Introduction
The incidence of thyroid carcinoma (TC) has been
increasing worldwide, with the papillary TC (PTC) histological
subtype accounting for 84% of all cases (1,2).
Epidemiological studies suggest that, in >40% of the cases, the
causative factors of PTC were manageable, including exposure to
radiation, obesity, cigarette smoking and unbalanced nutrition
(3). However, the accurate
identification of controllable risk factors for PTC remains
incomplete.
Iodine is used in the metabolism of thyroid gland
hormones. A number of epidemiological studies have attempted to
elucidate the association between excessive iodine intake and the
risk of developing PTC. For example, Lee et al (4) found that the median urinary iodine
concentration (UIC) and food frequency questionnaire score in
patients with PTC were significantly higher compared with those in
healthy control subjects. Zhou et al (5) discovered that UIC was higher in
patients with nodular goiter and PTC compared with the general
population. Based on a pair-matching case-control study design,
Zhang et al (6) demonstrated
that UIC was associated with PTC risk. Zhao et al (7) indicated that a higher than average UIC
was associated with an increased risk of larger tumors in female
patients with PTC. Furthermore, Zhao et al (8) and Wang et al (9) observed that UIC was associated with
lymph node metastasis in patients with PTC, and Kim et al
(10) identified UIC as an
independent predictor of BRAF mutations in PTC. However, the
aforementioned results were obtained based on random UIC
measurements, which only reflect iodine status at specific time
points, and cannot be used to independently represent chronic
iodine exposure. In TC ecology research, further studies evaluated
iodine exposure according to the water iodine levels of the
domicile districts of patients; however, this design lacks
individual iodine exposure assessment (11–13).
Furthermore, Santos et al (14) used reservoir water iodine content
combined with UIC to evaluate chronic iodine exposure in thyroid
histology pattern research. Zhang et al (15) investigated iodized salt consumption
combined with UIC to estimate long-term iodine intake. However,
this ecology-based integrated assessment design of iodine exposure
(which utilized water iodine or iodized salt consumption combined
with the UIC) is rarely applied for the analysis of
clinicopathological characteristics in PTC.
In the present study, UIC was innovatively combined
with water iodine values to determine the intrinsic association
between iodine exposure and the clinical characteristics of PTC.
Ecological integrated assessment was performed to determine whether
there is an association of excessive chronic iodine exposure with
capsular invasion and extrathyroid metastases. Further exploration
of the controllable risk factors of PTC may provide novel insights
and a reference for regional primary preventive strategies, and may
aid the identification of biomarkers for the early diagnosis of
PTC.
Materials and methods
Study population
The present study involved patients with thyroid
nodules who underwent thyroidectomy at the Department of Thyroid
Surgery, The Second Hospital of Shandong University (Jinan, China),
between July 2019 and December 2019. The exclusion criteria were as
follows: i) Patients with a family history of TC; ii) history of
radiation exposure during childhood; iii) recent use of therapeutic
iodine (2 months); iv) use of antithyroid drugs or thyroid hormone
therapy; and v) patients with kidney or liver dysfunction, and/or
other systemic diseases. The pathohistological type of the thyroid
nodules was determined by a pathologist at the Department of
Pathology. Finally, after excluding patients with medullary,
follicular and undifferentiated TC, 151 patients with thyroid
nodules remained, including 97 PTC and 54 non-PTC patients. The
Research Ethics committee of The Second Hospital of Shandong
University approved the present study and allowed oral consent to
be obtained from patients [approval no. KYLL-2019(KJ)P-0084]. All
the patients were informed of the purpose of the study and
volunteered to participate in this research by oral consent.
Iodine exposure assessment
A fasting urine specimen was collected by clinical
professionals between 6:00 and 7:00 a.m., and the UIC was
determined using an iodine determination kit (cat. no. 160031;
Xiangyang Wentes Health Technology Co., Ltd.) and a matching iodine
detector instrument (OTT-I-P; Xiangyang Wentes Health Technology
Co., Ltd.). During testing, the experimental environment was kept
free of iodine, and the external and internal quality controls were
processed according to the standard protocols of The Second
Hospital of Shandong University. The reference materials were
certified lyophilized human reference urine iodine
(GBW09108-GBW09110) produced by the National Reference Laboratory
for Iodine Deficiency Disorders. The urinary creatinine
concentration was determined using the Analyzer A25 (BioSystems
S.A.). Both UIC and urinary creatinine were processed within 2 h
after collection. According to the recommendations of the World
Health Organization (16), the
iodine nutritional status was categorized into three degrees as
follows: i) Low UIC (<100 µg/l), iodine-deficient; ii) adaptive
UIC (100–299 µg/l), iodine-adequate; and iii) high UIC (≥300 µg/l),
iodine-excessive.
The environmental water iodine data for domicile
districts of the Shandong province were consulted (17). According to the grading standard for
water iodine (17), Shandong was
divided into three groups as follows: i) Historically
iodine-deficient regions (median water iodine levels <10 µg/l);
ii) historically iodine-adaptive regions (median water iodine
levels 10–150 µg/l); and iii) historically iodine-excessive regions
(median water iodine levels >150 µg/l).
Clinical characteristics
In line with previously published standards
(18), patients with thyroid nodules
were categorized into four groups according to Thyroid Imaging
Reporting and Data System (TIRADS) grading as follows: Group 1,
TIRADS 2 and 3; group 2, TIRADS 4a; group 3, TIRADS 4b; and group
4, TIRADS 4c and 5. Descriptions of nodule multifocality and
bilaterality were obtained from ultrasound reports. For cases with
multiple reports, the latest ultrasound report and the highest
TIRADS grade were selected.
