Introduction
Breast cancer (BC) is the most common cancer and the
major cause of cancer-associated mortalities among women worldwide
(1). A total of 2,088,849 new cases
and 626.679 mortalities were reported globally for BC in 2018
(2). It was also reported that the
5-year survival rate of patients with BC during 2007–2013 was 90%
in the USA. The standard treatment modalities for BC include
surgery, radiation, chemotherapy, targeted therapy and hormone
therapy (3–5). Ductal carcinoma in situ (DCIS)
is the earliest stage of BC and refers to a heterogeneous group of
precursor lesions (6). Approximately
20–25% of patients with BC are diagnosed with DCIS (7). While not all DCIS cases progress to
invasive BC, DCIS is usually excised as its potential for invasion
remains difficult to predict (8).
Previous studies have focused on analyzing the
molecular differences between DCIS and invasive BC and the
potential progression mechanisms (9–12). The
upregulation of erb-b2 receptor tyrosine kinase 2 (ERBB2) is
considered to be an early step in the progression of DCIS (13,14).
Shah et al (13) revealed
that the downregulation of Rap1Gap, a GTPase-activating protein,
promotes the progression of DCIS to invasive ductal carcinoma by
activating the extracellular signal-regulated
kinase/mitogen-activated protein kinase signaling pathway. In the
phosphoinositide 3-kinase signaling pathway alterations have also
been reported to be associated with the progression of DCIS
(14). Additionally, the
upregulation of cyclin D1, MYC proto-oncogene bHLH transcription
factor and ERBB2, have previously been observed in DCIS (15). The upregulation of matrix
metalloproteinase (MMPs), including MMP2, and downregulation of
cadherin 1 are frequently observed in the progression from DCIS to
invasive BC via the epithelial-mesenchymal transition (9,16).
Previous studies have revealed that certain biomarkers, including
the ki67 proliferation marker, the tumor protein p53 tumor
suppressor gene and the estrogen receptor, may serve as prognostic
predictors in DCIS (17,18). However, further investigation of the
molecular features and differences between DCIS and normal breast
tissues is required. Therefore, the aim of the present study was to
identify key genes involved in DCIS and to explore their prognostic
values by integrating bioinformatics tools.
Materials and methods
Gene expression level analysis of DCIS
and normal breast tissue samples
The gene expression profiles of 46 DCIS and three
normal breast tissues in the GSE59248 dataset (19) were downloaded from the Gene
Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/). This dataset was
analyzed using the Agilent-028004 SurePrint G3 Human GE 8×60K
Microarray platform (Agilent Technologies, Inc.). The online tool
GEO2R (www.ncbi.nlm.nih.gov/geo/geo2r) was subsequently used
to identify the differentially expressed genes (DEGs) between DCIS
and normal breast tissues using the Benjamini and Hochberg false
discovery rate (20). The cut-off
criteria for the selection of DEGs were an adjusted P<0.05 and a
|log2 fold-change|>2.
Functional enrichment analyses of the
DEGs
Gene Ontology (GO) (http://geneontology.org) and Kyoto Encyclopedia of
Genes and Genomes (KEGG) analyses were performed to explore the
biological functions of the DEGs. GO functional analysis, which
includes biological processes, cellular components and molecular
functions, was performed using the Protein Analysis Through
Evolutionary Relationships (PANTHER) classification system
(16). KEGG pathways enrichment
analysis was performed using the Database for Annotation,
Visualization and Integrated Discovery (DAVID; version 6.8)
(https://david.ncifcrf.gov) (17). The criteria for significance were
P<0.05 and the number of enriched DEGs in pathways ≥5.
Construction of the protein-protein
interaction (PPI) network
To further explore the associations between the
identified DEGs, a PPI network was constructed using the Search
Tool for the Retrieval of Interacting Genes/Proteins (STRING)
database (https://string-db.org) (18) and visualized using Cytoscape software
(version 3.6.1) (21). In the PPI
network, the nodes and edges represent proteins and their
interactions, respectively. The genes in the PPI network with the
highest connectivity were considered hub genes. The potential hub
genes were screened from the entire PPI network using the cytoHubba
plug-in and the maximal clique centrality algorithm (22,23).
Survival analysis of hub genes with
the log-rank test
To evaluate the prognostic values of the identified
hub genes in patients with DCIS, an overall survival (OS) analysis
was carried out using the Kaplan-Meier plotter (https://kmplot.com/analysis/), which is a web-based
tool to assess the effect of 54,675 genes on survival in 21 cancer
types (24).
