Introduction
Worldwide, renal cell carcinoma (RCC) represents the
sixth most frequently diagnosed cancer in men and the 10th in
women, accounting for 5 and 3% of all oncological diagnoses,
respectively, and there >140,000 RCC-associated deaths yearly
(1). Although kidney cancer is often
treated by chemotherapy, the side effects of chemotherapy are not
well tolerated by a number of patients (2). Therefore, novel antitumor drugs with
low toxicity and high efficiency are urgently required.
Ursolic acid (UA) is a pentacyclic triterpenoid
carboxylic acid (3) known to have
antitumor effects on various types of malignant tumors, such as
lung cancer, breast cancer and hepatocellular carcinoma (4–6). One of
the most important antitumor functions of UA is the inhibition of
the invasiveness of numerous types of cancer cells. UA has been
demonstrated to significantly suppress the invasive phenotype of
human gastric cancer cells (7,8) and
inhibit cell migration, invasion and activity of MMP-2 and −9 in
non-small cell lung cancer cells (9,10).
Additionally, UA has inhibitory effects on the growth and
metastatic ability of osteosarcoma cells by suppressing epidermal
growth factor receptor (11,12). However, to the best of our knowledge,
no previous studies have examined the effects of UA on the
invasiveness of renal cancer cells.
NLR family pyrin domain-containing 3 (NLRP3)
inflammasomes are multi-protein complexes composed of the intrinsic
intracellular immune receptor NLRP3, the adaptor protein
apoptosis-associated speck-like protein containing a CARD (ASC) and
protease caspase-1 (13). The
assembly of this complex can induce the maturation and secretion of
pro-inflammatory factors, such as IL-1β and IL-18, thus promoting
the development of an inflammatory response (14). Additionally, NLRP3 is associated with
tumor development NLRP3 signaling activation in macrophages can
contribute to colorectal cancer cell migration and invasion
(15). NLRP3 inflammasomes serve a
vital role in the regulation of the proliferation and migration of
lung cancer A549 cells (16).
Furthermore, it has been reported that liver X receptor α promotes
the metastasis of renal cell cancer via suppression of the
expression levels of NLRP3 inflammasomes (17). Whether UA has a role in regulation of
the NLRP3 inflammasome remains to be investigated.
The present study aimed to explore the effects of UA
on A498 cells and resolve the underlying mechanisms to identify a
potential therapeutic agent for renal cancer treatment. The cell
viability assay and cell invasion assay were performed to
demonstrate that the cell viability and invasiveness of renal
cancer cells were decreased following UA exposure. Western blotting
was performed to detect the activation of the NLRP3 inflammasome
and expression of MMP-2. MCC950 was used to inhibit the activation
of NLRP3. Furthermore, it was concluded that UA inhibited the
invasiveness of A498 cells via NLRP3 activation.
Materials and methods
Cell culture and regents
A498 cells were purchased from the American Type
Culture Collection. Cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2. The medium was
changed every 2 days. Cells were used for experiments in their
logarithmic growth phase. The MTS reagent kit was obtained from
Promega Corporation. Primary antibodies for NLRP3 (cat. no.
ab263899; 1:1,000), caspase-1 (cat. no. ab207802; 1:1,000), IL-1β
(cat. no. ab216995; 1:1,000), cleaved IL-1β p15 (cat. no. ab33774;
1:1,000), MMP-2 (cat. no. ab181286; 1:1,000) and GAPDH (cat. no.
ab181602; 1:1,000) were purchased from Abcam. Secondary
HRP-conjugated goat anti-rabbit IgG antibodies were purchased from
Beyotime Company, Shanghai, China. (cat. no. A0208; 1:10,000). UA
was purchased from Tianjin Jinyao Amino Acid Co., Ltd., and MCC950
was purchased from MedChemExpress.
Cell cytotoxicity assay
A498 cells were seeded into a 96-well microplate and
cultured until the cells reached 70% confluency. Cells were
cultured with medium containing UA (0, 0.05, 0.5 and 5 µM) for
different periods of time (12, 24 and 48 h). Subsequently, 10 µl
MTS was added and incubated at 37°C for 2 h. The absorbance value
of each well was detected at 490 nm using a microplate reader
(BioTek Instruments, Inc).
