Introduction
Globally, the morbidity and mortality of lung cancer
is ranked first among cancers (1).
Non-small cell lung cancer (NSCLC) is cytological subtype of lung
cancer. More than 85% of patients with lung cancer are NSCLC
patients (2). According to
statistics, more than 1.3 million people succumb to lung cancer
every year worldwide. In recent years, medical treatments of NSCLC
have been continuously improved, however, the 5-year survival rate
of patients with advanced NSCLC is less than 20% (3). At present, the treatments of lung
cancer include radiotherapy, surgery and chemotherapy. Numerous
NSCLC patients present with advanced stage when they are diagnosed,
causing difficulty in treatment, high recurrence rate of NSCLC and
poor prognosis (4). In order to
improve the diagnosis and treatment of NSCLC, it is crucial to
identify useful biomarkers that are used for early diagnosis and
prognosis monitoring of NSCLC.
MicroRNA (miRNA) is an RNA whose length is equal to
the length of 19 to 24 nucleotides, which exists in a variety of
tissues and blood and can regulate the expression of numerous genes
in the body (5). Aberrant expression
of miRNAs may lead to tumorigenesis (6) and can affect the occurrence and
progression of tumors. In addition, it can also be used to monitor
the prognosis of cancers (7). miRNAs
that exist in blood circulation are potential medical markers of
tumors (8). The expression of miR-25
has been revealed to be upregulated in numerous tumors, and play an
important role in regulating tumorigenesis. According to a study by
Kondo et al (9) on the
expression and the clinical effects of miR-25 in patients with
breast cancer, inhibiting the expression of miR-25 could
significantly attenuate the proliferation ability of breast cancer
cells. Furthermore, miR-25 was revealed to be a potential
diagnostic factor of breast cancer. It is speculated that miR-25
may be specifically expressed in NSCLC and has a monitoring
function. miR-365 is a microRNA, which is similar to miR-25. A
study by Zhang et al (10)
indicated that low expression of miR-365 was revealed in human
gastric cancer tissues and mouse gastric cancer models. In
addition, overexpression of miR-365 could significantly inhibit the
proliferation ability and tumorigenic ability of gastric cancer
cells. A study by Han et al (11) revealed that high expression of
miR-365 was exhibited in breast cancer tissues, and miR-365 was
associated with the TNM stage of patients with breast cancer, and
that the proliferation and invasion abilities of breast cancer
cells could be suppressed by downregulating the expression level of
miR-365. In a study by Zhang et al (12), it was revealed that miR-365 played a
role in suppressing breast cancer. It is unknown whether miR-365
can suppress NSCLC or not.
Based on the aforementioned, miR-365 and miR-25 have
been revealed to be aberrantly expressed in various cancers and
involved in the progression of these cancers. Therefore, it is
surmised that these two miRNAs may also be aberrantly expressed in
NSCLC and be related to the progression of the disease. However,
there are few studies concerning the clinical role of miR-365 and
miR-25 in NSCLC. To explore the clinical role of these two miRNAs
in NSCLC, in the present study, the relationship between the
expression of miR-25 and miR-365 in serum of NSCLC patients and
their clinicopathological parameters was analyzed by detecting the
expression of miR-25 and miR-365 in serum of NSCLC patients and
comparing NSCLC patients with healthy volunteers in the control
group. Furthermore, the relationship among miR-25, miR-365 and the
postoperative 5-year survival rate of NSCLC patients was analyzed
to identify new minimally invasive biological clinical factors for
diagnosis, treatment and prognosis of NSCLC.
Materials and methods
Study subjects
In total, 180 patients, who were diagnosed with
NSCLC at the Department of Pathology of Shenzhen Longhua District
Central Hospital from July 2011 to December 2013, were used as the
experimental group. The diagnosis of patients was based on WHO
pathological histological diagnosis criteria. The study subjects
had not received antitumor therapy such as radiotherapy and
chemotherapy prior to enrollment in the study. The seventh edition
of TNM staging diagnosis criteria for lung cancer was used, which
was published by the International Association for the Study of
Lung Cancer (13). Among the 180
patients, there were 108 males and 72 females. There were 82
patients >50 years old, and 98 patients were <50 years old.
There were 99 patients with a history of smoking. As for the
pathological grade, 56 patients were in low grade, 68 patients in
middle grade, and 56 patients in high grade. There were 78 patients
with lymph node metastasis and 102 patients with no lymph node
metastasis. There were 88 patients with peripheral infiltration and
92 patients without peripheral infiltration. According to
pathological stage, 43 patients were in stage I, 48 patients in
stage II, 39 patients in stage III, and 50 patients in stage IV.