Diagnoses were reached using surgical slides from
the Department of Pathology, and the histological type of TC was
determined. Characteristics including primary tumor size, location,
capsular invasion, extrathyroid metastasis and lymph node status
were evaluated based on the National Comprehensive Carcinoma
Network guidelines (version 2; 2014) for TC recommendations
(https://www.nccn.org). Primary and secondary
pathological changes were examined and described in detail in the
associated pathological reports. The comprehensive assessment of
PTC was performed by the Departments of Thyroid Surgery and
Pathology, and the demographic and pathological characteristics of
each patient were obtained from medical records.
Statistical analysis
All data were entered by two researchers and
cross-checked using EpiData 3.0 software (https://www.epidata.dk) for quality control.
Descriptive analyses of basic characteristics are presented either
as the mean ± standard deviation or as percentages. χ2,
Student's t-test and F-test, and one-way ANOVA (with Bonferroni's
test for multiple comparisons) were used for comparative analysis.
McNemar's test was used for self-matching comparative analyses.
Univariate and multivariate logistic regression analyses were
performed using SPSS software, version 22.0 (IBM Corp.).
Collinearity diagnostics results indicated that there was no
multicollinearity for each variable in multiple conditional
logistic regression models. A receiver operating characteristic
(ROC) curve was generated using R software, version 3.5.1
(https://www.r-project.org), and Sigma
Plot, version 14.0 (Systat Software, Inc.) and R were used for data
presentation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Association between UIC and PTC risk
in patients with thyroid nodules
Basic characteristics
The data of 151 patients with thyroid nodules were
analyzed. The patients included 41 men and 110 women, and their
mean age ± SD was 49.2±13.0 years (age range, 17–75 years). The
comparison of basic characteristics, including general demographic
information, nodule ultrasound data and UIC, between PTC and
non-PTC patients is displayed in Table
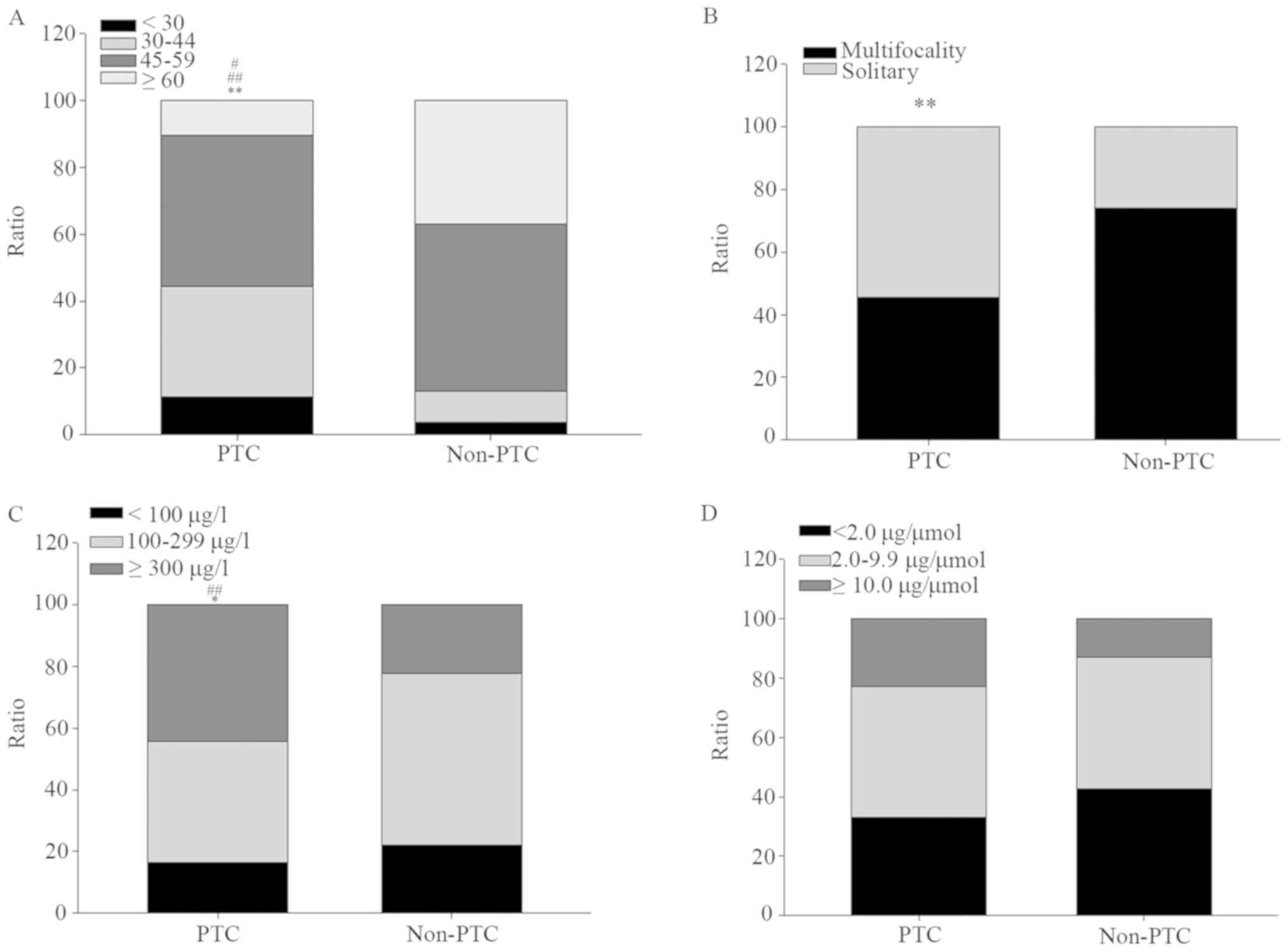
I and Fig. 1. Not a few PTC
patients (32.0%) were located in 30–40 years group, far more than
that in non-PTC patients (9.3%); by contrast, few PTC patients
(10.3%) were aged over 60 years, far less than that in non-PTC
patients (37.0%) (Fig. 1A). Almost
half (45.4%) of PTC patients manifested as multifocality of
nodules, significantly lower than that in non-PTC patients with
74.1% (Fig. 1B). Approximately half
of the PTC patients (44.3%) exhibited a high UIC (≥300 µg/l), which
was significantly higher compared with that of the non-PTC patients
(22.2%); by contrast, the proportion of PTC patients with adaptive
UICs (100–299 µg/l) was 39.2%, which was markedly lower compared
with that of the non-PTC patients (55.6%) (Fig. 1C). No significantly difference was
showed between PTC and non-PTC patients for UIC/creatinine
(Fig. 1D).
