Introduction
Although advances in multimodal therapies including
surgery, radiotherapy, and chemotherapy are being made, the
prognosis for glioblastomas, the most common primary brain tumor in
adults and classified as having WHO grade IV malignancy, has not
improved adequately for more than 30 years. In 2009, the EORTC/NCIC
reported final results indicating the benefits of concomitant and
adjuvant temozolomide (TMZ: a relatively new alkylating agent) in
addition to standard postoperative radiotherapy for glioblastomas
(1). Subsequently, concomitant
radiotherapy with TMZ followed by adjuvant TMZ chemotherapy has
become the current and global standard treatment for malignant
gliomas, especially glioblastomas. Even though such treatments show
a survival benefit in glioblastoma patients, the prognosis deriving
from these therapies remains unsatisfactory.
Ribavirin, first reported in 1972 by Sidwell et
al (2) as an anti-viral agent,
is a nucleic acid analog. To date, ribavirin has served as a
therapeutic agent widely used against RNA and DNA viruses, and is
in particular one of the standard agents for chronic hepatitis C
combined with interferon (3). On the
other hand, we and others have recently observed an anti-tumor
effect of ribavirin on various tumor cells, including breast
cancer, acute myeloid leukemia, and atypical teratoid/rhabdoid
tumors, which is thought to be mediated via
inosine-5′-monophosphate dehydrogenase (IMPDH), eukaryotic
translation initiation factor 4E (eIF4E), histone methyltransferase
enhancer of zeste homolog 2 (EZH2), extracellular regulated protein
kinases (ERK), and/or mitogen-activated protein kinase interacting
protein kinase 1 (MNK1) (3–11). To date, there have been only a few
studies on the anti-tumor effect of ribavirin against glioma cells.
Volpin et al (9) reported an
anti-tumor effect of ribavirin including in combination with TMZ
and irradiation on glioma cells and glioma stem-like cells in
vivo and in vitro. More recently, we demonstrated an
effect of ribavirin on apoptosis induction, cell cycle arrest, p53
pathway activation, and DNA damage (double-strand breaks: DSBs) in
malignant glioma cell lines (10).
In the present study, we obtained further data by
examining the effect of ribavirin in combination with TMZ and
interferon-beta (IFN-β) on malignant glioma cells. The reasons for
using TMZ and IFN-β in combination with ribavirin were that TMZ is
a standard chemotherapeutic agent for glioblastomas as mentioned
above, and IFN-β exhibits pleiotrophic biological activities
against neoplasias (12–15) and acts as a drug sensitizer enhancing
the anti-tumor effect when administered in combination with
nitrosoureas (alkylating agents) against malignant gliomas
(16). Furthermore, a synergistic
anti-tumor effect between TMZ and IFN-β has been demonstrated in
malignant glioma cells (17–19), and a significant anti-tumor effect of
ribavirin in combination with IFN-α (grouped to type I IFNs, the
same as IFN-β) has been observed in hepatoma cells and renal
carcinoma cells (20,21). Based on the findings of the present
study, the combination of these three agents could exert a
synergistic anti-tumor effect in malignant glioma cells and provide
an experimental basis for rational clinical treatments in
glioblastoma patients.
Materials and methods
Cell lines and cell culture
Human malignant glioma cells of the A-172 (cell no.
JCRB0228, lot no. 021999), AM-38 (cell no. IFO50492, lot no.
12082003), T98G (cell no. IFO50303, lot no. 1007), U-251MG (cell
no. IFO50288, lot no. 12132002), and YH-13 (cell no. IFO50493, lot
no. 1164) cell lines were obtained from Health Science Research
Resources Bank (Osaka, Japan), and U-87MG (glioblastoma of unknown
origin; catalog no.: HTB-14, lot no. 2497162) and U-138MG (catalog
no. HTB-16, lot no. 1104428) were procured from the American Type
Culture Collection. It has been confirmed by us that
O6-methylguanine-DNA methyltransferase (MGMT: a
key factor of alkylating agents) is expressed in T98G, U-138MG and
YH-13 by RT-PCR and western blot analysis in a previous study
(22). Consistent with an earlier
report (23), it was also confirmed
that T98G (237 Met→Ile) and U-251MG (273 Arg→His)
have a point mutation of the p53 gene. These cell lines were
cultured in Dulbecco's modified Eagle's medium (Nissui
Pharmaceutical) containing 10% fetal calf serum (Life Technologies)
using plastic culture flasks (Corning®) in a standard
humidified incubator at 37°C under a 5% CO2, 95% air
atmosphere.
Cell culture growth studies
Malignant glioma cell proliferation was evaluated
using a Coulter Counter (Beckman Coulter) to determine the cell
numbers in 24-well plates (Iwaki). Each well was seeded with
1×104 cells and cultured for 24 h prior to treatment to
allow adherence of the cells to the plate. The culture medium was
replenished with fresh medium containing ribavirin (0.1–100 µM;
Sigma) alone, or in combination with TMZ (10 µM; LKT Laboratories)
and IFN-β (10 IU/ml; Toray), and the cells were cultured for 72 h.
