Introduction
Breast cancer (BC) is a major cause of mortality in
women worldwide, with an estimated 2,090,000 newly diagnosed cases
and 626,679 deaths in 2018 (1).
Epidemiological studies have demonstrated that the incidence and
mortality rates of BC in China are increasing (2). Westernized lifestyles and changes in
fertility patterns may be a cause of the increased incidence of BC
(3–5), and the lack of early diagnosis and
effective adjuvant therapies may contribute to higher
mortality.
In the 19th century, Rudolf Virchow first proposed
the hypothesis that inflammation is associated with cancer
(6). Since then, numerous studies
have explored the relationship between them. Inflammation is now
recognized as a hallmark of cancer (7), which promotes the occurrence (8), development and metastasis of cancer
(9,10). Various systemic inflammatory markers,
including single markers, such as C-reactive protein (11), erythrocyte sedimentation rate
(12), neutrophils (13), lymphocytes (10) and fibrinogen (14), and prognostic systems, such as the
neutrophil-to-lymphocyte ratio (NLR) (15) and Glasgow prognostic score (16), have been demonstrated to be
associated with the prognosis of different types of cancer. Studies
have demonstrated that an elevated NLR is associated with poor
outcomes in various types of cancer, including hepatocellular
carcinoma (17), esophageal cancer
(18) and BC (19). In addition, a meta-analysis of 18
studies demonstrated that high NLR is associated with shorter
survival compared with low NLR in patients with BC (20).
In various malignant tumors, the coagulation system
is activated and promotes tumor progression and metastasis
(21,22). Fibrinogen, a 340-kDa glycoprotein
synthesized by the liver, is considered to predict the prognosis of
various malignancies (14,21). Elevated fibrinogen levels are
associated with poor outcomes in gynaecological cancer (23), lung cancer (24) and BC (25).
Previous studies using a scoring system based on the
combination of fibrinogen concentration and
neutrophil-to-lymphocyte ratio (F-NLR) have reported that a high
F-NLR score is associated with poor outcomes in different types of
cancer (26–29). However, to the best of our knowledge,
the prognostic value of F-NLR in BC remains unknown. Therefore, the
present study aimed to investigate the prognostic role of this
biomarker combination in resectable BC.
Materials and methods
Patients
The present study retrospectively analyzed patients
with resectable BC who received curative surgery at Peking Union
Medical College Hospital (Beijing, China) between July 2012 and May
2014. The Ethics Committee on Human Research at Peking Union
Medical College Hospital approved the study. Due to the
retrospective nature of the present study, the committee waived the
requirement for individual patient consent. The inclusion criteria
were as follows: i) Female patients aged 18–80 years; ii) confirmed
pathology of stage I–III BC; and iii) received radical surgery and
standard adjuvant therapy, such as chemotherapy, radiotherapy,
endocrine therapy, herceptin therapy. The exclusion criteria were:
i) Metastatic or inflammatory BC; ii) neoadjuvant therapy; iii)
neutrophil count >10×109/l for patients of untreated
status; and iv) blood coagulation disorders, autoimmune disease,
hematological disease or infectious disease. Finally, 906 patients
were included in the present study based on the inclusion and
exclusion criteria.
Clinicopathological parameters
The clinicopathological information and laboratory
data for these patients were collected from their medical records.
The TNM stage of BC was determined according to the AJCC Cancer
Staging Manual (7th edition) (30).
Hormone receptor status and human epidermal growth factor receptor
2 (HER2) status were assessed by immunohistochemistry according to
recommendations in the American Society of Clinical
Oncology/College of American Pathologists guidelines (31). When estrogen and/or progesterone
receptors were determined to be positive using
immunohistochemistry, the case was defined as hormone
receptor-positive, whereas it was defined as negative when both
hormone receptors were negative. HER2 positivity was defined as an
immunohistochemistry result of 3+ or 2+ confirmed by further
fluorescence in situ hybridization experiments. According to
the hormone receptor and HER2 status, molecular subtypes of BC were
categorized as follows: Luminal A-like (hormone receptor-positive,
HER2-negative and Ki-67 ≤14%), luminal B-like (hormone
receptor-positive, HER2-negative and Ki-67 >14%; or hormone
receptor-positive and HER2-positive), HER2 (hormone
receptor-negative and HER2-positive) or triple-negative (hormone
receptor-negative and HER2-negative). Lymphovascular invasion was
interpreted on hematoxylin and eosin staining slides of tumor
specimens. When tumor cells existed in a definite endothelial-lined
space, it was defined as lymphovascular invasion.
Clinical treatment and follow-up
All patients received radical BC surgery. Breast
management included lumpectomy or mastectomy, and axillary
management included sentinel lymph node biopsy or axillary lymph
node dissection. According to the National Comprehensive Cancer
Network guidelines (32), patients
received standard adjuvant therapy after surgery as appropriate.
Follow-up was conducted every 6 months in the 2 years after surgery
and annually thereafter. The last date of observation was July 31,
2019.
F-NLR definition and evaluation
Routine blood sampling was performed 3 days before
surgery to obtain the following indices: Neutrophil count,
lymphocyte count and plasma fibrinogen level. The NLR was
determined by dividing the neutrophil count by the lymphocyte
count. The cut-off values of the NLR and preoperative fibrinogen
concentration were determined using receiver operating
characteristic curve (ROC) analysis. Based on the ROC analysis, the
optimal cut-off values of NLR and fibrinogen for the prediction of
disease-free survival (DFS) were 2.39 and 3.21 g/l, with areas
under the curve (AUCs) of 0.778 (95% CI, 0.730–0.827) and 0.584
(95% CI, 0.522–0.646), respectively (Fig. 1A). The optimal cut-off values of NLR
and fibrinogen for the prediction of overall survival (OS) were
2.20 and 3.21 g/l, with AUCs of 0.829 (95% CI, 0.792–0.866) and
0.582 (95% CI, 0.506–0.658), respectively (Fig. 1B). In terms of the highest Youden
index, the optimal cut-off values of NLR and fibrinogen were 2.20
and 3.21 g/l, respectively. The sensitivity and specificity of NLR
for the prediction of DFS were 83.8 and 61.7%, and for the
prediction of OS were 95.4 and 60.8%, respectively. The sensitivity
and specificity of fibrinogen for the prediction of DFS were 41.4
and 78.6%, whereas those for the prediction of OS were 41.5 and
77.8%, respectively. Based on the cut-off values for NLR and
fibrinogen, the cohort was divided into three groups which were
assigned different F-NLR scores as follows: Score 2, fibrinogen
>3.21 g/l and NLR >2.20; score 1, fibrinogen >3.21 g/l or
NLR >2.20; and score 0, fibrinogen ≤3.21 g/l and NLR ≤2.20.