Cell invasion assay
Cell invasion was assessed using a Transwell assay
(pore size, 8 µm; Corning Inc.). Briefly, cells were treated with
0.5 uM UA or 50 nM MCC950 at 37°C for 24 h. Then, 5×104
treated cells were seeded in the upper chamber with serum-free
medium. The cells were attached to porous polycarbonate membranes,
which were previously coated with Matrigel basement membrane matrix
at 37°C for 30 min. The lower chamber was filled with DMEM with 15%
FBS. Cells that failed to attach to the polycarbonate membranes
were removed with a cotton swab. Subsequently, cells were incubated
for 30 h at 37°C, and the invading cells were fixed with 4%
paraformaldehyde at room temperature for 30 min. Subsequently,
cells were stained with crystal violet dissolved in 1% SDS for 30
min at 20°C. The number of cells visible in three randomly selected
visual fields under an Olympus CX23 light microscope
(magnification, ×200; Olympus Corporation) was recorded.
Western blot analysis
A498 cells were lysed using lysis buffer containing
protease and phosphate inhibitors (Beyotime Institute of
Biotechnology) on ice for 30 min, and then the cell lysis products
were centrifuged at speed in 12,700 × g at 4°C for 10 min and the
supernatant was collected. The total concentration was measured
using a BCA protein assay kit (Thermo Fisher Scientific, Inc.) and
total protein was boiled for 5 min. Next, 30 µg protein/lane was
loaded onto a 10% gel, resolved using SDS-PAGE and transferred onto
PVDF membranes. The membranes were blocked with 5% bovine serum
albumin (Beyotime Institute of Biotechnology) at room temperature
