Introduction
Breast cancer (BC) is the most prevalent malignancy
in females worldwide (1). Recently,
in addition to conventional medication therapies, including
chemotherapy, endocrine therapy, and anti-human epidermal growth
factor 2 (HER2) drugs, several molecular-targeting drugs and
immunotherapies have been developed for patients with BC. However,
despite these various therapeutic strategies, it remains difficult
to achieve a cure for patients with metastatic disease due to the
pleiotropic properties of BC (2).
Therefore, the development of novel biomarkers or therapeutic
targets focused on these diverse activities or pathways in BC cells
is required to improve a patients' prognoses.
In our previous study on gastric cancer,
transcriptome analysis was used to identify marginal zone B and B1
cell-specific protein (MZB1) as a potential novel prognostic
biomarker (3). Previous reports
regarding MZB1 as a prognostic marker were inconsistent possibly as
a result of the organ-specific function of MZB1 (4,5). MZB1 is
a molecular chaperone that cooperates with other chaperones,
including glucose-regulated proteins (GRPs) in the endoplasmic
reticulum (6). When cancer cells
undergo endoplasmic reticulum stress, which may be brought on by
hypoxia and a lack of nutrients, an increase in misfolded proteins
occurs and activates the unfolded protein response (UPR), which
leads to cancer progression (7–9). In BC,
UPR-related molecules, including X-box-binding protein 1 (XBP1) and
GRP78, have been reported to be upregulated in tumor specimens from
patients with more advanced BC (10,11). In
addition, our previous study reported that Derlin 3 (DERL3), which
is upregulated in the UPR pathway, contributes toward cancer
progression, and high DERL3 expression levels indicate poor
survival in patients with BC (12).
Although no clinically available biomarker or drug has targeted
these UPR-related pathways or molecules, these results suggested
the promising possibility of using these UPR-related molecules as
novel biomarkers or drug targets. However, there have been no
reports that refer to the activity of MZB1 in BC.
The present study investigated the significance of
MZB1 expression and evaluated whether MZB1 may be a biomarker in
BC.
Materials and methods
Sample collection
Thirteen BC cell lines (BT-20, BT-474, BT-549,
HCC1419, HCC1954, Hs578T, MCF7, MDA-MB-231, MDA-MB-361, MDA-MB-415,
MDA-MB-468, SK-BR-3 and ZR-75-1) and two non-cancerous breast
epithelial cell lines (MCF-10A and MCF-12A) were used in the
present study. BT-549, HCC1419, HCC1954 and Hs578T cell lines were
purchased from the Japanese Collection of Research Bioresources
Cell Bank (Osaka, Japan), and BT-474, MCF-7 and MCF-12A were gifted
by Professor David Sidransky of Johns Hopkins University
(Baltimore, MD, USA). All other cell lines were purchased from the
American Type Culture Collection. All cell lines were cultured in
RPMI-1640 medium (Sigma-Aldrich; Merck KGaA), supplemented with 10%
fetal bovine serum and incubated in an atmosphere with 5%
CO2 at 37°C (13).
Human samples were resected from 114 patients with
BC who had undergone surgery at Nagoya University Hospital between
March 2002 and May 2007. The selected patients were those whose
surveillance data for more than five years after surgery were
available. All patients were females, and the median age was 53
years (range, 32–78 years). Primary BC and non-cancerous specimens
and clinical data were collected from these patients. Clinical
specimens were resected to ~1.5 mm in diameter and frozen
immediately at −80°C. Non-cancerous specimens were resected >3
cm away from the edge of the tumor (12). The resected BC specimens were
diagnosed histologically as BC and classified using the Union for
International Cancer Control (UICC) staging system for BC (8th
edition) (14). Adjuvant medication
therapy was determined by physician discretion considering each
patient's general condition, pathological features, and subtype
(12,13).
The present study complied with the Declaration of
Helsinki and was approved by the Nagoya University Hospital
Institutional Review Board (approval number: 2019–0028).
Participants provided written informed consent for the use of their
clinical samples and data.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
MZB1, DNA damage inducible transcript 3 (DDIT3),
DERL3, and XBP1 mRNA expression levels were evaluated
using RT-qPCR. RNA was extracted from cell lines
(8.0×106 cells per cell line) and from 114 patient BC
and non-cancerous specimens using the RNeasy Mini kit (Qiagen
GmbH). cDNA was synthesized as previously described (12,13,15).
GAPDH mRNA levels were evaluated for normalizing MZB1,
DDIT3, DERL3, and XBP1 mRNA expression levels. The
primers specific for each gene were as follows: MZB1:
Forward, 5′-CTCACAGGCCCAGGACTTAG-3′ and reverse,
5′-TGTGGCTGACACCTTCTCTG-3′, which generated a 219-bp product;
DDIT3: Forward, 5′-AGCGACAGAGCCAAAATCAG-3′ and reverse,
5′-TGCTTTCAGGTGTGGTGATG-3′, which generated a 88-bp product;
DERL3: Forward, 5′-CTCACTTTCCAGGCACCGT-3′ and reverse,
5′-TAGTAGATATGGCCCACCGC-3′, which generated a 110-bp product
(12); XBP1: Forward,
5′-CAGACTACGTGCGCCTCTGC-3′ and reverse, 5′-CTTCTGGGTAGACCTCTGGG-3′,
which generated a 208-bp product (12); and GAPDH: Forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′,
which generated a 226-bp product (3). SYBR Green PCR core reagent kit (Thermo
Fisher Scientific, Inc.) was used for RT-qPCR with these cycling
conditions: One cycle at 95°C for 10 min, followed by 40 cycles at
95°C for 5 sec and 60°C for 60 sec, using an ABI StepOnePlus
real-time PCR System (Thermo Fisher Scientific, Inc.). The
2−ΔΔCt method was used for PCR quantification (16). All samples were assayed in
triplicate. The mRNA expression levels of MZB1, DDIT3,
DERL3, and XBP1 in each sample were obtained from the
value divided by the GAPDH value for normalization (12,13,15).
