Introduction
Lung cancer is the most common malignant tumor. The
morbidity and mortality rates rank first among all malignant
tumors, seriously threatening human health (1). Lung cancer includes small cell lung
cancer (SCLC) and non-small cell lung cancer (NSCLC), the latter of
which is dominant (2). The early
clinical symptoms of NSCLC are atypical, so the patients are in the
middle and advanced stage once diagnosed, with unsatisfactory
efficacy and poor prognosis. In recent years, the efficacy of
specific gene- or protein-targeted therapies for middle and
advanced NSCLC has greatly improved. However, the prognosis of
NSCLC is still very poor and the 5-year survival rate has not
improved. Therefore, study on the prognostic factors of NSCLC would
be helpful for effective judgment of the prognosis and the
formulation of individualized treatment plan (3). Metastasis is the main cause of death of
patients (4,5). Metastasis-associated protein 1 (MTA1)
plays an important role in tumor metastasis and often serves as a
potential indicator for malignant potential in clinic (6). Some studies have reported that MTA1
protein expression is associated with invasiveness, metastasis and
clinical outcome in patients with NSCLC (7–9).
Computed tomography (CT) examination is a conventional examination
method for NSCLC, which can fully reflect the pathophysiological
and molecular biological characteristics of tumors, and it has
important value in guiding the diagnosis and therapeutic evaluation
(10,11). However, the relationship between MTA1
protein expression and CT features has not been investigated so
far. It was hypothesized that there was some correlation between
the expression of MTA1 protein and CT features. Therefore, actively
exploring the associations between the imaging features of NSCLC
and molecular markers can provide a reliable theoretical basis for
targeted therapy of NSCLC.
Patients and methods
General data
A total of 98 elderly patients with NSCLC treated in
Shandong Provincial Hospital (Shandong, China) from July 2012 to
June 2014 were selected. Inclusion criteria: i) Patients meeting
the diagnostic criteria for NSCLC, ii) those undergoing CT
examination, and iiii) those without mental disorders and receiving
no surgery and chemoradiotherapy. Exclusion criteria: i) Patients
with failure of major organs, such as heart, liver and kidney, ii)
those with an allergic history of contrast agent, or iii) those who
underwent drug therapy or surgical therapy previously (Table I).
 | Table I.General data of patients. |
Table I.
General data of patients.
| Characteristics | Patients |
|---|
| Total no. | 98 |
| Mean age (years) | 68.74±5.86 |
| Sex
(male/female) | 69/29 |
| Tissue type [n
(%)] |
|
| Squamous
carcinoma | 53 (54.08) |
|
Adenocarcinoma | 45 (45.92) |
| Clinical stage [n
(%)] |
|
| I–II | 34 (34.69) |
|
III–IV | 64 (65.31) |
The study was approved by the Ethics Committee of
Shandong Provincial Hospital. Written informed consents were
obtained from the patients and/or guardians.
CT examination
Before the examination, all patients underwent solid
fasting for 6 h, and they were informed of relevant precautions.
The patients removed any metal objects, raised both hands to place
them on the both sides of the headrest. The dual-source CT machine
(Siemens AG) was used for plain scan (tube voltage: 120 kV,
rotation time: 0.5 sec, field of view: 414 mm, and slice thickness:
5 mm) from apex of the lung to costophrenic angle of patients under
a supine position. Then the patients drank properly, and were
injected with 50 ml of iohexol (350 mg/ml) at a rate of 6 ml/s
using the automatic high-pressure injector also under the supine
position. After injection for 7 sec, the perfusion scan was
performed once per second for a total of 31 times under BODY PCT
mode (tube current: 110 mAs, matrix: 512×512, collimation: 2×32×1.2
mm, rotation time: 0.28 sec, and tube voltage: 80 kV). Image
analysis and processing: The scanning images were transferred to
the processing workstation, and the clear perfusion slice with less
moving artifact was analyzed and processed by software. Finally,
the blood flow (BF), blood volume (BV), permeability surface (PS)
and mean transit time (MTT) were calculated.