Statistical analysis
SPSS software (version 22.0; IBM Corp.) was used to
perform the statistical analysis. The data were presented with mean
± SD. The associations between the clinicopathological parameters
and hub gene expression were analyzed using the χ2 test.
When the sample size was <40, the Fisher's exact test was
applied. P<0.05 was considered to indicate a statistically
significant difference. The α level was 0.05.
Results
DEGs between DCIS and normal tissue
samples
A total of 316 DEGs, including 32 upregulated and
284 downregulated genes, were identified in the GSE59248 dataset.
The GSE59248 dataset included the gene expression profiles of 46
DCIS and 3 normal breast tissue samples.
Functional enrichment analyses of the
DEGs
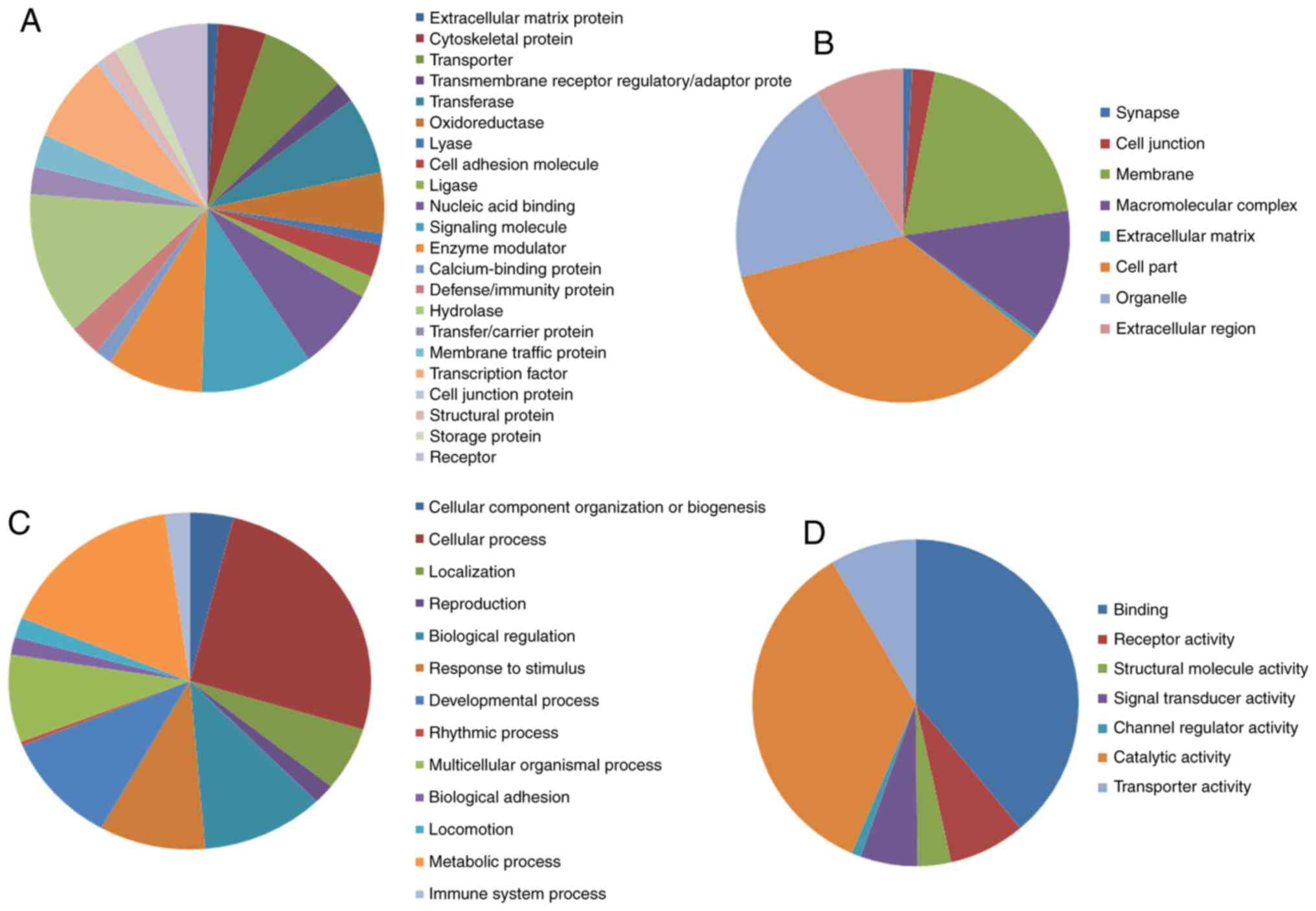
The analytical results of the PANTHER classification
system revealed that the identified DEGs could be classified into
22 protein categories, including signaling molecules, transporters,
hydrolases, enzyme modulators and transcription factors (Fig. 1A; Table
I). The GO analysis revealed that these DEGs were significantly
