Introduction
Osteosarcoma is a malignant tumor derived from the
skeletal system, often occurring in bone tissues, and it is the
most common malignant tumor in the skeletal system, with more than
90% of cases being highly malignant (1). Osteosarcoma is highly prevalent in
children or adolescents, and frequently occurs at the sites of bone
turnover and rapid bone growth, especially the metaphysis of long
bone of adolescents, and the morbidity rate of osteosarcoma in
males is approximately twice that in females (2). The pathogenesis of osteosarcoma is
complex, and the genetic factors, environmental factors and
radiative factors are all closely related to the occurrence and
development of osteosarcoma (1,2). Yu
et al (3) studied and found
that the occurrence of osteosarcoma in children may be closely
associated with the mutation of genes controlling the longitudinal
growth or maturation of bone in stem cells. Research evidence of
Wang et al (4) confirmed that
chromosomal recombination can occur in most patients with
osteosarcoma, and gene mutation is an important factor influencing
the occurrence and development of osteosarcoma. MicroRNAs
(miRNAs/miRs) are a type of endogenous non-coding RNAs that affect
the mRNA function through complementary binding to the untranslated
region (UTR) of mRNAs. The correlation between miRNAs and tumors
has attracted increasingly more attention from researchers
(5). There is much research evidence
that miRNAs are involved in the proliferation, invasion, migration
and apoptosis of a variety of tumor cells. For example, Cao et
al (6) found that miR-19a can
significantly enhance the sensitivity of non-small cell lung cancer
to chemotherapy drugs. Tan et al (7) showed that downregulation of the
expression of miR-19a can obviously reduce the proliferation,
invasion and migration, and promote the apoptosis of pancreatic
cancer cells. The Janus kinase 2 (JAK2)/signal transducer and
activator of transcription 3 (STAT3) signaling pathway is closely
related to mitochondrial-mediated apoptosis. Zhang et al
(8) demonstrated that inhibition of
the activation of the JAK2/STAT3 signaling pathway can effectively
suppress the proliferation of lung cancer cells. At present, there
are few studies on the effects of miR-19a on the JAK2/STAT3
signaling pathway and osteosarcoma cells. Therefore, the present
study aimed to explore the regulatory effect of miR-19a on
osteosarcoma cells through in vitro experiments, and further
analyze the effects of the JAK2/STAT3 signaling pathway on the
proliferation and apoptosis of osteosarcoma cells.
Materials and methods
Reagents
The reagents, kits and antibodies included: Adult
SaOS-2 osteosarcoma cells (Kunming Cell Bank, Chinese Academy of
Sciences, Kunming, China), RNA extraction kit (Takara), SYBR Premix
Ex Taq kit and Prime Script RT reagent Kit (Takara), Dulbecco's
modified Eagle's medium (DMEM) (Gibco, Rockville, MD, USA), fetal
bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.), TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.),
Radioimmunoprecipitation assay (RIPA) lysis buffer (Wuhan Guge
Biotech), methyl thiazolyl tetrazolium (MTT) kit (Wuhan Boster
Biological Technology Co., Ltd.), apoptosis assay kit (R&D
Systems, Inc.), reactive oxygen species (ROS) assay kit (Beyotime
Institute of Biotechnology), and mouse anti-human caspase-3 (cat.
no. ab13847; Abcam), cleaved caspase-3 (cat. no. ab32042; Abcam),
B-cell lymphoma-2 (Bcl-2) (cat. no. ab32124; Abcam), Bcl-2
associated X protein (Bax) (cat. no. ab32503; Abcam),
phosphorylated (p-)JAK2 (cat. no. ab32101; Abcam), p-STAT3 (cat.
no. ab76315; Abcam), JAK2 (cat. no. ab108596; Abcam), STAT3 (cat.
no. ab68153; Abcam) and myeloid cell leukemia-1 (Mcl-1) (cat. no.
ab32087; Abcam) antibodies. Other reagents of unspecified sources
are listed in the manuscript.
Construction of osteosarcoma cell
lines with low expression of miR-19a
The expression of miR-19a in the SaOS-2 osteosarcoma
cell line was knocked down by GenePharma using lentiviral
transfection. At 48 h after transfection, the successfully
transfected cells were selected to extract the total RNA. The
expression level of miR-19a in cells was verified via polymerase
chain reaction (PCR), and the transfection efficiency was
determined. The cell lines with low expression of miR-19a were
selected for stable subculture as the miR-19a-inhibitor group. The
blank plasmid group (NC-inhibitor group) and blank control group
(Control group) were also set up.
Detection of miR-19a expression level
in cells via qPCR
The cells in the logarithmic growth phase in each
group were collected and centrifuged at 10,500 × g for 10 min, the
supernatant was discarded, and appropriate amount of TRIzol was
added to extract the total RNA. The total RNA concentration in each
group was determined using the nucleic acid-protein quantometer
(NanoDrop 2000), and the optical density (OD) value was 1.8-2.0.
