Introduction
Esophageal cancer is one of the most common cancers,
and esophageal squamous cell carcinoma (ESCC) is the major type of
esophageal cancer (1–3). Due to late diagnosis, recurrence and
metastasis, the overall survival time of patients with ESCC is not
satisfactory (1,4). Therefore it is important to identify
the molecular mechanisms and develop novel strategies for ESCC
therapy (5,6). Recently, some oncogenes have been
reported to be responsible for the growth and metastasis of ESCC,
and some of them have been suggested to be potential therapeutic
targets (7,8). For instance, microRNA (miR)-130b
promotes the progression of ESCC by targeting SASH1, and is
suggested to be a therapeutic target for ESCC (7).
Long non-coding (lnc)RNAs, a type of non-coding RNA
containing >200 nucleotides, have been demonstrated to play
crucial roles in different physiological and pathological
processes, such as cell viability, proliferation, migration,
invasion, apoptosis, and tumorigenesis (9,10).
Furthermore, some lncRNAs are upregulated or downregulated and have
promotor or suppressor functions in malignant tumors, including
ESCC (11–13). For example, lncRNA TINCR was higher
in ESCC tissues compared with that in adjacent normal tissues, and
knockdown of lncRNA TINCR expression inhibits the proliferation,
migration and invasion of ESCC cells (13). In addition, lncRNA MEG3 induces ESCC
cell apoptosis via endoplasmic reticulum stress (14).
Cervical carcinoma expressed PCNA regulatory lncRNA
(CCEPR) is localized to chromosome 10q21.1 and has been frequently
upregulated in several common types of cancer, such as gastric,
liver, cervical, lung, colorectal and bladder cancers (15–20).
Overexpression of CCEPR predicts a poor prognosis for cervical
cancer (19). Furthermore, several
studies have shown that CCEPR plays oncogenic roles by regulating
tumor cell proliferation, apoptosis, migration and invasion
(16,17). For example, Liao et al
(16) showed that CCEPR promoted the
proliferation, metastasis and invasion of non-small lung cancer
cells, while Peng et al (17)
found that knockdown of CCEPR using shRNA significantly induced
growth arrest and cell apoptosis in hepatocellular carcinoma.
However, to the best of our knowledge, the expression and exact
role of CCEPR in ESCC have not been previously reported.
Therefore, the aim of the present study was to
investigate the expression level of CCEPR in ESCC. In addition, to
determine the function of CCEPR in regulating the malignant
phenotypes of ESCC cells in vitro.
Materials and methods
Tissue collection
ESCC and adjacent non-tumor tissues (at least 3 cm
from the primary tumor) were collected from 56 patients with ESCC
who underwent surgical resection from September 2011 to April 2013.
Patients with ESCC who received preoperative chemo- or radiotherapy
were excluded from the study. All of the tissues were stored at
−80°C until further use. The present study was approved by the
Ethics Committee of the First People's Hospital of Chenzhou City
(Chenzhou, China). Written informed consent was provided by all the
participants prior to the study. The follow-up time was 5 years
from surgical resection.
Cell culture and transfection
The human Het-1A esophageal epithelial cell line,
and four human ESCC cell lines, TE-9, ECA109, KYSE150 and EC9706,
were obtained from the Cell Bank of the Chinese Academy of
Sciences. The cell lines were cultured in DMEM with 10% FBS (both
from Thermo Fisher Scientific, Inc.) at 37°C in a humidified
incubator with 5% CO2. For cell transfection, the TE-9
and EC9706 cells were transfected with 100 nM CCEPR short hairpin
(sh)RNA1 (cat. no. n352462), CCEPR shRNA2 (cat. no. n352463), or
negative control (NC) shRNA (cat. no. 4457289) using
Lipofectamine® 2000 (all from Thermo Fisher Scientific,
Inc.). Reverse transcription-quantitative PCR (RT-qPCR) was
performed to examine the mRNA expression level of CCEPR, 48 h
following transfection.
RT-qPCR
TRIzol® reagent (Thermo Fisher
Scientific, Inc.) was used to extract total RNA from tissues and
cell lines. The total RNA was then reversed transcribed into cDNA
using a High Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.). The mRNA expression levels were then determined
using a SYBR® Green Real-time PCR Master Mix (Toyobo
Life Science) on a Roche 480 Real-Time PCR system (Roche
Diagnostics). The following thermocycling conditions were used:
Initial denaturation at 95°C for 3 min, followed by 40 cycles at
95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec. The relative
expression was determined with the 2−ΔΔCq method
(21). The primer sequences used
were as follows: CCEPR forward, 5′-AAGGTCCCAGGATACTCGC-3′ and
reverse, 5′-GTGTCGTGGACTGGCAAAAT-3′; GAPDH forward,
5′-CTGGGCTACACTGAGCACC-3′ and reverse,
5′-AAGTGGTCGTTGAGGGCAATG-3′.
Cell Counting Kit-8 (CCK-8)
assays
Transfected TE-9 and EC9706 cells (5,000 cells/well)
were seeded in 96-well plates, then cultured for 0, 24, 48 and 72
h. Next, 10 µl CCK-8 solution (Thermo Fisher Scientific, Inc.) was
added to the cells and incubated at 37°C for 2 h, according to the
manufacturer's instructions. The absorbance (450 nm) was determined
using a microplate reader (Bio-Rad Laboratories, Inc.).
Colony formation assay
Transfected TE-9 and EC9706 cells (300 cells/well in
6-well plates) were cultured in DMEM supplemented with 10% FBS for
2 weeks. Then, the cells were stained with a 0.5% crystal violet
solution at room temperature for 5 min. The colony numbers
(containing >50 cells) were counted.
Cell apoptosis assay
Transfected TE-9 and EC9706 cells were washed with
Dulbecco's phosphate (DPBS; Thermo Fisher Scientific, Inc.) and
fixed in 75% ethanol at 4°C for 3 h. After washing with PBS, twice,
the cells were stained with both FITC Annexin V and propidium
iodide (both from BD Biosciences) at room temperature for 30 min.