We selected the incubation conditions as 10 µM TMZ and/or 10 IU/ml
IFN-β, because these values represent clinically achievable
concentrations of TMZ and IFN-β (19,24).
Furthermore, in order to assess whether the effect of the
combination of these agents, TMZ, IFN-β, and/or ribavirin, was
synergistic, the ribavirin was set at a clinically relevant
concentration of 10 µM (25). The
proliferated cells were harvested with trypsin-EDTA solution
(Invitrogen; Thermo Fisher Scientific, Inc.) and the numbers
counted. The cell culture growth experiments were repeated a
minimum of four times each.
Synergism of drug combination
treatment
Moreover, to confirm whether the anti-tumor effect
of the combination with TMZ, IFN-β, and ribavirin was synergistic,
a combination index (CI) was also calculated using the Chou-Talalay
method (26). In the present study,
we decided to calculate the CI at 50% inhibition of cell
proliferation (IC50). Two malignant glioma cell lines,
A-172 and U-251MG, were therefore employed to calculate the CI,
because their IC50 values had been obtained on the basis
of dose response curves. The IC50 of TMZ, IFN-β, and
ribavirin for A-172 was 52.4 µM, 57.5 IU/ml, and 53.6 µM, and for
U-251 was 22.5 µM, 26.4 IU/ml, and 257.7 µM, respectively (7,15,22).
Based on the Chou-Talalay method, CI values of less than 1 are
indicative of synergism (the smaller the value, the greater the
degree of synergy), those equal to 1 indicate additivity, and those
of more than 1 are interpreted as antagonism. Values of CI <
0.4, between 0.4 and 0.8, and >0.8 indicate strong, medium and
slight synergism, respectively (26,27). An
additive effect is defined as a situation in which the final effect
is equal to the sum of the effects of the drugs. Drug interactions
are interpreted as antagonistic if they lead to a decrease in the
effects of one or both drugs (26,27).
Assessment of apoptosis
From the results for the growth inhibitory effect
and CI, U-251MG cells were subjected to further experiments.
Apoptosis was analyzed by flow cytometry, using dual staining with
an Annexin V-FITC/PI Apoptosis Detection Kit (BD Biosciences).
Cells were seeded in 6-well plates (Iwaki) at 1×106
cells and incubated for 24 h to adhere. The culture medium was then
replenished with fresh medium containing ribavirin, TMZ, IFN-β, TMZ
and IFN-β, or TMZ, IFN-β and ribavirin for 72 h (ribavirin, 10 µM;
TMZ, 10 µM; IFN-β, 10 IU/ml). The cells were next washed in PBS and
harvested using trypsin-EDTA solution. Following centrifugation and
washing in PBS, the solution was agitated with 100 ml of binding
buffer (Wako Pure Chemical Industries, Ltd.), into which 5 µl of
Annexin V Alexa Fluor 488 conjugate (Life Technologies; Thermo
Fisher Scientific, Inc.) and 10 µl of PI (Miltenyi Biotech) were
added, and incubated at room temperature for 10 min. An additional
400 µl of binding buffer was added to give a total sample volume of
500 µl. The fluorescence was measured with an FACSCalibur flow
cytometer (Becton-Dickinson). The apoptotic cells were analyzed
using Flowjo software (BioLegend). The experiments were repeated
three times to confirm reproducibility.
Statistical analysis
Appropriate comparisons were made employing one-way
analysis of variance followed by the Tukey-Kramer method among
multiple comparisons using the software Stat View (Ver. 5.0; SAS
Institute Inc.). Data were expressed as the means ± standard error
and were considered significant at P<0.05.
Results
Anti-tumor effects of a combination of
ribavirin with TMZ and IFN-β
We have previously demonstrated an anti-tumor effect
of ribavirin on malignant glioma cell lines (7). In that study, seven malignant glioma
cell lines were exposed to 0.1–1,000 µM of ribavirin and treated
for 72 h, and it was found that ribavirin inhibited the growth of
all seven cell lines in a dose-dependent manner (7).