for 2 h, incubated with primary antibodies at 4°C overnight, washed
with TBS containing 0.1% Tween 20 and incubated with secondary
antibody at room temperature for 2 h. The immunoreactivity was
visualized by chemiluminescence agent (EMD Millipore) and
quantified using Quantity One software version 6.0 (Bio-Rad
Laboratories Inc.).
Statistical analysis
All experiments were performed in triplicate and
data are presented as the mean ± SD. Statistical significance was
analyzed using one-way ANOVA followed by Dunnett's test when
comparing differences between the UA and control groups and Tukey's
test was performed to compare differences among all groups. All
statistical analyses were performed using GraphPad Prism v5.01
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
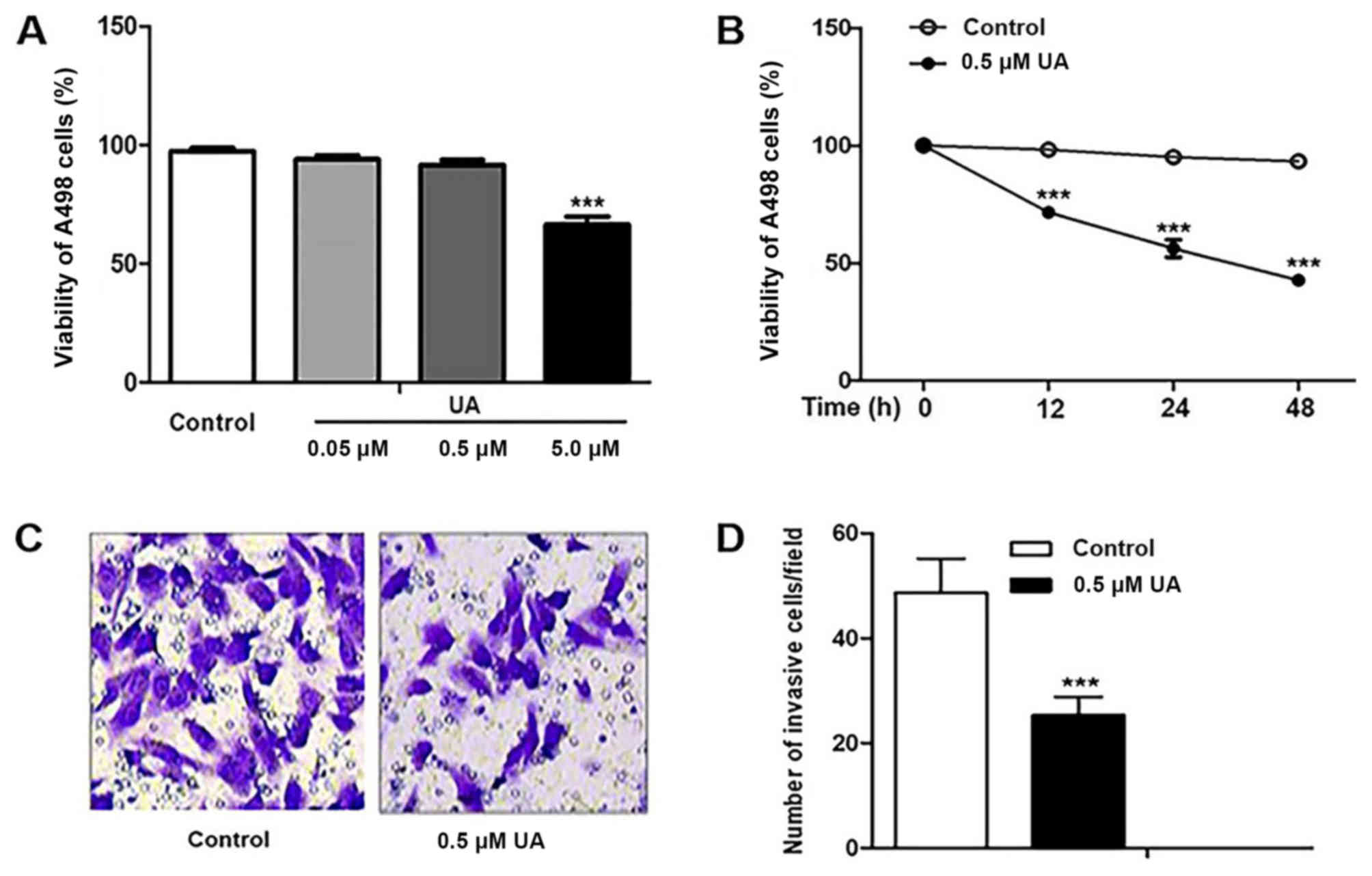
UA treatment decreases the
proliferation and invasiveness of A498 cells
The proliferation of A498 cells was assayed
following UA treatment at different concentrations (0, 0.05, 0.5
and 5 µM) for 12 h. Treatment with 5 µM UA, but not 0.05 and 0.5 µM
UA, significantly decreased the proliferation of A498 cells
compared with untreated cells (Fig.
1A). Additionally, A498 cells were treated with 5 µM UA for 12,
24 and 48 h. The proliferation of A498 cells decreased in a
time-dependent manner compared with that of untreated cells
(Fig. 1B). To avoid the effect of
proliferation on cell invasiveness, the effect of UA on
invasiveness was analyzed at a concentration of 0.5 µM. The results
of the Transwell invasion assay revealed that there were
significantly fewer invasive cells following UA treatment compared
with no treatment (Fig. 1C and
D).