Detection of MTA1 expression
The expression of MTA1 in lung tissue specimens was
determined using immunohistochemistry. The paraffin-embedded
tissues were sliced into 4 μm-thick sections using a
microtome (Leica Microsystems GmbH), baked at 60°C for 2 h,
deparaffinized with xylene and soaked in gradient ethanol and
distilled water for 5 min. Thereafter, the sections were added with
50 μl of 3% hydrogen peroxide solution, and incubated at
20°C for 10 min to block the activity of endogenous peroxidase.
After washing with phosphate buffered saline (PBS) 3 times (5
min/time), the sections were incubated with 50 μl of primary
antibodies MTA1 (1:300; cat. no. ab71153; Abcam) at 4°C overnight,
and then incubated again with secondary antibodies (1:500; cat. no.
ab7090; Abcam) at 20°C for 10 min, followed by color development
using diaminobenzidine (DAB) kit (Beijing ZSGB Biotechnology Co.,
Ltd.), hematoxylin counterstaining for 2 min and sealing with
neutral balsam.
Evaluation of indexes
Two senior radiologists conducted the imaging
analysis. CT perfusion parameters included BV, BF, capillary
permeability (PMB) and time to peak (TTP).
Expression of MTA1 in lung tissues
determined using immu-nohistochemistry
Ten fields of view (magnification, ×200) were
randomly selected in each section to detect the percentage of
positive cells (brown yellow cells), and the percentage point (PP)
was given: i) 0 point: no positive cells; ii) 1 point: percentage
of positive cells <5%; iii) 2 points: >5% to ≤20% positive
cells; iv) 3 points: percentage of positive cells >20%. Besides,
the staining intensity (SI) was also scored: i) 0 point: no
staining; ii) 1 point: light yellow; iii) 2 points: brown yellow;
iv) 3 points: dark brown. Finally, the immunoreactivity score (IRS)
was calculated: IRS = PPxSI. IRS >4 points indicates high
expression, while IRS ≤4 points indicates low expression (12).
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 software (SPSS Inc., Chicago, IL, USA) was used for data
processing. Measurement data were expressed as mean ± standard
deviation (mean ± SD), and t-test was performed. Enumeration data
were expressed as case (n) or percentage (%), and χ2
test was performed. P<0.05 indicates a statistically significant
difference.
Results
Expression of MTA1 in carcinoma
tissues and para-carcinoma normal tissues
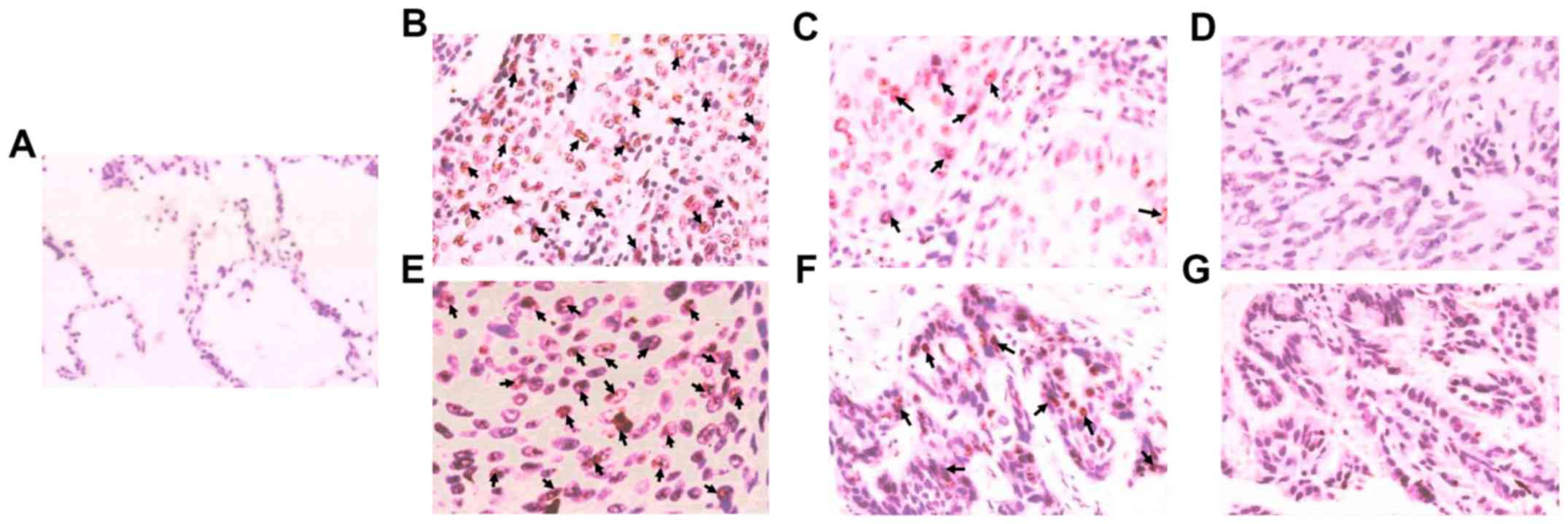
As shown by immunohistochemistry in Fig. 1, the positive expression rate of MTA1
in 98 patients was 60.20% (59/98), and MTA1 was mainly expressed in
the nucleus of carcinoma tissues. The positive expression of MTA1
was higher in poorly differentiated carcinoma than in moderately
differentiated and well differentiated carcinoma, and the MTA1
expression was very low in para-carcinoma normal tissues.
