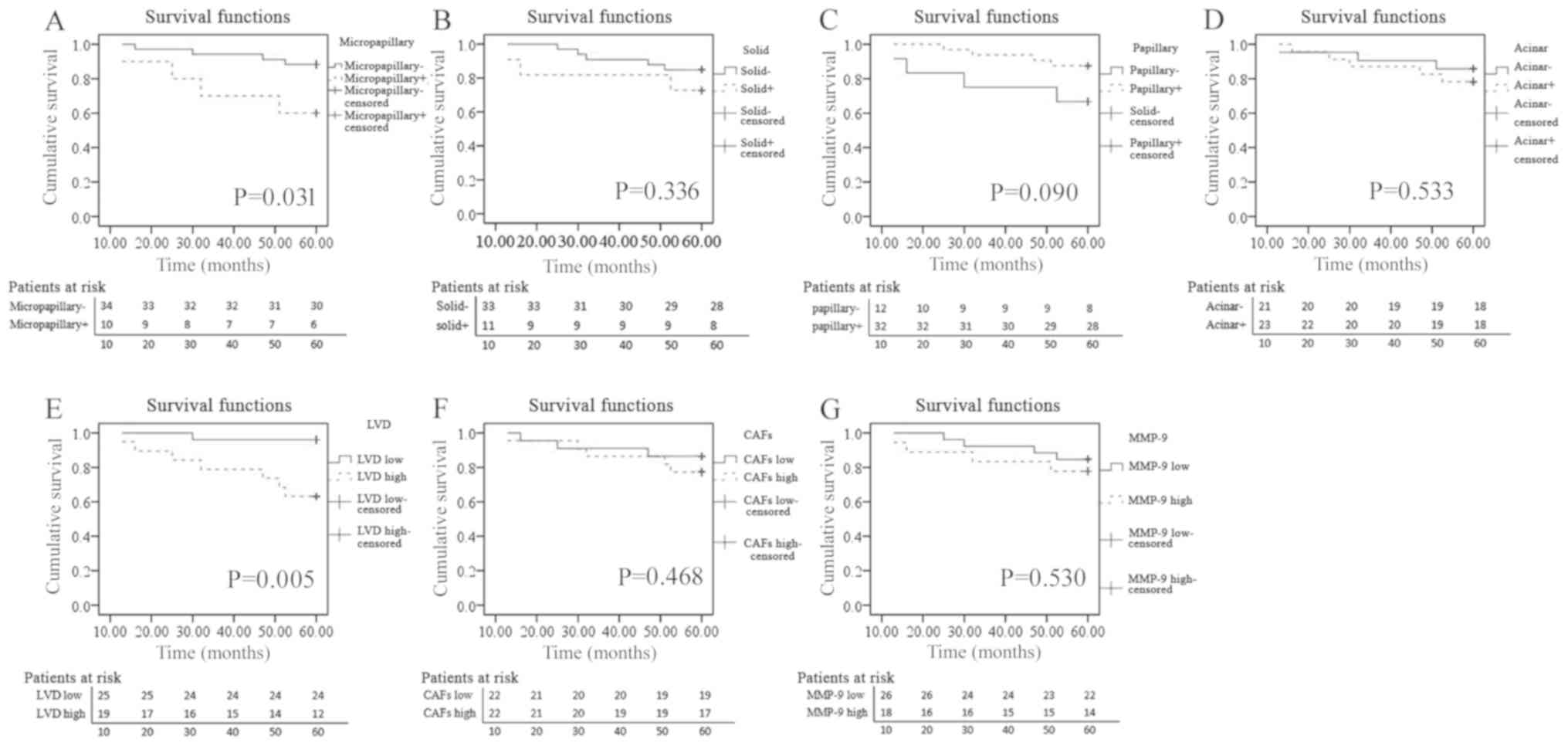 | Figure 3.Association between OS and different
parameters. Patients were classified into high or low groups based
on the median scores of CAFs, MMP-9 and LVD (6.0, 13.5 and 5.0,
respectively), and positive or negative invasive subtype groups
based on whether the invasive subtype is >5%. Association
between OS and (A) micropapillary component (P=0.031), (B) solid
component (P=0.336), (C) papillary component (P=0.090), (D) acinar
component (P=0.533), (E) LVD (P=0.005), (F) CAFs (P=0.468) and (G)
MMP-9 (P=0.530). OS, overall survival; CAFs, cancer-associated
fibroblasts; MMP-9, matrix metalloproteinase-9; LVD, lymphatic
vessel density. |
Discussion
The contribution of CAFs to the development of lung
adenocarcinoma has been supported by clinical evidence and
experimental models (13,27,28).