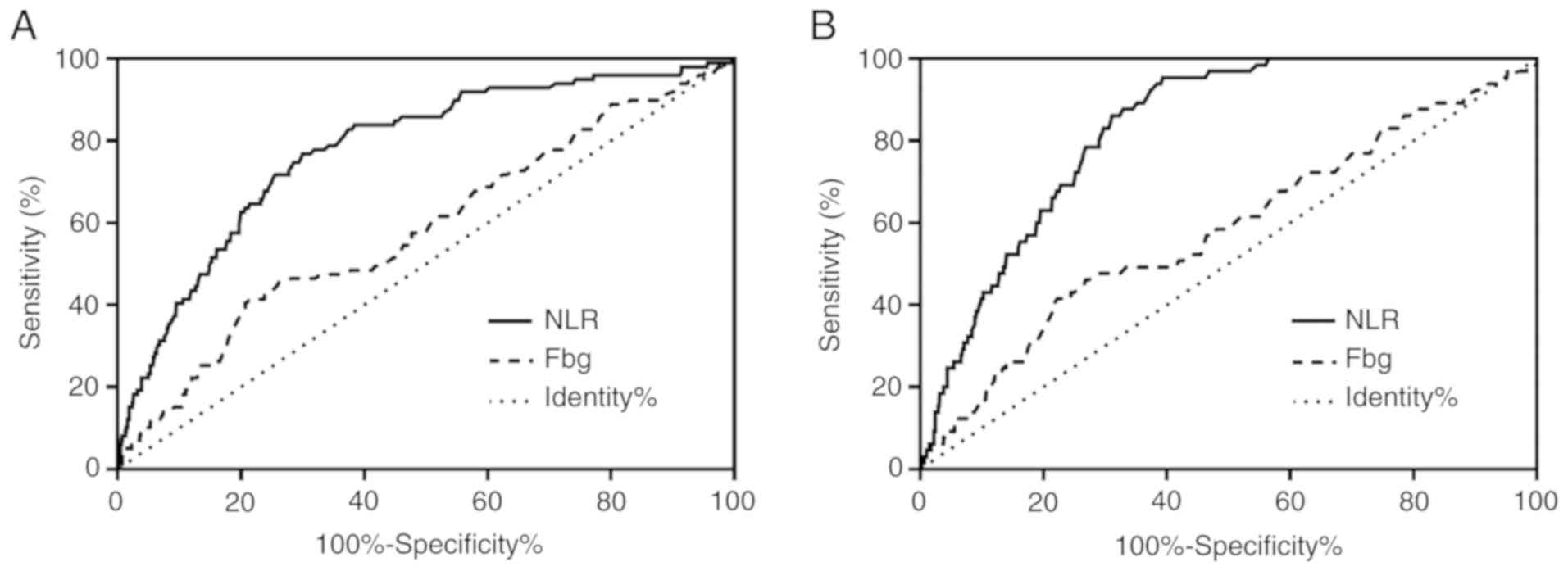 | Figure 1.(A) ROC curves of NLR and plasma
fibrinogen levels for the prediction of the disease-free survival
of patients with resectable breast cancer. NLR: AUC, 0.778; 95% CI,
0.730–0.827; P<0.001. Fibrinogen: AUC, 0.584; 95% CI,
0.522–0.646; P=0.007. (B) ROC curves of NLR and plasma fibrinogen
levels for the prediction of the overall survival of patients with
resectable breast cancer. NLR: AUC, 0.829; 95% CI, 0.792–0.866;
P<0.001. Fibrinogen: AUC, 0.582; 95% CI, 0.506–0.658; P=0.027.
AUC, area under the curve; Fbg, fibrinogen; NLR,
neutrophil-to-lymphocyte ratio; ROC, receiver operating
characteristic. |
Statistical analysis
A two-tailed χ2 test was used to evaluate
the associations between F-NLR and clinicopathological
characteristics. DFS and OS were calculated using the Kaplan-Meier
method, and differences in survival rates among the groups were
compared by log-rank tests. Prognostic factors were analyzed using
the Cox proportional hazards model. The hazard ratio was estimated
with 95% CI. SPSS version 19.0 (IBM Corp.) was used for data
analysis. P<0.05 (two-sided) was considered to indicate a
statistically significant difference.
Results
Clinicopathological characteristics of
patients with BC
As shown in Table I,
a total of 906 patients were enrolled in the present study. The
mean age of the patients was 50±12 years (range, 22–80 years).
There were 494 (54.5%) patients aged ≤50 years, and 491 (54.2%)
patients with T1 stage tumors. There were 385 (42.5%) patients with
lymph node positivity, including 163 (18.0%) patients with N2 and
N3 stage tumors. According to the AJCC guidelines, 362 (40.0%)
patients had stage II BC, whereas 168 (18.5%) patients had stage
III BC. Most patients (700; 77.3%) had a positive hormone receptor
status, and 249 (27.5%) patients were HER2 positive. Based on the
F-NLR scoring system, the whole cohort was divided into three
groups as follows: Score 0, 444 (49.0%) patients; score 1, 291
(32.1%) patients; and score 2, 171 (18.9%) patients. The median and
mean follow-up times of survivors were 57 months (range, 5–83
months) and 53.9 months, respectively. The 5-year DFS and OS rates
for the entire cohort were 89.0 and 92.2%, respectively.
 | Table I.Clinical baseline characteristics of
the patients with resectable breast cancer. |
Table I.
Clinical baseline characteristics of
the patients with resectable breast cancer.
| Characteristic | Value |
|---|
| Age, years (mean ±
SD) | 50±12 |
| Age, n (%) |
|
| >50
years | 412 (45.5) |
| ≤50
years | 494 (54.5) |
| Tumor stage, n
(%) |
|
| T1 | 491 (54.2) |
| T2 | 344 (38.0) |
| T3 | 56 (6.2) |
| T4 | 15 (1.6) |
| N stage, n (%) |
|
| N0 | 521 (57.5) |
| N1 | 222 (24.5) |
| N2 | 89 (9.8) |
| N3 | 74 (8.2) |
| TNM stage, n
(%) |
|
| I | 376 (41.5) |
| II | 362 (40.0) |
|
III | 168 (18.5) |
| Hormone receptor, n
(%) |
|
|
Positive | 700 (77.3) |
|
Negative | 206 (22.7) |
| Estrogen receptor,
n (%) |
|
|
Positive | 692 (76.4) |
|
Negative | 214 (23.6) |
| Progesterone
receptor, n (%) |
|
|
Positive | 622 (68.7) |
|
Negative | 284 (31.3) |
| HER2, n (%) |
|
|
Positive | 249 (27.5) |
|
Negative | 657 (72.5) |
| Histologic grade, n
(%) |
|
| 1 | 129 (14.2) |
| 2 | 540 (59.6) |
| 3 | 237 (26.2) |
| Ki-67, n (%) |
|
|
>14% | 528 (58.3) |
|
≤14% | 378 (41.7) |
| Lymphovascular
invasion, n (%) |
|
|
Positive | 184 (20.3) |
|
Negative | 722 (79.7) |
| F-NLR score, n
(%) |
|
| 0 | 444 (49.0) |
| 1 | 291 (32.1) |
| 2 | 171 (18.9) |
Association between F-NLR score and
clinicopathological characteristics
As shown in Table
II, age (P<0.001), tumor size (P=0.001), nodal positivity
(P=0.029), TNM stage (P=0.002) and lymphovascular invasion
(P<0.001) were significantly different among the three F-NLR
score groups.