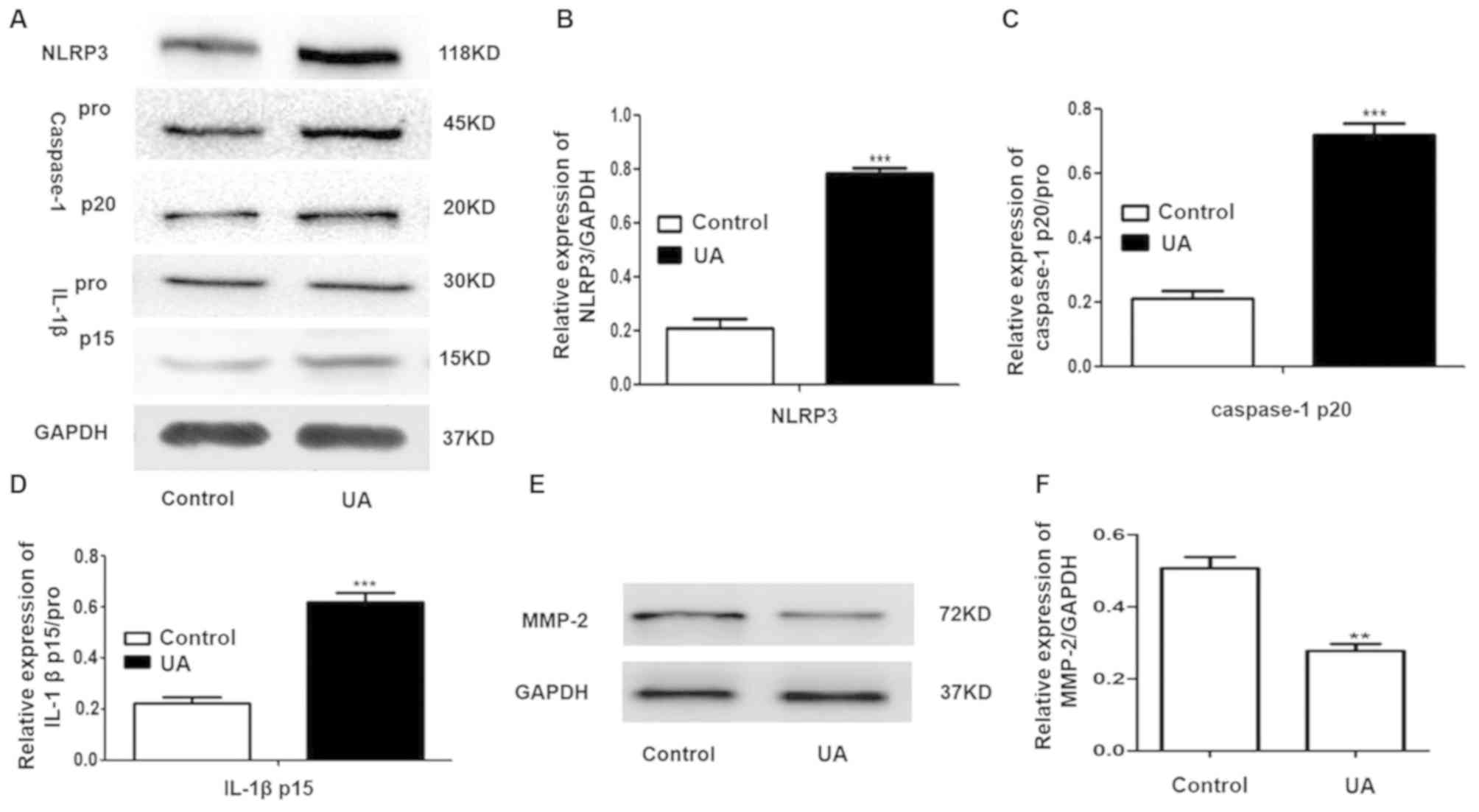
UA treatment significantly increases
the expression levels of NLRP3, -caspase-1 p20 and IL-1βp15 and
decreases MMP-2 expression in A498 cells
Caspase-1 pro exists in the cytoplasm as an inactive
proenzyme and caspase-1 pro is cleaved to caspase p20 when
caspase-1 is activated, which subsequently actives and cleaves the
pro-IL-1β into IL-1β p15 (18). To
investigate the effect of UA on the expression levels of NLRP3 and
MMP-2 in A498 cells, A498 cells were treated with 0.5 µM UA for 12
h. The results revealed that UA treatment significantly increased
the levels of NLRP3, caspase-1p20 and IL-1βp15 compared with the
control (Fig. 2A-D). Additionally,
UA treatment significantly decreased MMP-2 expression compared with
the control (Fig. 2E and F).