However, these previous studies have rarely examined non-invasive
lesions, which may represent the initial stage of lung
adenocarcinoma. Little is known about how CAFs differ in
non-invasive and invasive lesions. Kawase et al (14) reported that CAFs were only detected
in invasive adenocarcinoma and none were detected in non-invasive
adenocarcinoma. The present study revealed that there were fewer
CAFs in the non-invasive component than in the invasive component.
Additionally, CAFs displayed a significant increase in both
non-invasive and invasive components during the progression from
AIS to IAC. It was hypothesized that CAFs may provide a more
tumor-promoting microenvironment while progressing from AIS to IAC.
However, Matsubara et al (29) revealed completely opposite results:
Myofibroblasts positive for α-SMA, which were named subepithelial
myofibroblasts, were present in the non-invasive components, but
scanty in the invasive components. The retention of subepithelial
myofibroblasts in invasive lung adenocarcinoma was associated with
an excellent prognosis in patients (29). Therefore, the specific phenotype,
morphology and location of CAFs require further exploration.
Kodate et al (30) revealed that 1/11 cases of
bronchioloalveolar carcinoma expressed MMP-9 in the non-invasive
component. Kanomata et al (31) investigated the mRNA expression levels
of MMP-9 in different areas of mixed-type lung adenocarcinoma using
in situ hybridization; the mRNA expression levels and the
activity of MMP-9 were significantly higher in both non-invasive
and invasive carcinomas compared with in tumor-free lung tissues.
Consistent with these findings, the present study revealed that the
expression levels of MMP-9 were lower in the non-invasive component
compared with in the invasive component. The expression levels of
MMP-9 steadily increased in both the non-invasive and invasive
components while progressing from AIS to IAC. The current results
suggested that the increase in the proportion of CAFs and the
expression levels of MMP-9 may be an early event before
adenocarcinomas become invasive during tumor progression. The
non-invasive component was morphologically similar, but had
different invasive potential during progression from AIS to
IAC.
Stromal fibroblast-type cells are the most important
source of MMPs in mixed bronchioloalveolar carcinoma (31). Using an experimental mouse model,
Taguchi et al (32)
demonstrated that MMP-9 was expressed in fibroblasts but not in
tumor cells. The co-culture of fibroblasts with tumor cells
enhanced the expression levels and proteinase activity of MMP-9
(32). In the present study, CAFs in
the non-invasive and invasive components were strongly correlated
with the expression levels of MMP-9. The aforementioned findings
combined with the results of the present study indicated that CAFs
may induce MMP-9 expression and promote tumor aggressiveness and
progression in the earliest event of lung adenocarcinoma.
Previous studies have revealed that CAFs and MMP-9
influence lymphangiogenesis in ovarian cancer (33), oral squamous cell carcinoma (34) and breast cancer (35,36).
Chen et al (37) demonstrated
that the abundance of CAFs in lung adenocarcinoma was associated
with a higher LVD. This finding was extended in the present study:
The LVD increased only in the invasive component while progressing
from MIA to IAC, and a positive correlation with CAFs and MMP-9 was
detected in the invasive component but not in the non-invasive
component. Although further exploration is required for the
underlying mechanism, the results of the present study indicated
that histopathological patterns should be considered as a
prerequisite for exploring the role of CAFs and/or MMP-9 in
lymphangiogenesis. Compared with CAFs and MMP-9, the LVD seemed to
be a later phenomenon in the progression from AIS to IAC. Once the
adenocarcinoma progressed to be invasive, the LVD began to reveal
its important role in tumor invasion and metastasis.