The exclusion criteria was as follows: i) Patients with other tumor
diseases except NSCLC; ii) patients with severe heart, liver, lung
and other organ dysfunctions; iii) patients with incomplete
clinical data. Volunteers (n=90), who took a health examination at
the Outpatient Department during the same period, were used as the
control group. These patients did not have basic diseases such as
hypertension, hyperlipemia, and diabetes. General clinical data of
the patients in the two groups and clinicopathological data of the
patients in the experimental group were recorded. The patients,
volunteers and their family members were informed, and an informed
consent form was signed. This study was approved by the Medical
Ethics Committee of the Affiliated Hospital of Guangdong Medical
University.
Instruments and reagents
A real-time fluorescence quantitative PCR instrument
(model no. 7300) was purchased from ABI; Thermo Fisher Scientific,
Inc. A spectrophotometer (model no. DR5000) was purchased from
Hach. A high-speed refrigerated centrifuge (model no. 5418) was
purchased from Eppendorf. DEPC water was obtained from
Sigma-Aldrich; Merck KGaA. A TRIzol kit was purchased from BioTeke
Corporation. A reverse transcription kit was purchased from TaKaRa
Bio, Inc. Internal reference primers of miR-25, miR-365 and U6
small nuclear RNA (RNU6B) were designed and synthesized by
GeneCopoeia, Inc. The primer sequences are presented in Table I.
 | Table I.Primer sequences of miR-25, miR-365
and U6. |
Table I.
Primer sequences of miR-25, miR-365
and U6.
| Gene | Forward primer | Reverse primer |
|---|
| miR-25 |
5′-ATCCAGTGCGTGTCGTG-3′ |
5′-TGCTCATTGCACTTGTCTC-3′ |
| miR-365 |
5′-CGTAATGCCCCTAAAAAT-3′ |
5′-GTGCAGGGTCCGAGGT-3′ |
| U6 |
5′-ATTGGAACGATACAGAGAAG-3′ |
5′-GGAACGCTTCACGAATTTG-3′ |
qRT-PCR
The peripheral blood of the patients in the
experimental and control groups was collected by biochemical
coagulation tubes, then placed in sterile blood collection tubes
with a volume of 5 ml. Then, the serum was separated from the blood
by a centrifuge and was stored in a refrigerator at −80°C. Serum
samples of the patients in the two groups, which were stored in the
refrigerator, were removed. Then the temperature of the serum
samples was equilibrated with indoor temperature until they
completely dissolved. The serum samples (500 µl) were transferred
to new EP tubes. Then total RNA was extracted from the serum
according to the instructions of the TRIzol serum extraction kits.
Next, the concentration and purity of the extracted total RNA were
detected by a DR5000 UV–VIS spectrophotometer. Lastly, 2 µl of
total RNA was collected, and was reversely-transcribed into cDNA
according to the instructions of the TaKaRa reverse transcription
kits. cDNA was stored at −20°C. U6 was used as an internal
reference gene. The reaction system was as follows: 10 µl of PCR
Premix, 2 µl of upstream primers (10X), 2 µl of downstream primers
(10X), 5 µl of dd water (Rnase- and Dnase-free). The PCR
amplification cycle conditions were as follows: 90°C for 5 min,
90°C for 5 sec, 60°C for 30 sec, 72°C for 5 sec, 40 cycles. The PCR
reaction conditions were as follows: Pre-denaturation at 94°C for 3
min. The cycle parameters were: 95°C for 60 sec, 95°C for 30 sec,
60°C for 90 sec, 40 cycles. Three replicate wells were detected for
each sample miRNA. The data of the results were analyzed by
2−ΔΔCq (14).
Follow-up
The 180 patients were followed-up by telephone or
visits. The follow-up was carried out trimonthly for 5 years. The
deadline of the follow-up was January 2019. The overall survival
period was from the first day after surgery to the date of the last
follow-up or to the date of death of the patients.
Statistical analysis
SPSS 21.0 statistical software (EASYBIO) was used to
analyze the data. A Chi-square test was used to compare counting
data, such as sex, age and weight, between the two groups. The
relative expression levels of miR-365 and miR-25 in the two groups
were compared by t-test. The relationship between the relative
expression levels of miR-365, miR-25 and the clinicopathological
parameters of NSCLC patients was analyzed by t-test. The comparison
between miR-365, miR-25 and multigroup mean values of pathological
grade, TNM stage was carried out by one-way ANOVA. Then pairwise
comparison was carried out by Dunnett's t-test. Kaplan-Meier curves
were used to establish the survival curves of the patients with
high expression and low expression of miR-365 and miR-25. A
log-rank test was used to evaluate the difference of the survival
curves of the patients in the two groups. When P<0.05, the
difference was considered to be statistically significant.
Results
Comparison of general clinical data
between the two groups
There was no significant difference between the
patients in the experimental group and the subjects in the control
group in terms of sex, age, height, weight, educational level,
residence, exercise habits, smoking, and drinking (P>0.05;
Table II).
 | Table II.Comparisons between the clinical data
of the patients in the experimental and control groups [n (%)]. |
Table II.