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections (4-µm
thick) were constructed from blocks of resected specimens from 114
BC patients. The resected specimens were fixed with 10% formalin
for 48 h. The slides were heated for 2 min for antigen retrieval
with 1 mM EDTA buffer. The blocking procedure was not conducted.
The MZB1 rabbit polyclonal antibody (cat. no. 11454-1-AP;
ProteinTech, Inc.), which was diluted at 1:100, was used for
immunohistochemistry, and sections were incubated for 1 h at room
temperature (3). Then, SignalStain
Boost IHC Detection reagent (cat. no. 8114; Cell Signaling
Technology, Inc.) was used for the secondary antibody, and sections
were incubated for 30 min at room temperature. The entire cancerous
area of each section was observed using an upright light microscope
(Olympus Corporation; ×100 and ×400 magnification). The staining
intensity of the cytoplasm in cancer cells was evaluated and the
intensity was divided into three groups: ‘Negative’, ‘weak’, and
‘strong’. Subsequently, ‘weak’ and ‘strong’ were combined and
defined as ‘positive’.
Statistical analysis
Differences in the levels of MZB1 mRNA
between two groups were evaluated using a Mann-Whitney test.
Correlations between MZB1, DDIT3, DERL3, and XBP1
mRNA levels were analyzed using Spearman's rank correlation test.
The associations between mRNA or protein expression levels of MZB1
and patient clinicopathological factors were analyzed using the
χ2 test. The Kaplan-Meier method was utilized for
evaluating disease-free survival (DFS) and overall survival (OS)
rates, and the survival curves were compared using the log-rank
test. For multivariate regression analysis, the Cox proportional
hazards model was utilized to identify prognostic factors. Other
than MZB1 positivity, the variables were those considered to affect
breast cancer prognosis, including age, tumor size, lymph node
metastasis, and biological statuses. Next, variables for which
P<0.05 were entered into the final model. JMP 12 (SAS Institute,
Inc.) was employed for the statistical analysis, and P<0.05 was
considered to indicate a statistically significant difference.
Results
MZB1 mRNA expression levels in BC cell
lines
MZB1 mRNA expression in 13 BC cell lines and
two non-cancerous cell lines from the mammary gland were evaluated
(Fig. 1). The estrogen receptor
(ER), progesterone receptor (PgR), and HER2 statuses of the cell
lines have been evaluated in previous studies (17,18).
MZB1 mRNA was detectable in four ER-positive BC cell lines,
but not in the other nine BC and both non-cancerous cell lines.
When MZB1 mRNA expression levels were compared between
ER-positive and ER-negative BC cell lines, MZB1 mRNA
expression levels in ER-positive cell lines were significantly
higher than those in ER-negative BC cell lines (P=0.009).
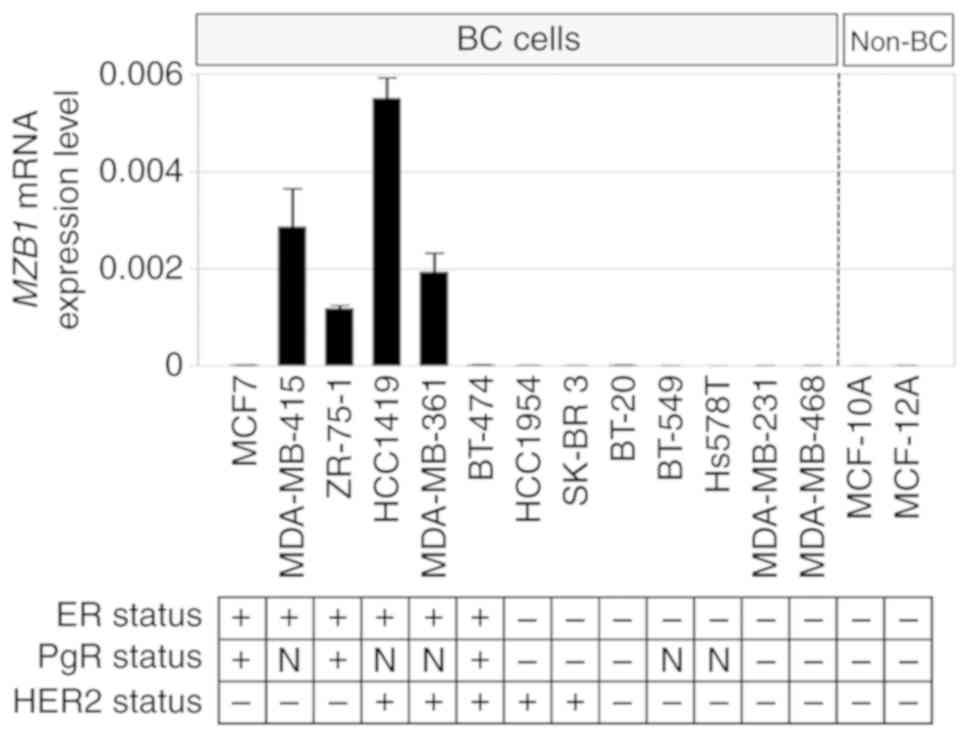 | Figure 1.Assessment of MZB1 mRNA
expression levels in cell lines. Bar graphs show MZB1 mRNA
levels in 13 BC cell lines and two non-cancerous breast cell lines.