 | Table I.Basic characteristics of PTC and
non-PTC among patients with thyroid nodules. |
Table I.
Basic characteristics of PTC and
non-PTC among patients with thyroid nodules.
| Characteristic | PTC (n=97) | Non-PTC (n=54) | t/χ2
value | P-value |
|---|
| Agea, years | 45.5±12.5 | 55.9±11.2 | −5.065 | <0.001 |
| Sexb |
|
|
|
|
|
Female | 73 (75.3) | 37 (68.5) | 0.796 | 0.372 |
|
Male | 24 (24.7) | 17 (31.5) |
|
|
| Smoking
habitb |
|
|
|
|
|
Smoker | 9 (9.3) | 6 (11.1) | 0.130 | 0.718 |
|
Non-smoker | 88 (90.7) | 48 (88.9) |
|
|
| BMIb, kg/m2 |
|
|
|
|
|
<24.0 | 47 (48.5) | 31 (57.4) | 1.114 | 0.291 |
|
≥24.0 | 50 (51.5) | 23 (42.6) |
|
|
| Blood
pressurea, mm/Hg |
|
|
|
|
|
Systolic | 78.4±8.4 | 81.1±9.7 | −1.514 | 0.132 |
|
Diastolic | 129.3±10.6 | 133.3±11.9 | −1.732 | 0.086 |
| TIRADS
gradeb |
|
|
|
|
|
≥4c | 44 (45.4) | 5 (9.2) | 73.922 | <0.001 |
| 4b | 26 (26.8) | 3 (5.6) |
|
|
| 4a | 21 (21.6) | 9 (16.7) |
|
|
| ≤3 | 6 (6.2) | 37 (68.5) |
|
|
|
Multifocalityb |
|
|
|
|
|
Yes | 44 (45.4) | 40 (74.1) | 11.587 | 0.001 |
| No | 53 (54.6) | 14 (25.9) |
|
|
|
Locationb |
|
|
|
|
|
Bilateral | 37 (38.1) | 20 (37.0) | 0.018 | 0.893 |
|
Unilateral | 60 (61.9) | 34 (63.0) |
|
|
| UICb, µg/l |
|
|
|
|
|
<100 | 16 (16.5) | 12 (22.2) | 7.335 | 0.026 |
|
100-299 | 38 (39.2) | 30 (55.6) |
|
|
|
≥300 | 43 (44.3) | 12 (22.2) |
|
|
| Urinary
creatininea,
µmol/l | 109.0±79.4 | 111.9±63.6 | −0.228 | 0.820 |
|
UIC/creatinineb, µg/µmol |
|
|
|
|
|
<2.0 | 32 (33.0) | 23 (42.6) | 2.584 | 0.275 |
|
2.0–9.9 | 43 (44.3) | 24 (44.4) |
|
|
|
≥10.0 | 22 (22.7) | 7 (13.0) |
|
|
Logistic regression analysis
A lower age [univariate analysis: Odds ratio
(OR)=5.186; 95% CI: 2.217–12.134; multivariate analysis: OR=5.192;
95% CI: 2.215–12.171 (both P<0.001)], multifocality of nodules
[univariate analysis: OR=0.291; 95% CI: 0.140–0.602 (P=0.001);
multivariate analysis: OR=0.334; 95% CI: 0.154–0.724 (P=0.005)],
and high UIC [univariate analysis: OR=3.359; 95% CI: 1.297–8.701
(P=0.013); multivariate analysis: OR=3.987; 95% CI: 1.355–11.736
(P=0.012)] were identified as predictors of PTC risk in patients
with thyroid nodules (Table
II).
 | Table II.Logistic regression analysis for
predictors of papillary thyroid carcinoma risk in patients with
thyroid nodules. |
Table II.
Logistic regression analysis for
predictors of papillary thyroid carcinoma risk in patients with
thyroid nodules.