The cell apoptosis rate was examined using a FACScan flow cytometer
and analyzed using the Accuri C6 system software v1.0 (both from BD
Biosciences).
Cell migration assay
For the cell migration assay, transfected TE-9 and
EC9706 cells in 12-well plates (5×105 cells/well) were
cultured at 37°C, to ~95% confluence. A 100 µ sterile pipette tip
was used to scratch the cells to generate a wound. The cells were
washed with DPBS and were added to each well with serum-free DMEM.
At this time point (indicated as 0 h), images of the wound were
obtained, using a light microscope (×40). Then, the cells were
incubated at 37°C for 24 h, and images of the wound were obtained
again, under a light microscope (×40). Wound closure rate is
calculated based on wound area relative to the original size using
ImageJ (version 1.8; National Institutes of Health).
Cell invasion assay
Matrigel-coated Transwell chambers (BD Biosciences,
USA) were used to perform the cell invasion assay. The precoating
was performed at room temperature for 30 min. In brief, transfected
TE-9 and EC9706 cells (50,000 cells/well) in 400 µl serum-free DMEM
were added to the upper chamber. Next, 300 µl DMEM with 10% FBS was
added to the lower chamber. After incubation at 37°C for 24 h, the
invading cells were stained with a 0.1% crystal violet solution at
room temperature for 10 min. Then, images were obtained using a
light microscope (×200).
Western blot analysis
Total protein was extracted from the tissues and
cells using a RIPA buffer (Thermo Fisher Scientific, Inc.). The
protein concentrations were determined with a Pierce bicinchoninic
acid protein assay kit (Thermo Fisher Scientific, Inc.). The
proteins were separated using 10% SDS-PAGE and then transferred
onto polyvinylidene fluoride membranes (Thermo Fisher Scientific,
Inc.). After blocking with 5% skimmed milk overnight at 4°C, the
membranes were incubated with the primary antibodies against
caspase-3 (cat. no. ab13847; 1:500), Bcl2 (cat. no. ab32124;
1:200), Bax (cat. no. ab32503; 1:500), matrix metalloproteinase
[(MMP)2; cat. no. ab97779; 1:200], MMP9 (cat. no. ab38898; 1:500),
N-cadherin (cat. no. ab76057; 1:500), vimentin (cat. no. ab45939;
1:500), E-cadherin (cat. no. ab227639; 1:200), and GAPDH (cat. no.
ab9485; 1:500) at room temperature for 5 h and then with the
HRP-conjugated goat anti-rabbit secondary antibody (cat. no.
ab6721; 1:10,000) at room temperature for 2 h (all antibodies were
obtained from Abcam). A western lightning™ chemiluminescence