To assess whether or not a combination of ribavirin
with TMZ and IFN-β could produce a more profound anti-tumor effect
as compared to ribavirin alone in malignant glioma cell lines,
cells were incubated in culture medium containing various
concentrations (0–100 IU/ml) of ribavirin alone or ribavirin with
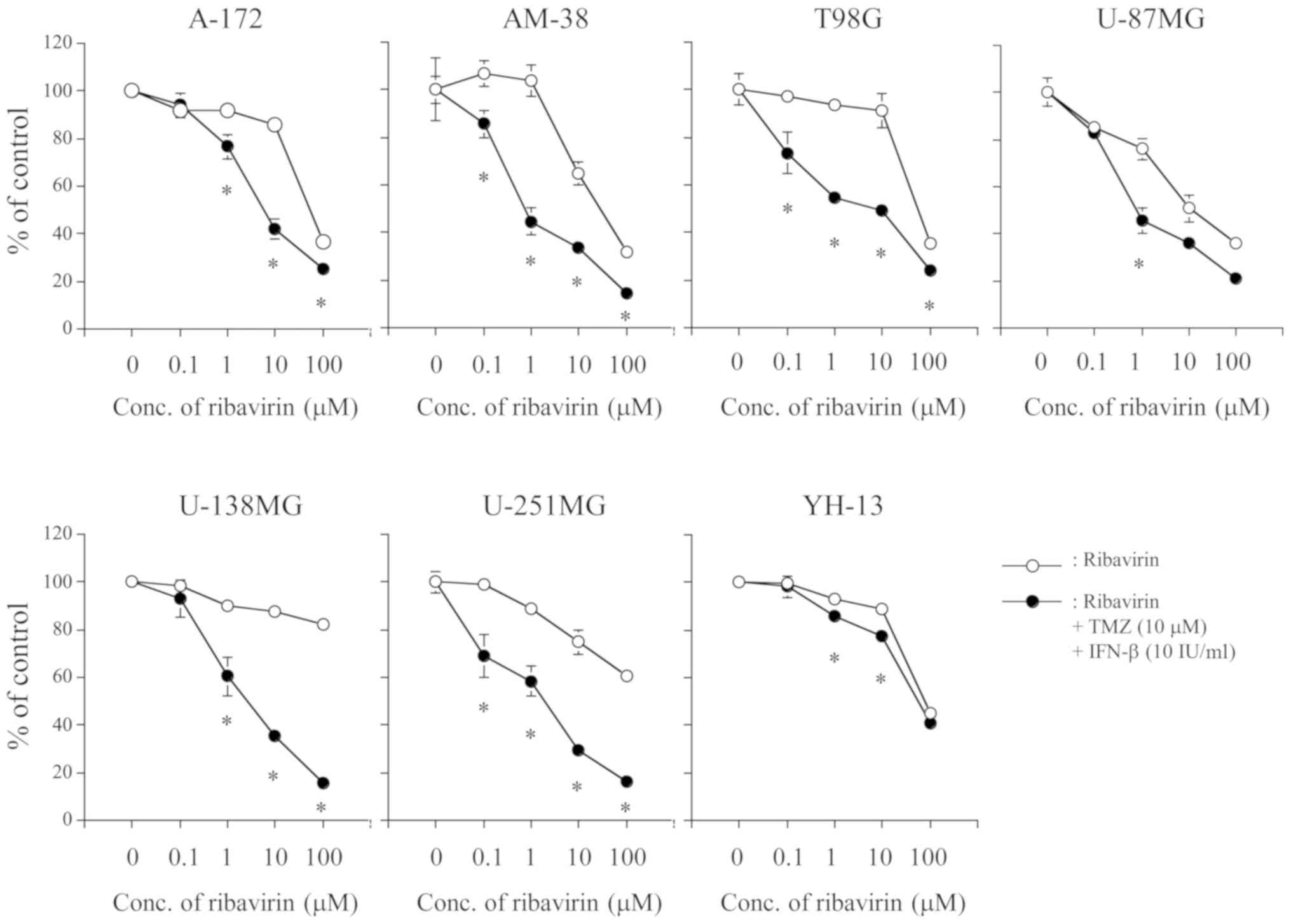
TMZ (10 µM) and IFN-β (10 IU/ml) for 72 h. As shown in Fig. 1, the combination of ribavirin with
TMZ (10 µM) and IFN-β (10 IU/ml) revealed a significant cell growth
inhibitory effect with a ribavirin dose-dependency in all seven
malignant glioma cell lines. Such a cell growth inhibitory effect
was also observed at a relative low concentration of ribavirin, 0.1
and 1 µM. Furthermore, it was evident at 0.1 µM of ribavirin in
AM-38, T98G, U-87MG, and U-251MG cells.