involved in cellular components such as ‘cell part’, ‘organelle’,
‘membranes’ and ‘macromolecular complex’ (Fig. 1B; Table
I). In terms of biological processes, the DEGs are mainly
associated with ‘metabolic process’, ‘cellular process’,
‘biological regulation’, ‘developmental process’ and ‘response to
stimulus’ (Fig. 1C; Table I). Regarding molecular functions, the
DEGs were mainly associated with ‘catalytic activity’, binding,
transporter activity and signal transducer activity (Fig. 1D; Table
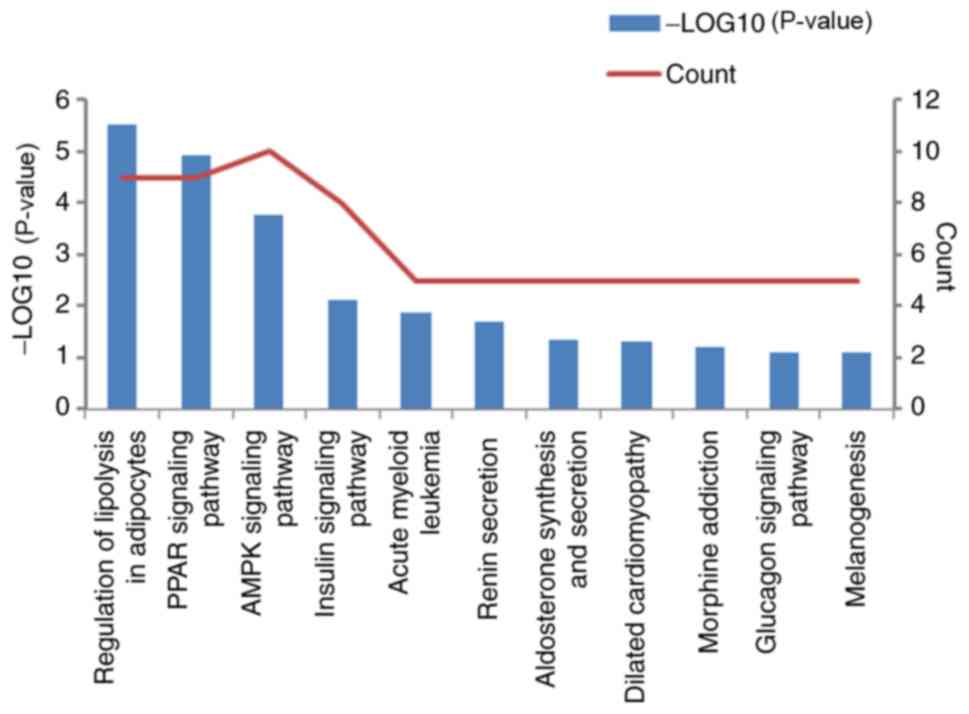
I). The analysis of KEGG pathways using DAVID indicated that
the DEGs are mainly enriched in pathways such as ‘PPAR signaling
pathway’, ‘AMPK signaling pathway’, ‘regulation of lipolysis in
adipocytes’ and ‘glucagon signaling pathway’ (Fig. 2).
 | Table I.Functional enrichment analyses of the
differentially expressed genes in ductal carcinoma in situ
using the Protein Analysis Through Evolutionary Relationships
classification system. The top eight items with its counting
percentage in each category were present. |
Table I.