MZB1 expression was detected in four estrogen
receptor-positive BC cell lines, but it was not detected in the
other nine BC and non-cancerous mammary cell lines. The ER, PgR,
and HER2 statuses of the cell lines were referred from the previous
studies (17,18). BC, breast cancer cell lines; non-BC,
non-cancerous breast cell lines; ER, estrogen receptor; HER2, human
epidermal growth factor 2; PgR, progesterone receptor; N, no
previous data available; MZB1, marginal zone B and B1 cell-specific
protein. |
Patient characteristics
The UICC stage distribution of 114 patients was as
follows: stage 0, six patients; stage I, 29 patients; stage II, 56
patients; and stage III, 23 patients. T stage was distributed as
follows: Tis (ductal carcinoma in situ), six patients; T1,
43 patients; T2, 54 patients; T3, six patients; and T4, five
patients. Half of the patients had lymph node metastasis. The
median follow-up duration was 123 months (range, 8–191 months) or
until death. ER, PgR, and HER2 statuses, determined from
immunohistochemistry tests in primary tumors, were as follows:
ER-positive, n=86; ER-negative, n=28; PgR-positive, n=76;
PgR-negative, n=38; HER2-positive, n=25; HER2-negative, n=80 (data
missing for nine patients); triple-negative, n=12; and
non-triple-negative, n=101 (data missing for one patient). Patients
whose tumor expressed at least one of ER, PgR, or HER2 were defined
as ‘non-triple-negative’. As eight patients out of nine whose HER2
statuses were unknown showed ER-positivity, they were categorized
as non-triple-negative.
Association between MZB1 mRNA
expression levels and patient clinicopathological factors
MZB1 is expressed, not only in BC cells, but
also in the cellular components of non-cancerous specimens (e.g.
mammary cells and lymphocytes). To evaluate MZB1 mRNA
expression levels in clinical BC samples, the ‘MZB1 C/N
ratio’, a ratio of MZB1 mRNA expression levels between BC
and adjacent non-cancerous specimens, was adopted aiming to reduce
the effects of MZB1 expression on non-cancerous tissues.
There were no significant differences between Tis/T1 (n=49) and
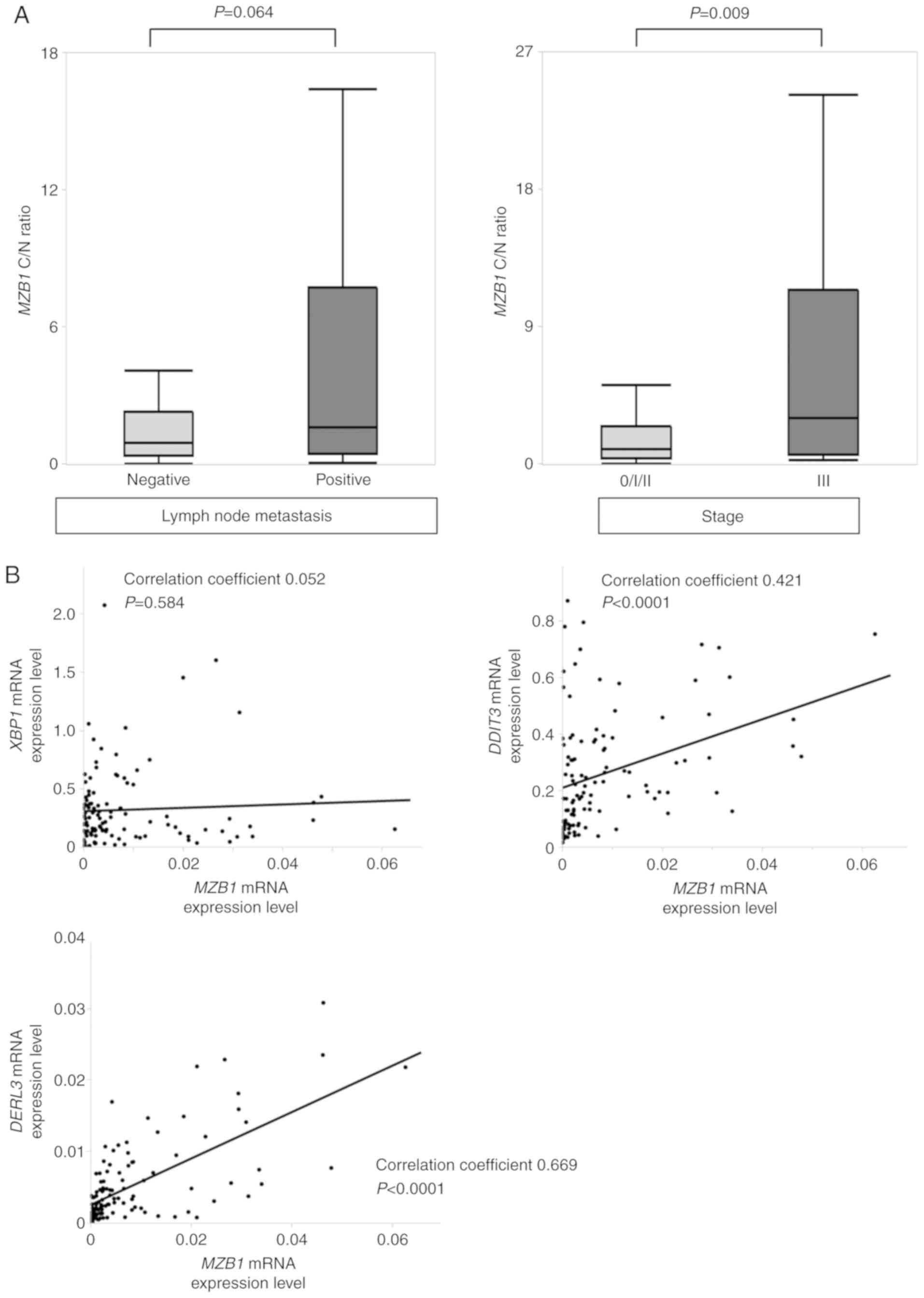
T2/T3/T4 patients (n=65; P=0.134). However, in patients with lymph
node metastasis (n=57), the MZB1 C/N ratio tended to be
higher than in lymph node-negative patients (n=57; P=0.064), and
stage III patients (n=23) had a significantly higher MZB1
C/N ratio compared with stage 0/I/II patients (n=91; P=0.009;
Fig. 2A). Patients whose MZB1
C/N ratios were higher than three were placed into a ‘high
MZB1 group’ (n=33), and the remaining patients were
designated as ‘others’ (n=81). The high MZB1 group was
associated with lymph node metastasis (P=0.007) and a more advanced