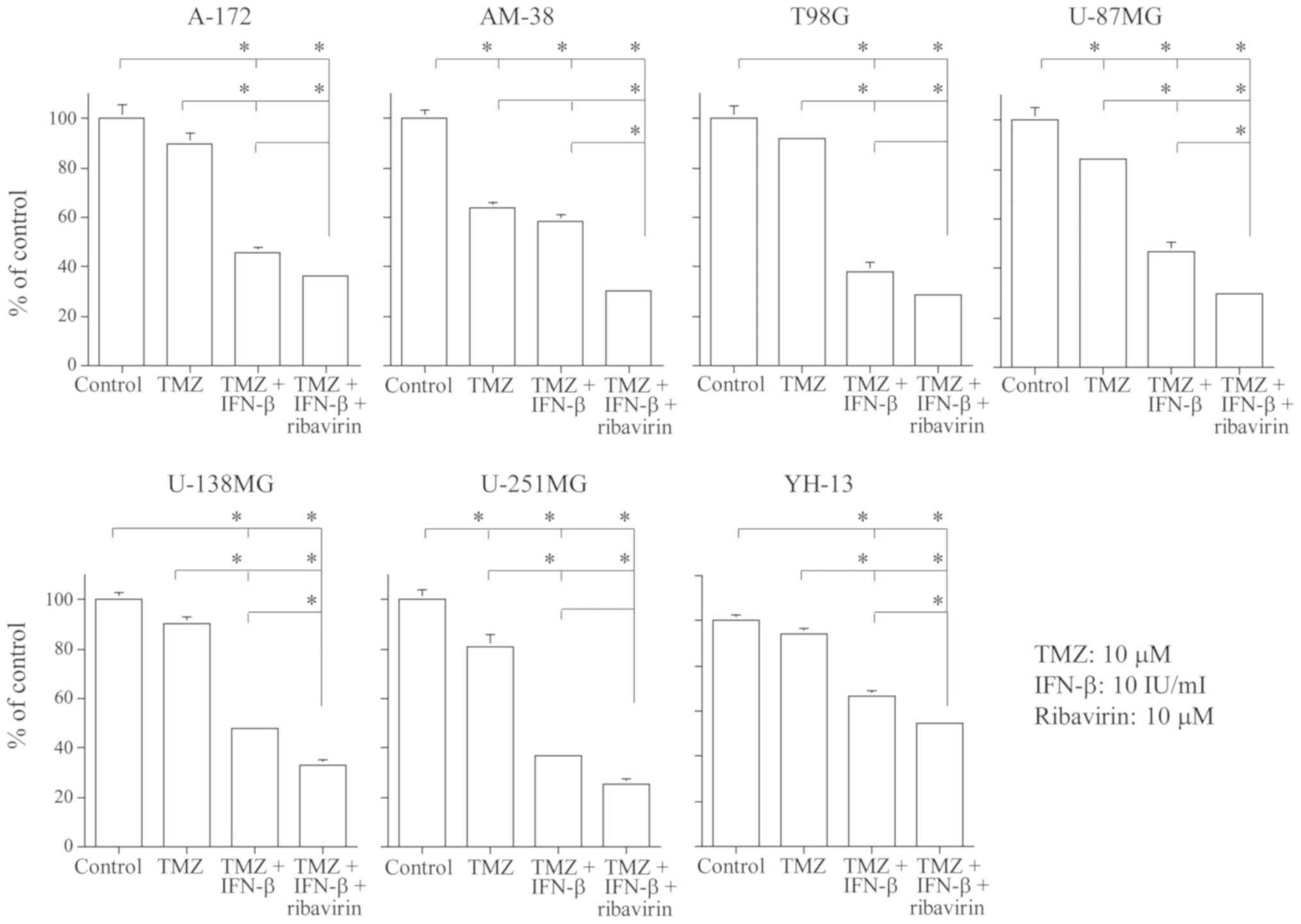
In addition, we examined whether the combination of
TMZ, IFN-β, and ribavirin displayed an enhanced cell growth
inhibitory effect as compared to the control, TMZ alone, or TMZ and
IFN-β. As demonstrated in Fig. 2,
the cell growth inhibitory effect at 72 h was significantly higher
in the group treated with TMZ (10 µM), IFN-β (10 IU/ml), and
ribavirin (10 µM) as compared to the group treated with TMZ alone
in all seven malignant glioma cell lines. Furthermore, the
combination of TMZ, IFN-β and ribavirin exerted a significantly
enhanced growth inhibitory effect as compared to TMZ and IFN-β in
the AM-38, U-87MG, U-138MG, and YH-13 cells.
Drug synergy analysis of a combination
of ribavirin with TMZ and IFN-β
Our results indicated that the combination of TMZ,
IFN-β and ribavirin displayed significant growth inhibition in
malignant glioma cells. When TMZ, IFN-β, and ribavirin were applied
in combination, a synergistic effect was detected as analyzed by
the Chou-Talalay method in malignant glioma cells. The CI value was
0.68 (medium synergism) in A-172 and 0.98 (slight synergism) in
U-251MG at the IC50 level, respectively.
Apoptosis induced by a combination of
TMZ, IFN-β, and ribavirin in U-251MG cells
The induction of apoptosis by TMZ, IFN-β, ribavirin,
TMZ and IFN-β, or TMZ, IFN-β and ribavirin in U-251MG cells was
examined by Annexin V/PI double staining and evaluated using flow
cytometry. The distribution of apoptotic cells (Annexin V-positive,
early-stage apoptosis; Annexin V/PI-positive, late-stage apoptosis)
after 72 h treatment is illustrated in Fig. 3. In particular, the proportion of
Annexin V/PI positive cells (late-stage apoptosis) following
treatment with three agents (TMZ, IFN-β and ribavirin; mean=10.56%)
was higher than that for each single agent (means: TMZ, 5.57%;
IFN-β, 5.74%; and ribavirin, 5.57%, respectively). The results
indicated that the apoptotic cells after treatment with three
agents tend to be increased, showing an elevation of more than 2%
between each treatment, in U-251MG cells. However, the fact that
statistically significant differences were not found among the
treatments might reflect the small numbers in each group.