The reverse transcription system was prepared, and the reaction
conditions are as follows: 37°C for 15 min and 85°C for 5 sec. The
total RNA was reversely transcribed into complementary
deoxyribonucleic acid (cDNA), and stored for later use. Then the
qPCR system was prepared in strict accordance with the instructions
of kit, and the reaction conditions were as follows: 95°C for 30
sec, 95°C for 5 sec, 60°C for 35 sec for a total of 35 cycles. With
U6 as an internal reference, the relative expression level of
miR-19a in each group was calculated by 2−ΔΔCq (9). The primers were synthesized by
Invitrogen; Thermo Fisher Scientific, Inc., and the primer
sequences are shown in Table I.
 | Table I.PCR primers. |
Table I.
PCR primers.
|
| Sequence |
|---|
| miR-19a | F:
5′-TCATCACGCTGTGCAAATCT-3′ |
|
| R:
5′-TATGGTTGTTCTGCTCTCTGTCTC-3′ |
| U6 | F:
5′-ATTGGAACGATACAGAGAAGATT-3′ |
|
| R:
5′-GGAACGCTTCACGAATTTG-3′ |
Detection of cell proliferation
ability using MTT assay
The cells in the logarithmic growth phase in each
group were collected, inoculated into a 96-well plate
(1×104 cells/well), and cultured using complete medium
with 5% CO2 under a constant temperature of 37°C. Then
20 µl of MTT was added into each well after 12, 24, 36, 48, 60 and
72 h respectively, followed by culture in an incubator for another
4 h. After the supernatant was discarded, 150 µl of dimethyl
sulfoxide (DMSO) (Wuxi Yangshan Biochemical Co., Ltd.) was added
into each well and shaken gently. Finally, the optical density (OD)
value of the cells in each group was measured at a wavelength of
450 nm using a microplate reader, based on which the cell
proliferation level was calculated.
Detection of apoptosis level via flow
cytometry
The cells in the logarithmic growth phase in each
group were collected, inoculated into a 6-well plate
(1×105 cells/well), and cultured using complete medium
with 5% CO2 under a constant temperature of 37°C. After
24 h, the supernatant was discarded, and the cells were washed
twice with pre-cooled phosphate-buffered saline (PBS) and
centrifuged using a centrifuge at 950 × g for 10 min to prepare the
cell suspension. Then the cells were resuspended, centrifuged,
added with fluorescence solution and incubated in the dark at room
temperature for 15 min, followed by detection of the apoptosis
level via flow cytometry strictly according to the instructions of
the apoptosis kit.
Determination of ROS level in each
group using DCFH-DA
The ROS level in each group was determined using
DCFH-DA. The cells in the logarithmic growth phase in each group
were collected, inoculated into the 6-well plate (1×105
cells/well), and cultured using complete medium with 5%
CO2 under a constant temperature of 37°C. After 24 h,
the reactive oxygen species (ROS) level in each group was detected
strictly according to the instructions of the ROS assay kit: An
appropriate amount of DCFH-DA (10 µM) was added into the 6-well
plate, and incubated in the incubator in the dark at 37°C for 20
min. After the medium was discarded, the cells were washed with
pre-cooled PBS and washing solution, and observed under a confocal
microscope (excitation wavelength of 488 nm, and emission
wavelength of 525 nm). The higher the ROS level, the higher the
brightness.
Detection of protein expression level
using western blotting
The cells in the logarithmic growth phase in each
group were collected, inoculated into the 6-well plate
(1×105 cells/well), and cultured using complete medium
with 5% CO2 under a constant temperature of 37°C. After
24 h, the supernatant was discarded, and the cells in each group
were collected for later use. The total protein was extracted from
cells in each group, and the total protein concentration was
determined using the bicinchoninic acid (BCA) protein
quantification kit (Wuhan Boster Biological Technology Co., Ltd.).
After the sodium dodecyl sulphate (SDS) 10% gel was prepared, the
protein was subjected to electrophoresis, transferred (40 mg) onto
polyvinylidene fluoride (PVDF) membranes (Millipore), and sealed
with freshly-prepared 5% skim milk powder for 1 h. Then the
corresponding target bands were cut, incubated with caspase-3
(dilution: 1/2,000), Bcl-2 (dilution: 1/2,000), Bax (dilution:
1/2,000), p-JAK2 (dilution: 1/2,000), p-STAT3 (dilution: 1/2,000),
JAK2 (dilution: 1/1,000), STAT3 (dilution: 1/2,000), Mcl-1
(dilution: 1/1,000) and GAPDH (dilution: 1/500) antibodies at 4°C
overnight, washed with Tris-buffered saline with
Tween®20 (TBST) for 3 times, incubated again with
horseradish peroxidase-conjugated secondary antibodies (dilution:
1/2,000; cat. no. ab6721; Abcam) at room temperature for 1 h, and
washed again with TBST for 3 times. Finally, bands were exposed by
enhanced chemiluminescence (ECL) detection kit (Amersham
Biosciences, and analyzed by ImageJ Software (version 1.38;
National Institutes of Health), and the protein bands were scanned
to calculate the protein expression levels in each group.
Statistical analysis
The data are expressed as mean ± standard deviation
and were analyzed using Statistical Product and Service Solutions
(SPSS) 22.0 software (IBM, Corp.). One-way analysis of variance was
adopted for the data in each group. The homogeneity test of
variance was performed. Bonferroni's method was adopted for the
pairwise comparison in the case of homogeneity of variance, while
Welch's method was used in the case of heterogeneity of variance.
P<0.05 suggested that the difference was statistically
significant.