Association between MTA1 and
clinicopathological features of patients
The expression of MTA1 was associated with the
degree of differentiation, stage and lymph node metastasis
(P<0.05), but not with the age, sex or tissue type (P>0.05)
(Table II).
 | Table II.Association between MTA1 and
clinicopathological features of patients [n (%)]. |
Table II.
Association between MTA1 and
clinicopathological features of patients [n (%)].
| Item | No. | High expression
MTA1 | Low expression
MTA1 | χ2 | P-value |
|---|
| Age, years |
|
|
|
|
|
|
<75 | 55 | 33 (60.00) | 22 (40.00) | 0.001 | 0.983 |
| ≥75 | 43 | 25 (58.14) | 18 (41.86) |
|
|
| Sex |
|
|
|
|
|
| Male | 69 | 41 (59.42) | 28 (40.58) | 0.023 | 0.879 |
|
Female | 29 | 17 (58.62) | 12 (41.38) |
|
|
| Tissue type |
|
|
|
|
|
| Squamous
carcinoma | 53 | 31 (58.49) | 22 (41.51) | 0.001 | 0.954 |
|
Adenocarcinoma | 45 | 27 (60.00) | 18 (40.00) |
|
|
| Degree of
differentiation |
|
|
|
|
|
| Well
differentiated | 23 | 6 (26.09) | 17 (73.91) | 24.262 | <0.001 |
|
Moderately differentiated | 30 | 14 (46.67) | 16 (53.33) |
|
|
| Poorly
differentiated | 45 | 38 (84.44) | 7 (15.56) |
|
|
| Lymph node
metastasis |
|
|
|
|
|
| + | 63 | 51 (80.95) | 12 (19.05) | 32.127 | <0.001 |
| − | 35 | 7 (20.00) | 28 (80.00) |
|
|
| Clinical stage |
|
|
|
|
|
| I–II | 34 | 9 (26.47) | 25 (73.53) | 21.037 | <0.001 |
|
III–IV | 64 | 49 (76.56) | 15 (23.44) |
|
|
Association between CT image features
and MTA1 expression
The expression of MTA1 was related to the spicule
sign, pleural indentation sign and lymph node metastasis sign
(P<0.05) (Table III).
 | Table III.MTA1 expression in patients with
different CT image features [n (%)]. |
Table III.
MTA1 expression in patients with
different CT image features [n (%)].
| CT image
features | No. | High expression
MTA1 | Low expression
MTA1 | χ2 | P-value |
|---|
| Lobulation sign | 87 | 51 (58.62) | 36 (41.38) | 0.001 | 0.994 |
| No lobulation
sign | 11 | 7 (63.64) | 4 (36.36) |
|
|
| Spicule sign | 60 | 50 (83.33) | 10 (16.67) | 34.824 | 0.983 |
| No spicule
sign | 38 | 8 (21.05) | 30 (78.95) |
|
|
| Pleural indentation
sign | 61 | 52 (85.25) | 9 (14.75) | 42.617 | 0.983 |
| No pleural
indentation sign | 37 | 6 (16.22) | 31 (83.78) |
|
|
| Lymph node
metastasis sign | 63 | 51 (80.95) | 12 (19.05) | 21.037 | <0.001 |
| No lymph node
metastasis sign | 35 | 7 (20.00) | 28 (80.00) |
|
|
Association between CT perfusion
parameters and MTA1 expression
There were no obvious differences in BV, BF and TTP
between MTA1 high expression group and MTA1 low expression group.