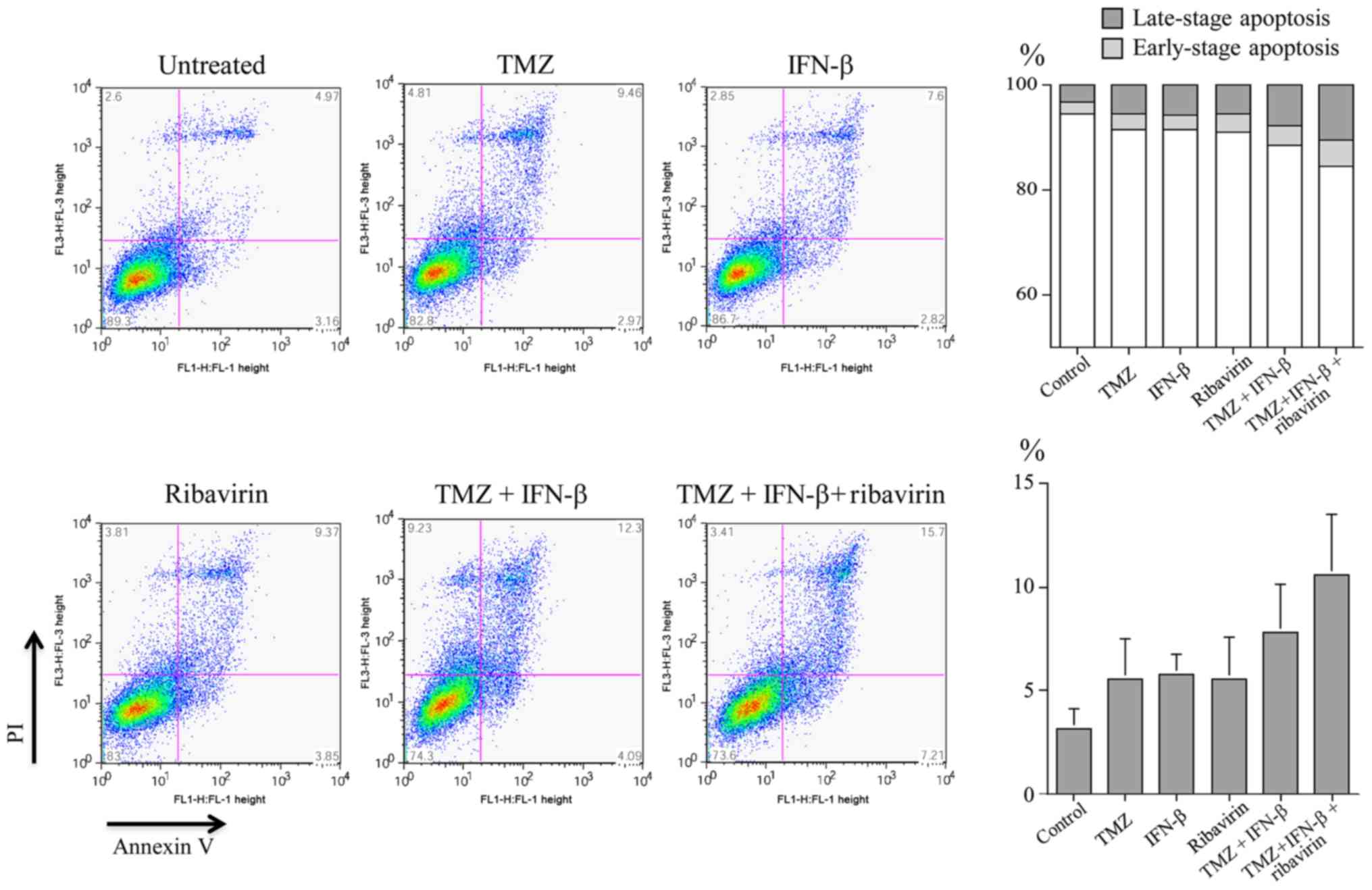 | Figure 3.Induction of apoptosis by TMZ, IFN-β,
ribavirin, TMZ and IFN-β, or TMZ, IFN-β and ribavirin in U251MG
cells. The ratio of detection of Annexin V-positive and Annexin
V/PI-positive cells, indicating early-stage apoptosis, and
late-phase apoptosis or necrosis, respectively, was measured by
fluorescence-activated cell sorting after 72 h of treatment. The
percentage of Annexin V-positive cells and the percentage of
Annexin V/PI-positive cells was increased as shown at the bottom
right and top right, respectively. The mean distributions of
apoptotic cells with each drug and the combination are illustrated
on the right. IFN-β, interferon-β; TMZ, temozolomide. |
Discussion
TMZ has become the current first-line
chemotherapeutic agent, but it does not provide satisfactory
benefits for glioblastoma patients. Further studies are needed to
improve the clinical therapeutic efficacy and to establish a
therapeutic strategy for glioblastomas. On the other hand, we
demonstrated a dose-dependent anti-tumor efficacy of ribavirin for
malignant glioma cell lines (7).