reagent plus kit (Thermo Fisher Scientific, Inc.) was used to
visualize the bands, while the ImageJ software v1.48 (National
Institutes of Health) was used to determine the protein expression
level.
Statistical analysis
The data are expressed as the mean ± SD and the
experiments were repeated 3 times. SPSS v19.0 (IBM Corp.) was used
for statistical analysis. A one-way ANOVA followed by Tukey's post
hoc test was used to analyze the differences between more than two
groups, while an unpaired Student's t-test was used to analyze the
differences between 2 groups. Based on the median expression value
(2.372) of CCEPR, the patients were divided into a high and a low
CCEPR expression level group. The associations between CCEPR mRNA
expression levels and the clinicopathological characteristics of
patients with ESCC were analyzed using a χ2 test. The
survival analysis was performed using Kaplan-Meier analysis and
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Upregulation of CCEPR is associated
with ESCC progression
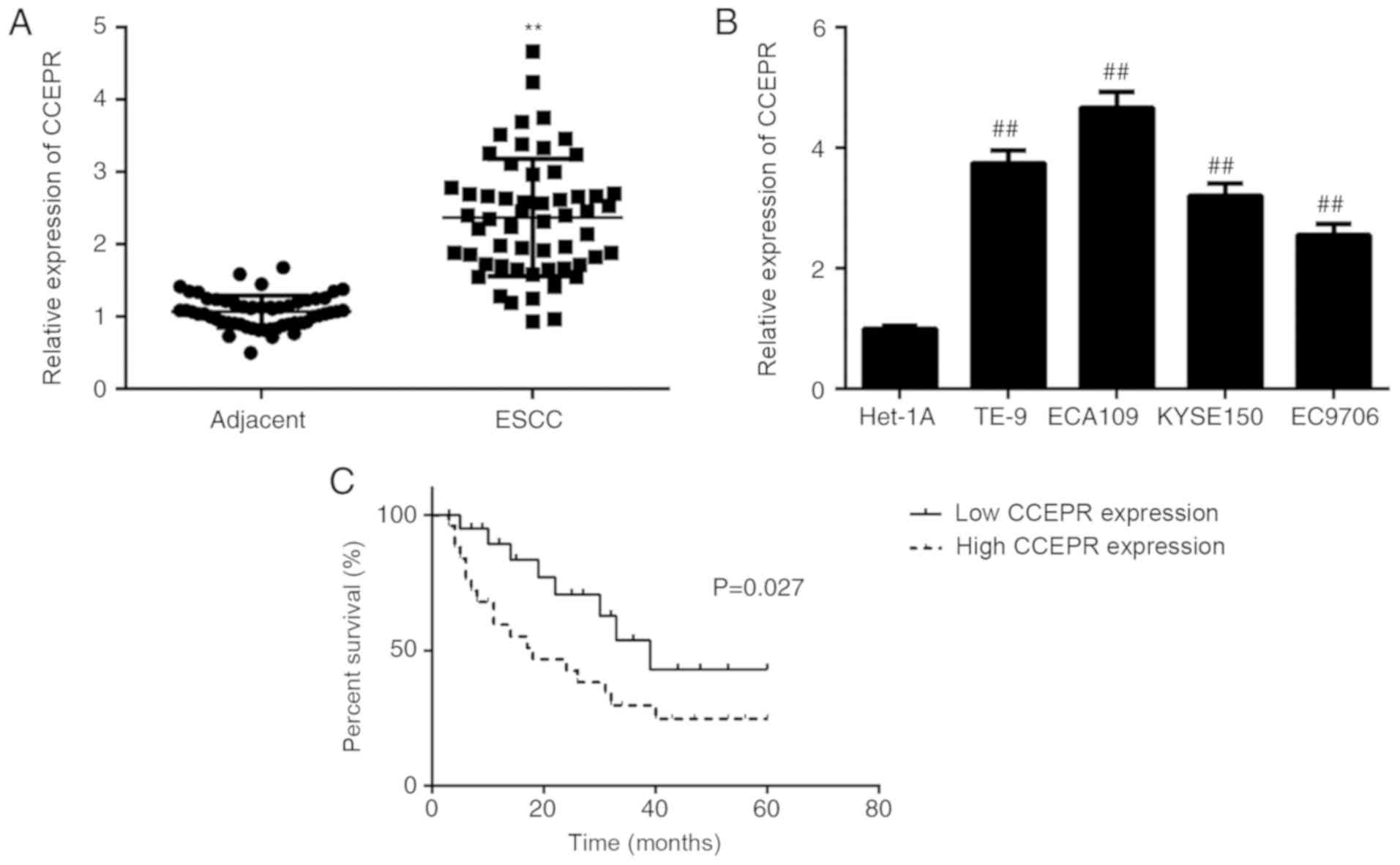
In the present study, the mRNA expression levels of
CCEPR in ESCC tissues was determined using RT-qPCR. As shown in
Fig. 1A, the expression levels of
CCEPR were significantly higher in ESCC tissues compared with that
in adjacent non-tumor tissues. In addition, the CCEPR mRNA
expression level was also significantly higher in ESCC cell lines
compared with that in the Het-1A cell line (Fig. 1B).
We hypothesized that the increased mRNA expression
of CCEPR might be involved in the development and progression of
ESCC. To investigate this hypothesis, the clinical significance of
CCEPR mRNA expression in ESCC was determined. Based on the median
expression value (2.372) of CCEPR, the patients were divided into a
high and a low CCEPR expression level groups. As shown in Table I, a χ2 test data indicated
that high CCEPR expression was significantly associated with
advanced TNM stage, as well as lymph node metastasis, suggesting
that overexpression of CCEPR may play an important role in ESCC
progression. Subsequently, the overall survival time was
significantly shorter for patients with ESCC and high CCEPR mRNA
expression levels compared with that in patients with low CCEPR
expression levels (Fig. 1C). Thus,
upregulation of CCEPR may predict poor prognosis in ESCC.
 | Table I.Association between cervical carcinoma
expressed PCNA regulatory lncRNA mRNA expression level and
clinicopathological characteristics in patients with esophageal
squamous cell carcinoma. |
Table I.