 | Table II.Association between F-NLR scores and
clinicopathological factors in patients with resectable breast
cancer. |
Table II.
Association between F-NLR scores and
clinicopathological factors in patients with resectable breast
cancer.
|
|
| F-NLR score, n |
|
|---|
|
|
|
|
|
|---|
| Variables | All, n | 0 | 1 | 2 | P-value |
|---|
| Age, years |
|
|
|
| <0.001 |
|
>50 | 412 | 180 | 131 | 101 |
|
|
≤50 | 494 | 264 | 160 | 70 |
|
| Tumor size, cm |
|
|
|
| 0.001 |
|
>2 | 415 | 180 | 137 | 98 |
|
| ≤2 | 491 | 264 | 154 | 73 |
|
| Nodal
positivity |
|
|
|
| 0.029 |
|
Negative | 521 | 267 | 171 | 83 |
|
|
Positive | 385 | 177 | 120 | 88 |
|
| TNM stage |
|
|
|
| 0.002 |
| I | 376 | 209 | 114 | 53 |
|
| II | 362 | 169 | 119 | 74 |
|
|
III | 168 | 66 | 58 | 44 |
|
| Hormone
receptor |
|
|
|
| 0.270 |
|
Positive | 700 | 353 | 217 | 130 |
|
|
Negative | 206 | 91 | 74 | 41 |
|
| HER2 |
|
|
|
| 0.760 |
|
Positive | 249 | 123 | 76 | 50 |
|
|
Negative | 657 | 321 | 215 | 121 |
|
| Ki-67, % |
|
|
|
| 0.226 |
|
>14 | 528 | 246 | 177 | 105 |
|
|
≤14 | 378 | 198 | 114 | 66 |
|
| Lymphovascular
invasion |
|
|
|
| <0.001 |
|
Positive | 184 | 64 | 67 | 53 |
|
|
Negative | 722 | 380 | 224 | 118 |
|
| Histologic
grade |
|
|
|
| 0.070 |
| 1 | 129 | 68 | 32 | 29 |
|
| 2 | 540 | 271 | 167 | 102 |
|
| 3 | 237 | 105 | 92 | 40 |
|
| Molecular
subtype |
|
|
|
| 0.640 |
| Luminal
A-like | 291 | 152 | 90 | 49 |
|
| Luminal
B-like | 409 | 201 | 127 | 81 |
|
|
HER2 | 96 | 45 | 33 | 18 |
|
|
Triple-negative | 110 | 46 | 41 | 23 |
|
Univariate and multivariate analyses
for DFS
The univariate analysis demonstrated that tumor size
(P<0.001), nodal positivity (P<0.001), TNM stage
(P<0.001), hormone receptor status (P=0.015), HER2 status
(P=0.005), Ki-67 index (P=0.039), lymphovascular invasion
(P<0.001), histologic grade (P=0.018) and F-NLR score
(P<0.001) were significantly associated with DFS (Table III). Multivariate analysis
demonstrated that F-NLR score (P<0.001), as well as TNM stage
(P=0.005) and lymphovascular invasion (P=0.001), could
independently predict DFS (Table
III).
 | Table III.Univariate and multivariate analysis
of clinical characteristics in relation to disease-free
survival. |
Table III.
Univariate and multivariate analysis
of clinical characteristics in relation to disease-free
survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | Patients, n | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years
(≤50/>50) | 494/412 | 0.886 | 0.595–1.320 | 0.552 |
|
|
|
| Tumor size, cm
(≤2/>2) | 491/415 | 2.602 | 1.707–3.965 | <0.001 | 1.104 | 0.686–1.776 | 0.684 |
| Nodal positivity
(negative/positive) | 521/385 | 3.371 | 2.195–5.175 | <0.001 | 1.284 | 0.686–2.404 | 0.434 |
| TNM stage
(I/II/III) | 376/362/168 | 2.669 | 2.032–3.506 | <0.001 | 1.889 | 1.213–2.943 | 0.005 |
| Hormone receptor
(positive/negative) | 700/206 | 1.683 | 1.105–2.565 | 0.015 | 1.251 | 0.797–1.963 | 0.331 |
| HER2
(negative/positive) | 657/249 | 1.786 | 1.194–2.673 | 0.005 | 1.395 | 0.921–2.112 | 0.116 |
| Ki-67, %
(≤14/>14) | 378/528 | 1.560 | 1.024–2.377 | 0.039 | 1.082 | 0.694–1.687 | 0.726 |
| Lymphovascular
invasion (negative/positive) | 722/184 | 3.201 | 2.148–4.769 | <0.001 | 2.034 | 1.350–3.065 | 0.001 |
| Histologic grade
(1/2/3) | 129/540/237 | 1.473 | 1.068–2.030 | 0.018 | 1.193 | 0.839–1.696 | 0.325 |
| F-NLR score
(0/1/2) | 444/291/171 | 2.584 | 2.004–3.332 | <0.001 | 2.279 | 1.758–2.954 | <0.001 |
Univariate and multivariate analyses
for OS
The univariate analysis revealed that tumor size
(P<0.001), nodal positivity (P<0.001), TNM stage
(P<0.001), hormone receptor status (P=0.013), HER2 status
(P=0.001), Ki-67 index (P=0.007), lymphovascular invasion
(P<0.001) and F-NLR score (P<0.001) were significantly
associated with OS (Table IV).
Multivariate analysis demonstrated that F-NLR score (P<0.001),
as well as TNM stage (P=0.040) and lymphovascular invasion
(P<0.001), could independently predict OS (Table IV).
 | Table IV.Univariate and multivariate analysis
of clinical characteristics in relation to overall survival. |
Table IV.