Recently, Volpin et al (9)
showed that ribavirin (30 µM) inhibited cell proliferation and
migration, and increased cell arrest and cell death in glioma
cells, potentially through modulation of the elF4E, EZH2, and ERK
pathways. Furthermore, they indicated that ribavirin combined with
TMZ (100 µM) and irradiation (5 Gy) could potentially enhance the
anti-tumor response in glioma cells and glioma stem-like cells, and
that animals treated with a combination of ribavirin, irradiation,
and TMZ had a significantly increased survival time (9). More recently, we found that ribavirin
(10 µM) exerts an anti-tumor effect on malignant glioma cells via
the biological processes of induction of DSBs, cell cycle arrest in
G0/G1, and both exogenous and endogenous
apoptosis (10). Moreover, such
effects might not be dependent on MGMT expression, which is closely
correlated with resistance to TMZ treatment (10).
In the present study, the combination of ribavirin
with TMZ and IFN-β (clinically achievable concentrations) revealed
a more profound cell growth inhibitory effect as compared to
ribavirin alone with a ribavirin dose-dependency, which was
observed from a relatively low concentration of ribavirin, in all
seven malignant glioma cell lines (Fig.
1), suggesting that ribavirin has some anti-tumor effect as a
medical drug. Although TMZ (10 µM) did not show a cell growth
inhibitory effect in A-172, T98G, U-138MG, and YH-13, the
combination of ribavirin with TMZ and IFN-β did exhibit a
significant cell growth inhibitory effect in these cell lines. Out
of the four cells, U-138MG and YH-13 displayed a significant cell
growth inhibitory effect when treated with ribavirin, TMZ, and
IFN-β as compared to the effect without ribavirin (Fig. 2). These findings suggested that the
combination of ribavirin with TMZ and IFN-β revealed a more
significant cell growth inhibitory effect in not only TMZ sensitive
cells but also in TMZ resistant cells. Furthermore, in this study,
the anti-tumor efficacy of a combination of these three agents on
A-172 and U-251MG cells indicated a synergistic interaction when
assessed by the Chou-Talalay method [A-172 and U-251MG were used in
the analysis because the IC50 for each drug had been
obtained (7,22)]. Such a combination of ribavirin with
TMZ and IFN-β might therefore be considered as an effective
treatment in certain glioblastoma cells, although, to the best of
our knowledge, no report has yet described the detailed effect of
such a combination on glioma cell lines and other cell lines.
Although the mechanism underlying the anti-viral and
anti-tumor effects of ribavirin has not yet been fully elucidated,
several participating/possible processes have been mentioned above.
Kast et al (28) pointed out
the following major mechanisms of action, particularly related to
the anti-viral action: i) actual intermingling within the viral
RNA; ribavirin enters the cell via a nucleoside transport
mechanism, subsequently inhibiting/altering viral RNA synthesis,
ii) structural analogy to GTP; incorporation into the cell
passively, then binding/inhibiting RNA polymerase/RNA synthesis,
iii) immune clearance; immune-stimulation by up-regulation of
cytokines to shift the Th1/2 cell balance to Th1 dominance, iv)
inhibition of eIF4E; thereby inhibiting mRNA capping and
translation initiation, v) modulation of IFN-related gene
expression, vi) inhibition of IMPDH; with consequent depletion of
intracellular GTP, and vii) RNA mutagen; following
triphosphorylation, ribavirin triphosphate is incorporated into
replicating RNA viral RNA polymerases with consequent induction of
viral mutagenesis. These processes are attractive as factors in the
repurposing of the anti-viral ribavirin as an anti-tumor agent, and
also in the mechanism underlying the synergistic effect of
ribavirin in combination with other agents. In particular, the
modulation of IFN-related gene expression is thought to be a
potential factor in the synergistic effect of ribavirin with IFN
(13,19), since IFN exerts a priming effect on
the ribavirin-induced IFN-related gene (29). Further studies investigating the
molecular mechanisms are clearly needed to reaffirm the efficacy of
these combinations.
In the present study, the flow cytometry analysis
might indicate that apoptosis induction represented one possible
biological process associated with the synergistic anti-tumor
effect of triple combination treatment, because the apoptotic cells
after such triple treatment tended to be increased in U-251MG,
although no statistically significant differences were observed.
Schlosser et al (20)
demonstrated that ribavirin and IFN enhanced apoptosis and caspase
activation in hepatoma cells. Further, Teng et al (21) showed that ribavirin in combination
with IFN could significantly inhibit the cell proliferation and
migration, induce apoptosis, arrest the cell cycle, and decrease
IL-10 production in renal carcinoma cells. On the basis of these
findings, we propose that ribavirin in combination with TMZ and
IFN-β could increase apoptosis in glioblastoma cells.
Finally, and very importantly, we need briefly to
mention other effects of the drug combination used in this study.