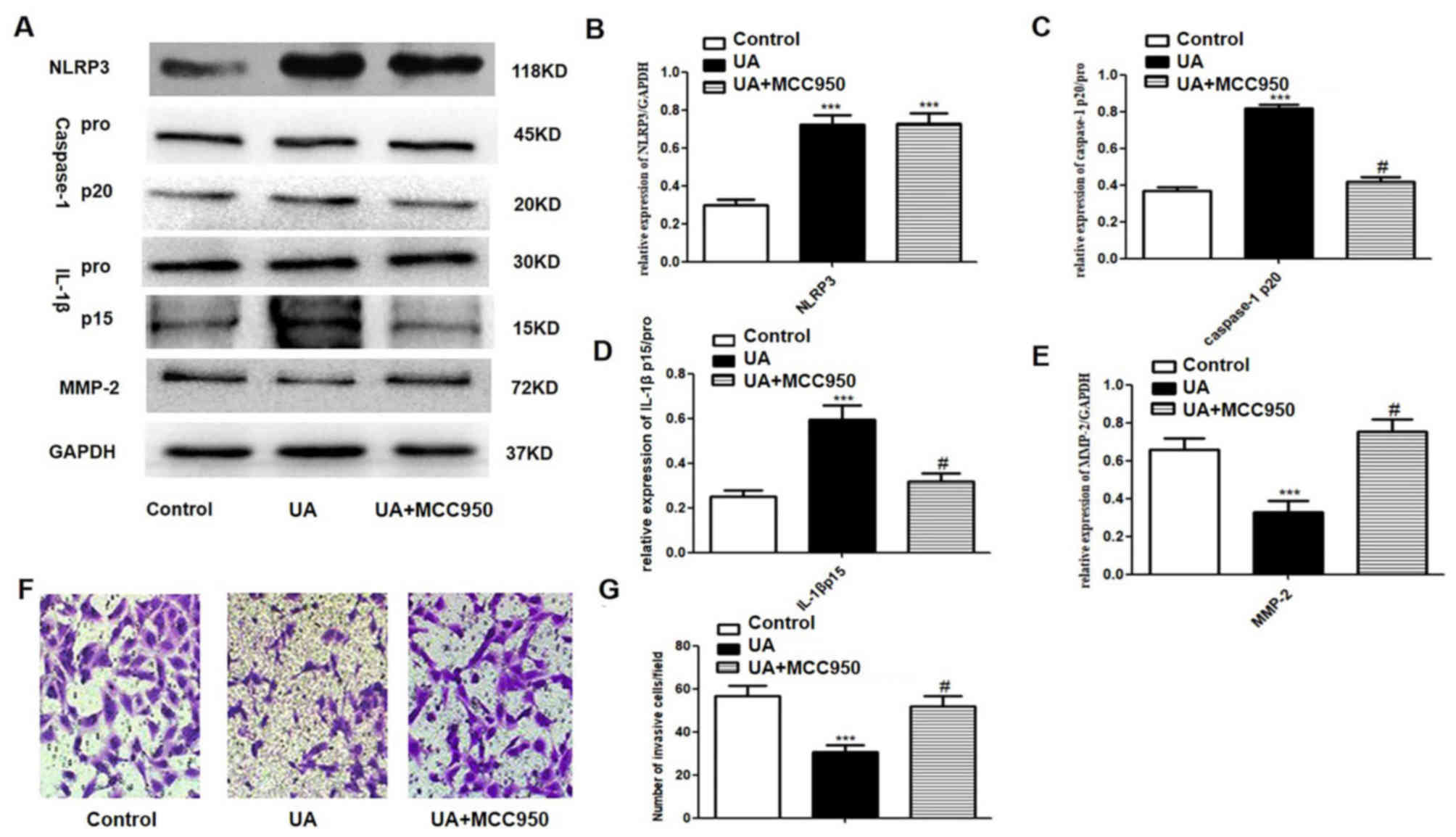
Increased NLRP3, caspase-1p20 and
IL-1βp15 expression and decreased cell invasiveness in response to
UA are abrogated by MCC950 treatment
A498 cells were exposed to MCC950, an NLRP3 receptor
antagonist, to assess the role of NLRP3 inflammasomes and MMP-2
following UA treatment. A concentration of 50 nM MCC950 was used to
block the NLRP3 receptor (19).
MCC950 treatment after UA treatment had no impact on of NLRP3
expression; however, significantly decreased caspase-1p20 and
IL-1βp15 protein expression and increased MMP-2 protein expression
compared with UA groups (Fig. 3A-E).