PMB was higher in MTA1 high expression group than that in MTA1 low
expression group (P<0.05) (Table
IV).
 | Table IV.Comparison of CT perfusion parameters
in patients with different MTA1 expression. |
Table IV.
Comparison of CT perfusion parameters
in patients with different MTA1 expression.
| Groups | No. | BF
(ml•min-1•100g-1) | BV (ml•100g-1) | TTP (sec) | PMB
(ml•min-1•100g-1) |
|---|
| Low expression
MTA1 | 40 | 27.84±3.05 | 6.38±1.72 | 24.78±3.14 | 17.52±3.65 |
| High expression
MTA1 | 58 | 28.18±3.52 | 6.59±1.86 | 25.53±3.02 | 32.48±3.15 |
| t-test |
|
0.41 |
0.28 |
0.86 | 33.512 |
| P-value |
| >0.05 | >0.05 | >0.05 | <0.001 |
Survival status of patients with
different MTA1 expression
Patients with low expression of MTA1 had
significantly longer survival time and a remarkably higher 5-year
survival rate than those with high expression of MTA1 (P<0.05)
(Table V).
 | Table V.Comparison of 5-year follow-up
conditions between the groups. |
Table V.
Comparison of 5-year follow-up
conditions between the groups.
| Groups | No. | 5-year survival [n
(%)] | Mean survival time
(months) |
|---|
| Low expression
MTA1 | 40 | 26 (65.00) | 59.85±7.38 |
| High expression
MTA1 | 58 | 24 (41.38) | 44.52±6.63 |
|
χ2/t |
| 4.383 | 9.614 |
| P-value |
| 0.036 | <0.001 |
Discussion
NSCLC is a malignant tumor originating from the
bronchial mucous epithelium, its pathogenesis is not fully clear.
It is generally believed that smoking, occupational carcinogens,
air pollution, ionizing radiation, diet and nutrition, hereditary
and genetic changes are the major precipitating factors, under the
influence of which the carcinogens will cause damage to lung
tissues. Due to the low immunity of the elderly, it is difficult
for the self-defense and scavenging system of the body's immune
mechanism to completely eliminate these carcinogens, so that the
inflammatory response is induced, and abnormal changes are caused
in the microenvironment of lungs, ultimately leading to the
formation of malignant tumors (13–15). Due
to atypical early symptoms, NSCLC is usually diagnosed in the
middle and advanced stage, during which the tumor is prone to
metastasis and patients have poor prognosis (16).
MTA1 is a recently discovered protein associated
with tumor metastasis, and it belongs to the tumor
metastasis-associated protein family (17). Related studies have confirmed that
the expression level of MTA1 is extremely low in normal tissues but
obviously high in malignant tumor tissues, and it has a close
association with tumor metastasis (18). In this study, the positive expression
rate of MTA1 in 98 patients was 60.20% (59/98), and MTA1 was mainly
expressed in the nucleus of carcinoma tissues. The positive
expression of MTA1 was higher in poorly differentiated carcinoma
than that in moderately differentiated and well differentiated
carcinoma, and the MTA1 expression was very low in para-carcinoma
normal tissues. This is because MTA1 can be detected in the nucleus
of cancer tissues due to its high hydrophilicity and no
transmembrane feature. In this study, the expression of MTA1 was
associated with the degree of differentiation, stage and lymph node
metastasis (P<0.05), but not with age, gender or tissue type
(P>0.05). The reason is that MTA1 can inhibit ER gene
transcription, thereby promoting down-regulation of ER expression,
and facilitating the proliferation, invasion and metastasis of
tumor cells, which make the prognosis of patients poorer and
shorten their survival time.