Comparisons between the clinical data
of the patients in the experimental and control groups [n (%)].
| Characteristics | Experimental group
(n=180) | Control group
(n=90) | χ2 | P-value |
|---|
| Sex |
|
| 0.124 | 0.725 |
| Male | 108 (60.00) | 56 (62.22) |
|
|
|
Female | 72 (40.00) | 34 (37.78) |
|
|
| Average age
(years) |
|
| 1.069 | 0.301 |
| ≤50 | 98 (54.44) | 43 (47.78) |
|
|
|
>50 | 82 (45.56) | 47 (52.22) |
|
|
| Height (cm) |
|
| 1.896 | 0.580 |
|
<160 | 78 (43.33) | 50 (55.56) |
|
|
| ≥160 | 102 (56.67) | 40 (44.44) |
|
|
| Weight (kg) |
|
| 0.189 | 0.664 |
|
<55 | 79 (43.89) | 37 (41.11) |
|
|
|
≥55 | 101 (56.11) | 53 (58.89) |
|
|
| Educational
level |
|
| 1.434 | 0.231 |
| ≤High
school | 89 (49.44) | 38 (42.22) |
|
|
|
>High school | 91 (50.56) | 52 (57.78) |
|
|
| Residence |
|
| 0.119 | 0.731 |
|
City | 90 (50.00) | 43 (47.78) |
|
|
|
Countryside | 90 (50.00) | 47 (52.22) |
|
|
| Exercise
habits |
|
| 2.983 | 0.084 |
|
Yes | 76 (50.00) | 48 (53.33) |
|
|
| No | 104 (50.00) | 42 (46.67) |
|
|
| Smoking |
|
| 0.007 | 0.931 |
|
Yes | 99 (42.22) | 49 (54.44) |
|
|
| No | 81 (57.78) | 41 (45.56) |
|
|
| Drinking |
|
| 3.023 | 0.082 |
|
Yes | 96 (53.33) | 58 (64.44) |
|
|
| No | 84 (46.67) | 32 (35.56) |
|
|
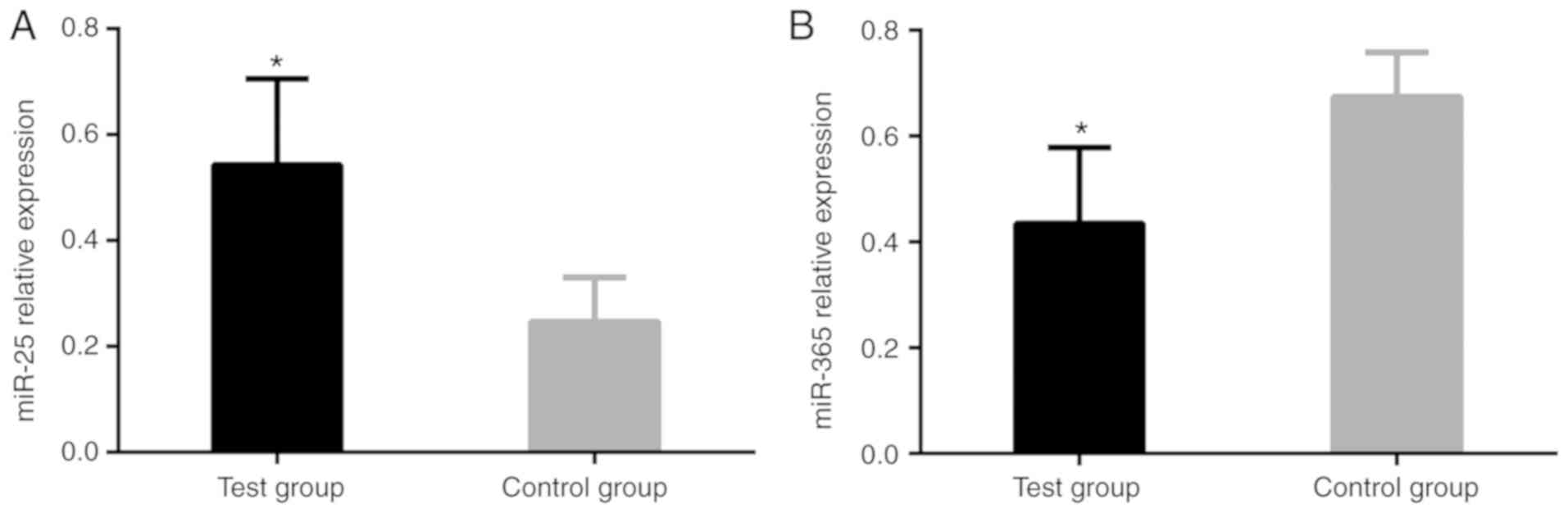
Comparisons between the relative
expression levels of miR-25 and miR-365 in the serum of the
patients in the experimental and control groups
The relative expression level of miR-25 of the
patients in the experimental group was significantly higher than
that of the patients in the control group. The difference was
statistically significant (P<0.001). The relative expression
level of miR-365 of the patients in experimental group was
significantly lower than that of the patients in the control group.