In the Transwell invasion assay, the number of invasive UA-exposed
A498 cells was significantly increased following treatment with
MCC950 when compared with UA groups (Fig. 3F and G).
Discussion
The present study revealed that UA exposure
decreased the proliferation and invasiveness of A498 cells, and
significantly decreased MMP-2 production and induced activation of
NLRP3 inflammasome, and this effect could be reversed by MCC950
treatment.
UA is a type of triterpenoid extracted from natural
herbs (3), which has been reported
to have antitumor effects (4–6).
Exposure to UA has been associated with decreased proliferation of
cancer cells (20,21). Xavier et al (20) demonstrated that UA has an
antiproliferative effect in human colorectal cancer cells. Zhang
et al (21) reported that UA
inhibits proliferation by inactivating Wnt/β-catenin signaling in
human osteosarcoma cells. Li et al (22) revealed that UA suppresses renal
cancer cell viability. The present study revealed that UA decreased
the proliferation of A498 cells in a dose- and time-dependent
manner. Additionally, it has been reported that UA decreases the
invasiveness of breast cancer cells (5). The present study revealed that UA
inhibited the invasiveness of A498 cells.
Incidence rates of renal cell cancer, which accounts
for 85% of kidney cancer cases in adults (23). Patients with renal cell cancer
usually lack obvious symptoms (24)
and ~17% of newly diagnosed patients already have local invasions
or have progressed to stage IV with distant metastases (25). Cancer metastasis is one of the
characteristics of malignant tumors, and involves MMPs (26). Nam et al (8) reported that UA significantly decreased
MMP-2 expression in gastric cancer and this may be responsible for
the anti-invasive activity of UA. MMP-2 is a proteolytic enzyme
that is capable of degrading structural components of the
extracellular matrix that contribute to tumor invasion (27). In the present study, UA exposure
decreased MMP-2 expression in A498 cells. Therefore, UA may have
decreased the invasiveness of carcinoma cells via the suppression
of MMP-2 expression.
NLRP3 inflammasomes are an activating platform of
caspases, consisting of NLRP3, ASC and caspase-1 (13). The activation of NLRP3 inflammasomes
is closely associated with tumor formation (28). Inhibition of the NLRP3 inflammasome
in the tumor microenvironment leads to suppression of the
metastatic potential of melanoma cells (29). Suppressing the expression levels of
the NLRP3 inflammasome promotes metastasis of renal cell cancer
cells (17). The activation of the
inflammasome is a critical step to secrete mature IL-1β through
stepwise reactions to activate caspase-1 (30). The present study demonstrated that UA
treatment increased the expression levels of NLRP3, cleaved
caspase-1 and IL-1β, indicating that UA may activate the NLRP3
inflammasome.
It reported that the combination of MCC950 and NLRP3
results in the conformational change of NLRP3, affecting the
function of the Walker B motif in the nucleotide-binding fold and
then inhibiting the NLRP3 pathway without affecting the expression
of NLRP3 (31). Studies of the
effect of MCC950 on tumors have been previously conducted (19,32).
MCC950 markedly attenuates cancer-induced bone pain (32). NLRP3 inflammasome blockade by MCC950
delays tumorigenesis of head and neck squamous cell carcinoma
(19). In the present study, MCC950
significantly inhibited the expression levels of caspase-1p20 and
IL-1βp15, and increased MMP-2 expression in UA-treated A498 cells.
Additionally, the number of invasive UA-exposed A498 cells was
significantly increased by MCC950.