Functional enrichment analyses of the
differentially expressed genes in ductal carcinoma in situ
using the Protein Analysis Through Evolutionary Relationships
classification system. The top eight items with its counting
percentage in each category were present.
| A, Protein
categories |
|---|
|
|---|
| Term | Percentage |
|---|
| Hydrolase | 12.60 |
| Signaling
molecule | 10.20 |
| Enzyme
modulator | 8.70 |
| Transcription
factor | 7.80 |
| Transporter | 7.80 |
| Nucleic acid
binding | 7.30 |
| Transferase | 6.80 |
| Receptor | 6.80 |
|
| B, Cellular
component |
|
| Term |
Percentage |
|
| Cell part | 35.50 |
| Organelle | 20.30 |
| Membrane | 19.50 |
| Macromolecular
complex | 12.60 |
| Extracellular
region | 8.70 |
| Cell junction | 2.20 |
| Synapse | 0.90 |
| Extracellular
matrix | 0.40 |
|
| C, Biological
process |
|
| Term |
Percentage |
|
| Cellular
process | 25.80 |
| Metabolic
process | 16.70 |
| Biological
regulation | 11.00 |
| Developmental
process | 10.60 |
| Response to
stimulus | 9.50 |
| Multicellular
organismal process | 8.40 |
| Localization | 6.10 |
| Cellular component
organization or biogenesis | 3.90 |
|
| D, Molecular
function |
|
| Term |
Percentage |
|
| Binding | 38.90 |
| Catalytic
activity | 35.10 |
| Transporter
activity | 8.50 |
| Receptor
activity | 7.60 |
| Signal transducer
activity | 5.70 |
| Structural molecule
activity | 3.30 |
| Channel regulator
activity | 0.90 |
PPI network and hub genes
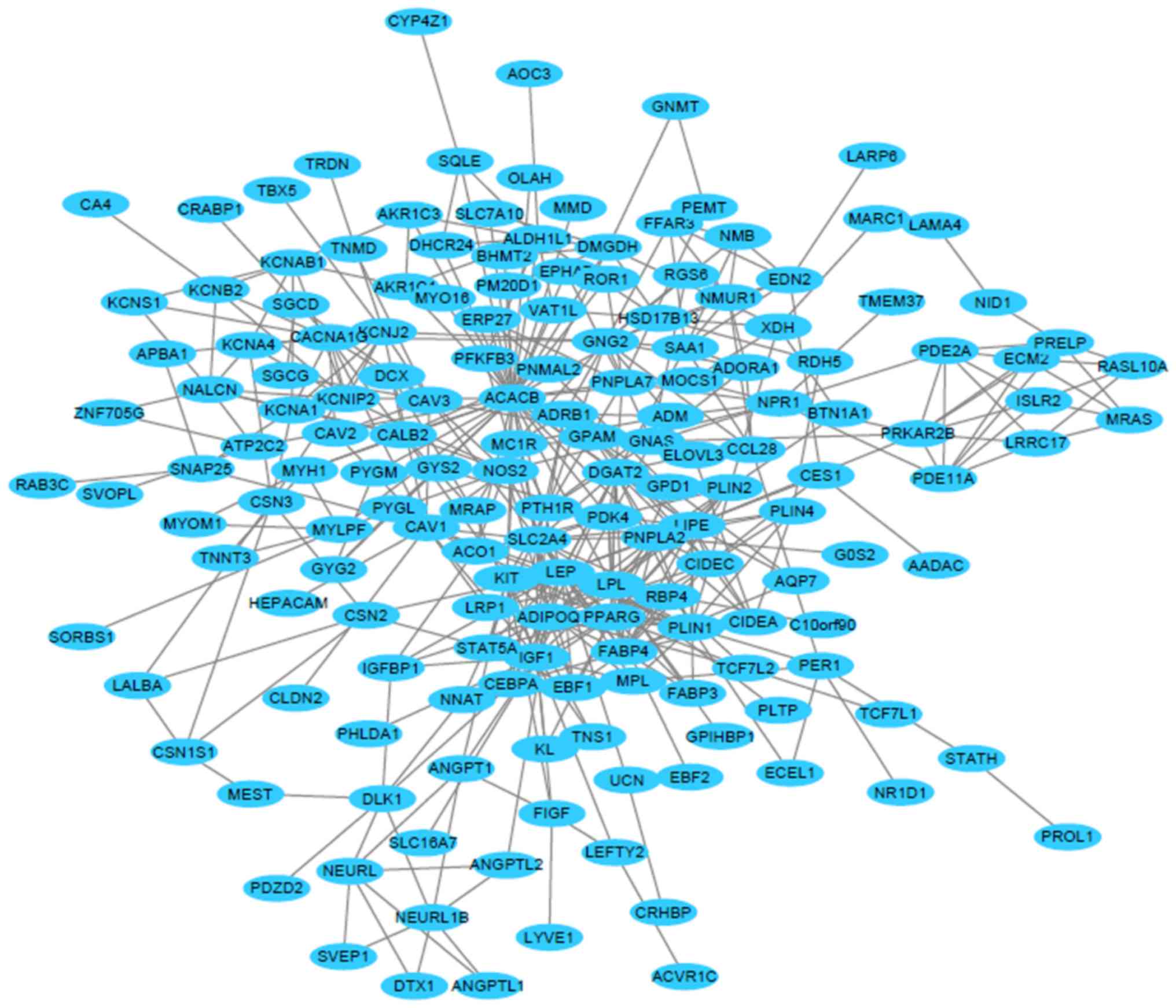
In the present study, the STRING database was used
to construct a PPI network to visualize the protein-protein
interactions between the DEGs (Fig.