Results
Construction of osteosarcoma cell
lines with low expression of miR-19a
The expression of miR-19a in SaOS-2 osteosarcoma
cell lines was downregulated using lentiviral transfection. After
successful transfection, the expression level of miR-19a in each
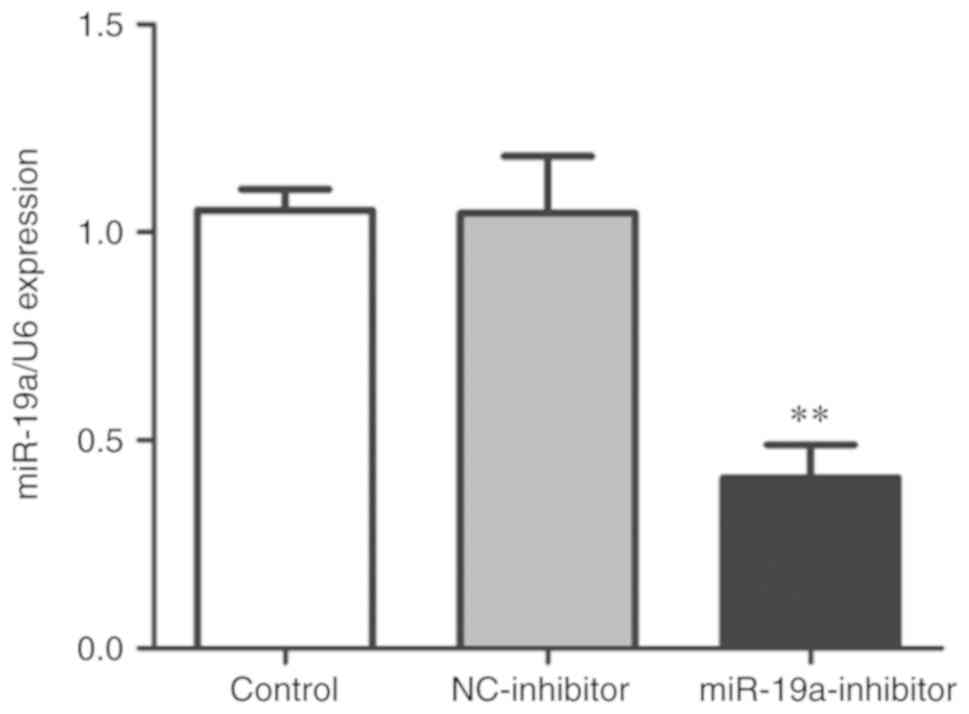
group was detected via qPCR. As shown in Fig. 1, the expression level of miR-19a in
the miR-19a-inhibitor group was significantly lower than that in
the control group and NC-inhibitor group (P<0.01), indicating
that the transfection was successful and subsequent experiments
could be performed.
Effect of miR-19a on proliferation of
osteosarcoma cells
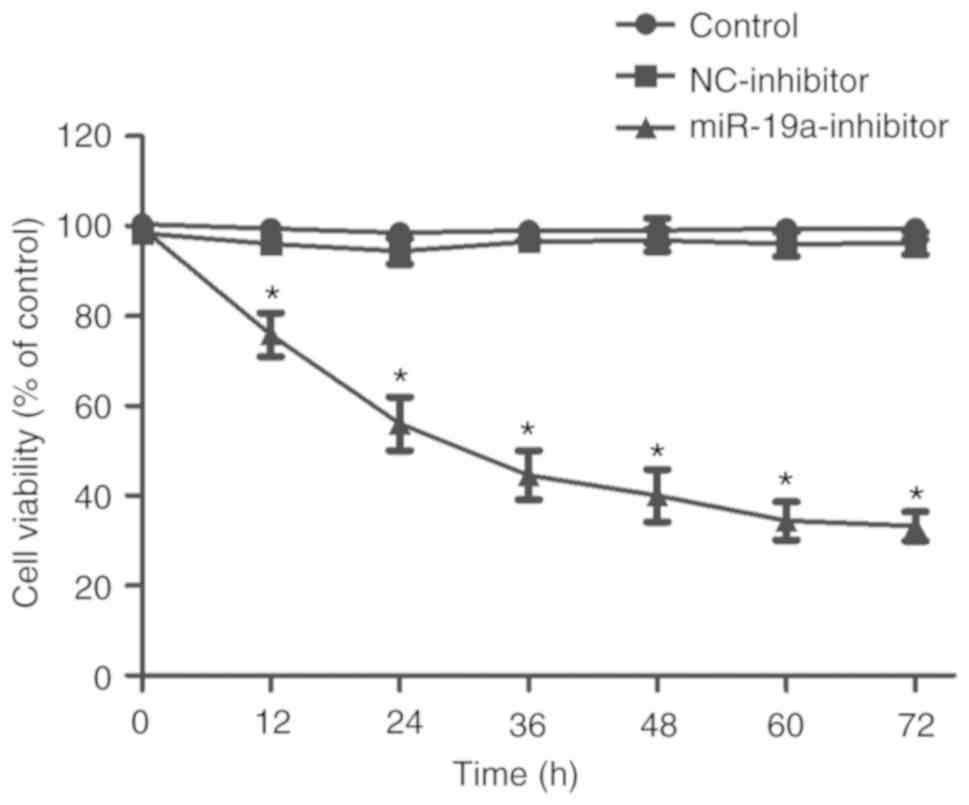
The effect of miR-19a downregulation on the
proliferation of SaOS-2 cells was detected using MTT assay. As
shown in Fig. 2, the cell
proliferation ability in the miR-19a-inhibitor group was
significantly weaker than that in the control group and
NC-inhibitor group (P<0.01), and the proliferation ability in
the miR-19a-inhibitor group became weaker with time.