Predominant histopathological patterns can reflect
the prognosis in patients with lung adenocarcinoma. Patients with
solid- or micropapillary-predominant patterns have worse survival
rates compared with patients with acinar- or papillary- or
lepidic-predominant patterns (38,39).
Yanagawa et al (40) revealed
that patients with solid and/or micropapillary patterns had a
poorer prognosis compared with those without solid and/or
micropapillary patterns, even if their patterns were not
predominant. The results of the present study were consistent with
the aforementioned findings, demonstrating that patients with ≥5%
micropapillary pattern had a poorer OS rate compared with those
with <5% micropapillary pattern in stage IA lung adenocarcinoma.
However, no significant association was detected between the solid
pattern and OS in the present study. Additionally, the current
study demonstrated that CAFs, MMP-9 and LVD were all significantly
higher in the solid + micropapillary group than in the acinar +
papillary group, further supporting the involvement of CAFs, MMP-9
and LVD in tumor invasiveness. However, within stage IA IAC, only
the LVD had a significant association with OS. The present result
indicated that LVD may be a useful prognostic indicator while
selecting therapeutic strategies for patients with early-stage lung
adenocarcinoma after surgery. Increased careful follow-up and
individual treatments are warranted in cases displaying a high LVD
in the invasive component.
The present study had some limitations. First, the
sample size was relatively small, and it was a retrospective study
conducted at a single institution, which may lead to selection
bias. Second, the staining scores of CAFs, MMP-9 and LVD were
evaluated using IHC, which provided only a semi-quantitative
assessment of protein expression status; additionally, during
fixing, processing or staining, the target protein may have been
lost (31). Therefore, the use of
enzyme-linked immunosorbent assay or western blotting with fresh
specimens is required in future studies. Third, this was a
preliminary study that did not use cell lines or animal models to
investigate signaling molecules to further support the results.
Additionally, the association between the proportion of subtypes
and prognosis was not assessed. Finally, expanding the study to
include stage IB-IIA lung adenocarcinoma may have been more
conducive to continuous observation of tumor invasive
manifestations. These unresolved issues require further study in
the future.
In conclusion, the present study revealed that CAFs,
MMP-9 and LVD were involved in tumor progression from AIS to IAC.
The increase in the proportion of CAFs and the expression levels of
MMP-9 may be an early event before adenocarcinomas become invasive.
CAFs had a strong correlation with MMP-9 in both non-invasive and
invasive components. The LVD increased from MIA to IAC and was
strongly associated with CAFs and MMP-9 only in the invasive
component. Within stage IA IAC, only the LVD was a prognostic
marker for OS. The current findings provided novel insights into
the biology of the tumor microenvironment in the early progression
of lung adenocarcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Bureau of Science and Technology of Zhoushan (grant no.
2017B31112).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CC, BZ and WL designed and performed the study,
analyzed the data and wrote the manuscript. ZC, JW, YZ, YW, TD and
HL made substantial contributions to the design of the study,
acquisition of data, interpretation of data and revision of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Zhoushan Hospital (Zhoushan, China). Informed
consent from patients was exempted due to the retrospective nature
of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
National Lung Screening Trial Research
Team, ; Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD,
Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM and Sicks JD:
Reduced lung-cancer mortality with low-dose computed tomographic
screening. N Engl J Med. 365:395–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger K, Yatabe Y, Powell CA, Beer D, Riely G,
Garg K, et al: International association for the study of lung
Cancer/American thoracic Society/European respiratory society
international multidisciplinary classification of lung
adenocarcinoma. Proc Am Thorac Soc. 8:381–385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noguchi M: Stepwise progression of
pulmonary adenocarcinoma-clinical and molecular implications.