3). The network consisted of a total of 315 nodes and 474
edges. The local clustering coefficient of the PPI network was
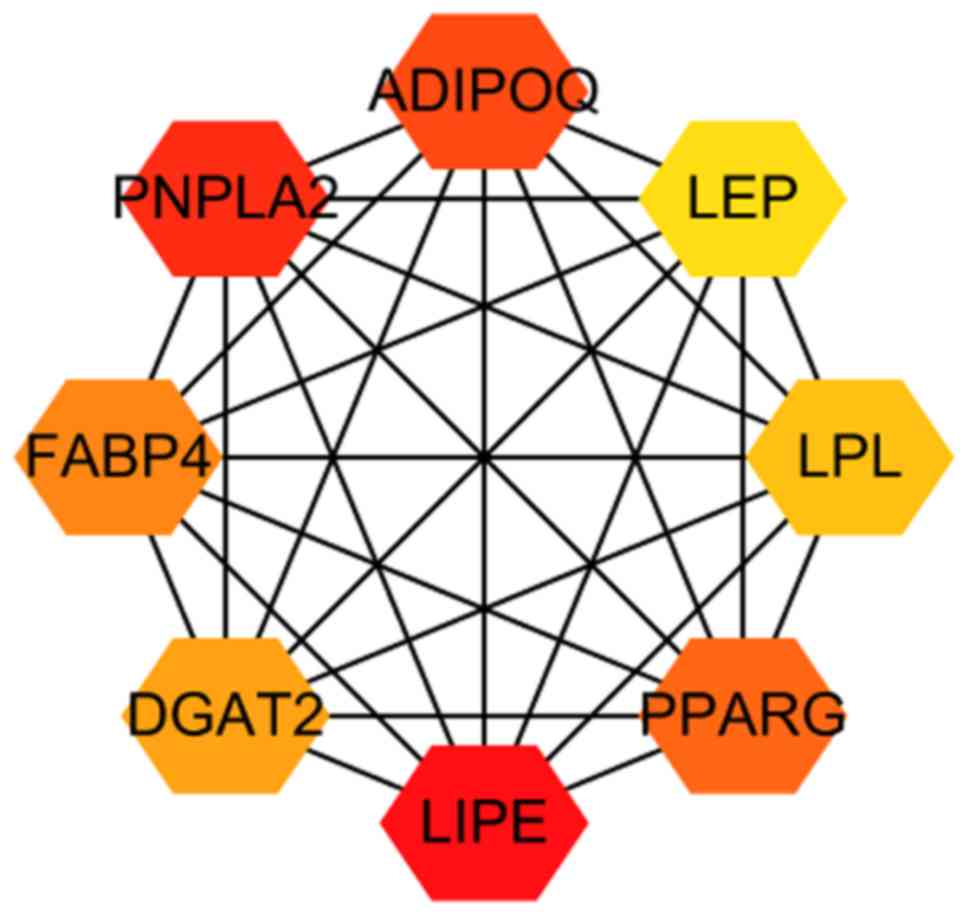
0.374 and the average node degree was 3.01. The top eight hub genes
were identified from the PPI network using the cytoHubba plugin and
the maximal clique centrality algorithm. The hub genes were as
follows: Lipase E hormone sensitive type (LIPE), patatin-like
phospholipase domain-containing 2 (PNPLA2), adiponectin (ADIPOQ),
peroxisome proliferator activated receptor γ (PPARG), fatty
acid-binding protein 4 (FABP4), diacylglycerol O-acyltransferase 2
(DGAT2), lipoprotein lipase (LPL) and leptin (LEP; Fig. 4). The above mentioned hub genes were
downregulated in DCIS compared with normal breast tissue (Fig. 5).