Effect of miR-19a on apoptosis of
osteosarcoma cells
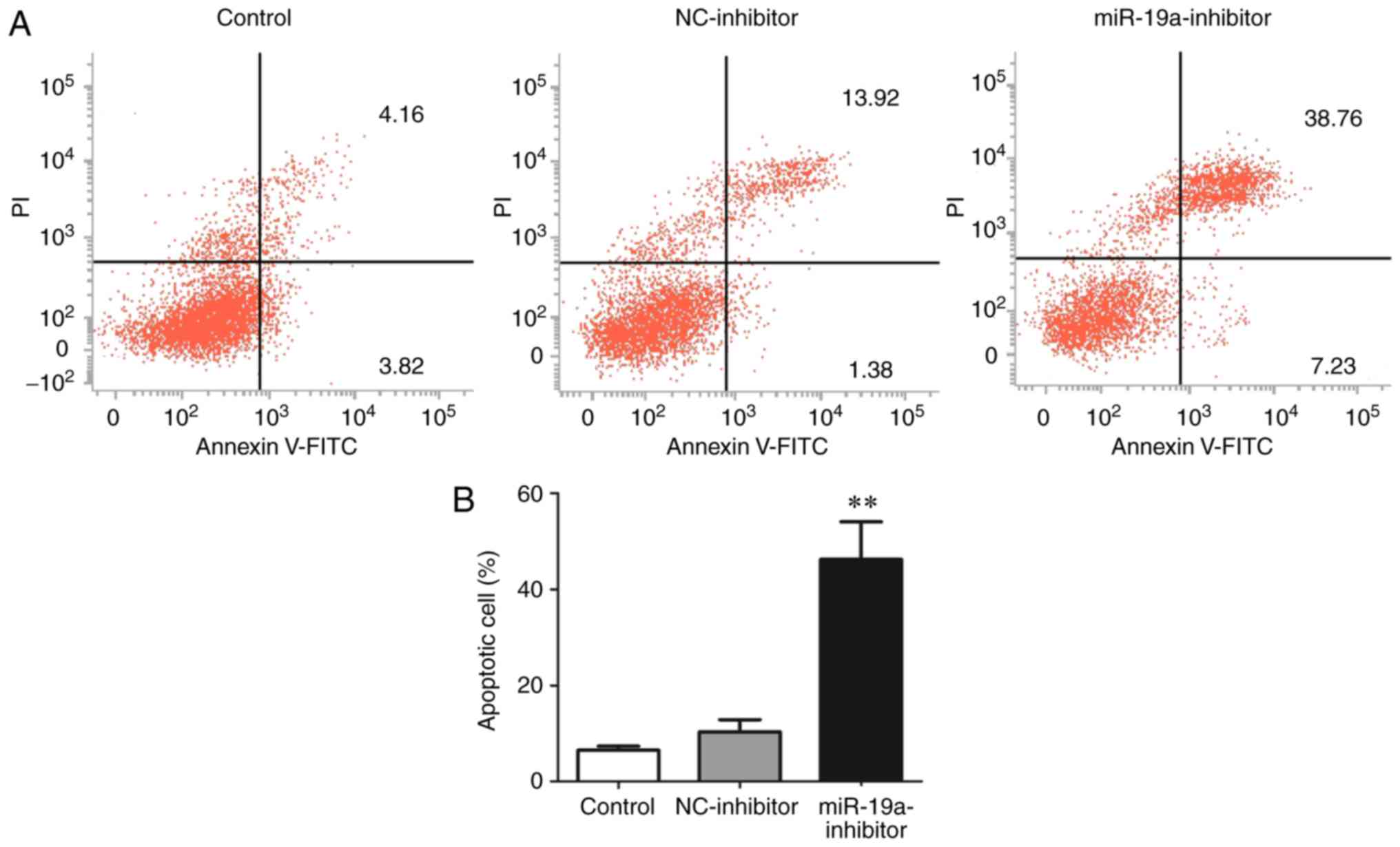
The apoptosis level in each group was detected
through flow cytometry. The results showed that the
miR-19a-inhibitor group had an obviously higher apoptosis level
(38.76%) than the control group (4.16%) and NC-inhibitor group
(13.92%) (P<0.01) (Fig. 3).