The difference was statistically significant (P<0.001; Table III and Fig. 1).
 | Table III.Comparisons between the relative
expression levels of miR-25 and miR-365 in the serum of the
patients in the experimental and control groups (mean ± SD). |
Table III.
Comparisons between the relative
expression levels of miR-25 and miR-365 in the serum of the
patients in the experimental and control groups (mean ± SD).
| Group | n | Relative expression
level of miR-25 | Relative expression
level of miR-365 |
|---|
| Experimental
group | 180 | 0.543±0.163 | 0.435±0.143 |
| Control group | 90 | 0.246±0.084 | 0.674±0.084 |
| t-value |
| 17.32 | 14.64 |
| P-value |
|
<0.001 |
<0.001 |
Relationship between the expression of
miR-25 and clinicopathological features
qRT-PCR was used to detect the expression of miR-25
in the serum of patients with various clinicopathological
characteristics. There was no difference in the relative expression
level of miR-25 of the patients in the experimental group although
the patients had different ages, sex, history of smoking and
pathological types (P>0.05). The relative expression level of
miR-25 of patients with peripheral infiltration was significantly
higher than that of patients without peripheral infiltration
(P<0.05). There was a significant difference in the relative
expression level of miR-25 in different pathological grades
(P<0.05). The relative expression level of miR-25 of patients
whose tumor diameter was <3.0 cm was significantly lower than
that of patients whose tumor diameter was ≥3.0 cm (P<0.05).
There was a significant difference in the relative expression level
of miR-25 in different TNM stages (P<0.05). The relative
expression level of miR-25 of patients with lymph node metastasis
was significantly higher than that of patients without lymph node
metastasis (P<0.05; Table
IV).
 | Table IV.Relationship between the relative
expression level of miR-25 and clinicopathological features
(x¯ ± SD). |
Table IV.
Relationship between the relative
expression level of miR-25 and clinicopathological features
(x¯ ± SD).
| Pathological
parameters | n | Relative expression
level of miR-25 | t/F-value | P-value |
|---|
| Sex |
|
| 1.179 | 0.240 |
|
Male | 108 | 0.524±0.076 |
|
|
|
Female | 72 | 0.538±0.081 |
|
|
| Age (years) |
|
| 0.414 | 0.679 |
|
≤50 | 98 | 0.546±0.082 |
|
|
|
>50 | 82 | 0.541±0.079 |
|
|
| History of
smoking |
|
| 0.496 | 0.621 |
|
Yes | 99 | 0.554±0.091 |
|
|
| No | 81 | 0.561±0.098 |
|
|
| Peripheral
infiltration |
|
| 8.844 | <0.001 |
|
Yes | 88 | 0.542±0.075 |
|
|
| No | 92 | 0.455±0.056 |
|
|
| Pathological
grades |
|
| 54.643 | <0.001 |
|
High | 56 | 0.435±0.048 |
|
|
|
Middle | 68 |
0.489±0.056a |
|
|
|
Low | 56 |
0.547±0.065a,b |
|
|
| Tumor diameter |
|
| 9.069 | <0.001 |
| <3.0
cm | 83 | 0.485±0.076 |
|
|
| ≥3.0
cm | 97 | 0.597±0.088 |
|
|
| TNM stages |
|
| 89.623 | <0.001 |
| I | 43 | 0.445±0.043 |
|
|
| II | 48 |
0.502±0.053c |
|
|
|
III | 39 |
0.570±0.057c,d |
|
|
| IV | 50 |
0.623±0.066c–e |
|
|
| Lymph node
metastasis |
|
| 13.781 | <0.001 |
|
Yes | 78 | 0.613±0.078 |
|
|
| No | 102 | 0.466±0.065 |
|
|
| Pathological
type |
|
| 1.803 | 0.073 |
|
Squamous carcinoma | 33 | 0.513±0.081 |
|
|
|
Adenocarcinoma | 147 | 0.549±0.108 |
|
|
Relationship between the expression of
miR-365 and clinicopathological features
qRT-PCR was used to detect the expression of miR-365
in the serum of patients with various clinicopathological
characteristics. There was no significant difference in the
relative expression level of miR-365 of the patients in the
experimental group although the patients had different ages, sex,
history of smoking and pathological types (P>0.05). The relative
expression level of miR-365 of the patients with peripheral
infiltration was significantly lower than that of patients without
peripheral infiltration (P<0.05). There was a significant
difference in the relative expression level of miR-365 in different
pathological grades (P<0.05). The relative expression level of
miR-365 of patients whose tumor diameter was <3.0 cm was
significantly higher than that of patients whose tumor diameter was
≥3.0 cm (P<0.05). There was a significant difference in the
relative expression level of miR-365 in different TNM stages
(P<0.05). The relative expression level of miR-365 of patients
with lymph node metastasis was significantly lower than that of
patients without lymph node metastasis (P<0.05; Table V).
 | Table V.Relationship between the relative
expression level of miR-365 and clinicopathological features
(x¯ ± SD). |
Table V.