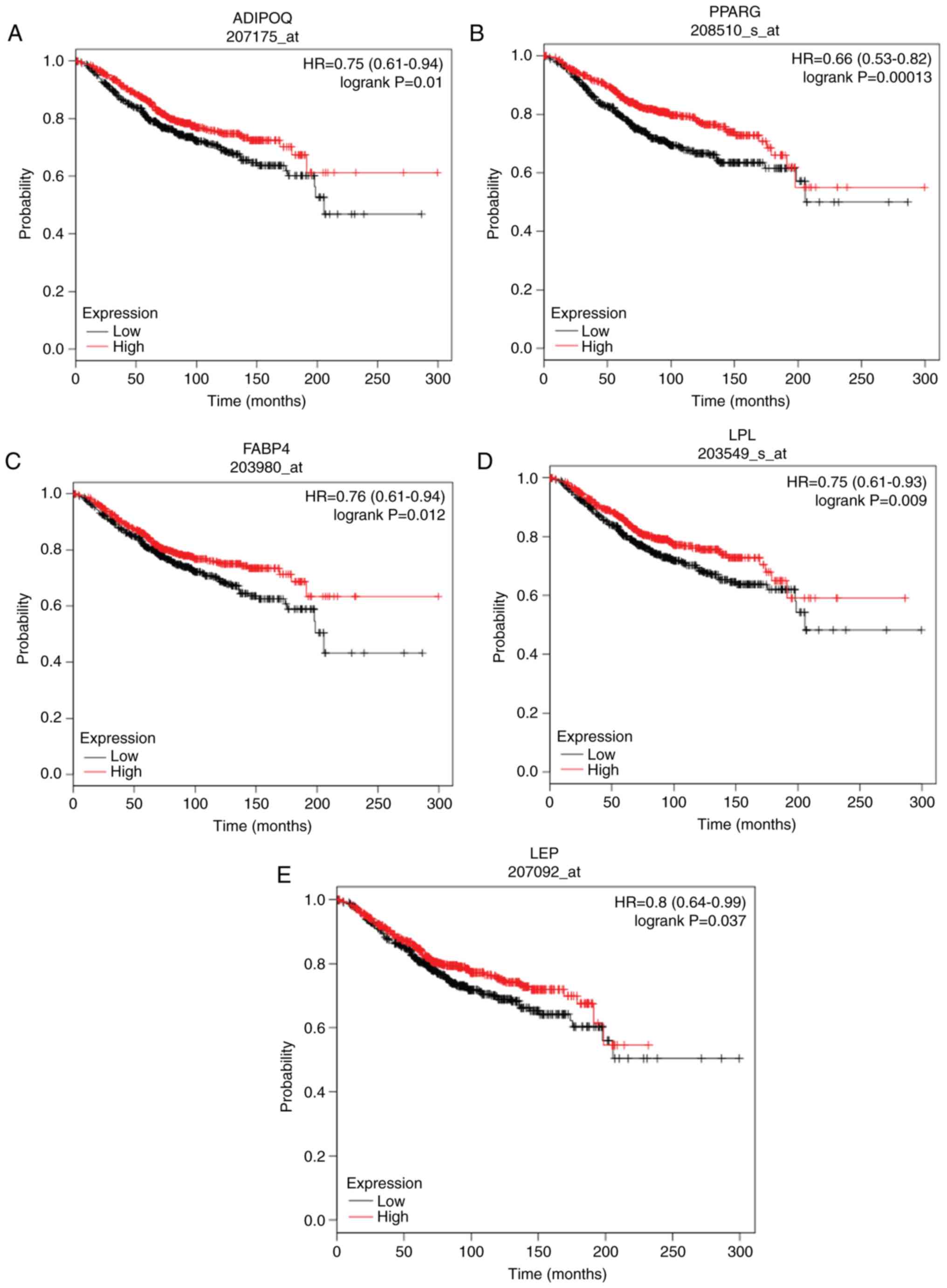 | Figure 5.Overall survival analysis of (A)
ADIPOQ, (B) PPARG, (C) FABP4, (D) LPL and (E) LEP in ductal
carcinoma in situ was performed using the Kaplan-Meier
plotter. Red and black represent high and low expression,
respectively. ADIPOQ, adiponectin; PPARG, peroxisome proliferator
activated receptor γ; FABP4, fatty acid-binding protein 4; LPL,
lipoprotein lipase; LEP, leptin; HR, hazard ratio. |
Survival analysis results of the hub
genes
The prognostic values of the identified hub genes in
patients with DCIS were investigated using the Kaplan-Meier
plotter. Only the low expression of ADIPOQ [hazard ratio (HR)=0.75;
95% confidence interval (CI), 0.61–0.94; log-rank P=0.01], PPARG
(HR=0.66; 95% CI, 0.53–0.82; log-rank P=0.0013), FABP4 (HR=0.76;
95% CI, 0.61–0.94; log-rank P=0.012), LPL (HR=0.75; 95% CI,
0.61–0.93; log-rank P=0.009) and LEP (HR=0.8; 95% CI, 0.64–0.99;
log-rank P=0.037) were significantly associated with poor OS in
patients with DCIS (Fig. 5). The
associations between the hub gene expression levels and
clinicopathological parameters of patients with DCIS are presented
in Table II. The expression levels
of LIPE, PNPLA2 and DGAT2 did not significantly influence the
prognosis of patients with DCIS (Fig.