|
| Univariate
analysis | Multivariate
analysisa |
|---|
|
|
|
|
|---|
| Characteristic | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Female | 1.00 |
|
| 1.00 |
|
|
|
Male | 1.398 | 0.669–2.919 | 0.373 | 1.405 | 0.643–3.071 | 0.394 |
| Age, years |
|
|
|
|
|
|
|
>45 | 1.00 |
|
| 1.00 |
|
|
|
≤45 | 5.186 | 2.217–12.134 | <0.001 | 5.192 | 2.215–12.171 | <0.001 |
| Smoking habit |
|
|
|
|
|
|
|
Smoker | 1.00 |
|
| 1.00 |
|
|
|
Non-smoker | 0.604 | 0.206–1.767 | 0.357 | 0.848 | 0.248–2.892 | 0.792 |
| BMI,
kg/m2 |
|
|
|
|
|
|
|
<4.0 | 1.00 |
|
| 1.00 |
|
|
|
≥24.0 | 1.765 | 0.901–3.458 | 0.098 | 1.914 | 0.959–3.821 | 0.066 |
| Multifocality | 0.291 | 0.140–0.602 | 0.001 | 0.334 | 0.154–0.724 | 0.005 |
| Bilaterality | 0.774 | 0.388–1.541 | 0.465 | 0.979 | 0.478–2.135 | 1.010 |
| UIC, µg/l |
|
|
|
|
|
|
|
100-299 | 1.00 |
|
| 1.00 |
|
|
|
<100 | 2.546 | 1.135–5.712 | 0.023 | 2.252 | 0.943–5.375 | 0.067 |
|
≥300 | 3.359 | 1.297–8.701 | 0.013 | 3.987 | 1.355–11.736 | 0.012 |
| UIC/creatinine,
µg/µmol |
|
|
|
|
|
|
|
2.0–9.9 | 1.00 |
|
| 1.00 |
|
|
|
<2.0 | 1.845 | 0.696–4.887 | 0.218 | 2.107 | 0.722–6.148 | 0.173 |
|
≥10.0 | 2.167 | 0.779–6.033 | 0.139 | 2.516 | 0.822–7.698 | 0.106 |
Diagnostic value analysis
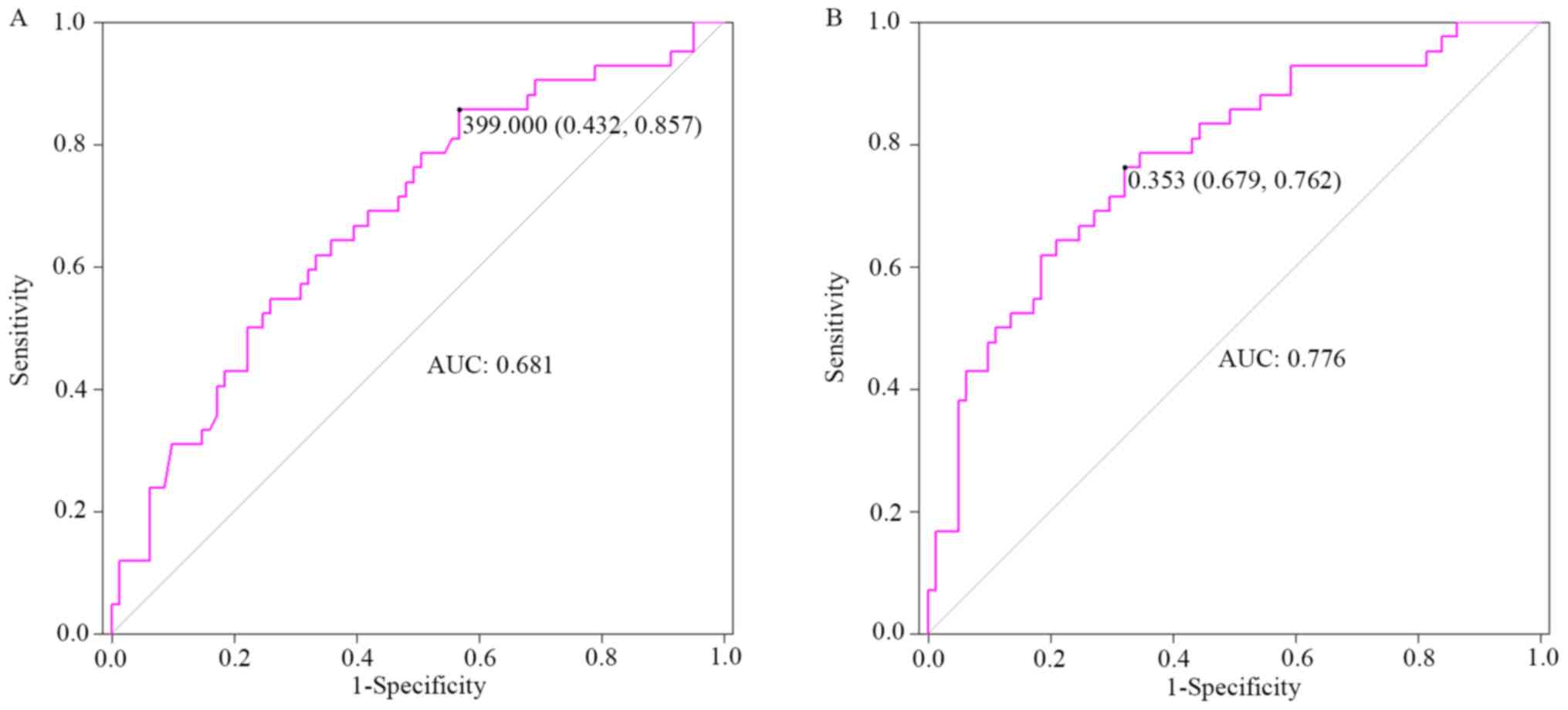
The area under the ROC curve (95% CI), cut-off
value, sensitivity and specificity were 0.681 (0.581–0.782), 399.0
µg/l (UIC) (0.432, 0.857) for univariate analysis, and 0.776
(0.687–0.864), 0.353 (prediction probability) µg/l (0.679, 0.762)
for multivariate analysis, after adjusting potential covariates,
such as sex, age, smoking habits and body mass index (BMI)
(Fig. 2).
Analysis of UIC and
clinicopathological characteristics
Compared with a low UIC, adaptive and high UICs were
both significantly associated with capsular invasion of PTC
(P<0.05). Compared with an adaptive UIC, only a high UIC was
significantly associated with extrathyroid metastasis (P<0.05).
No notable association was observed between UIC and age, sex,
smoking habits, BMI, tumor size, complications or lymph node status
in patients with PTC (Table
III).
 | Table III.Association between UIC and
clinicopathological characteristics among patients with papillary
thyroid carcinoma. |
Table III.