In summary, the present study demonstrated that UA
may decrease the invasiveness of renal cancer A498 cells via the
activation of the NLRP3 inflammasome to suppress the expression
levels of MMP-2. UA may be used as a potential therapeutic agent
for renal cancer treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YMC contributed to the study design and contributed
to data analysis, BXT and WYC contributed to performing experiments
and MSZ contributed to data analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Capitanio U, Bensalah K, Bex A, Boorjian
SA, Bray F, Coleman J, Gore JL, Sun M, Wood C and Russo P:
Epidemiology of renal cell carcinoma. Eur Urol. 75:74–84. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao QC, Zhu WS, Feng W, Lee SS, Leung AW,
Shen J, Gao L and Xu C: A Review of resveratrol as a potent
chemoprotective and synergistic agent in cancer chemotherapy. Front
Pharmacol. 9:15342019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Traore-Coulibaly M, Ziegler HL, Olsen CE,
Hassanata MK, Pierre GI, Nacoulma OG, Guiguemdé TR and Christensen
SB: 19alpha-Hydroxy-3-oxo-ursa-1,12-dien-28-oic acid, an
antiplasmodial triterpenoid isolated from Canthium multiflorum. Nat
Prod Res. 23:1108–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SH, Ryu HG, Lee J, Shin J, Harikishore
A, Jung HY, Kim YS, Lyu HN, Oh E, Baek NI, et al: Ursolic acid
exerts anti-cancer activity by suppressing vaccinia-related kinase
1-mediated damage repair in lung cancer cells. Sci Rep.
5:145702015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang Q, Liu Y, Li T, Yang X, Zheng G, Chen
H, Jia L and Shao J: A novel co-drug of aspirin and ursolic acid
interrupts adhesion, invasion and migration of cancer cells to
vascular endothelium via regulating EMT and EGFR-mediated signaling
pathways: Multiple targets for cancer metastasis prevention and
treatment. Oncotarget. 7:73114–73129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong H, Yang X, Xie J, Xiang L, Li Y, Ou
M, Chi T, Liu Z, Yu S, Gao Y, et al: UP12, a novel ursolic acid
derivative with potential for targeting multiple signaling pathways
in hepatocellular carcinoma. Biochem Pharmacol. 93:151–162. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim ES and Moon A: Ursolic acid inhibits
the invasive phenotype of SNU-484 human gastric cancer cells. Oncol
Lett. 9:897–902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nam H and Kim MM: Ursolic acid induces
apoptosis of SW480 cells via p53 activation. Food Chem Toxicol.
62:579–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruan JS, Zhou H, Yang L, Wang L, Jiang ZS,
Sun H and Wang SM: Ursolic acid attenuates TGF-β1-induced
epithelial-mesenchymal transition in NSCLC by targeting integrin
αVβ5/MMPs signaling. Oncol Res. 27:593–600. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Meng X and Dong Y: Ursolic acid
nanoparticles inhibit cervical cancer growth in vitro and
in vivo via apoptosis induction. Int J Oncol. 50:1330–1340.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pei Y, Zhang Y, Zheng K, Shang G, Wang Y,
Wang W, Qiu E and Zhang X: Ursolic acid suppresses the biological
function of osteosarcoma cells. Oncol Lett. 18:2628–2638.
2019.PubMed/NCBI
|
|
12
|
Sohn EJ, Won G, Lee J, Yoon SW, Lee I, Kim
HJ and Kim SH: Blockage of epithelial to mesenchymal transition and
upregulation of let 7b are critically involved in ursolic acid
induced apoptosis in malignant mesothelioma cell. Int J Biol Sci.
12:1279–1288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun L, Ma W, Gao W, Xing Y, Chen L, Xia Z,
Zhang Z and Dai Z: Propofol directly induces caspase-1-dependent
macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell
Death Dis. 10:5422019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shao BZ, Wang SL, Pan P, Yao J, Wu K, Li
ZS, Bai Y and Linghu EQ: Targeting NLRP3 Inflammasome in
inflammatory bowel disease: Putting out the fire of inflammation.
Inflammation. 42:1147–1159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng Q, Geng Y, Zhao L, Li R, Zhang Z, Li
K, Liang R, Shao X, Huang M, Zuo D, et al: NLRP3 inflammasomes in
macrophages drive colorectal cancer metastasis to the liver. Cancer
Lett. 442:21–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Kong H, Zeng X, Liu W, Wang Z, Yan
X, Wang H and Xie W: Activation of NLRP3 inflammasome enhances the
proliferation and migration of A549 lung cancer cells. Oncol Rep.