Relationship between the relative
expression level of miR-365 and clinicopathological features
(x¯ ± SD).
| Pathological
parameters | n | Relative expression
level of miR-365 | t/F-value | P-value |
|---|
| Sex |
|
| 0.438 | 0.662 |
|
Male | 108 | 0.429±0.088 |
|
|
|
Female | 72 | 0.435±0.093 |
|
|
| Age (years) |
|
| 0.557 | 0.579 |
|
≤50 | 98 | 0.438±0.076 |
|
|
|
>50 | 82 | 0.432±0.067 |
|
|
| History of
smoking |
|
| 0.424 | 0.672 |
|
Yes | 99 | 0.432±0.087 |
|
|
| No | 81 | 0.438±0.103 |
|
|
| Peripheral
infiltration |
|
| 8.910 | <0.001 |
|
Yes | 88 | 0.367±0.055 |
|
|
| No | 92 | 0.457±0.078 |
|
|
| Pathological
grades |
|
| 46.536 | <0.001 |
|
High | 56 | 0.489±0.056 |
|
|
|
Middle | 68 |
0.439±0.076a |
|
|
|
Low | 56 |
0.377±0.045a,b |
|
|
| Tumor diameter |
|
| 6.363 | <0.001 |
| <3.0
cm | 83 | 0.484±0.087 |
|
|
| ≥3.0
cm | 97 | 0.398±0.094 |
|
|
| TNM stages |
|
| 33.257 | <0.001 |
| I | 43 | 0.499±0.056 |
|
|
| II | 48 |
0.459±0.063c |
|
|
|
III | 39 |
0.410±0.077c,d |
|
|
| IV | 50 |
0.375±0.060c–e |
|
|
| Lymph node
metastasis |
|
| 9.984 | <0.001 |
|
Yes | 78 | 0.381±0.077 |
|
|
| No | 102 | 0.486±0.064 |
|
|
| Pathological
type |
|
| 1.236 | 0.218 |
|
Squamous carcinoma | 33 | 0.445±0.056 |
|
|
|
Adenocarcinoma | 147 | 0.465±0.089 |
|
|
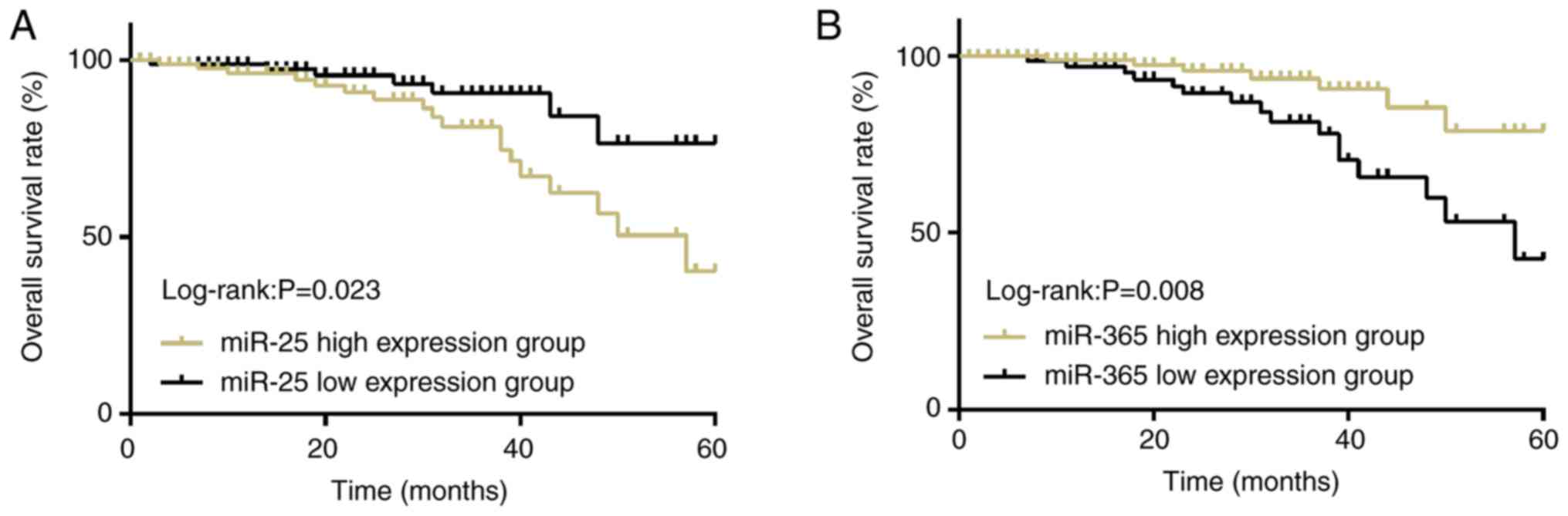
Survival condition of NSCLC
patients
According to a critical value, which was the median
of the relative expression levels of miR-25 and miR-365 in the
serum of the patients in the experimental group, there were 88
patients (≥0.543) in the miR-25 high-expression group and 92
patients in miR-25 low-expression group (<0.543). There were 102
patients (≥0.435) in the miR-365 high expression group and 78
patients (<0.435) in the miR-365 low expression group. The
5-year overall survival rate of the patients in the miR-25
high-expression group [43.18% (38/88)] was significantly lower than
that of the patients in the miR-25 low-expression group [75.00%
(69/92); P<0.05]. The 5-year overall survival rate of the
patients in the miR-365 high-expression group [76.47% (78/102)] was
significantly higher than that of the patients in miR-365
low-expression group [43.59% (34/78); P<0.05; Fig. 2].