Association between UIC and
clinicopathological characteristics among patients with papillary
thyroid carcinoma.
|
| UIC, µg/l |
|
|---|
|
|
|
|
|---|
| Characteristic | <100 | 100-299 | ≥300 | F/χ2
value | P-value |
|---|
| Numbera | 16 (16.5) | 38 (39.2) | 43 (44.3) |
|
|
| Ageb, years | 46.3±15.1 | 46.1±12.0 | 44.6±12.2 | 0.192 | 0.825 |
| Sexa,c |
|
|
|
|
|
|
Female | 13 (81.2) | 27 (71.1) | 33 (76.7) | 0.728 | 0.695 |
|
Male | 3 (18.8) | 11 (28.9) | 10 (23.3) |
|
|
| Smoking
habita,c |
|
|
|
|
|
|
Smoker | 2 (12.5) | 2 (5.3) | 5 (11.6) | 1.293 | 0.544 |
|
Non-smoker | 14 (87.5) | 36 (94.7) | 38 (88.4) |
|
|
| BMIa, kg/m2 |
|
|
|
|
|
|
<24.0 | 8 (50.0) | 17 (44.7) | 22 (51.2) | 0.352 | 0.839 |
|
≥24.0 | 8 (50.0) | 21 (55.3) | 21 (48.8) |
|
|
| Water
iodinea,c,d, µg/l |
|
|
|
|
|
|
≥10.0 | 6 (37.5) | 16 (42.1) | 35 (81.4) | 17.787 | 0.001 |
|
2-10 | 8 (50.0) | 15 (39.5) | 6 (13.9) |
|
|
|
<2 | 2 (12.5) | 7 (18.4) | 2 (4.7) |
|
|
| TIRADS
gradesa,c |
|
|
|
|
|
|
≥4c | 9 (56.3) | 16 (42.2) | 19 (44.2) | 2.425 | 0.658 |
| 4b | 2 (12.5) | 11 (28.9) | 13 (30.2) |
|
|
|
≤4a | 5 (31.2) | 11 (28.9) | 11 (25.6) |
|
|
|
Multifocalitya |
|
|
|
|
|
|
Yes | 5 (31.3) | 23 (60.5) | 16 (37.2) | 5.964 | 0.051 |
| No | 11 (68.7) | 15 (39.5) | 27 (62.8) |
|
|
| Primary tumor
sizea, cm |
|
|
|
|
|
|
>1.0 | 6 (38.5) | 12 (26.7) | 17 (36.6) | 0.570 | 0.752 |
|
≤1.0 | 10 (61.5) | 26 (73.3) | 26 (63.4) |
|
|
| Capsular
invasiona,e |
|
|
|
|
|
|
Invasion | 6 (37.5) | 28 (73.7) | 33 (76.7) | 9.029 | 0.011 |
| No
invasion | 10 (62.5) | 10 (26.3) | 10 (23.3) |
|
|
|
Locationa,c |
|
|
|
|
|
|
Bilateral | 2 (12.5) | 19 (50.0) | 16 (37.2) | 7.464 | 0.024 |
|
Unilateral | 14 (87.5) | 19 (50.0) | 27 (62.8) |
|
|
| Extrathyroid
metastasisa,d |
|
|
|
|
|
|
Yes | 10 (62.5) | 16 (42.1) | 30 (69.8) | 6.504 | 0.039 |
| No | 6 (37.5) | 22 (57.9) | 13 (30.2) |
|
|
|
Complicationsa,c |
|
|
|
|
|
|
Yes | 12 (75.0) | 21 (55.3) | 27 (62.8) | 1.947 | 0.378 |
| No | 4 (25.0) | 17 (44.7) | 16 (37.2) |
|
|
| Lymph
nodesa,c |
|
|
|
|
|
| Central
district area | 13 (81.3) | 30 (78.9) | 34 (79.1) | 0.042 | 0.979 |
|
Non-central area | 3 (18.7) | 8 (21.1) | 9 (20.9) |
|
|
| Central
metastasisa |
|
|
|
|
|
|
Yes | 10 (62.5) | 14 (36.8) | 26 (60.5) | 5.428 | 0.066 |
| No | 6 (37.5) | 24 (63.2) | 17 (39.5) |
|
|
Assessment of excessive chronic iodine
exposure
The water iodine data of the domicile districts of
patients with PTC were utilized and combined with the UIC to
conduct ecology integrated assessment of chronic iodine exposure.
Among patients with PTC who exhibited high UICs, 81.4% were from
historically non-iodine deficient regions (Table III). Among those residing in
historically iodine-excessive regions, 66.7% exhibited a high UIC,
whereas only 7.4% had low UIC values (Table IV). An association between high UIC
and historically iodine-excessive regions was also noted. Thus, in
the present study, high UIC was found to independently reflect
excessive chronic iodine exposure in patients with PTC.
 | Table IV.Assessment of UIC combined with water
iodine in patients with papillary thyroid carcinoma. |
Table IV.
Assessment of UIC combined with water
iodine in patients with papillary thyroid carcinoma.
|
| Residence,
region |
|---|
|
|
|
|---|
| UICa, µg/l |
Iodine-deficient |
Iodine-adaptive |
Iodine-excessive |
|---|
| <100 | 10 (25.0) | 4 (13.3) | 2 (7.4) |
| 100-299 | 22 (55.0) | 9 (30.0) | 7 (25.9) |
| ≥300 | 8 (20.0) | 17 (56.7) | 18 (66.7) |
| Total | 40 (100.0) | 30 (100.0) | 27 (100.0) |
Pre- and postoperative alterations in
UIC in patients with PTC
There were no significant changes in the UIC grading
between pre- and postoperative samples (P>0.05; Table V and Fig.