UICC pathological stage (P=0.006; Table
I). As MZB1 mRNA expression levels were detected in only
ER-positive cells among BC cell lines, MZB1 mRNA expression
levels were evaluated in ER-positive patients (n=86). Stage III
patients (n=13) had a significantly higher MZB1 C/N ratio
than stage 0/I/II patients (n=73; P=0.003). Furthermore, the high
MZB1 group (n=28) was associated with lymph node metastasis
(P=0.004) and a more advanced UICC pathological stage (P=0.002),
compared with others (n=58). In summary, MZB1 mRNA
expression was associated with lymph node metastasis and a more
advanced stage in patients with ER-positive BC.
 | Table I.Association between MZB1 mRNA
expression and clinicopathological characteristics in 114 patients
with breast cancer. |
Table I.
Association between MZB1 mRNA
expression and clinicopathological characteristics in 114 patients
with breast cancer.
| Clinicopathological
parameter | High MZB1
group (n=33) | Other (n=81) | P-value |
|---|
| Age |
|
| 0.246 |
| ≤60
years | 23 | 47 |
|
| >60
years | 10 | 34 |
|
| Histology |
|
| 0.401 |
| Ductal
carcinoma in situ | 0 | 6 |
|
|
Invasive ductal carcinoma | 31 | 68 |
|
|
Invasive lobular
carcinoma | 1 | 3 |
|
|
Other | 1 | 4 |
|
| UICC T factor |
|
| 0.081 |
|
Tis/T1 | 10 | 39 |
|
|
T2/T3/T4 | 23 | 42 |
|
| Node status |
|
| 0.007a |
|
Negative | 10 | 47 |
|
|
Positive | 23 | 34 |
|
| UICC pathological
stage |
|
| 0.006a |
|
0/I/II | 21 | 70 |
|
|
III | 12 | 11 |
|
| ER status |
|
| 0.136 |
|
Positive | 28 | 58 |
|
|
Negative | 5 | 23 |
|
| PgR status |
|
| 0.381 |
|
Positive | 20 | 56 |
|
|
Negative | 13 | 25 |
|
| HER2 status |
|
| 0.944 |
|
Positive | 8 | 17 |
|
|
Negative | 25 | 55 |
|
|
Unknown | 0 | 9 |
|
|
Triple-negative |
|
| 0.312 |
|
Yes | 2 | 10 |
|
| No | 31 | 70 |
|
|
Unknown | 0 | 1 |
|
| Adjuvant
therapy |
|
| 0.032a |
|
Endocrine therapy alone | 12 | 33 |
|
|
Chemotherapy alone | 5 | 15 |
|
|
Endocrine and
chemotherapy | 16 | 21 |
|
|
None | 0 | 12 |
|
As MZB1 has been reported to exist in the
endoplasmic reticulum of cancer cells (5), the correlations between mRNA expression
levels of MZB1, XBP1, and DDIT3, UPR-related
molecules (11,19), were evaluated. Although there was no
significant correlation between mRNA expression levels of
MZB1 and XBP1 (correlation coefficient, 0.052;
P=0.584; Fig. 2B), MZB1 mRNA
expression was significantly correlated with that of DDIT3
(correlation coefficient, 0.421; P<0.0001; Fig. 2B). Furthermore, when MZB1 and
DERL3 mRNA levels were evaluated, there was a significant
correlation (correlation coefficient, 0.669; P<0.0001; Fig. 2B). These results suggested that MZB1
serves some role associated with UPR pathways.