Association between cervical carcinoma
expressed PCNA regulatory lncRNA mRNA expression level and
clinicopathological characteristics in patients with esophageal
squamous cell carcinoma.
|
|
| CCEPR mRNA expression
level |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Number (n=56) | High (n=28) | Low (n=28) | P-value |
|---|
| Age, years |
|
|
| 0.778 |
|
<55 | 19 | 9 | 10 |
|
| ≥55 | 37 | 19 | 18 |
|
| Sex |
|
|
| 0.577 |
|
Male | 36 | 17 | 19 |
|
|
Female | 20 | 11 | 9 |
|
| Grade |
|
|
| 0.064 |
| Well
and moderate | 42 | 18 | 24 |
|
|
Poor | 14 | 10 | 4 |
|
| Lymph node
metastasis |
|
|
| 0.032a |
|
Negative | 30 | 11 | 19 |
|
|
Positive | 26 | 17 | 9 |
|
| TNM stage |
|
|
| 0.029a |
|
I–II | 34 | 13 | 21 |
|
|
III–IV | 22 | 15 | 7 |
|
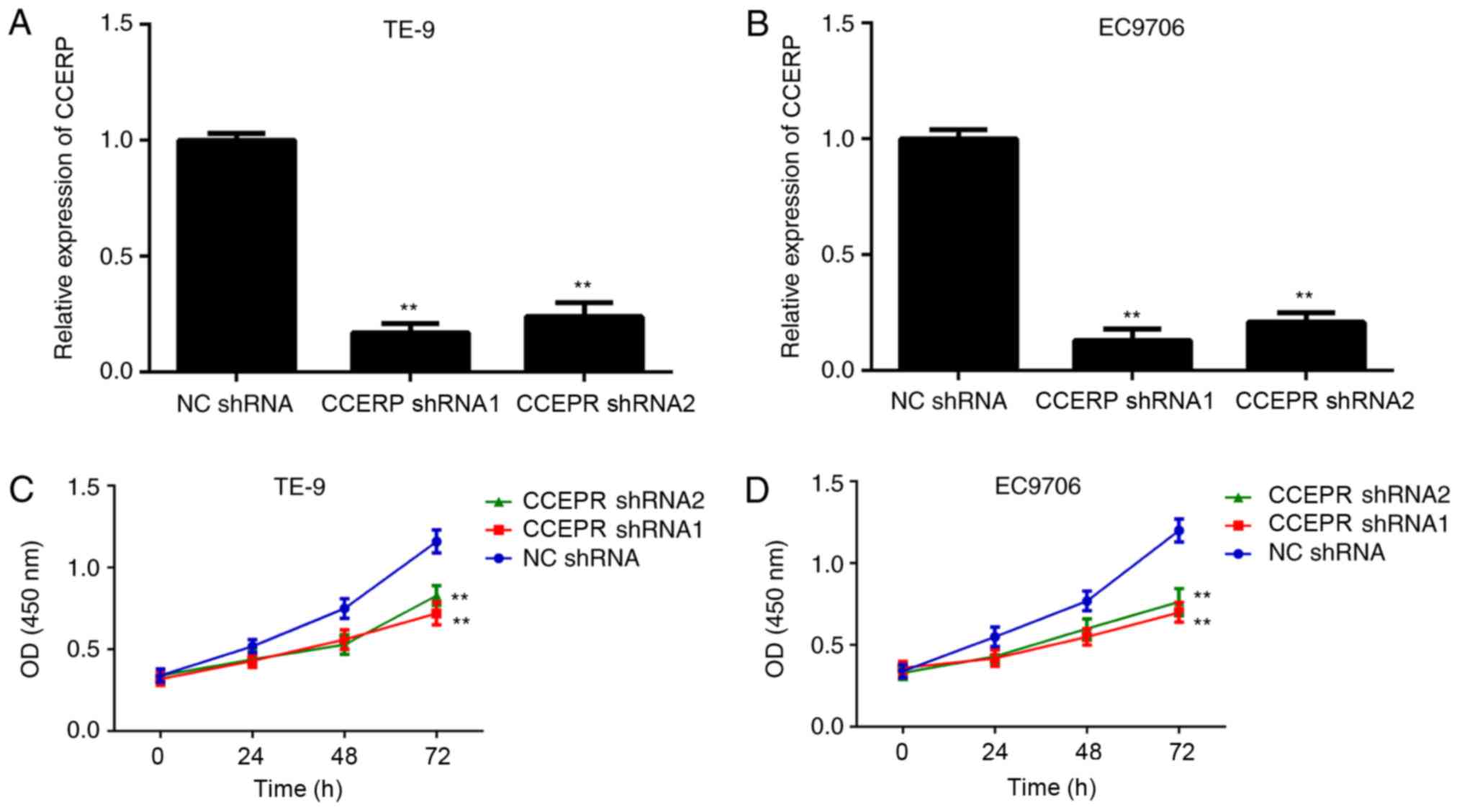
Knockdown of CCEPR expression
suppresses ESCC cell proliferation
Then, the function of CCEPR in regulating ESCC cells
in vitro was investigated. As CCEPR was significantly
upregulated in the ESCC cell lines, and the TE-9 and EC9706 cell
lines showed the highest mRNA expression levels of CCEPR, the TE-9
and EC9706 cell lines were transfected with two shRNAs to
downregulate CCEPR expression. CCEPR was downregulated in the CCEPR
shRNA 1 and shRNA 2 groups compared with that in the NC shRNA
group, in both cell lines (Fig. 2A and
B). A CCK-8 assay was then performed to determine the effects
of CCEPR downregulation on ESCC cell proliferation and the results
showed that the number of TE-9 and EC9706 cells was significantly
lower in the CCEPR shRNA groups compared with that in the NC shRNA
group (Fig. 2C and D). Thus, CCEPR
may promote ESCC cell proliferation.
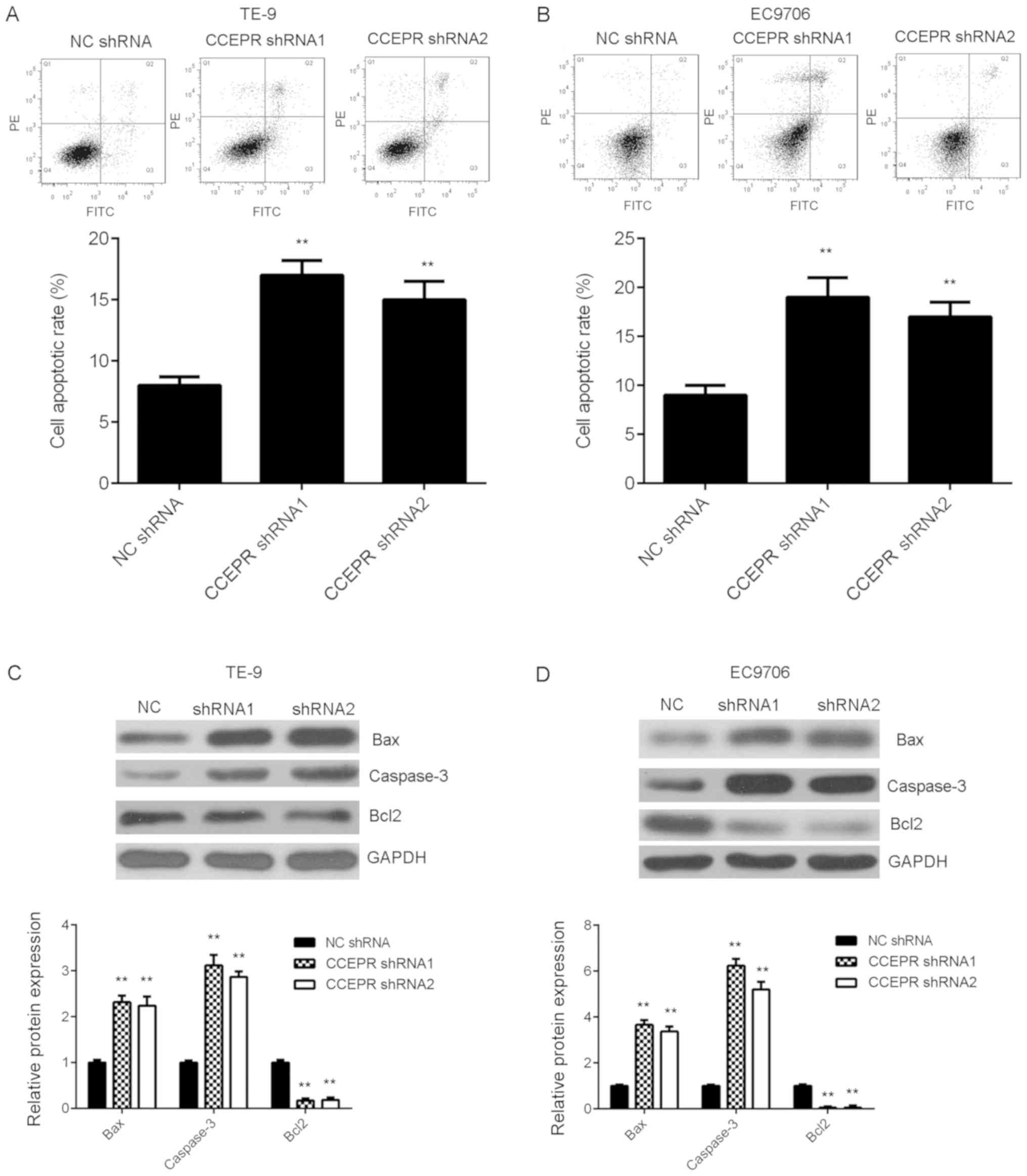
Knockdown of CCEPR induces cell
apoptosis
The reduced cell proliferation caused by CCEPR
downregulation may be due to increased cell apoptosis. Thus, flow
cytometry was performed to examine the rate of cell apoptosis in
each experimental group. As shown in Fig. 3A and B, apoptosis of both the ESCC
cell lines transfected with 2 types of shRNA was significantly
increased following silencing of CCEPR mRNA expression compared
with that in the NC group. Subsequently, the protein expression
levels of several key apoptotic-related genes, including
pro-apoptotic Bax and caspase-3 and anti-apoptotic Bcl2 were
investigated. Knockdown of CCEPR significantly increased the
protein expression levels of Bax and caspase-3, while the protein
expression levels of Bcl2 was decreased in both ESCC cell lines
transfected with shRNA (Fig. 3C and
D).