S1) in patients with PTC. Among patients with a high
preoperative UIC, 90.0% also exhibited a high postoperative UIC,
regardless of the presence of carcinomatous foci. These findings
indicated that a high UIC did not result from the metabolic
properties of the PTC per se.
 | Table V.Pre- and postoperative UIC in
patients with papillary thyroid carcinoma. |
Table V.
Pre- and postoperative UIC in
patients with papillary thyroid carcinoma.
|
| Postoperative |
|
|
|---|
|
|
|
|
|
|---|
| UICa, µg/l | <300 | ≥300 | Total | P-value |
|---|
| Preoperative |
|
|
| 0.065b |
| <300 | 11 (55.0) | 9 (45.0) | 20 (50.0) |
|
| ≥300 | 2 (10.0) | 18 (90.0) | 20 (50.0) |
|
| Total | 13 (32.5) | 27 (67.5) | 40 (100.0) |
|
Discussion
The association between iodine intake and thyroid
diseases has been suggested to follow a U-shaped distribution
(19,20). However, few studies have determined
the specific role of chronic excessive iodine exposure in the
development of PTC. The results of the present study suggested that
almost half of the patients with PTC (44.3%) exhibited a high UIC
(≥300 µg/l), and that the proportion of patients with PTC with a
high UIC was significantly higher compared with that observed in
those without PTC (44.3 vs. 22.2%, respectively). Together with the
results of logistic regression and ROC curve analysis, it was
comprehensively hypothesized that a high UIC may serve as a
predictor or biomarker for PTC risk in patients with thyroid
nodules. This hypothesis is supported by other similar studies,
although there was no sufficient evidence to identify excessive
iodine as an independent predictor of PTC (4–12). The
UIC is also a widely accepted indicator of iodine nutritional
status (which is almost entirely dependent on iodine exposure,
including dietary intake) owing to the fact that >90% of
ingested iodine is excreted in the urine. Therefore, it was further
proposed that excessive iodine exposure was significantly
associated with the risk of PTC.
It is worth mentioning that the most innovative
characteristic of present study is the fact that the UIC was
combined with the water iodine levels of domicile districts, and
that this integrated evaluation assessed chronic iodine exposure
based on ecology. The data revealed that a substantial proportion
of patients with PTC (~70%) who resided in historically
iodine-excessive regions presented with high UICs; consistently,
the majority of PTC patients with high UIC (>80%) were from
historically non-iodine deficient regions. This suggested an
association between high UIC and iodine-excessive regions.
Therefore, to a large extent, a high UIC may independently reflect
excessive chronic iodine exposure in patients with PTC. Based on
the aforementioned findings, ecology integrated assessment was used
to investigate the association between chronic iodine exposure and
the clinicopathological characteristics of patients with PTC. The
results indicated that adaptive and high UIC values are associated
with capsular invasion of carcinoma, but that only high UIC is
significantly associated with extrathyroid metastasis. On the one
hand, high iodine concentrations may promote PTC cell proliferation
(represented by a higher iodine concentration) and manifest as
higher rates of proliferation. On the other hand, this regularity
only applies for iodine concentrations ≤1.0×10−3 mmol/l;
beyond this boundary, the rate of proliferation gradually decreases
(21). Importantly, the
concentration of iodine in the normal human thyroid gland ranges
between 1.0×10−6 and 1.0×10−5 mmol/l
(21), which is below the
aforementioned concentration. Therefore, for adaptive iodine levels
and beyond, it was hypothesized that the higher the concentration
of iodine in the thyroid gland (within a specified range,
1.0×10−3 mmol/l), the higher the rate of carcinoma cell
proliferation; however, for extremely high iodine levels
(≥1.0×10−3 mmol/l, for pathological thyroid gland), it
was speculated in the present study that the proliferative rate may
marginally decrease.
It should also be noted that the rate of
proliferation usually reflects the potential for capsular invasion
(9,21). In this respect, it is apparent that
both an adaptive and high UIC were equally significantly associated
with capsular invasion, and the difference between the two was not
statistically significant. Therefore, both adaptive and high UICs
were concluded to enhance the potential for capsular invasion in
patients with PTC. However, only high levels of iodine were able to
upregulate the expression of vascular endothelial growth factor
(VEGF)-A in W3 cells (21), MAPK in
TPC-1 cells (22), and AKT in TFC
(thyroid follicular cells) (23).
AKT and MAPK are involved in the PI3K/AKT/mTOR and MAPK signaling
pathways, respectively (24,25); both activate downstream VEGF
(26,27), which is responsible for angiogenic
activation and immune microenvironment disorders in carcinomas
(27,28), contributing to proliferation and
metastasis (29–35). It was therefore hypothesized that
only high concentrations of iodine can trigger the activation of
the PI3K/AKT/mTOR and MAPK pathways and alter VEGF-mediated
angiogenesis and immune reactions, thereby promoting the
proliferation and metastasis of PTC. This may explain why only a
high UIC was found to be significantly associated with capsular
invasion and extrathyroid metastasis. Consequently, it was
hypothesized that excessive chronic iodine exposure may promote
capsular invasion and extrathyroid metastasis in patients with
PTC.
Another innovation of the present study was the
self-matching design and random selection of 40 patients, and the
discovery that UIC grading does not differ significantly between
the pre- and postoperative period, regardless of the presence of
carcinomatous foci. This finding further excludes the possibility
that a higher UIC may be attributed to the metabolism of
carcinomatous foci. Therefore, it was concluded that a high UIC is
indicative of excessive iodine exposure rather than the metabolic
characteristics of the carcinoma per se, and this must be
considered as a risk factor for pathogenic PTC.