Discussion
In recent years, the morbidity and mortality of lung
cancer have been increasing. Currently, deaths caused by lung
cancer account for ~18% of cancer-related deaths worldwide, with
non-small cell lung cancer accounting for the vast majority
(15). Some risk factors that lead
to NSCLC are smoking, air pollution, ionizing radiation, and
genetic factors (16). The
probability that NSCLC patients have distant metastasis and local
lymph node metastasis is high. Moreover, NSCLC patients generally
have no evident clinical symptoms, as a result, the early diagnosis
of NSCLC patients is difficult. Numerous patients are in advanced
stage (III-B-IV) and have distant metastasis and local lymph node
metastasis when they are diagnosed, and are therefore not eligible
to receive radical surgery (17,18).
Thus, finding effective NSCLC prognostic markers is of great
significance for treating patients.
Numerous studies have revealed that the aberrant
expression of a great number of miRNAs is closely correlated with
cancer progression. miRNAs have been revealed to be involved in the
occurrence and progression of cancer by regulating the expression
of its target genes and cooperating with target genes (19–22).
Since miR-25 and miR-365 were identified, the medical community has
been carrying out some in-depth studies on them. More and more
scholars have revealed that miR-25 and miR-365 are aberrantly
expressed in various cancers (23–25), and
they could be used as new tumor markers. In a study by Chen et
al (26), it was revealed that
the expression level of miR-25 in lung cancer tissues was
significantly higher than that in normal tissues. Their study
confirmed that miR-25 may be an oncogene, which can control
apoptosis of lung cancer cells by targetedly regulating the
expression of tumor suppressor gene RGS3. In the present study, the
expression of miR-25 was also increased in the serum of NSCLC
patients. Possibly the progression of tumors can be suppressed by
inhibiting the expression of miR-25. A study by Xiang et al
(27) revealed that the relative
expression level of miR-25 in lung cancer tissues was significantly
higher than that in normal lung tissues, and that miR-25 could
control apoptosis, metastasis and invasion of lung cancer cells by
targetedly regulating FBXW7. Their study confirmed that miR-25 was
involved in the occurrence and progression of lung cancer. The
survival curves of NSCLC patients were analyzed in the present
study. The results revealed that the higher the relative expression
level of miR-25 was, the lower the 5-year survival rate of patients
was, and that the expression of miR-25 was related to lymph node
metastasis and peripheral infiltration. This result indicated that
miR-25 may also be involved in the occurrence and progression of
NSCLC. The expression of miR-365 has been revealed to be
downregulated in some malignant tumors such as malignant melanoma
(28), epidermal squamous cell
carcinoma (29), and colon cancer
(30). The expression of miR-365 was
also downregulated in the serum of NSCLC patients in the present
study. The result of this study was consistent with the results of
other studies, and it was confirmed that miR-365 plays the same
role in NSCLC. A study by Nie et al (31) revealed that low expression of miR-365
was exhibited in cancer tissues and serum of patients with
pancreatic cancer, and that miR-365 was closely related to distant
metastasis and clinical stage of patients with pancreatic cancer.