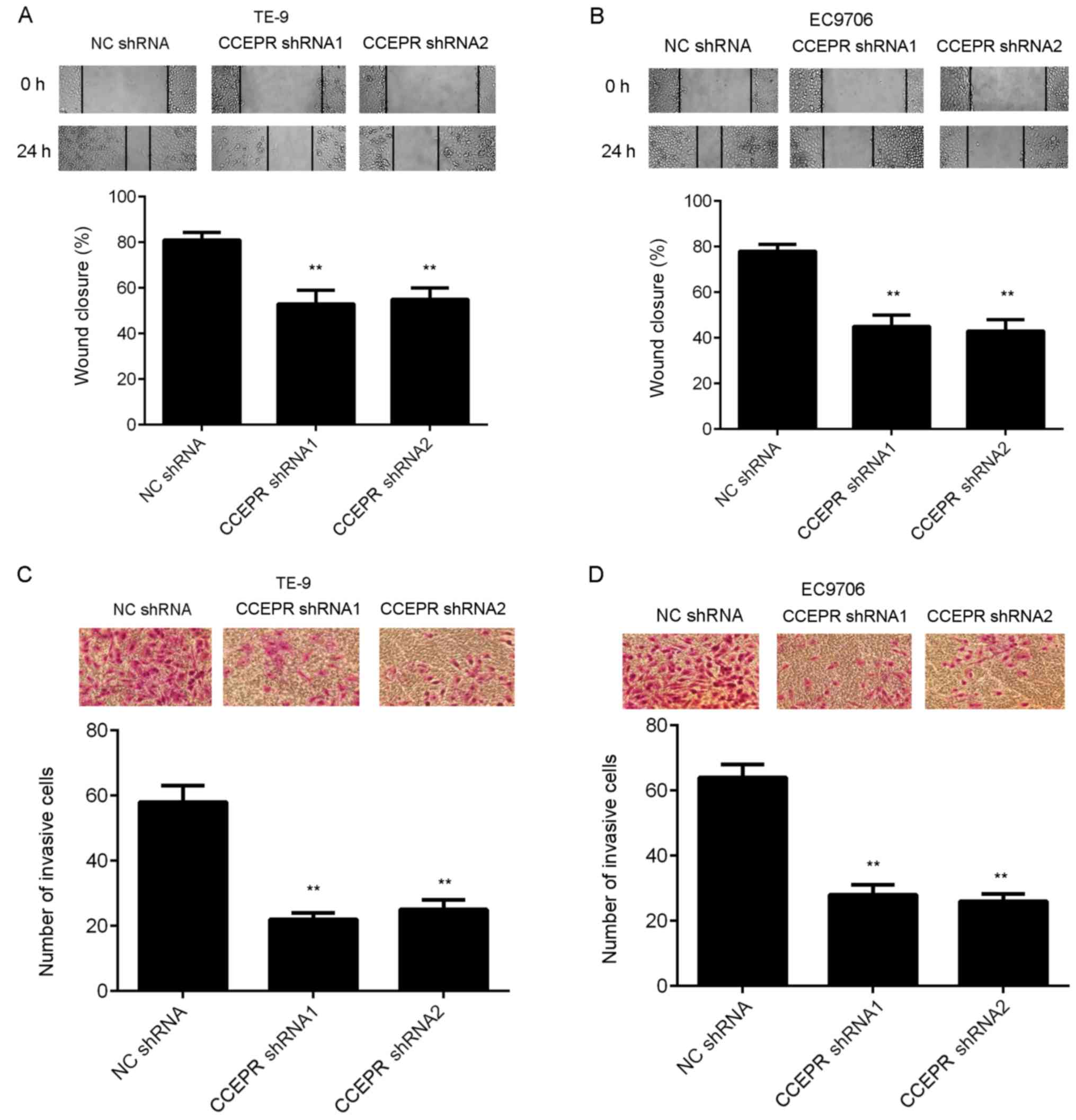
Inhibition of CCEPR suppresses ESCC
cell migration, invasion and epithelial-mesenchymal transition
(EMT)
The effects of CCEPR knockdown on the migratory and
invasive abilities of ESCC cells was subsequently investigated. The
results from the wound healing assay indicated that the migration
of TE-9 and EC9706 cells was significantly inhibited following
downregulation of CCEPR (Fig. 4A and
B). Similarly, silencing CCEPR expression also downregulated
the invasive abilities of both ESCC cell lines (Fig. 4C and D). The protein expression
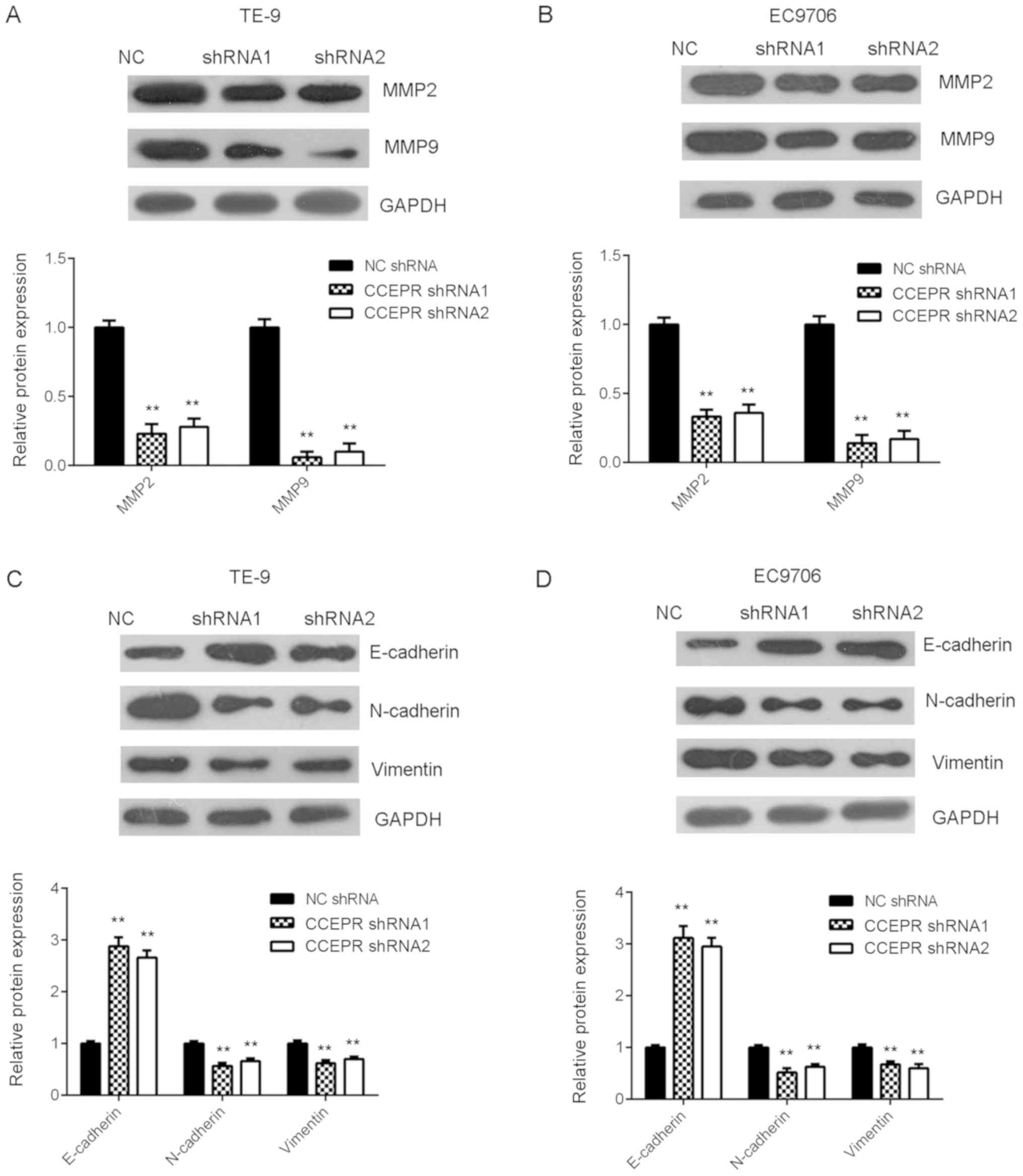
levels of MMP2 and MMP9 were then examined using western blot
analysis. As shown in Fig. 5A and B,
downregulation of CCEPR significantly decreased the protein
expression levels of MMP2 and MMP9. Then, the effects of CCEPR
downregulation on EMT in both the ESCC cell lines was investigated
and the results showed that silencing CCEPR increased the protein
expression levels of E-cadherin, while the protein expression
levels of N-cadherin and vimentin were decreased, indicating that
EMT was reduced (Fig. 5C and D).
Therefore, CCEPR may promote EMT in ESCC cell lines.
Discussion
A previous study has shown that overexpression of
CCEPR predicts poor prognosis for cervical cancer, and could
promote cervical cancer cell proliferation by upregulating
proliferating cell nuclear antigen (19). To the best of our knowledge, however,
the exact role of CCEPR in ESCC has not been previously reported.
Therefore, the present study investigated the mRNA expression level