Effect of miR-19a on the ROS level in
osteosarcoma cells
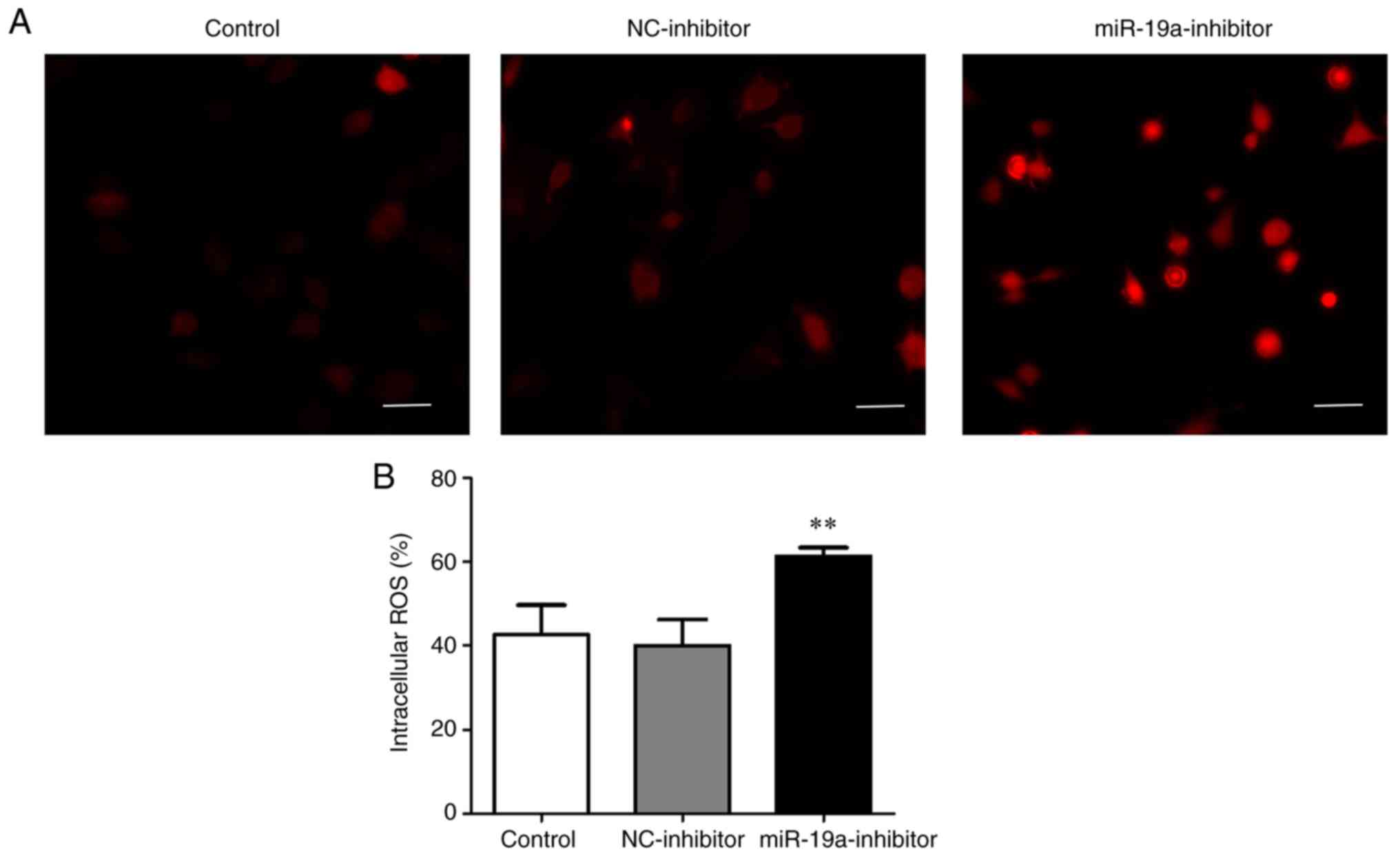
According to the detection results of ROS level in
each group using DCFH-DA, the content of ROS (60%) in the
miR-19a-inhibitor group was evidently higher than that in the
control group (42%) and NC-inhibitor group (40%) (P<0.01)
(Fig. 4).
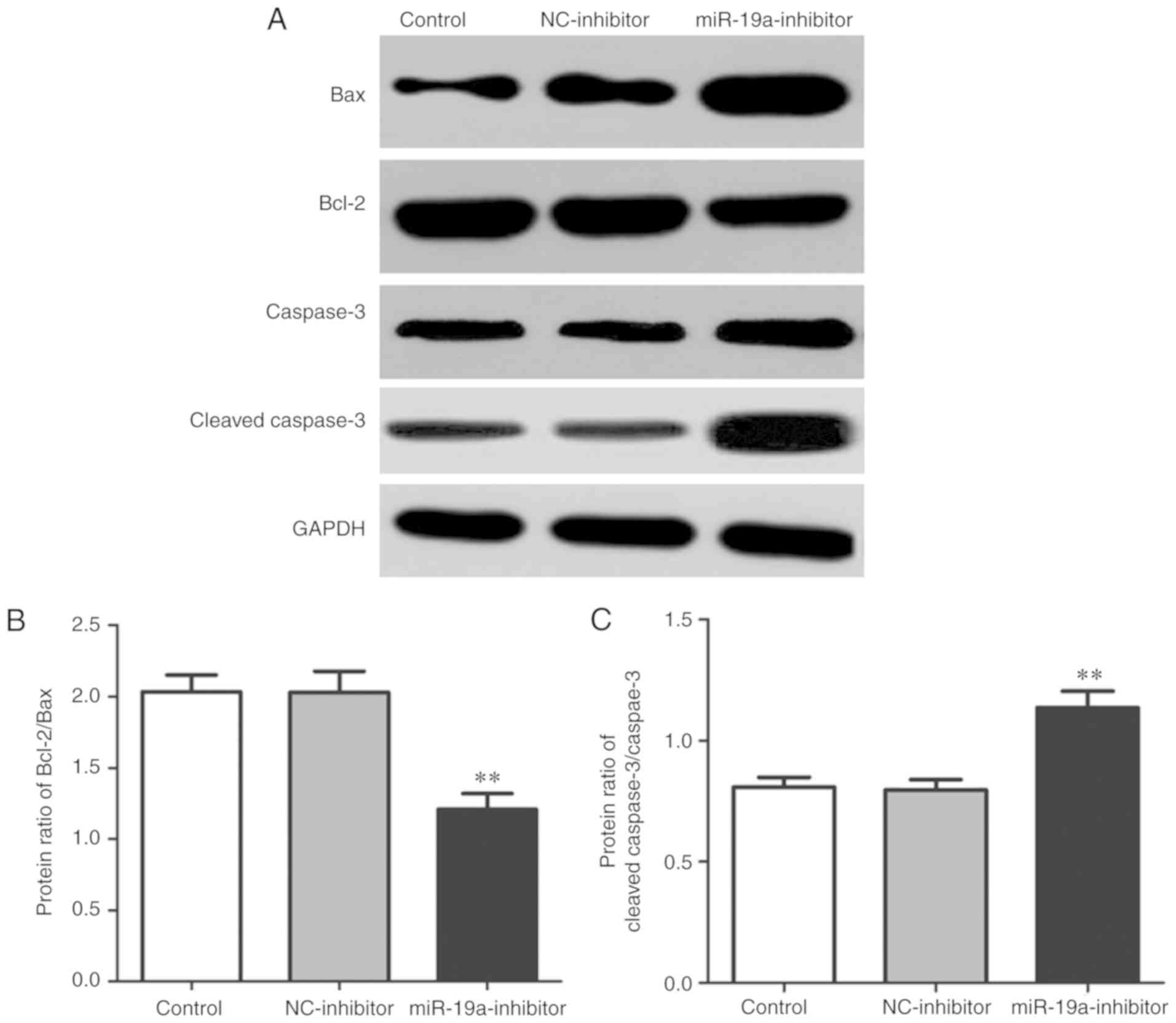
Effect of miR-19a on expression of
apoptosis-related proteins in osteosarcoma cells
Western blotting was performed to detect the
expression levels of apoptosis-related proteins in each group. The
results revealed that compared with those in the control group and
NC-inhibitor group, the ratio of expression of Bcl-2/Bax was
significantly decreased (P<0.01), while that of cleaved
caspase-3/caspase-3 was significantly increased in the
miR-19a-inhibitor group (P<0.01) (Fig. 5).
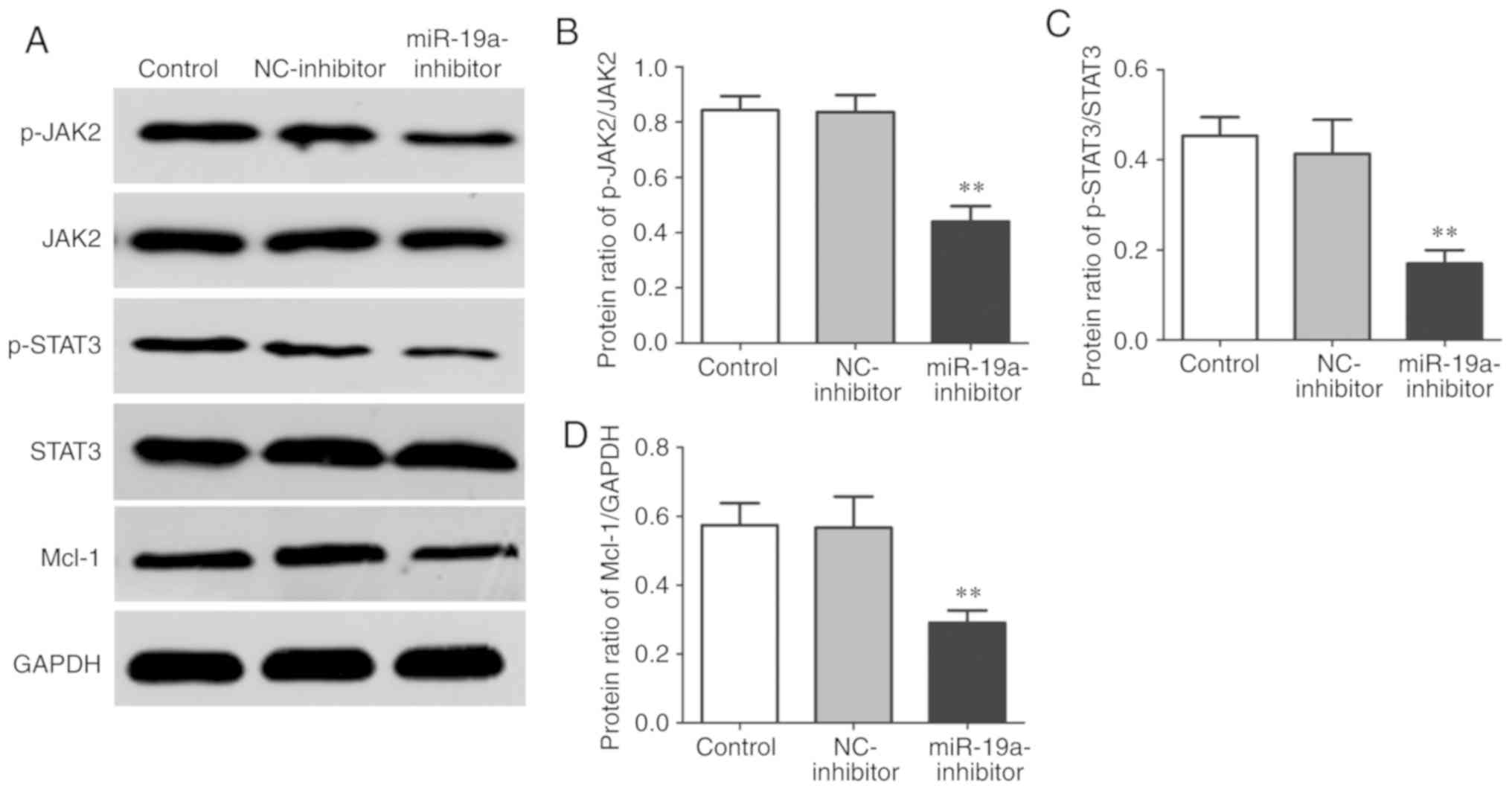
Effect of miR-19a on JAK2/STAT3 in
osteosarcoma cells
Western blotting was also performed to detect the
expression levels of JAK2/STAT3 signaling pathway-related proteins
in each group. It was found that the miR-19a-inhibitor group had
significantly lower protein expression ratio of p-JAK2/JAK,
p-STAT3/STAT3 and Mcl-1/GAPDH than the control group and
NC-inhibitor group (P<0.01) (Fig.
6).
Discussion
Osteosarcoma is a malignant tumor derived from such
osteocytes as fibroblasts, chondroblasts and osteoblasts, and has a
high grade of malignancy. Wu et al (10) found that osteosarcoma is prone to
blood, liver and lung metastases, and the incidence rate of
recurrence in situ is high, for which the mechanism is
closely related to gene mutation. Yin et al (11) demonstrated that miR-19a is a typical
cancer-promoting gene that can facilitate the proliferation of a