The conclusions would have been more powerful if the
study had included a larger prospective cohort from multiple
centers (where high iodine exposure and PTC tumorigenesis were
assessed over time), and more effort had been made to control the
selective bias of this particular hospital (e.g., Berkson bias).
Despite these limitations, however, convincing, manageable risk
factors for PTC were identified. Furthermore, the aim of the study
was to investigate the risk factors and their possible roles in
patients with PTC, rather than to assess the iodine nutritional
status of the general population. It is also not possible to
exclude undiagnosed PTC cases within the general population and,
therefore, in the present study, patients with thyroid nodules
included those in the non-PTC group. Based on these findings, it is
necessary to implement regional control strategies for iodine
supplementation in Shandong, where the iodine-deficient, -adaptive
and -excessive regions coexist. This may help to prevent invasion
and metastasis in patients with PTC, particularly those with
thyroid nodules.
Collectively, the results of the present study
indicated that excessive chronic iodine exposure may promote
capsular invasion and extrathyroid metastasis, and may be
significantly associated with the risk of PTC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Chengjun Zhou, Dr
Haitao Wang, Dr Xiaoying Wang and Dr Guoxin Teng, (Department of
Pathology, The Second Hospital of Shandong University, Jinan,
China) for their support with pathological diagnosis. The authors
would also like to thank Dr Shouluan Ding and Chunchun Shao,
(Evidence-Based Medicine Center, The Second Hospital of Shandong
University, Jinan, China) for statistical advice.
Funding
This project was supported by the Research
Development Fund of The Second Hospital of Shandong University
(grant no. 11681808).
Availability of materials and data
All data generated or analyzed in the present study
are available from the corresponding author upon reasonable
request.
Authors' contributions
FH, JL and HJ designed the study. FH, WC, YZ, MG,
JS, LS and LW collected and analysed the urine samples. FH, JX and
QX analysed the data. ZY and HJ interpreted the data and provided
academic guidance. FH drafted the manuscript, and JL, ZY and HJ
made further revisions. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of The Second Hospital of Shandong University;
ethics approval no.: [KYLL-2019(KJ)P-0084]. All patients were
informed of the purpose of the study and volunteered to
participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sui C, Liang N, Du R, He Q, Zhang D, Li F,
Fu Y, Dionigi G and Sun H: Time trend analysis of thyroid cancer
surgery in China: Single institutional database analysis of 15,000
patients. Endocrine. 68:617–628. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brito JP, Gionfriddo MR, Al Nofal A,
Boehmer KR, Leppin AL, Reading C, Callstrom M, Elraiyah TA, Prokop
LJ, Stan MN, et al: The accuracy of thyroid nodule ultrasound to
predict thyroid cancer: Systematic review and meta-analysis. J Clin
Endocrinol Metab. 99:1253–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitahara CM and Sosa JA: The changing
incidence of thyroid cancer. Nat Rev Endocrinol. 12:646–653. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JH, Song RY, Yi JW, Yu HW, Kwon H, Kim
SJ, Chai YJ, Choi JY, Moon JH, Lee KE, et al: Case-control study of
papillary thyroid carcinoma on urinary and dietary iodine status in
South Korea. World J Surg. 42:1424–1431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou Z, Zhang J, Jiang F, Xie Y, Zhang X
and Jiang L: Higher urinary bisphenol a concentration and excessive
iodine intake are associated with nodular goiter and papillary
thyroid carcinoma. Biosci Rep. 37:BSR201706782017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Fang C, Liu L, Liu X, Fan S, Li
J, Zhao Y, Ni S, Liu S and Wu Y: A case-control study of urinary
levels of iodine, perchlorate and thiocyanate and risk of papillary
thyroid cancer. Environ Int. 120:388–393. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao H, Li H and Huang T: High urinary
iodine, thyroid autoantibodies, and thyroid-stimulating hormone for
papillary thyroid cancer risk. Biol Trace Elem Res. 184:317–324.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao H, Li H and Huang T: High iodine
intake and central lymph node metastasis risk of papillary thyroid
cancer. J Trace Elem Med Biol. 53:16–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang F, Wang Y, Wang L, Wang X, Sun C,
Xing M and Zhao W: Strong association of high urinary iodine with
thyroid nodule and papillary thyroid cancer. Tumour Biol.
35:11375–11379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HJ, Park HK, Byun DW, Suh K, Yoo MH,
Min YK, Kim SW and Chung JH: Iodine intake as a risk factor for
BRAF mutations in papillary thyroid cancer patients from an
iodine-replete area. Eur J Nutr. 57:809–815. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vuong HG, Kondo T, Oishi N, Nakazawa T,
Mochizuki K, Inoue T, Tahara I, Kasai K, Hirokawa M, Tran TM and
Katoh R: Genetic alterations of differentiated thyroid carcinoma in
iodine-rich and iodine-deficient countries. Cancer Med.
5:1883–1889. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv C, Yang Y, Jiang L, Gao L, Rong S,
Darko GM, Jiang W, Gao Y and Sun D: Association between chronic
exposure to different water iodine and thyroid cancer: A
retrospective study from 1995 to 2014. Sci Total Environ.
609:735–741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bogdanova TI, Saenko VA, Hirokawa M, Ito
M, Zurnadzhy LY, Hayashi T, Rogounovitch TI, Miyauchi A, Tronko MD
and Yamashita S: Comparative histopathological analysis of sporadic
pediatric papillary thyroid carcinoma from Japan and Ukraine.
Endocr J. 64:977–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santos JE, Freitas M, Fonseca CP, Castilho
P, Carreira IM, Rombeau JL and Branco MC: Iodine deficiency a
persisting problem: Assessment of iodine nutrition and evaluation
of thyroid nodular pathology in Portugal. J Endocrinol Invest.