Univariate and multivariate analysis
of clinical characteristics in relation to overall survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | Patients, n | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years
(≤50/>50) | 494/412 | 0.660 | 0.397–1.098 | 0.110 |
|
|
|
| Tumor size, cm
(≤2/>2) | 491/415 | 2.957 | 1.732–5.046 | <0.001 | 1.213 | 0.680–2.163 | 0.513 |
| Nodal positivity
(negative/positive) | 521/385 | 4.801 | 2.696–8.549 | <0.001 | 1.720 | 0.771–3.838 | 0.185 |
| TNM stage
(I/II/III) | 376/362/1688 | 3.181 | 2.242–4.514 | <0.001 | 1.748 | 1.025–2.983 | 0.040 |
| Hormone receptor
(positive/negative) | 700/206 | 1.902 | 1.144–3.162 | 0.013 | 1.439 | 0.857–2.417 | 0.168 |
| HER2
(negative/positive) | 657/249 | 2.226 | 1.365–3.631 | 0.001 | 1.576 | 0.959–2.589 | 0.073 |
| Ki-67, %
(≤14/>14) | 378/528 | 2.145 | 1.233–3.730 | 0.007 | 1.604 | 0.916–2.809 | 0.098 |
| Lymphovascular
invasion (negative/positive) | 722/184 | 5.634 | 3.448–9.206 | <0.001 | 3.406 | 2.058–5.637 | <0.001 |
| Histologic grade
(1/2/3) | 129/540/237 | 1.461 | 0.983–2.172 | 0.060 |
|
|
|
| F-NLR score
(0/1/2) | 444/291/171 | 2.916 | 2.109–4.032 | <0.001 | 2.414 | 1.738–3.353 | <0.001 |
Survival analysis of F-NLR score
groups
Kaplan-Meier analyses and log-rank tests were used
to compare differences in survival among patients in the three
F-NLR score groups. The overall 5-year DFS and OS rates were 89.0
and 92.2%, respectively. The 5-year DFS rate in the group with an
F-NLR score of 2 was significantly lower than that in the groups
with a score of 1 or 0 (74.0 vs. 87.5 or 95.7%, respectively;
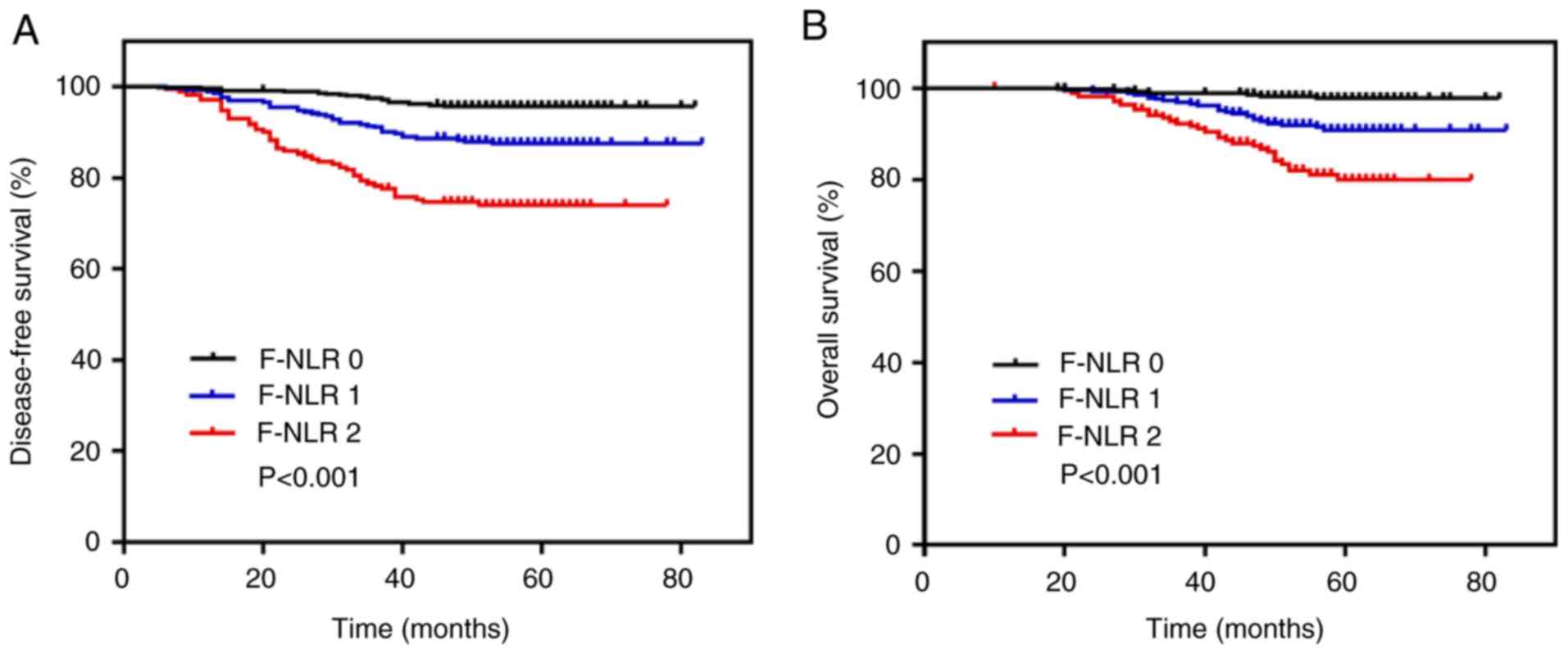
P<0.001; Fig. 2A). The 5-year OS
rate in the group with an F-NLR score of 2 was significantly lower
than that in the groups with a score of 1 or 0 (79.9 vs. 90.9 or
97.8%, respectively; P<0.001; Fig.
2B). Comparisons were also made in the 5-year DFS and OS rates
between two groups based on only low/high NLR or low/high
fibrinogen according to their cut-off values, and among the three
groups based on F-NLR scores (Table
SI). The results showed that the 5-year DFS rate in the group
with an F-NLR score of 2 (74.0%) was lower than that in the
NLR-high (78.7%) or fibrinogen-high groups (80.6%). The 5-year OS
rate in the group with an F-NLR score of 2 (79.9%) was also lower
than that in the NLR-high (83.1%) or fibrinogen-high groups
(86.0%).