variety of tumor cells, and promote the invasion and migration of
tumor cells through blood vessels and lymphatic vessels by
downregulating the expression of target proteins and binding to
vascular endothelial growth factor (VEGF) protein. Zhang et
al (12) demonstrated that the
expression level of miR-19a in peripheral blood of osteosarcoma
patients is obviously higher than that in normal people. In the
present study, it was found that downregulation of miR-19a
expression in osteosarcoma cells significantly suppressed the cell
proliferation ability and promoted apoptosis. Clinical evidence
suggests that the expression or activation of caspase-3, a typical
pro-apoptotic protein, is closely related to the occurrence and
development of various tumors, and it also affects the prognosis of
tumor patients (13). Bcl-2 is one
of the original oncogenes that mediate apoptosis and the protein
that maintains mitochondrial membrane homeostasis, which, through
keeping the stability and integrity of mitochondrial membrane
potential, inhibits the release of cytochrome c, thereby
suppressing apoptosis (14). In this
study, it was observed that the downregulation of miR-19a in
osteosarcoma cells significantly incrased expression of caspase-3,
decrease Bcl-2/Bax and effectively promote apoptosis.
The JAK/STAT signaling pathway is closely related to
maintenance of homeostasis. As a major functional protein in the
JAK family, JAK2 is able to regulate cell proliferation,
differentiation, apoptosis and migration (15). The increased phosphorylation level of
JAK2 protein can further activate the STAT family proteins, and
then the activated STAT3 proteins remarkably upregulate the
expression of anti-apoptotic proteins Bcl-2 and Mcl-1 (16). Wu et al (17) found that inhibiting the activation of