Assessment of MZB1 protein expression
status using immunohistochemistry
As mRNA extracted from cancer specimens may contain
mRNA derived from stromal cells, immunohistochemistry of MZB1 was
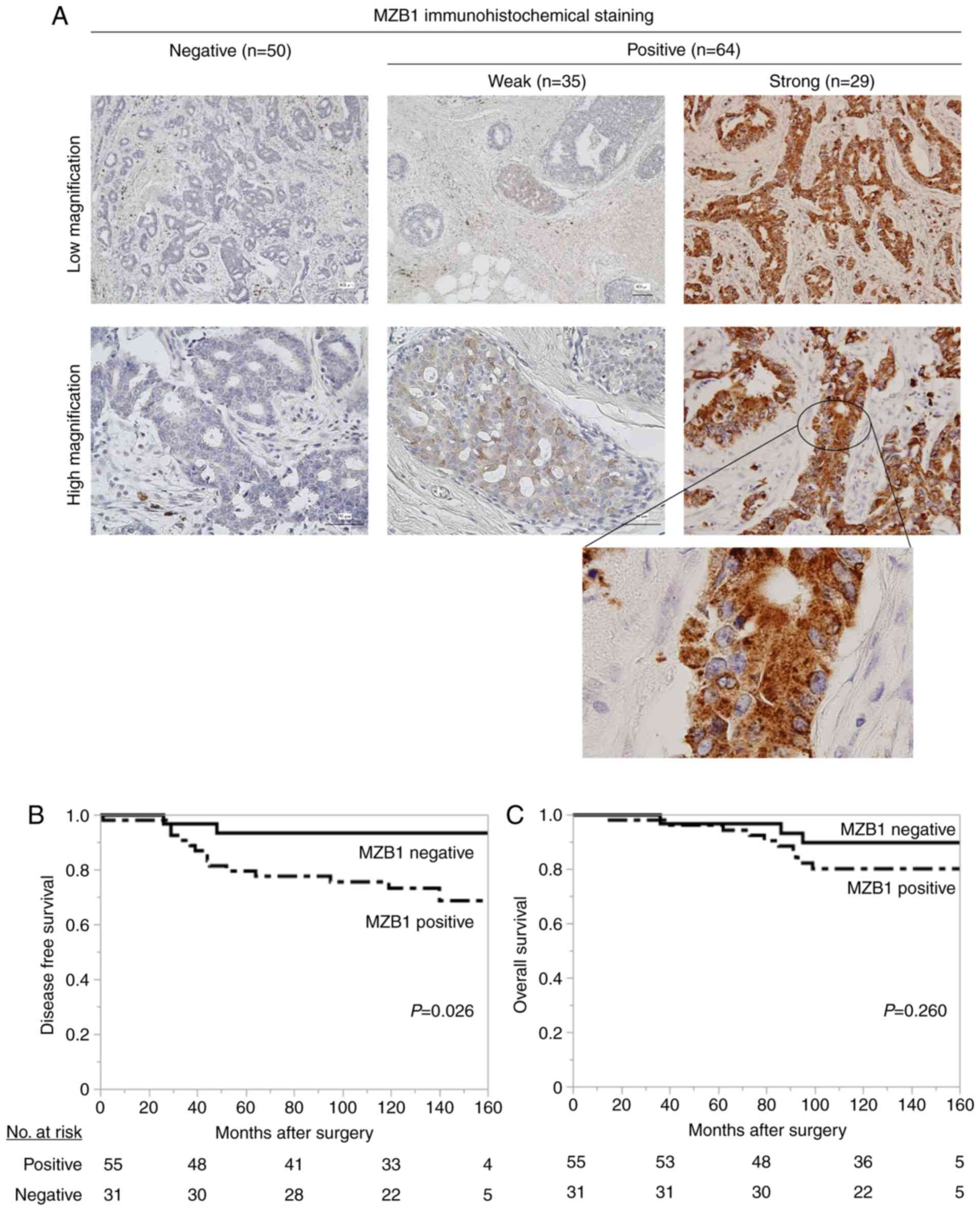
conducted to assess the significance of its expression in BC cells.
Among 114 patients with breast cancer, BC specimens from patients
did not express MZB1 (determined as negative), and those from 35
and 29 patients expressed MZB1 weakly and strongly, respectively
(determined as positive, Fig. 3A).
There was no significant association between MZB1 positivity and T
status (P=0.181), node status (P=0.706), or pathological stage
(P=0.326). However, patients' age (≤60-year-old; P=0.009),
ER-positivity (P=0.003), PgR-positivity (P=0.003), and
non-triple-negativity (P=0.023) were significantly associated with
MZB1 positivity (Table II). There
were no differences between MZB1 positive and negative groups
regarding DFS (P=0.478) or OS (P=0.996).
 | Table II.Association between
immunohistochemical MZB1 expression and clinicopathological
characteristics in 114 patients with breast cancer. |
Table II.
Association between
immunohistochemical MZB1 expression and clinicopathological
characteristics in 114 patients with breast cancer.