Cancer Metastasis Rev. 29:15–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scott WJ, Howington J, Feigenberg S,
Movsas B and Pisters K; American College of Chest Physicians, :
Treatment of non-small cell lung cancer stage I and stage II: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132 (3 Suppl):234S–242S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campbell I, Polyak K and Haviv I: Clonal
mutations in the cancer-associated fibroblasts: The case against
genetic coevolution. Cancer Res. 69:6765–6768. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishii G, Ochiai A and Neri S: Phenotypic
and functional heterogeneity of cancer-associated fibroblast within
the tumor microenvironment. Adv Drug Deliv Rev. 99:186–196. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feig C, Jones JO, Kraman M, Wells RJ,
Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL,
et al: Targeting CXCL12 from FAP-expressing carcinoma-associated
fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic
cancer. Proc Natl Acad Sci USA. 110:20212–20217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Egeblad M, Rasch MG and Weaver VM: Dynamic
interplay between the collagen scaffold and tumor evolution. Curr
Opin Cell Biol. 22:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito M, Ishii G, Nagai K, Maeda R, Nakano Y
and Ochiai A: Prognostic impact of cancer-associated stromal cells
in patients with stage I lung adenocarcinoma. Chest. 142:151–158.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawase A, Ishii G, Nagai K, Ito T, Nagano
T, Murata Y, Hishida T, Nishimura M, Yoshida J, Suzuki K and Ochiai
A: Podoplanin expression by cancer associated fibroblasts predicts
poor prognosis of lung adenocarcinoma. Int J Cancer. 123:1053–1059.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: A dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vandooren J, Van den Steen PE and
Opdenakker G: Biochemistry and molecular biology of gelatinase B or
matrix metalloproteinase-9 (MMP-9): The next decade. Crit Rev
Biochem Mol Biol. 48:222–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ebrahem Q, Chaurasia SS, Vasanji A, Qi JH,
Klenotic PA, Cutler A, Asosingh K, Erzurum S and Anand-Apte B:
Cross-talk between vascular endothelial growth factor and matrix
metalloproteinases in the induction of neovascularization in vivo.
Am J Pathol. 176:496–503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mira E, Lacalle RA, Buesa JM, de Buitrago
GG, Jiménez-Baranda S, Gómez-Moutón C, Martínez-A C and Mañes S:
Secreted MMP9 promotes angiogenesis more efficiently than
constitutive active MMP9 bound to the tumor cell surface. J Cell
Sci. 117:1847–1857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai J, Li R, Xu X, Zhang L, Wu S, Yang T,
Fang L, Wu J, Zhu X, Li M and Huang Y: URGCP promotes non-small
cell lung cancer invasiveness by activating the NF-κB-MMP-9
pathway. Oncotarget. 6:36489–36504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stacker SA, Achen MG, Jussila L, Baldwin
ME and Alitalo K: Lymphangiogenesis and cancer metastasis. Nat Rev
Cancer. 2:573–583. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kadota K, Huang CL, Liu D, Ueno M, Kushida
Y, Haba R and Yokomise H: The clinical significance of
lymphangiogenesis and angiogenesis in non-small cell lung cancer
patients. Eur J Cancer. 44:1057–1067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwakiri S, Nagai S, Katakura H, Takenaka
K, Date H, Wada H and Tanaka F: D2-40-positive lymphatic vessel
density is a poor prognostic factor in squamous cell carcinoma of
the lung. Ann Surg Oncol. 16:1678–1685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: WHO Classification of Tumours of the Lung,
Pleura, Thymus and Heart. 4th. Lyon, France: IARC Press; 2015
|
|
24
|
Hashimoto O, Yoshida M, Koma Y, Yanai T,
Hasegawa D, Kosaka Y, Nishimura N and Yokozaki H: Collaboration of
cancer-associated fibroblasts and tumour-associated macrophages for
neuroblastoma development. J Pathol. 240:211–223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ikemura S, Aramaki N, Fujii S, Kirita K,
Umemura S, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Kuwata T, et al:
Changes in the tumor microenvironment during lymphatic metastasis
of lung squamous cell carcinoma. Cancer Sci. 108:136–142. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu JM, Liu K, Liu JH, Jiang XL, Wang XL,
Chen YZ, Li SG, Zou H, Pang LJ, Liu CX, et al: CD163 as a marker of
M2 macrophage, contribute to predicte aggressiveness and prognosis
of Kazakh esophageal squamous cell carcinoma. Oncotarget.