Chemotherapy-induced toxicity is a major problem in anti-tumor
therapy. A cell growth inhibitory effect was also observed at a
relatively low concentration of ribavirin (0.1 and 1 µM) in some
glioma cell lines, AM-38, T98G, U-87MG, U-138MG, and U2-51MG,
combined with 10 µM TMZ and 10 IU/ml IFN-β: both concentrations are
clinically achievable (19,24). Ribavirin at 10 µM is considered a
clinically relevant concentration, because when administered at a
dose of 800 mg/day as a therapeutic agent for chronic hepatitis C,
the blood concentration of ribavirin reached 13 µM and the cerebral
spinal migration of ribavirin was 70% (10,25). In
addition, Casaos et al (11)
employed 50 µM ribavirin as a clinically appropriate concentration
in their in vitro experiments. A very low concentration of
ribavirin should help to reduce the adverse events of ribavirin,
such as anemia, and be easier for clinical use. In addition,
ribavirin is relatively inexpensive, and therefore beneficial in
relation to the rising medical costs particularly of tumor
treatment. Since the clinical use of triple combination therapy
could lead to combined toxicity in patients, further studies are
needed to investigate the extent of efficacy at various doses and
times of use of these combinations.
In conclusion, we have provided evidence that
ribavirin in combination with TMZ and IFN-β can induce synergistic
anti-tumor effects involving of cell growth inhibition in glioma
cells, and could be of potential importance in the clinical
setting.
Acknowledgements
The authors would like to thank Mr. Nobuo Miyazaki
(Toray Industries Inc., Tokyo, Japan) for his discussions. Some
parts of the present study have been included within a
Japanese-language thesis submitted for the Ph.D. degree of Yushi
Ochiai at Nihon University School of Medicine.
Funding
This work was supported in part by Grants-in-Aid for
Scientific Research from the Japan Society for the Promotion of
Science (grant no. 16K10772) and in part by a grant from the Health
Sciences Research Institute, Inc. (Yokohama, Japan) for the
Division of Companion Diagnostics, Department of Pathology and
Microbiology, Nihon University School of Medicine.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AY contributed to the experimental concept and
design. YO and ES developed the experimental design, performed most
of the experiments and some of the data analysis, and wrote part of
the draft manuscript. KS and AY also conducted part of the data
analysis and contributed to the writing of the manuscript. SYo, SYa
and AO were involved in the conception and design of the study,
undertook part of the experiments, analyzed the data, and
contributed to the writing of the draft manuscript. TU, YS, TN, HH
and YK supervised the study (including the experimental design),
performed some of the experiments, analyzed data, helped prepare
the draft manuscript, and proofread the manuscript. YO and KS
contributed equally to this work. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sidwell RW, Huffman JH, Khare GP, Allem
LB, Witkowski JT and Robins RK: Broad-spectrum antiviral activity
of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide.
Science. 177:705–706. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kohli A, Shaffer A, Sherman A and Kottilil
S: Treatment of hepatitis C: A systematic review. JAMA.
312:631–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kentsis A, Topisirovic I, Culjkovic B,
Shao L and Borden KL: Ribavirin suppresses eIF4E-mediated oncogenic
transformation by physical mimicry of the 7-methyl guanosine mRNA
cap. Proc Natl Acad Sci USA. 101:18105–18110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Assouline S, Culjkovic B, Cocolakis E,
Rousseau C, Beslu N, Amri A, Caplan S, Leber B, Roy DC, Miller WH
Jr and Borden KL: Molecular targeting of the oncogene eIF4E in
acute myeloid leukemia (AML): A proof-of-principle clinical trial
with ribavirin. Blood. 114:257–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borden KL and Culjkovic-Kraljacic B:
Ribavirin as an anti-cancer therapy: Acute myeloid leukemia and
beyond? Leuk Lymphoma. 51:1805–1815. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogino A, Sano E, Ochiai Y, Yamamuro S,
Tashiro S, Yachi K, Ohta T, Fukushima T, Okamoto Y, Tsumoto K, et
al: Efficacy of ribavirin against malignant glioma cell lines.
Oncol Lett. 8:2469–2474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De la Cruz-Hernandez E, Medina-Franco JL,
Trujillo J, Chavez-Blanco A, Dominguez-Gomez G, Perez-Cardenas E,
Gonzalez-Fierro A, Taja-Chayeb L and Dueñas-Gonzalez A: Ribavirin
as a tri-targeted antitumor repositioned drug. Oncol Rep.
33:2384–2392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Volpin F, Casaos J, Sesen J, Mangraviti A,
Choi J, Gorelick N, Frieche J, Lott T, Felder R, Scotland SJ, et
al: Use of an anti-viral drug, Ribavirin, as an anti-glioblastoma
therapeutic. Oncogene. 36:3037–3047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ochiai Y, Sano E, Okamoto Y, Yoshimura S,
Makita K, Yamamuro S, Ohta T, Ogino A, Tadakuma H, Ueda T, et al:
Efficacy of ribavirin against malignant glioma cell lines:
Follow-up study. Oncol Rep. 39:537–544. 2018.PubMed/NCBI
|
|
11
|
Casaos J, Huq S, Lott T, Felder R, Choi J,
Gorelick N, Peters M, Xia Y, Maxwell R, Zhao T, et al: Ribavirin as
a potential therapeutic for atypical teratoid/rhabdoid tumors.