| Clinicopathological
parameter | MZB1-positive group
(n=64) | MZB1-negative group
(n=50) | P-value |
|---|
| Age |
|
| 0.009a |
| ≤60
years | 46 | 24 |
|
| >60
years | 18 | 26 |
|
| Histology |
|
| 0.477 |
| Ductal
carcinoma in situ | 2 | 4 |
|
|
Invasive ductal carcinoma | 56 | 43 |
|
|
Invasive lobular
carcinoma | 2 | 2 |
|
|
Other | 4 | 1 |
|
| UICC T factor |
|
| 0.181 |
|
Tis/T1 | 24 | 25 |
|
|
T2/T3/T4 | 40 | 25 |
|
| Node status |
|
| 0.706 |
|
Negative | 31 | 26 |
|
|
Positive | 33 | 24 |
|
| UICC pathological
stage |
|
| 0.326 |
|
0/I/II | 49 | 42 |
|
|
III | 15 | 8 |
|
| ER status |
|
| 0.003a |
|
Positive | 55 | 31 |
|
|
Negative | 9 | 19 |
|
| PgR status |
|
| 0.003a |
|
Positive | 50 | 26 |
|
|
Negative | 14 | 24 |
|
| HER2 status |
|
| 0.878 |
|
Positive | 13 | 12 |
|
|
Negative | 43 | 37 |
|
|
Unknown | 8 | 1 |
|
|
Triple-negative |
|
| 0.023a |
|
Yes | 3 | 9 |
|
| No | 60 | 41 |
|
|
Unknown | 1 | 0 |
|
| Adjuvant
therapy |
|
| 0.175 |
|
Endocrine therapy alone | 27 | 18 |
|
|
Chemotherapy alone | 9 | 11 |
|
|
Endocrine and
chemotherapy | 24 | 13 |
|
|
None | 4 | 8 |
|
MZB1 mRNA expression levels were detected in
ER-positive BC cell lines, and high MZB1 C/N levels were
associated with a more advanced pathological stage in specimens
from patients with BC. Furthermore, MZB1 exhibited increased
expression in ER-positive patients (Table II). These results implied that there
is an association between MZB1 and ER-positive BC. In ER-positive
patients (n=86), DFS rates was significantly poorer in
MZB1-positive patients (n=55) compared with MZB1-negative patients
(n=31; 5-year DFS rates: MZB1-positive group, 80.0%; negative
group, 93.4%; P=0.026; Fig. 3B). By
contrast, OS in MZB1-positive patients did not differ from that in
MZB1-negative patients (5-year OS rates: MZB1-positive group,
94.5%; negative group, 96.8%; P=0.260; Fig. 3C). Multivariate analysis of DFS
identified ‘lymph node metastasis’ (HR, 7.81; 95% CI, 2.10–50.9;
P=0.001) and ‘MZB1 positivity’ (HR, 4.30; 95% CI, 1.21–27.4;
P=0.022) as independent prognostic factors (Table III).
 | Table III.Prognostic factors for disease-free
survival in 86 patients with estrogen receptor-positive breast
cancer. |
Table III.
Prognostic factors for disease-free
survival in 86 patients with estrogen receptor-positive breast
cancer.
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Variable | n | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age, >60
years | 31 | 0.89 | 0.28–2.39 | 0.820 |
|
|
|
| Tumor size, >2
cm | 46 | 4.48 | 1.46–19.4 |
0.007a | 2.19 | 0.69–9.79 | 0.197 |
| Node status
positive | 42 | 9.88 | 2.78–62.7 |
0.001a | 7.81 | 2.10–50.9 |
0.001a |
| HER2-positive | 9 | 2.95 | 0.82–8.49 | 0.091 |
|
|
|
| MZB1-positive | 55 | 4.58 | 1.29–29.1 |
0.016a | 4.30 | 1.21–27.4 |
0.022a |
Discussion
The present study has demonstrated the significance
of MZB1 expression, particularly in ER-positive BC. In cell lines,
MZB1 expression was only detectable in ER-positive BC cells,
but not in ER-negative or non-cancerous cells. In clinical samples,
a higher MZB1 C/N ratio was associated with lymph node
metastasis and a more advanced UICC stage in patients with
ER-positive BC. Notably, MZB1-positive expression, performed using
immunohistochemistry, indicated a poor prognosis, and was an
independent prognostic factor in patients with ER-positive BC.
In recent years, attention has been focused on the
endoplasmic reticulum stress response, including UPR in cancer
cells (19). UPR is involved in
protein-folding homeostasis against various stresses and is
enhanced in cancer cells, resulting in cancer progression (19). As UPR activates endoplasmic reticulum
chaperones that stabilize protein-folding to promote cancer cell
survival, proliferation, angiogenesis, metastasis, and therapy
resistance (20), UPR-related
pathways or molecules have been considered attractive candidates
for novel biomarkers or therapeutic targets.
MZB1 was originally identified as a molecule that
regulates the correct surface presentation and secretion of IgM,
which is located in the endoplasmic reticulum in B lymphocytes
(21,22). It has been reported that MZB1 is
involved in the stabilization and secretion of IgA and IgM as a
molecular chaperone in cooperation with other chaperones, including
GRP78 and GRP94 (6). Conversely, in
malignant tumor cells, the role of MZB1 has not been fully
clarified. Our previous study reported that DERL3, which is located
in the endoplasmic reticulum and is activated in the UPR pathway,
promotes BC progression, and that DERL3 expression was
associated with more aggressive clinical features in BC (12). Notably, MZB1 expression was
positively correlated with not only DDIT3 expression but
also DERL3 expression in the clinical BC specimens,
suggesting the possibility that MZB1 is somewhat associated with
UPR pathways in BC cells.
Several studies have reported inconsistent roles for
MZB1 in various malignancies. Our group previously demonstrated the
tumor-suppressive roles of MZB1 and showed that low MZB1
expression was an independent poor prognostic factor in gastric
cancer (3). In hepatocellular
carcinoma, patients with positive MZB1 expression using
immunohistochemistry experienced a better prognosis (5). Conversely, in chronic lymphocytic
leukemia, high MZB1 expression was associated with poorer
survival (4). In the present study,
MZB1 was considered to be associated with tumor-progression in BC,
because its C/N ratio was higher in patients with more advanced
disease. Furthermore, in ER-positive patients, MZB1 expression was
an independent prognostic factor that was indicative of poor DFS
rates. Although MZB1-positive patients tended to show poorer OS
rates, there was no significant difference. This may be because
metastatic BC patients, particularly those with ER positivity, have
various treatment options, which leads to long survival times
following recurrence. Notably, MZB1 expression was
detectable in only four ER-positive cell lines among thirteen BC
cell lines, and MZB1 was more likely to be expressed in ER-positive
clinical specimens, suggesting that MZB1 expression may be more
activated in the ER-positive BC subtype. However, it should be
noted that not all ER-positive BC cell lines and clinical specimens
expressed MZB1. In ER-positive BC, estrogen acting via estrogen
receptor α (ERα) induces activation of UPR components, including
inositol requiring enzyme 1 (IRE1) and GRP78, which confer
estrogen-ERα-induced cell proliferation and resistance to endocrine
therapy and chemotherapy (23,24). The
results of the present study suggested that MZB1 expression
reflects the activation of the UPR pathway in BC cells, leading to
a poor prognosis. However, the present study is not capable of
determining whether MZB1 serves any oncogenic role due to the lack
of mechanistic experiments.