CT examination has important value in the clinical
diagnosis and treatment of NSCLC, characterized by clear images and
short scanning time, and it is not influenced by surrounding
tissues and organs (19). CT
examination can be used to accurately observe the mass of NSCLC and
to assess whether there is enlargement of lymph nodes and
metastasis (20). With the
development of imaging genomics, the NSCLC tissues can be
quantitatively analyzed, so that the images can be converted into
researchable data to reveal the intrinsic relation between tumor
imaging features and molecular markers, which is of positive
significance in predicting related gene mutations, and can offer
strong support to targeted therapy of NSCLC (21). In this study, there was
overexpression of MTA1 in tumor tissues in elderly NSCLC patients
with spicule sign, pleural indentation sign and lymph node
metastasis sign in CT images, indicating that patients with high
expression of MTA1 are more prone to spicule sign, pleural
indentation sign and lymph node metastasis sign. CT perfusion
imaging technique can continuously scan the lesion area after
high-pressure intravenous injection of contrast agent, thereby
reflecting the hemodynamics of tumor tissues (22,23). In
this study, there were no obvious differences in BV, BF and TTP
between high expression MTA1 group and low expression MTA1 group.
PMB was higher in high expression MTA1 group than that in low
expression MTA1 group (P<0.05). The possible reason is that the
overexpression of MTA1 will regulate the cell transcription level
and affects tumor angiogenesis, but such a pro-angiogenic effect is
not significant enough to alter the CT perfusion parameters BV, BF
and TTP. However, in the case of MTA1 overexpression, the tumor is
more prone to hematogenous metastasis, and PMB is obviously
increased. As far as we know, MAT1 can only be detected in tissue,
but not in the blood. In clinic, the lung tissues of patients were
not always acquired for some reasons while CT examination was easy
to obtain. The results of this study provide more evidence for
targeted therapy of elderly NSCLC.
In conclusion, the expression of MTA1 has close
associations with the CT features, pathology and prognosis of
elderly NSCLC patients, which has positive guiding significance for
targeted therapy of elderly with NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NY, CL and JH designed the study and performed the
experiments. NY, CL and XH acquired the data. ZF, XH and FQ
analyzed the data. NY, CL and JH wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shandong Provincial Hospital (Shandong, China). Written informed
consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jamal-Hanjani M, Wilson GA, McGranahan N,
Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R,
Rosenthal R, et al TRACERx Consortium, : Tracking the evolution of
non-small-cell lung cancer. N Engl J Med. 376:2109–2121. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu L, Zhou XY, Zhang JQ, Wang GG, He J,
Chen YY, Huang C, Li L and Li SQ: LncRNA HULC promotes non-small
cell lung cancer cell proliferation and inhibits the apoptosis by
up-regulating sphingosine kinase 1 (SPHK1) and its downstream
PI3K/Akt pathway. Eur Rev Med Pharmacol Sci. 22:8722–8730.
2018.PubMed/NCBI
|
|
3
|
Peters S, Camidge DR, Shaw AT, Gadgeel S,
Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, et al
ALEX Trial Investigators, : Alectinib versus crizotinib in
untreated ALK-positive non-small-cell lung cancer. N Engl J Med.
377:829–838. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rotow J and Bivona TG: Understanding and
targeting resistance mechanisms in NSCLC. Nat Rev Cancer.
17:637–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hendriks LEL and Dingemans AC: Heat shock
protein antagonists in early stage clinical trials for NSCLC.
Expert Opin Investig Drugs. 26:541–550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma K, Fan Y, Dong X, Dong D, Guo Y, Wei X,
Ning J, Geng Q, Wang C, Hu Y, et al: MTA1 promotes epithelial to
mesenchymal transition and metastasis in non-small-cell lung
cancer. Oncotarget. 8:38825–38840. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu X, Guo Y, Li X, Ding Y and Chen L:
Metastasis-associated protein 1 nuclear expression is associated
with tumor progression and clinical outcome in patients with
non-small cell lung cancer. J Thorac Oncol. 5:1159–1166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hofer MD, Tapia C, Browne TJ, Mirlacher M,
Sauter G and Rubin MA: Comprehensive analysis of the expression of
the metastasis-associated gene 1 in human neoplastic tissue. Arch
Pathol Lab Med. 130:989–996. 2006.PubMed/NCBI
|
|
9
|
Sasaki H, Moriyama S, Nakashima Y,
Kobayashi Y, Yukiue H, Kaji M, Fukai I, Kiriyama M, Yamakawa Y and
Fujii Y: Expression of the MTA1 mRNA in advanced lung cancer. Lung
Cancer. 35:149–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swensen SJ: CT screening for lung cancer.