35:2053–2064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang K, Xu T, Ruan H, Xiao H, Liu J, Song
Z, Cao Q, Bao L, Liu D, Wang C, et al: LXRα promotes cell
metastasis by regulating the NLRP3 inflammasome in renal cell
carcinoma. Cell Death Dis. 10:1592019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Conos SA, Lawlor KE, Vaux DL, Vince JE and
Lindqvist LM: Cell death is not essential for caspase-1-mediated
interleukin-1 beta activation and secretion. Cell Death Differ.
23:1827–1838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang CF, Chen L, Li YC, Wu L, Yu GT,
Zhang WF and Sun ZJ: NLRP3 inflammasome activation promotes
inflammation-induced carcinogenesis in head and neck squamous cell
carcinoma. J Exp Clin Canc Res. 36:1162017. View Article : Google Scholar
|
|
20
|
Xavier CP, Lima CF, Preto A, Seruca R,
Fernandes-Ferreira M and Pereira-Wilson C: Luteolin, quercetin and
ursolic acid are potent inhibitors of proliferation and inducers of
apoptosis in both KRAS and BRAF mutated human colorectal cancer
cells. Cancer Lett. 281:162–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang RX, Li Y, Tian DD, Liu Y, Nian W,
Zou X, Chen QZ, Zhou LY, Deng ZL and He BC: Ursolic acid inhibits
proliferation and induces apoptosis by inactivating Wnt/β-catenin
signaling in human osteosarcoma cells. Int J Oncol. 49:1973–1982.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Zhang HX, Nie MX, Tian YL, Chen X,
Chen C, Chen H and Liu R: Ursolic acid derivative FZU-03,010
inhibits STAT3 and induces cell cycle arrest and apoptosis in renal
and breast cancer cells. Acta Biochim Biophys Sin (Shanghai).
49:367–373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lipworth L, Tarone RE, Lund L and
McLaughlin JK: Epidemiologic characteristics and risk factors for
renal cell cancer. Clin Epidemiol. 1:33–43. 2009.PubMed/NCBI
|
|
24
|
Yang KW, Xiong GY, Li XS, Tang Y, Tang Q,
Zhang CJ, He ZS and Zhou LQ: Prevalence of baseline chronic kidney
disease in 2,769 Chinese patients with renal cancer:
Nephron-sparing treatment is still underutilized. World J Urol.
32:1027–1031. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Capitanio U and Montorsi F: Renal cancer.
Lancet. 387:894–906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sandri S, Faiao-Flores F, Tiago M,
Pennacchi PC, Massaro RR, Alves-Fernandes DK, Berardinelli GN,
Evangelista AF, de Lima Vazquez V, Reis RM and Maria-Engler SS:
Vemurafenib resistance increases melanoma invasiveness and
modulates the tumor microenvironment by MMP-2 upregulation.
Pharmacol Res. 111:523–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zaki MH, Vogel P, Body-Malapel M, Lamkanfi
M and Kanneganti TD: IL-18 production downstream of the Nlrp3
inflammasome confers protection against colorectal tumor formation.
J Immunol. 185:4912–4920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee HE and Lee JY, Yang G, Kang HC, Cho
YY, Lee HS and Lee JY: Inhibition of NLRP3 inflammasome in tumor
microenvironment leads to suppression of metastatic potential of
cancer cells. Sci Rep. 9:122772019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Davis BK, Wen HT and Ting JP: The
inflammasome NLRs in immunity, inflammation, and associated
diseases. Annu Rev Immunol. 29:707–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Coll RC, Hill JR, Day CJ, Zamoshnikova A,
Boucher D, Massey NL, Chitty JL, Fraser JA, Jennings MP, Robertson
AAB and Schroder K: MCC950 directly targets the NLRP3
ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol.
15:556–559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen SP, Zhou YQ, Wang XM, Sun J, Cao F,
HaiSam S, Ye DW and Tian YK: Pharmacological inhibition of the
NLRP3 inflammasome as a potential target for cancer-induced bone
pain. Pharmacol Res. 147:1043392019. View Article : Google Scholar : PubMed/NCBI
|