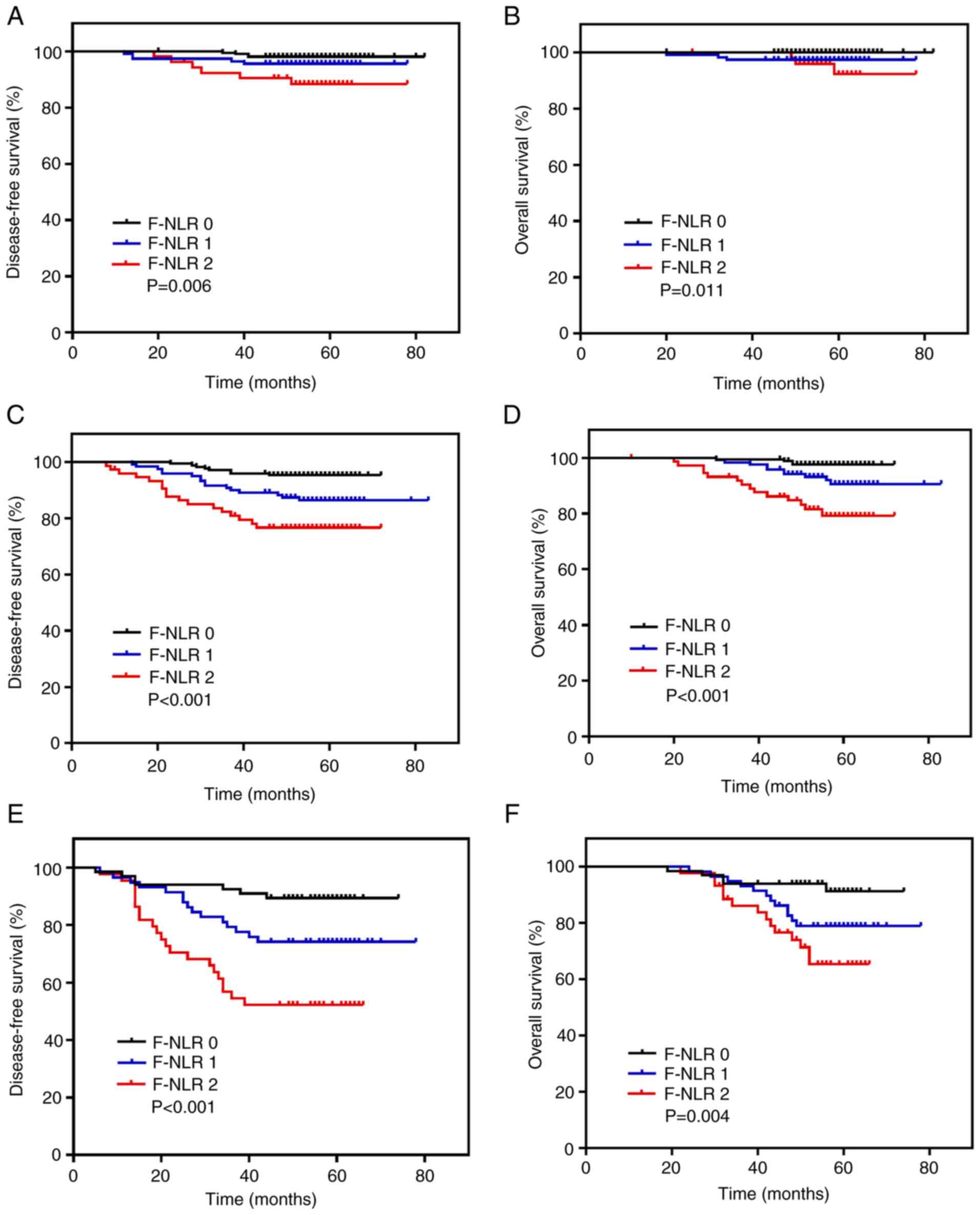
Further subgroup analysis revealed that the
prognostic effect of the preoperative F-NLR score for patients with
BC differed among TNM stages and molecular subtypes. When patients
were stratified according to TNM stage, the F-NLR score retained a
prognostic effect for the 5-year DFS in stages I (P=0.006; Fig. 3A), II (P<0.001; Fig. 3C) and III (P<0.001; Fig. 3E), as well as for the 5-year OS in
stages I (P=0.011; Fig. 3B), II
(P<0.001; Fig. 3D) and III
(P=0.004; Fig. 3F). When the F-NLR
score and TNM stage were stratified, the 5-year DFS ranged between
98.1% (F-NLR 0, TNM I; Table V) and
52.3% (F-NLR 2, TNM III; Table V),
and the 5-year OS ranged between 100.0% (F-NLR 0, TNM I; Table V) and 65.4% (F-NLR 2, TNM III;
Table V). When patients were
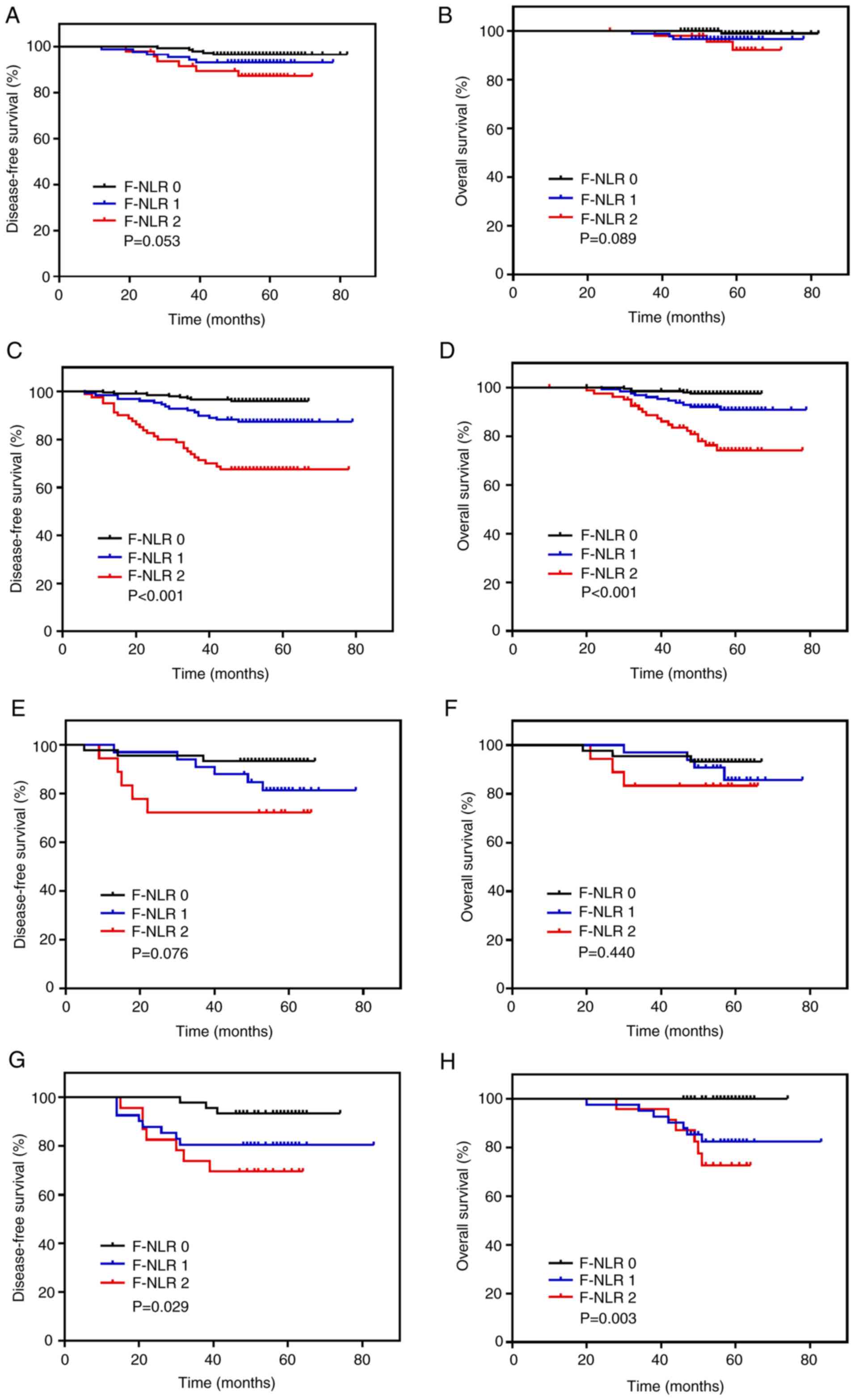
stratified on the basis of the molecular subtype of BC, the
prognostic effect of F-NLR was notable for DFS and OS in the
luminal B-like subtype (both P<0.001; Fig. 4C and D) and the triple-negative
subtype (DFS, P=0.029; OS, P=0.003; Fig.