AJR Am J Roentgenol. 179:833–836. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goss G, Tsai CM, Shepherd FA, Ahn MJ,
Bazhenova L, Crinò L, de Marinis F, Felip E, Morabito A, Hodge R,
et al: CNS response to osimertinib in patients with T790M-positive
advanced NSCLC: Pooled data from two phase II trials. Ann Oncol.
29:687–693. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jagadish N, Parashar D, Gupta N, Agarwal
S, Sharma A, Fatima R, Suri V, Kumar R, Gupta A, Lohiya NK, et al:
A novel cancer testis antigen target A-kinase anchor protein
(AKAP4) for the early diagnosis and immunotherapy of colon cancer.
OncoImmunology. 5:e10789652016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi Y, Sun Y, Yu J, Ding C, Wang Z, Wang
C, Wang D, Wang C, Wang Z, Wang M, et al: China experts consensus
on the diagnosis and treatment of advanced stage primary lung
cancer (2016 Version). (In Chinese). View Article : Google Scholar
|
|
14
|
Cui Y, Zhang F, Zhu C, Geng L, Tian T and
Liu H: Upregulated lncRNA SNHG1 contributes to progression of
non-small cell lung cancer through inhibition of miR-101-3p and
activation of Wnt/β-catenin signaling pathway. Oncotarget.
8:17785–17794. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Q, Wang Q, Wang SF, Jiao LJ, Zhang
RX, Zhong Y, Zhang J and Xu L: Oral Chinese herbal medicine as
maintenance treatment after chemotherapy for advanced
non-small-cell lung cancer: A systematic review and meta-analysis.
Curr Oncol. 24:269–276. 2017. View Article : Google Scholar
|
|
16
|
Vesel M, Rapp J, Feller D, Kiss E, Jaromi
L, Meggyes M, Miskei G, Duga B, Smuk G, Laszlo T, et al: ABCB1 and
ABCG2 drug transporters are differentially expressed in non-small
cell lung cancers (NSCLC) and expression is modified by cisplatin
treatment via altered Wnt signaling. Respir Res. 18:522017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia QP, Yan CY, Zheng XR, Pan X, Cao X and
Cao L: Upregulation of MTA1 expression by human papillomavirus
infection promotes CDDP resistance in cervical cancer cells via
modulation of NF-κB/APOBEC3B cascade. Cancer Chemother Pharmacol.
83:625–637. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bui-Nguyen TM, Pakala SB, Sirigiri DR,
Martin E, Murad F and Kumar R: Stimulation of inducible nitric
oxide by hepatitis B virus transactivator protein-HBx requires MTA1
coregulator. J Biol Chem. 292:47652017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li HS, Lv RQ and Liu L: Correlation of CT
indicators of NSCLC and pathological features and the expression
level of p53 and c-myc. Eur Rev Med Pharmacol Sci. 22:135–141.
2018.PubMed/NCBI
|
|
20
|
Lee J, Cui Y, Sun X, Li B, Wu J, Li D,
Gensheimer MF, Loo BW Jr, Diehn M and Li R: Prognostic value and
molecular correlates of a CT image-based quantitative pleural
contact index in early stage NSCLC. Eur Radiol. 28:736–746. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abel S, Hasan S, White R, Schumacher L,
Finley G, Colonias A and Wegner RE: Stereotactic ablative
radiotherapy (SABR) in early stage non-small cell lung cancer:
Comparing survival outcomes in adenocarcinoma and squamous cell
carcinoma. Lung Cancer. 128:127–133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carrascosa P and Capunay C: Myocardial CT
perfusion imaging for ischemia detection. Cardiovasc Diagn Ther.
7:112–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chu LL, Knebel RJ, Shay AD, Santos J,
Badawi RD, Gandara DR and Knollmann FD: CT perfusion imaging of
lung cancer: Benefit of motion correction for blood flow estimates.
Eur Radiol. 28:5069–5075. 2018. View Article : Google Scholar : PubMed/NCBI
|