the JAK2/STAT3 signaling pathway blocks the cell cycle of
pancreatic cancer cells and effectively inhibits the growth,
invasion and migration of tumor cells. Zhang et al (8) showed that inhibition of the JAK2/STAT3
signaling pathway can mediate the mitochondrial apoptotic pathway,
thus inducing apoptosis of rectal cancer cells. A large amount of
ROS produced during apoptosis is derived from mitochondria, and the
increase in ROS is directly related to apoptosis (18). Moreover, according to a study of Xu
et al (19), lowering the
phosphorylation level of STAT3 can effectively reduce the
mitochondrial membrane potential, increase the content of ROS in
cells, and induce mitochondrial apoptosis. In the present study,
the results revealed that downregulation of miR-19a expression in
osteosarcoma cells markedly increased the content of ROS in cells,
suppressed the JAK2/STAT3 signaling pathway in cells, and markedly
reduced the expression levels of p-JAK2, p-STAT3 and anti-apoptotic
protein Mcl-1. In addition, it was preliminarily found through flow
cytometry that miR-19a significantly increased the apoptosis level
of osteosarcoma cells, whose mechanism may also be to induce cell
cycle arrest and downregulate the expression of cyclin. Such an
uncertain mechanism is the deficiency in this study, thus further
verification is needed in future research. However, there is
insufficient evidence that JAK2/STAT3 is the direct target of
miR-19a. The relationship of the JAK2/STAT3 signaling pathway and
miR-19a and the potential target genes of miR-19a are still
unknown, which will be explored in our next research stufy. In
addition, only one cell line SaOS-2 was chosen for this study, and
additional osteosarcoma cell lines will be employed to further
investigate the effect of miR-19a.