Oncotarget. 9:8054–8067. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito R, Mizuno M, Hatano M, Kumabe T,
Yoshimoto T and Yoshida J: Two different mechanisms of apoptosis
resistance observed in interferon-β induced apoptosis of human
glioma cells. J Neurooncol. 67:273–280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshino A, Katayama Y, Yokoyama T,
Watanabe T, Ogino A, Ota T, Komine C, Fukushima T and Kusama K:
Therapeutic implication of interferon regulatory factor 1 (IRF-1)
and IRF-2 in diffusely infiltrating astrocytomas (DIA): Response to
(IFN)-beta in glioblastoma cells and prognostic value for DIA. J
Neurooncol. 74:249–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vannucchi S, Chiantore MV, Mangino G,
Percario ZA, Affabris E, Fiorucci G and Romeo G: Perspective in
biomolecular therapeutic intervention in cancer: From the early to
the new strategies with type 1 interferons. Curr Med Chem.
14:667–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshino A, Tashiro S, Ogino A, Yachi K,
Ohta T, Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Sano E and
Tsumoto K: Gene expression profiles predicting the response to
IFN-β and a combination of temozolomide and IFN-β in malignant
gliomas. Int J Oncol. 39:529–542. 2011.PubMed/NCBI
|
|
16
|
Yoshida J, Kajita Y, Wakabayashi T and
Sugita K: Long-term follow-up results of 175 patients with
malignant glioma: Importance of radical tumor resection and
post-operative adjuvant therapy with interferon, ACNU and
radiation. Acta Neurochir. 127:55–59. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Natsume A, Ishii D, Wakabayashi T, Tsuno
T, Hatano H, Mizuno M and Yoshida J: IFN-beta down-regulates the
expression of DNA repair gene MGMT and sensitizes resistant glioma
cells to temozolomide. Cancer Res. 65:7573–7579. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JA, Joe YA, Kim TG and Hong YK:
Potentiation of anti-glioma effect with combined temozolomide and
interferon-beta. Oncol Rep. 16:1253–1260. 2006.PubMed/NCBI
|
|
19
|
Yoshino A, Ogino A, Yachi K, Ohta T,
Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N and Sano
E: Effect of IFN-beta on human glioma cell lines with temozolomide
resistance. Int J Oncol. 35:139–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schlosser SF, Schuler M, Berg CP, Lauber
K, Schulze-Osthoff K, Schmahl FW and Wesselborg S: Ribavirin and
alpha interferon enhance death receptor-mediated apoptosis and
caspase activation in human hepatoma cells. Antimicrob Agents
Chemother. 47:1912–1921. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teng L, Ding D, Chen Y, Dai H, Liu G, Qiao
Z and An R: Anti-tumor effect of ribavirin in combination with
interferon-α on renal cell carcinoma cell lines in vitro. Cancer
Cell Int. 14:632014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshino A, Ogino A, Yachi K, Ohta T,
Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N, Sano E
and Tsumoto K: Gene expression profiling predicts response to
temozolomide in malignant gliomas. Int J Oncol. 36:1367–1377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wischhusen J, Naumann U, Ohgaki H,
Rastinejad F and Weller M: CP-31398, a novel p53-stabilizing agent,
induces p53-dependent and p53-independent glioma cell death.
Oncogene. 22:8233–8245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ostermann S, Csajka C, Buclin T, Leyvraz
S, Lejeune F, Decosterd LA and Stupp R: Plasma and cerebrospinal
fluid population pharmacokinetics of temozolomide in malignant
glioma patients. Clin Cancer Res. 10:3728–3736. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Naik GS and Tyagi MG: A pharmacological
profile of ribavirin and monitoring of its plasma concentration in
chronic hepatitis C infection. J Clin Exp Hepatol. 2:42–54. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren H, Tan X, Dong Y, Giese A, Chou TC,
Rainov N and Yang B: Differential effect of imatinib and synergism
of combination treatment with chemotherapeutic agents in malignant
glioma cells. Basic Clin Pharmacol Toxicol. 104:241–252. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kast RE, Skuli N, Cos S, Karpel-Massler G,
Shiozawa Y, Goshen R and Halatsch ME: The ABC7 regimen: A new
approach to metastatic breast cancer using seven common drugs to
inhibit epithelial-to-mesenchymal transition and augment
capecitabine efficacy. Breast Cancer. 9:495–514. 2017.PubMed/NCBI
|
|
29
|
Stewart WE, Gosser JB and Lockart RZ Jr:
Priming: A nonantiviral function of interferon. J Virol. 7:792–801.
1971. View Article : Google Scholar : PubMed/NCBI
|