4G and H). However, no differences in prognostic effect were
observed in the luminal A-like (DFS, P=0.053; OS, P=0.089; Fig. 4A and B) and HER2 subtypes (DFS,
P=0.076; OS, P=0.440; Fig. 4E and
F).
 | Table V.Association between F-NLR score and
5-year DFS or OS in patients with resectable breast cancer of
stages I, II and III. |
Table V.
Association between F-NLR score and
5-year DFS or OS in patients with resectable breast cancer of
stages I, II and III.
|
| 5-year DFS rate
(%) | 5-year OS rate
(%) |
|---|
|
|
|
|
|---|
| F-NLR | I | II | III | All | I | II | III | All |
|---|
| 0 | 98.1 | 95.3 | 89.4 | 95.7 | 100 | 97.6 | 91.3 | 97.8 |
| 1 | 95.6 | 86.4 | 74.1 | 87.5 | 97.4 | 90.6 | 79.0 | 90.9 |
| 2 | 88.4 | 76.7 | 52.3 | 74.0 | 92.2 | 79.2 | 65.4 | 79.9 |
| P-value | 0.006 | <0.001 | <0.001 | <0.001 | 0.011 | <0.001 | 0.004 | <0.001 |
Discussion
Previous studies have demonstrated that elevated
fibrinogen level, NLR and a high F-NLR score are associated with
tumor development and shorter survival in esophageal squamous cell
carcinoma (26,29), non-small cell lung cancer (NSCLC)
(27,33) and gastric cancer (28,34).
Based on these findings, the present study investigated the effect
of F-NLR on the prognosis of 906 patients with resectable BC.
Inflammation is considered to be a hallmark of
cancer (7). Cancer-associated
inflammation can be both local and systemic. Local inflammation is
mainly associated with the immune reaction in the tumor
microenvironment, whereas systemic inflammation causes
paraneoplastic symptoms through inflammatory mediators, such as
cytokines in the systemic circulation (35). Solid tumors have the ability to raise
immune cells and upregulate inflammatory cytokines, which then
influence tumor angiogenesis, development and distant metastasis
(8,36). The inflammatory mediator colony
stimulating factor 1 has been demonstrated to accelerate tumor cell
proliferation and promote tumor metastasis via the recruitment of
macrophages to pre-malignant areas (37). The oncogenic Ras is considered to
upregulate the expression of the pro-inflammatory cytokine
interleukin (IL)-8, leading to increased tumor volume and
angiogenesis in nude mouse models (38). Cancer-associated cytokines promote
the recruitment of myeloid cells to tumors that secrete IL-6 to
boost the transformation and tumorigenesis of BC cells (39). Myeloid-derived suppressor cells
(MDSCs), which are generated by cancer-associated myelopoiesis, may
persist in the circulation (40).
The concentration of circulating MDSCs is higher in patients with
malignant tumors than in healthy individuals and is associated with
advanced disease and distant metastasis (41,42). The
classic ‘Th-17-like’ inflammatory response to damaged epithelial
junctions in tumor cells exacerbates tumor growth and progression
(43). IL-22 and IL-32 are important
agents in the ‘Th-17 like’ inflammatory response and have recently
been demonstrated to be closely associated with tumor angiogenesis
(44,45). Collectively, these findings reveal a
complex relationship between cancer-associated inflammation,
tumorigenesis and tumor progression, which are mutually causal and
reinforcing. In the process of cancer-mediated myelopoiesis, the
number of circulating granulocytes is increased, with neutrophils
being most abundant (41).
Furthermore, a study demonstrated that neutrophils may be used as
an independent prognostic indicator for malignant tumors (46). Additionally, other studies have
demonstrated the prognostic utility of NLR in various types of
cancer (17–20).
A hypercoagulable state is much more likely to occur
in patients with cancer compared with healthy individuals and has
been associated with malignancy (47). Fibrinogen is frequently deposited in
the stroma of solid tumors (48).
The expression of fibrinogen can be upregulated by the inflammatory
cytokine IL-6 (49). The fibrinogen
synthesized and secreted by BC epithelial cells has been
demonstrated to mediate cell proliferation and form an
extracellular matrix that binds to tumor cell surfaces (50–52).
This process forms a solid framework around the cancer cells, which
may increase their adhesion, invasion and metastasis. Previous
studies (14,22) have reported that fibrinogen promotes
spontaneous metastasis, possibly by limiting the elimination of new
micrometastases by natural killer cells. Furthermore,
hyperfibrinogenemia has been demonstrated to be associated with
poor outcomes in various types of cancer (23–25).
The present study revealed a strong association
between high F-NLR score and larger tumor size, nodal positivity,
late TNM stage and lymphovascular invasion for resectable BC. These
findings are consistent with the results of previous studies on
gastric cancer (28) and NSCLC
(27,33). Notably, the F-NLR score was
identified as an independent predictor for DFS and OS in the
present study, which is consistent with previous studies on various
types of cancer (26–29,33–34).