of CCEPR in ESCC and it was found that CCEPR was upregulated in
ESCC tissues and cell lines, and upregulation of CCEPR was
associated with tumor progression and poor prognosis in ESCC.
Furthermore, knockdown of CCEPR inhibited cell proliferation,
migration, invasion, and EMT in ESCC cells, while inducing ESCC
cell apoptosis.
In recent years, a large number of lncRNAs have been
found to be increased or decreased in a variety of human
malignancies, and some specific lncRNAs have been reported to play
crucial roles during ESCC progression (22–25). For
example, lncRNA SNHG6 was upregulated in ESCC tissues and cell
lines and was associated with advanced TNM stage, lymph node
metastasis, and shorter survival time in patients with ESCC, and
SNHG6 promotes ESCC cell proliferation, migration and invasion
(26). In addition, overexpression
of lncRNA SNHG20 was found to promote ESCC cell growth and
metastasis by regulating the ATM-JAK-PD-L1 signaling pathway
(27). In the present study, CCEPR
mRNA expression levels were significantly higher in ESCC tissues
compared with that in adjacent non-tumor tissues. In addition,
CCEPR mRNA expression was also higher in ESCC cell lines compared
with that in esophageal epithelial Het-1A cell line. As no previous
study has revealed the clinical significance of CCEPR mRNA
expression in ESCC, a χ2 test was performed to
investigate the clinical significance of CCEPR mRNA expression
level in ESCC. Upregulation of CCEPR was significantly associated
with advanced TNM stage, as well as lymph node metastasis in ESCC.