The study also revealed that miR-365 could be used as an
independent prognostic factor of the overall survival rate of
patients with pancreatic cancer, and that overexpression of miR-365
could inhibit proliferation and invasion of pancreatic cancer
cells. Therefore, when the expression of miR-365 was downregulated,
tumor growth was observed. In the present study, the survival
curves of NSCLC patients revealed that the lower the relative
expression level of miR-365 was, the lower the 5-year survival rate
of the patients was, which confirmed that the downregulation of
miR-365 indicated worse prognosis. Therefore, the relative
expression level of miR-365 of NSCLC patients with peripheral
infiltration was significantly lower than that of NSCLC patients
without peripheral infiltration, which indicated that miR-365 was
involved in the occurrence and progression of NSCLC, and that
downregulation of miR-365 indicated proliferation of cancers.
miR-365 could be used as a potential molecular marker of NSCLC.
With the disclosure of the medical uses of miRNAs,
it is believed that miRNAs can provide a breakthrough and improve
the treatment of cancers in the near future. In the present study,
miR-365 and miR-25 were revealed to be aberrantly expressed in the
serum of NSCLC patients and associated with the prognosis of
patients, indicating that they have the potential to be therapeutic
targets for NSCLC. Previous studies have revealed that miR-25 and
miR-365 are involved in tumor development by regulating their
downstream target genes, for example, miR-365 could target Bcl-2 to
induce the apoptosis of HCC cells (32). miR-365 could also target the
volatilizing and anticancer effects of CYR61 in osteosarcoma
(33). miR-25 promoted the
development of liver cancer by inhibiting RhoGDI1 (34). These studies revealed that miR-365
and miR-25 play a significant role in the development of tumors.
However, this study, as a clinical study, did not conduct cell
experiments to explore the specific role and mechanism of miR-365
and miR-25 in NSCLC. In addition, due to the insufficient number of
qualified specimens of cancer tissues and adjacent tissues
obtained, their expression in tissues was not detected. It is hoped
that further cell research and the increase of the number of tissue
specimens can be achieved in the future.
In summary, the expression of miR-25 and miR-365 was
different in the serum of NSCLC patients, and these miRNAs could be
used as important tumor markers to evaluate the prognosis of NSCLC
patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DH conceived and designed the study and wrote the
manuscript. WO analyzed the general data of the patients and
compared the relative expression of miR-25 and miR-365 in the serum
of patients in the experimental and control groups. HT and MP
performed PCR analysis and analyzed the survival of patients. YO
and ZS analyzed the relationship between miR-25, miR-365 and
clinicopathological features and were responsible for the follow-up
of patients. All authors read and approved the final manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Guangdong Medical University which the
authors were previously affiliated to. Patients who participated in
this research, signed the informed consent and had complete
clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
International variation in lung cancer mortality rates and trends
among women. Cancer Epidemiol Biomarkers Prev. 23:1025–1036. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Midha A, Dearden S and McCormack R: EGFR
mutation incidence in non-small-cell lung cancer of adenocarcinoma
histology: A systematic review and global map by ethnicity
(mutMapII). Am J Cancer Res. 5:2892–2911. 2015.PubMed/NCBI
|
|
3
|
Zhou C, Liu D, Li J, Sun H, Zheng X, Wang
S, Hong G, Mallampati S, Sun H, Zhou X, et al: Chemotherapy plus
dendritic cells co-cultured with cytokine-induced killer cells
versus chemotherapy alone to treat advanced non-small-cell lung
cancer: A meta-analysis. Oncotarget. 7:86500–86510. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Novello S, Barlesi F, Califano R, Cufer T,
Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, et al:
Metastatic non-small-cell lung cancer: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 27
(Suppl 5):v1–v27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng CJ, Bahal R, Babar IA, Pincus Z,
Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM,
et al: MicroRNA silencing for cancer therapy targeted to the tumour
microenvironment. Nature. 518:107–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Christopher AF, Kaur RP, Kaur G, Kaur A,
Gupta V and Bansal P: MicroRNA therapeutics: Discovering novel
targets and developing specific therapy. Perspect Clin Res.
7:68–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mo MH, Chen L, Fu Y, Wang W and Fu SW:
Cell-free circulating miRNA biomarkers in cancer. J Cancer.
3:432–448. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo N, Toyama T, Sugiura H, Fujii Y and
Yamashita H: miR-206 Expression is down-regulated in estrogen
receptor alpha-positive human breast cancer. Cancer Res.
68:5004–5008. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Liu X, Jin H, Guo X, Xia L, Chen
Z, Bai M, Liu J, Shang X, Wu K, et al: miR-206 inhibits gastric
cancer proliferation in part by repressing cyclinD2. Cancer Lett.