This suggests that the F-NLR score could be used to classify the
survival risks of patients. Although the ROC curves indicate that
NLR has a much higher predictive efficiency than fibrinogen for DFS
and OS in patients with resectable BC, the effect of fibrinogen on
prognosis merits consideration, despite its relatively low
prediction efficiency. On the basis of survival analyses, the
present study found that the combination of NLR and fibrinogen
classified the whole cohort more distinctly and helped to screen
out the subgroup with the worst prognosis, which comprised those
patients in which NLR and fibrinogen were both high (F-NLR score
2). Greater attention should be focused on those patients, to
ensure that adequate adjuvant therapy and close follow-up are
applied. In the subgroup analysis of TNM stage in the present
study, the F-NLR score continued to exhibit an important prognostic
effect, which is similar to previous findings in gastric cancer
(28). The prognosis of patients in
each F-NLR group differed significantly according to disease stage.
Furthermore, in the molecular subgroup analysis, the predictive
significance of F-NLR was highest for the luminal B-like and
triple-negative BC subtypes.
The present study demonstrated that the F-NLR score
is a powerful prognostic index for patients with resectable BC.
However, the results should be considered in the context of several
limitations. First, the present study was a retrospective study
conducted at a single center and the statistical capacity was
limited by the small sample size. Second, the follow-up was of
short duration. Third, hematological indicators fluctuate under the
influence of the internal environment, which means the results of a
single blood test may not adequately reflect the condition of an
individual.
In conclusion, the preoperative F-NLR score was a
strong and independent unfavorable index of survival in patients
with resectable BC, which may be of great significance for the
identification of high-risk patients and provision of accurate
treatment. Furthermore, as a marker, the F-NLR score is simple and
inexpensive to analyze, suggesting that it is potentially
applicable to clinical practice. However, further research should
be conducted to confirm this.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by Fundamental Research
Funds for the Central Universities (grant no. 3332020001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC designed the present study, carried out the
experiments, collected and analyzed the data, and wrote the
manuscript. YZ, FM and YL assisted with data collection and
analysis, and also modified and revised the manuscript. QS
conceived the present study and was involved in drafting the
manuscript, coordinating the whole research process, and revising
and finalizing the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by Ethical
Committee/Institutional Review Board of Peking Union Medical
College Hospital. Due to the retrospective nature of the present
study, formal consent was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BC
|
breast cancer
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
F-NLR
|
combination of fibrinogen
concentration and neutrophil-to-lymphocyte ratio
|
|
AJCC
|
American Joint Committee on Cancer
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
ROC
|
receiver operating characteristic
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
AUC
|
area under the curve
|
|
HR
|
hazard ratio
|
|
NSCLC
|
non-small cell lung cancer
|
|
IL
|
interleukin
|
|
MDSCs
|
myeloid-derived suppressor cells
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goss PE, Strasser-Weippl K, Lee-Bychkovsky
BL, Fan L, Li J, Chavarri-Guerra Y, Liedke PER, Pramesh CS,
Badovinac-Crnjevic T, Sheikine Y, et al: Challenges to effective
cancer control in China, India, and Russia. Lancet Oncol.
15:489–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Varghese C and Shin HR: Strengthening
cancer control in China. Lancet Oncol. 15:484–485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Ji J, Wang JB, Niyazi M, Qiao YL and
Boffetta P: Attributable causes of breast cancer and ovarian cancer
in China: Reproductive factors, oral contraceptives and hormone
replacement therapy. Chin J Cancer Res. 24:9–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Virchow R: An address on the value of
pathological experiments. Br Med J. 2:198–203. 1881. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shrotriya S, Walsh D, Bennani-Baiti N,
Thomas S and Lorton C: C-reactive protein is an important biomarker
for prognosis tumor recurrence and treatment response in adult
solid tumors: A systematic review. PLoS One. 10:e01430802015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sengupta S, Lohse CM, Cheville JC,
Leibovich BC, Thompson RH, Webster WS, Frank I, Zincke H, Blute ML
and Kwon ED: The preoperative erythrocyte sedimentation rate is an
independent prognostic factor in renal cell carcinoma. Cancer.
106:304–312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uribe-Querol E and Rosales C: Neutrophils
in cancer: Two sides of the same coin. J Immunol Res.
2015:9836982015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Palumbo JS, Kombrinck KW, Drew AF, Grimes
TS, Kiser JH, Degen JL and Bugge TH: Fibrinogen is an important
determinant of the metastatic potential of circulating tumor cells.
Blood. 96:3302–3309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McNamara MG, Templeton AJ, Maganti M,
Walter T, Horgan AM, McKeever L, Min T, Amir E and Knox JJ:
Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract
cancer. Eur J Cancer. 50:1581–1589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McMillan DC, Crozier JE, Canna K, Angerson
WJ and McArdle CS: Evaluation of an inflammation-based prognostic
score (GPS) in patients undergoing resection for colon and rectal
cancer. Int J Colorectal Dis. 22:881–886. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mano Y, Shirabe K, Yamashita Y, Harimoto
N, Tsujita E, Takeishi K, Aishima S, Ikegami T, Yoshizumi T,
Yamanaka T and Maehara Y: Preoperative neutrophil-to-lymphocyte
ratio is a predictor of survival after hepatectomy for
hepatocellular carcinoma: A retrospective analysis. Ann Surg.
258:301–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yodying H, Matsuda A, Miyashita M,
Matsumoto S, Sakurazawa N, Yamada M and Uchida E: Prognostic
significance of neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio in oncologic outcomes of esophageal
cancer: A systematic review and meta-analysis. Ann Surg Oncol.
23:646–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wariss BR, de Souza Abrahão K, de Aguiar
SS, Bergmann A and Thuler LCS: Effectiveness of four inflammatory
markers in predicting prognosis in 2374 women with breast cancer.
Maturitas. 101:51–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Qu JK, Zhang J, Yan Y, Zhao XX,
Wang JZ, Qu HY, Liu L, Wang JS and Duan XY: Prognostic role of
pretreatment neutrophil to lymphocyte ratio in breast cancer
patients: A meta-analysis. Medicine (Baltimore). 96:e81012017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Satoh T, Matsumoto K, Tanaka YO, Akiyama
A, Nakao S, Sakurai M, Ochi H, Onuki M, Minaguchi T, Sakurai H and
Yoshikawa H: Incidence of venous thromboembolism before treatment
in cervical cancer and the impact of management on venous
thromboembolism after commencement of treatment. Thromb Res.