8:21526–21538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsu YL, Hung JY, Chiang SY, Jian SF, Wu
CY, Lin YS, Tsai YM, Chou SH, Tsai MJ and Kuo PL: Lung
cancer-derived galectin-1 contributes to cancer associated
fibroblast-mediated cancer progression and immune suppression
through TDO2/kynurenine axis. Oncotarget. 7:27584–27598. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Otomo R, Otsubo C, Matsushima-Hibiya Y,
Miyazaki M, Tashiro F, Ichikawa H, Kohno T, Ochiya T, Yokota J,
Nakagama H, et al: TSPAN12 is a critical factor for
cancer-fibroblast cell contact-mediated cancer invasion. Proc Natl
Acad Sci USA. 111:18691–18696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsubara D, Morikawa T, Goto A, Nakajima
J, Fukayama M and Niki T: Subepithelial myofibroblast in lung
adenocarcinoma: A histological indicator of excellent prognosis.
Mod Pathol. 22:776–785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kodate M, Kasai T, Hashimoto H, Yasumoto
K, Iwata Y and Manabe H: Expression of matrix metalloproteinase
(gelatinase) in T1 adenocarcinoma of the lung. Pathol Int.
47:461–469. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanomata N, Nakahara R, Oda T, Aoyagi Y,
Ishii G, Yokose T, Hasebe T, Nagai K, Yokozaki H and Ochiai A:
Expression and localization of mRNAs for matrix metalloproteinases
and their inhibitors in mixed bronchioloalveolar carcinomas with
invasive components. Mod Pathol. 18:828–837. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taguchi A, Kawana K, Tomio K, Yamashita A,
Isobe Y, Nagasaka K, Koga K, Inoue T, Nishida H, Kojima S, et al:
Matrix metalloproteinase (MMP)-9 in cancer-associated fibroblasts
(CAFs) is suppressed by omega-3 polyunsaturated fatty acids in
vitro and in vivo. PLoS One. 9:e896052014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei R, Lv M, Li F, Cheng T, Zhang Z, Jiang
G, Zhou Y, Gao R, Wei X and Lou J: Human CAFs promote
lymphangiogenesis in ovarian cancer via the Hh-VEGF-C signaling
axis. Oncotarget. 8:67315–67328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin NN, Wang P, Zhao D, Zhang FJ, Yang K
and Chen R: Significance of oral cancer-associated fibroblasts in
angiogenesis, lymphangiogenesis, and tumor invasion in oral
squamous cell carcinoma. J Oral Pathol Med. 46:21–30. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu QW, Yang QM, Huang YF, She HQ, Liang J,
Yang QL and Zhang ZM: Expression and clinical significance of
matrix metalloproteinase-9 in lymphatic invasiveness and metastasis
of breast cancer. PLoS One. 9:e978042014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cirri P and Chiarugi P: Cancer associated
fibroblasts: The dark side of the coin. Am J Cancer Res. 1:482–497.
2011.PubMed/NCBI
|
|
37
|
Chen L, Qin Y, Zhang T, Ding N, Chen Y,
Zhang Z and Guo C: Clinical significance of cancer-associated
fibroblasts and their correlation with microvessel and lymphatic
vessel density in lung adenocarcinoma. J Clin Lab Anal.
33:e228322019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshizawa A, Motoi N, Riely GJ, Sima CS,
Gerald WL, Kris MG, Park BJ, Rusch VW and Travis WD: Impact of
proposed IASLC/ATS/ERS classification of lung adenocarcinoma:
Prognostic subgroups and implications for further revision of
staging based on analysis of 514 stage I cases. Mod Pathol.
24:653–664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ujiie H, Kadota K, Chaft JE, Buitrago D,
Sima CS, Lee MC, Huang J, Travis WD, Rizk NP, Rudin CM, et al:
Solid predominant histologic subtype in resected stage I lung
adenocarcinoma is an independent predictor of early, extrathoracic,
multisite recurrence and of poor postrecurrence survival. J Clin
Oncol. 33:2877–2884. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yanagawa N, Shiono S, Abiko M, Katahira M,
Osakabe M and Ogata SY: The Clinical impact of solid and
micropapillary patterns in resected lung adenocarcinoma. J Thorac
Oncol. 11:1976–1983. 2016. View Article : Google Scholar : PubMed/NCBI
|

