332:94–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han JG, Jiang YD, Zhang CH, Yang YM, Pang
D, Song YN and Zhang GQ: A novel panel of serum
miR-21/miR-155/miR-365 as a potential diagnostic biomarker for
breast cancer. Ann Surg Treat Res. 92:55–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Zhang Z, Wang Q, Xing XJ and Zhao
Y: Overexpression of microRNA-365 inhibits breast cancer cell
growth and chemo-resistance through GALNT4. Eur Rev Med Pharmacol
Sci. 20:4710–4718. 2016.PubMed/NCBI
|
|
13
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L;
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions, : The
IASLC lung cancer staging project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng TY, Cramb SM, Baade PD, Youlden DR,
Nwogu C and Reid ME: The international epidemiology of lung cancer:
Latest trends, disparities, and tumor characteristics. J Thorac
Oncol. 11:1653–1671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuura N, Koh G, Konishi C, Minamino S,
Takahara Y, Harada H, Kodama K and Emoto M: Fulminant onset of
insulin-dependent diabetes with positive anti-GAD antibody titers
during treatment with nivolumab in a patient with NSCLC. Cancer
Immunol Immunother. 67:1417–1424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Sun Y and Chen H: Effect of tumor
size on prognosis of node-negative lung cancer with sufficient
lymph node examination and no disease extension. Onco Targets Ther.
9:649–653. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Helissey C, Champiat S and Soria JC:
Immune checkpoint inhibitors in advanced nonsmall cell lung cancer.
Curr Opin Oncol. 27:108–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu DD, Wu Y, Shen HY, Lv MM, Chen WX,
Zhang XH, Zhong SL, Tang JH and Zhao JH: Exosomes in development,
metastasis and drug resistance of breast cancer. Cancer Sci.
106:959–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lynch SM, McKenna MM, Walsh CP and McKenna
DJ: miR-24 regulates CDKN1B/p27 expression in prostate cancer.
Prostate. 76:637–648. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Z, Ma Y, Xia Q, Li Y, Li R, Chang W,
Chen J, Leng Z and Tao K: MicroRNA-155 expression inversely
correlates with pathologic stage of gastric cancer and it inhibits
gastric cancer cell growth by targeting cyclin D1. J Cancer Res
Clin Oncol. 142:1201–1212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zoni E, van der Horst G, van de Merbel AF,
Chen L, Rane JK, Pelger RC, Collins AT, Visakorpi T, Snaar-Jagalska
BE, Maitland NJ and van der Pluijm G: miR-25 modulates invasiveness
and dissemination of human prostate cancer cells via regulation of
αv- and α6-integrin expression. Cancer Res. 75:2326–2336. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li M, Liu L, Zang W, Wang Y, Du Y, Chen X,
Li P, Li J and Zhao G: miR365 overexpression promotes cell
proliferation and invasion by targeting ADAMTS-1 in breast cancer.
Int J Oncol. 47:296–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fassan M, Baffa R, Palazzo JP, Lloyd J,
Crosariol M, Liu CG, Volinia S, Alder H, Rugge M, Croce CM and
Rosenberg A: MicroRNA expression profiling of male breast cancer.
Breast Cancer Res. 11:R582009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Z, Wu Y, Meng Q and Xia Z: Elevated
microRNA-25 inhibits cell apoptosis in lung cancer by targeting
RGS3. In Vitro Cell Dev Biol Anim. 52:62–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiang J, Hang JB, Che JM and Li HC: MiR-25
is up-regulated in non-small cell lung cancer and promotes cell
proliferation and motility by targeting FBXW7. Int J Clin Exp
Pathol. 8:9147–9153. 2015.PubMed/NCBI
|
|
28
|
Bai J, Zhang Z, Li X and Liu H:
MicroRNA-365 inhibits growth, invasion and metastasis of malignant
melanoma by targeting NRP1 expression. Cancer Biomark. 15:599–608.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu X and Li Z: The role of miRNAs in
cutaneous squamous cell carcinoma. J Cell Mol Med. 20:3–9. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu Y, Zhao H, Rao M and Xu S:
MicroRNA-365 inhibits proliferation, migration and invasion of
glioma by targeting PIK3R3. Oncol Rep. 37:2185–2192. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nie J, Liu L, Zheng W, Chen L, Wu X, Xu Y,
Du X and Han W: microRNA-365, down-regulated in colon cancer,
inhibits cell cycle progression and promotes apoptosis of colon
cancer cells by probably targeting Cyclin D1 and Bcl-2.
Carcinogenesis. 33:220–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li M, Yang Y, Kuang Y, Gan X, Zeng W, Liu
Y and Guan H: miR-365 induces hepatocellular carcinoma cell
apoptosis through targeting Bcl-2. Exp Ther Med. 13:2279–2285.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Y, Chu H, Zhou Y, Wang J, Dong C and
Yin R: miR-365 functions as a tumor suppressor by directly
targeting CYR61 in osteosarcoma. Biomed Pharmacother. 98:531–537.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang C, Wang X, Su Z, Fei H, Liu X and Pan
Q: miR-25 promotes hepatocellular carcinoma cell growth, migration
and invasion by inhibiting RhoGDI1. Oncotarget. 6:36231–36244.
2015. View Article : Google Scholar : PubMed/NCBI
|