131:e127–e132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Palumbo JS, Talmage KE, Massari JV, La
Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M and Degen JL:
Platelets and fibrin(ogen) increase metastatic potential by
impeding natural killer cell-mediated elimination of tumor cells.
Blood. 105:178–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao K, Deng H, Qin Y, Liao W and Liang W:
Prognostic significance of pretreatment plasma fibrinogen and
platelet levels in patients with early-stage cervical cancer.
Gynecol Obstet Invest. 79:25–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jones JM, McGonigle NC, McAnespie M, Cran
GW and Graham AN: Plasma fibrinogen and serum C-reactive protein
are associated with non-small cell lung cancer. Lung Cancer.
53:97–101. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mei Y, Zhao S, Lu X, Liu H, Li X and Ma R:
Clinical and prognostic significance of preoperative plasma
fibrinogen levels in patients with operable breast cancer. PLoS
One. 11:e01462332016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kijima T, Arigami T, Uchikado Y, Uenosono
Y, Kita Y, Owaki T, Mori S, Kurahara H, Kijima Y, Okumura H, et al:
Combined fibrinogen and neutrophil-lymphocyte ratio as a prognostic
marker of advanced esophageal squamous cell carcinoma. Cancer Sci.
108:193–199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang W, Wang S, Zhang H, Zhang B and Wang
C: Prognostic significance of combined fibrinogen concentration and
neutrophil-to-lymphocyte ratio in patients with resectable
non-small cell lung cancer. Cancer Biol Med. 15:88–96. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu X, Liu Z, Lin E, Chen Y, Sun X and
Zhou Z: A cumulative score based on preoperative fibrinogen and the
neutrophil-lymphocyte ratio to predict outcomes in resectable
gastric cancer. Cancer Manag Res. 10:3007–3014. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arigami T, Okumura H, Matsumoto M,
Uchikado Y, Uenosono Y, Kita Y, Owaki T, Mori S, Kurahara H, Kijima
Y, et al: Analysis of the fibrinogen and neutrophil-lymphocyte
ratio in esophageal squamous cell carcinoma: A promising blood
marker of tumor progression and prognosis. Medicine (Baltimore).
94:e17022015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer;
New York, NY: 2010
|
|
31
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JMS, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American society of
clinical oncology/college of American pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
National Comprehensive Cancer Network, .
The NCCN Breast Cancer Clinical Practice Guidelines in Oncology
(version 1.2012) (EB/OL). https://www.nccn.org/professionals/physician_gls/default.aspx
|
|
33
|
Wang H, Zhao J, Zhang M, Han L, Wang M and
Xingde L: The combination of plasma fibrinogen and neutrophil
lymphocyte ratio (F-NLR) is a predictive factor in patients with
resectable non small cell lung cancer. J Cell Physiol.
233:4216–4224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arigami T, Uenosono Y, Matsushita D,
Yanagita S, Uchikado Y, Kita Y, Mori S, Kijima Y, Okumura H,
Maemura K, et al: Combined fibrinogen concentration and
neutrophil-lymphocyte ratio as a prognostic marker of gastric
cancer. Oncol Lett. 11:1537–1544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Diakos CI, Charles KA, McMillan DC and
Clarke SJ: Cancer-related inflammation and treatment effectiveness.
Lancet Oncol. 15:e493–e503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Coussens LM, Zitvogel L and Palucka AK:
Neutralizing tumor-promoting chronic inflammation: A magic bullet?
Science. 339:286–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin EY, Nguyen AV, Russell RG and Pollard
JW: Colony-stimulating factor 1 promotes progression of mammary
tumors to malignancy. J Exp Med. 193:727–740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sparmann A and Bar-Sagi D: Ras-induced
interleukin-8 expression plays a critical role in tumor growth and
angiogenesis. Cancer Cell. 6:447–458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rokavec M, Wu W and Luo JL: IL6-mediated
suppression of miR-200c directs constitutive activation of
inflammatory signaling circuit driving transformation and
tumorigenesis. Mol Cell. 45:777–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gabrilovich DI, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ohki S, Shibata M, Gonda K, Machida T,
Shimura T, Nakamura I, Ohtake T, Koyama Y, Suzuki S, Ohto H and
Takenoshita S: Circulating myeloid-derived suppressor cells are
increased and correlate to immune suppression, inflammation and
hypoproteinemia in patients with cancer. Oncol Rep. 28:453–458.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Diaz-Montero CM, Salem ML, Nishimura MI,
Garrett-Mayer E, Cole DJ and Montero AJ: Increased circulating
myeloid-derived suppressor cells correlate with clinical cancer
stage, metastatic tumor burden, and doxorubicin-cyclophosphamide
chemotherapy. Cancer Immunol Immunother. 58:49–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Grivennikov SI, Wang K, Mucida D, Stewart
CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung
KE, et al: Adenoma-linked barrier defects and microbial products
drive IL-23/IL-17-mediated tumour growth. Nature. 491:254–258.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Protopsaltis NJ, Liang W, Nudleman E and
Ferrara N: Interleukin-22 promotes tumor angiogenesis.
Angiogenesis. 22:311–323. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH
and Park MH: Interleukin 32, inflammation and cancer. Pharmacol
Ther. 174:127–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guthrie GJ, Charles KA, Roxburgh CS,
Horgan PG, McMillan DC and Clarke SJ: The systemic
inflammation-based neutrophil-lymphocyte ratio: Experience in
patients with cancer. Crit Rev Oncol Hematol. 88:218–230. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Goldenberg N, Kahn SR and Solymoss S:
Markers of coagulation and angiogenesis in cancer-associated venous
thromboembolism. J Clin Oncol. 21:4194–4199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Simpson-Haidaris PJ and Rybarczyk B:
Tumors and fibrinogen. The role of fibrinogen as an extracellular
matrix protein. Ann NY Acad Sci. 936:406–425. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Baumann H and Gauldie J: The acute phase
response. Immunol Today. 15:74–80. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sahni A, Simpson-Haidaris PJ, Sahni SK,
Vaday GG and Francis CW: Fibrinogen synthesized by cancer cells
augments the proliferative effect of fibroblast growth factor-2
(FGF-2). J Thromb Haemost. 6:176–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rybarczyk BJ and Simpson-Haidaris PJ:
Fibrinogen assembly, secretion, and deposition into extracellular
matrix by MCF-7 human breast carcinoma cells. Cancer Res.
60:2033–2039. 2000.PubMed/NCBI
|
|
52
|
Dahl M: Networking with fibrinogen: A
prerequisite for fibroblast growth factor-2 (FGF-2)-stimulated
tumor growth? J Thromb Haemost. 6:174–175. 2008. View Article : Google Scholar : PubMed/NCBI
|