Therefore, we hypothesized that overexpression of CCEPR may be
involved in the malignant progression of ESCC. To further
investigate this hypothesis, the association between CCEPR mRNA
expression levels and prognosis in patients with ESCC was
investigated and the results showed that patients with high CCEPR
mRNA expression levels had a shorter survival time compared with
than those with low CCEPR mRNA expression levels. These findings
suggested that high expression of CCEPR predicts poor prognosis in
patients with ESCC.
To further reveal the role of lncRNA CCEPR during
ESCC progression, in vitro experiments were performed to
identify the exact function of CCEPR in regulating ESCC cell
proliferation, apoptosis, migration and invasion. As the TE-9 and
EC9706 cell lines had the highest expression level of CCEPR, among
the four examined ESCC cell lines, these were selected for the
in vitro experiments. A total of 2 CCEPR-specific shRNAs
were then used to transfect TE-9 and EC9706 cells to reduce the
expression level of CCEPR. Knockdown of CCEPR significantly
suppressed ESCC cell proliferation and colony formation abilities,
while apoptosis was increased. Thus, CCEPR may promote ESCC cell
growth. Subsequently, silencing CCEPR significantly inhibited ESCC
cell migration and invasion. Thus, CCEPR may have promoting effects
on ESCC metastasis. MMP2 and MMP9, two key matrix
metalloproteinases, play key roles in cancer metastasis by
promoting tumor cell migration and invasion (28,29).
Consistent with the results from the cell migration and invasion
assays, the protein expression levels of MMP2 and MMP9 were
significantly reduced following CCEPR knockdown, suggesting that
MMP2 and MMP9 may be involved in CCEPR-mediated ESCC cell
metastasis. EMT is characterized by epithelial phenotype loss and
mesenchymal phenotype acquisition (30). EMT has been demonstrated to play a
promoting role during tumor metastasis, and inhibition of EMT could
suppress the migratory and invasive abilities of various cancer
cells such as cholangiocarcinoma and ovarian cancer (31,32).
Furthermore, some lncRNAs have been reported to be associated with
EMT in different types of cancer (33,34). For
example, lncRNA SNHG6 promoted lung cancer cell migration and
invasion by increasing EMT (33).
E-cadherin, N-cadherin and vimentin are the most representative EMT
markers (30). E-cadherin is an
important epithelial marker, while N-cadherin and vimentin are two
key mesenchymal markers (30). The
present study showed that knockdown of CCEPR significantly
increased the protein expression levels of E-cadherin and reduced
the protein expression levels of N-cadherin and vimentin in ESCC
cell lines, indicating that EMT was suppressed. These findings
suggested that the inhibitory effects of CCEPR downregulation on
ESCC metastasis in vitro may be by inhibiting EMT.
In conclusion, the findings in the present study
indicated that upregulation of CCEPR was associated with tumor
progression and poor prognosis in ESCC and that knockdown of CCEPR
expression significantly suppressed the malignant phenotypes of 2
ESCC cell lines. Therefore, the lncRNA CCEPR may be used as a
promising therapeutic target for ESCC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW designed the study, and wrote and revised the
manuscript. HZ collected the clinical tissues and clinical data,
and analyzed the clinical data. LZ, HO, WL, XL performed the
experiments and analyzed the data. All approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of First People's Hospital of Chenzhou City. Written
informed consent was provided by all participants.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Yu H, Zhang Y, Zhang X, Zheng G,
Gao Y, Wang C and Zhou L: A functional TNFAIP2 3′-UTR rs8126
genetic polymorphism contributes to risk of esophageal squamous
cell carcinoma. PLoS One. 9:e1093182014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He Y, Mingyan E, Wang C, Liu G, Shi M and
Liu S: CircVRK1 regulates tumor progression and radioresistance in
esophageal squamous cell carcinoma by regulating
miR-624-3p/PTEN/PI3K/AKT signaling pathway. Int J Biol Macromol.
125:116–123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Y, Ma Y, Peng H, Gong L, Xiao M, Xiang
L, He D and Cao K: MiR-130b promotes the progression of oesophageal
squamous cell carcinoma by targeting SASH1. J Cell Mol Med.
23:93–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu X, Li W, Xia Z, Xie L, Ma X, Liang Q,
Liu L, Wang J, Zhou X, Yang Y and Liu H: Targeting MCL-1 sensitizes
human esophageal squamous cell carcinoma cells to cisplatin-induced
apoptosis. BMC Cancer. 17:4492017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hua Y, Zhao K, Tao G, Dai C and Su Y:
MiR-25 promotes metastasis via targeting FBXW7 in esophageal
squamous cell carcinoma. Oncol Rep. 38:3030–3038. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Rugeebah A, Alanazi M and Parine NR:
MEG3: An oncogenic long non-coding RNA in different cancers. Pathol
Oncol Res. 25:859–874. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao M, Wang J, Xi X, Tan N and Zhang L:
SNHG12 promotes angiogenesis following ischemic stroke via
regulating miR-150/VEGF pathway. Neuroscience. 390:231–240. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bo H, Fan L, Gong Z, Liu Z, Shi L, Guo C,
Li X, Liao Q, Zhang W, Zhou M, et al: Upregulation and
hypomethylation of lncRNA AFAP1AS1 predicts a poor prognosis and
promotes the migration and invasion of cervical cancer. Oncol Rep.
41:2431–2439. 2019.PubMed/NCBI
|
|
12
|
Lv D, Sun R, Yu Q and Zhang X: The long
non-coding RNA maternally expressed gene 3 activates p53 and is
downregulated in esophageal squamous cell cancer. Tumour Biol.