In conclusion, the present study demonstrated that
downregulation of miR-19a effectively promotes apoptosis and
inhibits the proliferation of osteosarcoma cells. The related
mechanism of action may be that it inhibits the JAK2/STAT3
signaling pathway, increases the ROS level in cells, activates the
mitochondrial apoptotic pathway, and promotes the expression levels
of apoptosis-related proteins.
Acknowledgements
Not applicable.
Funding
Not funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JC and ZC designed the study and performed the
experiments, JC collected the data, ZC analyzed the data, JC and ZC
prepared the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Lee CM, Lee J, Nam MJ, Choi YS and Park
SH: Tomentosin displays anti-carcinogenic effect in human
osteosarcoma MG-63 cells via the induction of intracellular
reactive oxygen species. Int J Mol Sci. 20:1508–1519. 2019.
View Article : Google Scholar
|
|
2
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu X, Hu L, Li S, Shen J, Wang D, Xu R and
Yang H: Long non-coding RNA Taurine upregulated gene 1 promotes
osteosarcoma cell metastasis by mediating HIF-1α via miR-143-5p.
Cell Death Dis. 10:2802019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Shen J, Sun W, Zhang T, Zuo D,
Wang H, Wang G, Xu J, Yin F, Mao M, et al: Antitumor activity of
Raddeanin A is mediated by Jun amino-terminal kinase activation and
signal transducer and activator of transcription 3 inhibition in
human osteosarcoma. Cancer Sci. 110:1746–1759. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mishra S, Yadav T and Rani V: Exploring
miRNA based approaches in cancer diagnostics and therapeutics. Crit
Rev Oncol Hematol. 98:12–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao X, Lai S, Hu F, Li G, Wang G, Luo X,
Fu X and Hu J: miR-19a contributes to gefitinib resistance and
epithelial mesenchymal transition in non-small cell lung cancer
cells by targeting c-Met. Sci Rep. 7:29392017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan Y, Yin H, Zhang H, Fang J, Zheng W, Li
D, Li Y, Cao W, Sun C, Liang Y, et al: Sp1-driven up-regulation of
miR-19a decreases RHOB and promotes pancreatic cancer. Oncotarget.
6:17391–17403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Lu P, Guo X, Liu T, Luo X and Zhu
YT: Inhibition of JAK2/STAT3 signaling pathway protects mice from
the DDP-induced acute kidney injury in lung cancer. Inflamm Res.
68:751–760. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu H, He Y, Chen H, Liu Y, Wei B, Chen G
and Lin H and Lin H: LncRNA THOR increases osteosarcoma cell
stemness and migration by enhancing SOX9 mRNA stability. Febs Open
Bio. 9:781–790. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin Q, Wang PP, Peng R and Zhou H: MiR-19a
enhances cell proliferation, migration and invasion through
improving lymphangiogenesis via targeting thrombospondin-1 in
colorectal cancer. Biochem Cell Biol. 3:12–27. 2019.
|
|
12
|
Zhang B, Liu Y and Zhang J: Silencing of
miR-19a-3p enhances osteosarcoma cells chemosensitivity by
elevating the expression of tumor suppressor PTEN. Oncol Lett.
17:414–421. 2019.PubMed/NCBI
|
|
13
|
Zhang Z, Wang M, Zhou L, Feng X, Cheng J,
Yu Y, Gong Y, Zhu Y, Li C, Tian L and Huang Q: Increased HMGB1 and
cleaved caspase-3 stimulate the proliferation of tumor cells and
are correlated with the poor prognosis in colorectal cancer. J Exp
Clin Cancer Res. 34:512015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Groner B and von Manstein V: Jak Stat
signaling and cancer: Opportunities, benefits and side effects of
targeted inhibition. Mol Cell Endocrinol. 451:1–14. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Cui HZ, Xu CM, Sun ZW, Tang ZK and
Chen HL: RUNX3 protects against acute lung injury by inhibiting the
JAK2/STAT3 pathway in rats with severe acute pancreatitis. Eur Rev
Med Pharmacol Sci. 23:5382–5391. 2019.PubMed/NCBI
|
|
17
|
Wu L, Li J, Liu T, Li S, Feng J, Yu Q,
Zhang J, Chen J, Zhou Y, Ji J, et al: Quercetin shows anti-tumor
effect in hepatocellular carcinoma LM3 cells by abrogating
JAK2/STAT3 signaling pathway. Cancer Med. 8:4806–4820. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akhtar S, Achkar IW, Siveen KS,
Kuttikrishnan S, Prabhu KS, Khan AQ, Ahmed EI, Sahir F, Jerobin J,
Raza A, et al: Sanguinarine induces apoptosis pathway in multiple
myeloma cell lines via inhibition of the JaK2/STAT3 signaling.
Front Oncol. 9:2852019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Y, Zhang Q, Zhou J, Li Z, Guo J and
Wang W and Wang W: Down-regulation of SOX18 inhibits laryngeal
carcinoma cell proliferation, migration, and invasion through
JAK2/STAT3 signaling. Biosci Rep. 39:558–569. 2019. View Article : Google Scholar
|