Recently, although chemotherapy, endocrine therapy,
anti-HER2 drugs, molecular-targeting drugs, and immunotherapy have
been used in the treatment of patients with metastatic BC, no drugs
that target pathways related to the endoplasmic reticulum stress
response are available. However, in preclinical research, the UPR
pathway and molecular chaperones are receiving attention as
potential novel therapeutic targets. For example, ganetespib, which
inhibits heat shock protein 90 (HSP90), also known as GRP94, was
shown to suppress MAPK, AKT, and mTOR pathways in BC (25), and it has been clinically tested
(26). In addition, other studies
have shown the synergistic effects of combining conventional
chemotherapy with UPR inhibitors (19,26).
‘Targeting UPR’ has been recognized as one of the novel therapeutic
strategies (19), and the present
study may aid in understanding the UPR pathways in BC.
There are certain limitations to the present study.
As mentioned earlier, as this study did not evaluate the function
of MZB1, it remains uncertain how MZB1 works in BC cells.
Additionally, MZB1 is expressed not only in BC cells but also in
normal mammary and stromal cells (e.g. lymphocytes), which may
cause discrepancies depending on the analytic methods. Although
RT-qPCR in BC cell lines and immunohistochemistry in clinical
samples evaluated the status of MZB1 expression in BC cells,
MZB1 mRNA expression levels in clinical samples did not
exclude those derived from stromal cells. For example, in the
present study, MZB1 mRNA expression levels in ER-positive
patients were not higher than those in ER-negative patients, unlike
the results of BC cell lines and immunohistochemistry. Finally,
although the present study showed that MZB1 positivity was a poor
prognostic marker in our cohort, it should be validated in
different cohorts to determine the utility of MZB1 as a novel
biomarker.
In conclusion, the present study has raised the
possibility of MZB1 acting as a prognostic marker in patients with
ER-positive BC. Pathways related to the endoplasmic reticulum
stress response are considered attractive targets to develop novel
biomarkers and therapeutic strategies.
Acknowledgements
The authors would like to thank Professor David
Sidransky, Director of the Otolaryngology Department of Johns
Hopkins University School of Medicine (Baltimore, MD, USA) for
providing the BT-474, MCF-7, and MCF-12A cell lines.
Funding
The present study was supported by JSPS KAKENHI of
Japan (grant no. JP7119K16795) and Hibino Foundation of Japan
(grant no. 2600006536).
Availability of data and materials
The data used and/or analyzed during the present
study can be obtained from the corresponding author upon reasonable
request.
Authors' contributions
MS and MK conceived and designed the study. MW, MS,
TIn, and TIc conducted the experiments. MW and MS analyzed the data
and wrote the manuscript. MS, NM, YT, DT, NT, and TK contributed
the acquisition and interpretation of patient data. TIn, TIc, IS,
NM, YT, DT, NT, MK, TK, YK, and MN reviewed and revised the
manuscript. The final manuscript was read and approved by all
authors.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Nagoya University Graduate School of Medicine
(reference number: 2019-0028). Written informed consent was
obtained from participants for the use of samples and data.
Patient consent for publication
Participants in this study granted written informed
consent for publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BC
|
breast cancer
|
|
DDIT3
|
DNA damage inducible transcript 3
|
|
DERL3
|
Derlin 3
|
|
DFS
|
disease-free survival
|
|
ER
|
estrogen receptor
|
|
ERα
|
estrogen receptor α
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
GRP
|
glucose-regulated protein
|
|
HER2
|
human epidermal growth factor 2
|
|
HSP
|
heat shock protein
|
|
IRE1
|
inositol requiring enzyme 1
|
|
MZB1
|
marginal zone B and B1 cell-specific
protein
|
|
OS
|
overall survival
|
|
PgR
|
progesterone receptor
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
UICC
|
Union for International Cancer
Control
|
|
XBP1
|
X-box binding protein 1
|
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, regional, and national cancer incidence, mortality, years
of life lost, years lived with disability, and Disability-adjusted
life-years for 32 cancer groups, 1990 to 2015 a systematic analysis
for the global burden of disease study. JAMA Oncol. 3:524–548.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iwata H: Future treatment strategies for
metastatic breast cancer: Curable or incurable? Breast Cancer.
19:200–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanda M, Tanaka C, Kobayashi D, Tanaka H,
Shimizu D, Shibata M, Takami H, Hayashi M, Iwata N, Niwa Y, et al:
Epigenetic suppression of the immunoregulator MZB1 is associated
with the malignant phenotype of gastric cancer. Int J Cancer.
139:2290–2298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herold T, Mulaw MA, Jurinovic V, Seiler T,
Metzeler KH, Dufour A, Schneider S, Kakadia PM, Spiekermann K,
Mansmann U, et al: High expression of MZB1 predicts adverse
prognosis in chronic lymphocytic leukemia, follicular lymphoma and
diffuse large B-cell lymphoma and is associated with a unique gene
expression signature. Leuk Lymphoma. 54:1652–1657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsumura S, Imoto I, Kozaki K, Matsui T,
Muramatsu T, Furuta M, Tanaka S, Sakamoto M, Arii S and Inazawa J:
Integrative array-based approach identifies MZB1 as a frequently
methylated putative tumor suppressor in hepatocellular carcinoma.