40:185–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang YL, Li P, Liu ZY, Yi JP, Chen Y,
Zhang M and Lin Q: Does relatively low iodine intake contribute to
thyroid cancer? An ecological comparison of epidemiology. Medicine
(Baltimore). 98:e175392019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
World Health Organization, UNICEFICCIDD, .
Assessment of iodine deficiency disorders and monitoring their
elimination, a guide for programme managers Third edition (updated
1st September 2008). 32–33
|
|
17
|
Gao J, Zhang ZJ, Wang ZL, Bian JC, Wang
JB, Jiang W, Wang XM and Jiang QW: Spatial distribution
characteristics and influencing factors of iodine in drinking water
in Shandong Province between year 2008 and 2010. Zhonghua Yu Fang
Yi Xue Za Zhi. 47:18–22. 2013.(In Chinese). PubMed/NCBI
|
|
18
|
Horvath E, Majlis S, Rossi R, Franco C,
Niedmann JP, Castro A and Dominguez M: An ultrasonogram reporting
system for thyroid nodules stratifying cancer risk for clinical
management. J Clin Endocrinol Metab. 94:1748–1751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zimmermann MB and Boelaert K: Iodine
deficiency and thyroid disorders. Lancet Diabetes Endocrinol.
3:286–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitro SD, Rozek LS, Vatanasapt P,
Suwanrungruang K, Chitapanarux I, Srisukho S, Sriplung H and Meza
R: Iodine deficiency and thyroid cancer trends in three regions of
Thailand, 1990–2009. Cancer Epidemiol. 43:92–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiang J, Wang X, Wang Z, Wu Y, Li D, Shen
Q, Sun T, Guan Q and Wang Y: Effect of different iodine
concentrations on well-differentiated thyroid cancer cell behavior
and its inner mechanism. Cell Biochem Biophys. 71:299–305. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Yang H, Si Y, Hu D, Yu Y, Zhang Y,
Gao M and Zhang H: Iodine promotes tumorigenesis of thyroid cancer
by suppressing mir-422a and up-regulating MAPK1. Cell Physiol
Biochem. 43:1325–1336. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu C, Wu F, Mao C, Wang X, Zheng T, Bu L,
Mou X, Zhou Y, Yuan G, Wang S and Xiao Y: Excess iodine promotes
apoptosis of thyroid follicular epithelial cells by inducing
autophagy suppression and is associated with Hashimoto thyroiditis
disease. J Autoimmun. 75:50–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang F, Sun Y, Gao H, Wu H and Wang Z:
Carbon disulfide induces embryo loss by perturbing the expression
of the mTOR signalling pathway in uterine tissue in mice. Chem Biol
Interact. 300:8–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao X, Hou P, Xin H, Zhang Y, Zhou A, Lai
C and Xie J: A glucogalactomanan polysaccharide isolated from
Agaricus bisporus causes an inflammatory response via the ERK/MAPK
and IKB/NFKB pathways in macrophages. Int J Biol Macromol.
151:1067–1073. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geng J, Li X, Zhou Z, Wu CL, Dai M and Bai
X: EZH2 promotes tumor progression via regulating VEGF-A/AKT
signaling in non-small cell lung cancer. Cancer Lett. 359:275–287.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsai HC, Cheng SP, Han CK, Huang YL, Wang
SW, Lee JJ, Lai CT, Fong YC and Tang CH: Resistin enhances
angiogenesis in osteosarcoma via the MAPK signaling pathway. Aging
(Albany NY). 11:9767–9777. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wheeler KC, Jena MK, Pradhan BS, Nayak N,
Das S, Hsu CD, Wheeler DS, Chen K and Nayak NR: VEGF may contribute
to macrophage recruitment and M2 polarization in the decidua. PLoS
One. 13:e01910402018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song J, Qiu W, Deng X, Qiu Z, Fan Y and
Yang Z: A somatic mutation of RasGRP3 decreases
Na+/I− symporter expression in metastases of
radioactive iodine-refractory thyroid cancer by stimulating the Akt
signaling pathway. Am J Cancer Res. 8:1847–1855. 2018.PubMed/NCBI
|
|
30
|
Subarnbhesaj A, Miyauchi M, Chanbora C,
Mikuriya A, Nguyen PT, Furusho H, Ayuningtyas NF, Fujita M,
Toratani S, Takechi M, et al: Roles of VEGF-Flt-1 signaling in
malignant behaviors of oral squamous cell carcinoma. PLoS One.
12:e01870922017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Sun R, Zou J, Ying Y and Luo Z: Dual
roles of the AMP-activated protein kinase pathway in angiogenesis.
Cells. 8:7522019. View Article : Google Scholar
|
|
32
|
Shakya G, Balasubramanian S, Hoda M and
Rajagopalan R: Inhibition of metastasis and angiogenesis in Hep-2
cells by wheatgrass extract-an in vitro and in silico approach.
Toxicol Mech Methods. 28:205–218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Welsh SJ, Rizos H, Scolyer RA and Long GV:
Resistance to combination BRAF and MEK inhibition in metastatic
melanoma: Where to next? Eur J Cancer. 62:76–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hegde PS, Wallin JJ and Mancao C:
Predictive markers of anti-VEGF and emerging role of angiogenesis
inhibitors as immunotherapeutics. Semin Cancer Biol. 52:117–124.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Turkowski K, Brandenburg S, Mueller A,
Kremenetskaia I, Bungert AD, Blank A, Felsenstein M and Vajkoczy P:
VEGF as a modulator of the innate immune response in glioblastoma.
Glia. 66:161–174. 2018. View Article : Google Scholar : PubMed/NCBI
|