2016.(Epub ahead of print). View Article : Google Scholar
|
|
13
|
Xu Y, Qiu M, Chen Y, Wang J, Xia W, Mao Q,
Yang L, Li M, Jiang F, Xu L and Yin R: Long noncoding RNA, tissue
differentiation-inducing nonprotein coding RNA is upregulated and
promotes development of esophageal squamous cell carcinoma. Dis
Esophagus. 29:950–958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang ZL, Chen RP, Zhou XT, Zhan HL, Hu
MM, Liu B, Wu GD and Wu LF: Long non-coding RNA MEG3 induces cell
apoptosis in esophageal cancer through endoplasmic reticulum
stress. Oncol Rep. 37:3093–3099. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu G, Zhang Y, Li N, Zhang JB and Xu R:
LncRNA CCHE1 in the proliferation and apoptosis of gastric cancer
cells. Eur Rev Med Pharmacol Sci. 22:2631–2637. 2018.PubMed/NCBI
|
|
16
|
Liao Y, Cheng S, Xiang J and Luo C: lncRNA
CCHE1 increased proliferation, metastasis and invasion of non-small
lung cancer cells and predicted poor survival in non-small lung
cancer patients. Eur Rev Med Pharmacol Sci. 22:1686–1692.
2018.PubMed/NCBI
|
|
17
|
Peng W and Fan H: Long noncoding RNA CCHE1
indicates a poor prognosis of hepatocellular carcinoma and promotes
carcinogenesis via activation of the ERK/MAPK pathway. Biomed
Pharmacother. 83:450–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhan Y, Li Y, Guan B, Chen X, Chen Z, He
A, He S, Gong Y, Peng D, Liu Y, et al: Increased expression of long
non-coding RNA CCEPR is associated with poor prognosis and promotes
tumorigenesis in urothelial bladder carcinoma. Oncotarget.
8:44326–44334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Wang CX, Sun XX, Wang C, Liu TF
and Wang DJ: Long non-coding RNA CCHE1 overexpression predicts a
poor prognosis for cervical cancer. Eur Rev Med Pharmacol Sci.
21:479–483. 2017.PubMed/NCBI
|
|
20
|
Gaballah HH, Gaber RA, Elrashidy MA,
Elshahat DA, Hablus MA and Ebeid AM: Expression of long non-coding
RNA CCHE1 in colorectal carcinoma: Correlations with
clinicopathological features and ERK/COX-2 pathway. Mol Biol Rep.
46:657–667. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Liang Y, Wu Y, Chen X, Wang K, Li
J, Guan X, Xiong G, Yang K and Bai Y: Upregulation of a novel
lncRNA LINC01980 promotes tumor growth of esophageal squamous cell
carcinoma. Biochem Biophys Res Commun. 513:73–80. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong Z, Liang X, Wu X, Kang X, Guo Y, Shen
S, Liang J and Guo W: Promoter hypermethylation-mediated
downregulation of tumor suppressor gene SEMA3B and lncRNA
SEMA3B-AS1 correlates with progression and prognosis of esophageal
squamous cell carcinoma. Clin Exp Metastasis. 36:225–241. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui Z, Luo Z, Lin Z, Shi L, Hong Y and Yan
C: Long non-coding RNA TTN-AS1 facilitates tumorigenesis of
papillary thyroid cancer through modulating miR-153-3p/ZNRF2 axis.
J Gene Med. 21:e30832019. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang ZW, Chen JJ, Xia SH, Zhao H, Yang
JB, Zhang H, He B, Jiao J, Zhan BT and Sun CC: Long intergenic
non-protein coding RNA 319 aggravates lung adenocarcinoma
carcinogenesis by modulating miR-450b-5p/EZH2. Gene. 650:60–67.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Li R, Ding X, Zhang K and Qin W:
Upregulation of long non-coding RNA SNHG6 promote esophageal
squamous cell carcinoma cell malignancy and its diagnostic value.
Am J Transl Res. 11:1084–1091. 2019.PubMed/NCBI
|
|
27
|
Zhang C, Jiang F, Su C, Xie P and Xu L:
Upregulation of long noncoding RNA SNHG20 promotes cell growth and
metastasis in esophageal squamous cell carcinoma via modulating
ATM-JAK-PD-L1 pathway. J Cell Biochem. 2019.(Epub ahead of
print).
|
|
28
|
Dahal U, Kang L and Gupta M: RNA m6A
methyltransferase METTL3 regulates invasiveness of melanoma cells
by matrix metallopeptidase 2. Melanoma Res. 29:382–389. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen W, Zhong X, Wei Y, Liu Y, Yi Q, Zhang
G, He L, Chen F, Liu Y and Luo J: Retraction note to: TGF-β
regulates survivin to affect cell cycle and the expression of EGFR
and MMP9 in glioblastoma. Mol Neurobiol. 54:75512017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh M, Yelle N, Venugopal C and Singh
SK: EMT: Mechanisms and therapeutic implications. Pharmacol Ther.
182:80–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia XL, Xue D, Xiang TH, Xu HY, Song DK,
Cheng PG and Wang JQ: Overexpression of long non-coding RNA CRNDE
facilitates epithelial-mesenchymal transition and correlates with
poor prognosis in intrahepatic cholangiocarcinoma. Oncol Lett.
15:4105–4112. 2018.PubMed/NCBI
|
|
32
|
Hua W, Zhao Y, Jin X, Yu D, He J, Xie D
and Duan P: METTL3 promotes ovarian carcinoma growth and invasion
through the regulation of AXL translation and epithelial to
mesenchymal transition. Gynecol Oncol. 151:356–365. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang R, Xiao G, Wang M, Li X, Li Y, Hui
Z, Sun X, Qin S, Zhang B, Du N, et al: SNHG6 functions as a
competing endogenous RNA to regulate E2F7 expression by sponging
miR-26a-5p in lung adenocarcinoma. Biomed Pharmacother.
107:1434–1446. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang W, Shan Z, Zhou X, Peng L, Zhi C,
Chai J, Liu H, Yang J and Zhang Z: Knockdown of lncRNA GHET1
inhibits osteosarcoma cells proliferation, invasion, migration and
EMT in vitro and in vivo. Cancer Biomark. 23:589–601. 2018.
View Article : Google Scholar : PubMed/NCBI
|