Clin Cancer Res. 18:3541–3551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenbaum M, Andreani V, Kapoor T, Herp S,
Flach H, Duchniewicz M and Grosschedl R: MZB1 is a GRP94
cochaperone that enables proper immunoglobulin heavy chain
biosynthesis upon ER stress. Genes Dev. 28:1165–1178. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cox JS, Shamu CE and Walter P:
Transcriptional induction of genes encoding endoplasmic reticulum
resident proteins requires a transmembrane protein kinase. Cell.
73:1197–1206. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hochachka PW, Buck LT, Doll CJ and Land
SC: Unifying theory of hypoxia tolerance: Molecular/metabolic
defense and rescue mechanisms for surviving oxygen lack. Proc Natl
Acad Sci USA. 93:9493–9498. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koumenis C: ER stress, hypoxia tolerance
and tumor progression. Curr Mol Med. 6:55–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernandez PM, Tabbara SO, Jacobs LK,
Manning FC, Tsangaris TN, Schwartz AM, Kennedy KA and Patierno SR:
Overexpression of the glucose-regulated stress gene GRP78 in
malignant but not benign human breast lesions. Breast Cancer Res
Treat. 59:15–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujimoto T, Onda M, Nagai H, Nagahata T,
Ogawa K and Emi M: Upregulation and overexpression of human X-box
binding protein 1 (hXBP-1) gene in primary breast cancers. Breast
Cancer. 10:301–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shibata M, Kanda M, Tanaka H, Umeda S,
Miwa T, Shimizu D, Hayashi M, Inaishi T, Miyajima N, Adachi Y, et
al: Overexpression of Derlin 3 is associated with malignant
phenotype of breast cancer cells. Oncol Rep. 38:1760–1766. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shibata M, Kanda M, Shimizu D, Tanaka H,
Umeda S, Hayashi M, Inaishi T, Miyajima N, Adachi Y, Takano Y, et
al: Expression of regulatory factor X1 can predict the prognosis of
breast cancer. Oncol Lett. 13:4334–4340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cserni G, Chmielik E, Cserni B and Tot T:
The new TNM-based staging of breast cancer. Virchows Archi.
472:697–703. 2018. View Article : Google Scholar
|
|
15
|
Shibata M, Kanda M, Shimizu D, Tanaka H,
Umeda S, Miwa T, Hayashi M, Inaishi T, Miyajima N, Adachi Y, et al:
RASEF expression correlates with hormone receptor status in breast
cancer. Oncol Lett. 16:7223–7230. 2018.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Finn RS, Dering J, Conklin D, Kalous O,
Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, et al: PD
0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially
inhibits proliferation of luminal estrogen receptor-positive human
breast cancer cell lines in vitro. Breast Cancer Res. 11:R772009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Subik K, Lee JF, Baxter L, Strzepek T,
Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG and
Tang P: The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67
and AR by immunohistochemical analysis in breast cancer cell lines.
Breast Cancer (Auckl). 4:35–41. 2010.PubMed/NCBI
|
|
19
|
McGrath EP, Logue SE, Mnich K, Deegan S,
Jäger R, Gorman AM and Samali A: The unfolded protein response in
breast cancer. Cancers (Basel). 10:3442018. View Article : Google Scholar
|
|
20
|
Ansa-Addo EA, Thaxton J, Hong F, Wu BX,
Zhang Y, Fugle CW, Metelli A, Riesenberg B, Williams K, Gewirth DT,
et al: Clients and oncogenic roles of molecular chaperone
gp96/grp94. Curr Top Med Chem. 16:2765–2778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Belkaya S, Murray SE, Eitson JL, de la
Morena MT, Forman JA and van Oers NS: Transgenic expression of
microRNA-185 causes a developmental arrest of T cells by targeting
multiple genes including Mzb1. J Biol Chem. 288:30752–30762. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Flach H, Rosenbaum M, Duchniewicz M, Kim
S, Zhang SL, Cahalan MD, Mittler G and Grosschedl R: Mzb1 protein
regulates calcium homeostasis, antibody secretion, and integrin
activation in innate-like B cells. Immunity. 33:723–735. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rajapaksa G, Thomas C and Gustafsson JÅ:
Estrogen signaling and unfolded protein response in breast cancer.
J Steroid Biochem Mol Biol. 163:45–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andruska N, Zheng X, Yang X, Helferich WG
and Shapiro DJ: Anticipatory estrogen activation of the unfolded
protein response is linked to cell proliferation and poor survival
in estrogen receptor α-positive breast cancer. Oncogene.
34:3760–3769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friedland JC, Smith DL, Sang J, Acquaviva
J, He S, Zhang C and Proia DA: Targeted inhibition of Hsp90 by
ganetespib is effective across a broad spectrum of breast cancer
subtypes. Invest New Drugs. 32:14–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jhaveri K, Wang R, Teplinsky E,
Chandarlapaty S, Solit D, Cadoo K, Speyer J, D'Andrea G, Adams S,
Patil S, et al: A phase I trial of ganetespib in combination with
paclitaxel and trastuzumab in patients with human epidermal growth
factor receptor-2 (HER2)-positive metastatic breast cancer. Breast
Cancer Res. 19:892017. View Article : Google Scholar : PubMed/NCBI
|