S1).
 | Table II.The correlation analysis of the hub
genes' expression levels and the clinicopathological parameters of
Ductal Carcinoma in situ of the Breast (data from the GSE59248
dataset). |
Table II.
The correlation analysis of the hub
genes' expression levels and the clinicopathological parameters of
Ductal Carcinoma in situ of the Breast (data from the GSE59248
dataset).
|
|
| ADIPOQ
expression |
| LEP expression |
| LPL expression |
| FABP4
expression |
| PPARG
expression |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Clinical
parameter | No. of cases
(n) | High | Low | P-value | High | Low | P-value | High | Low | P-value | High | Low | P-value | High | Low | P-value |
|---|
| Age |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
≤55 | 19 | 12 | 7 | 0.314 | 14 | 5 | 0.049 | 12 | 7 | 1.00 | 9 | 10 | 0.220 | 12 | 7 | 0.314 |
|
>55 | 27 | 13 | 14 |
| 12 | 15 |
| 18 | 9 |
| 8 | 19 |
| 13 | 14 |
|
| Tumor size
(cm) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ≤3 | 22 | 14 | 8 | 0.678 | 14 | 8 | 0.242 | 15 | 7 | 0.417 | 12 | 10 | 0.419 | 12 | 10 | 0.419 |
|
>3 | 8 | 4 | 4 |
| 3 | 5 |
| 4 | 4 |
| 6 | 2 |
| 6 | 2 |
|
| ER |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Positive | 15 | 6 | 9 | 0.105 | 6 | 9 | 0.400 | 7 | 8 | 0.210 | 8 | 7 | 1.00 | 9 | 6 | 1.00 |
|
Negative | 9 | 7 | 2 |
| 6 | 3 |
| 7 | 2 |
| 5 | 4 |
| 5 | 4 |
|
| PR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Positive | 12 | 4 | 8 | 0.100 | 4 | 8 | 0.220 | 3 | 9 | 0.003 | 5 | 7 | 0.414 | 8 | 4 | 0.680 |
|
Negative | 12 | 9 | 3 |
| 8 | 4 |
| 11 | 1 |
| 8 | 4 |
| 6 | 6 |
|
| HER2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Positive | 9 | 6 | 3 | 0.387 | 5 | 4 | 1.00 | 7 | 2 | 0.080 | 6 | 3 | 0.660 | 6 | 3 | 0.660 |
|
Negative | 12 | 5 | 7 |
| 6 | 6 |
| 4 | 8 |
| 6 | 6 |
| 6 | 6 |
|
Discussion
DCIS is a heterogeneous disease and represents the
pre-invasive stage of BC (25).
Although the majority of patients with DCIS undergo breast excision
to remove the lesion, certain patients with DCIS may still develop
invasive BC (26). Therefore, it is
important to explore the molecular features of DCIS development and
to identify potential prognostic biomarkers.
The present study analyzed the gene expression data
of 46 DCIS and three normal breast tissues. A total of 316 DEGs
were identified, including 32 upregulated and 284 downregulated
genes. The identified DEGs could be classified into 22 protein
categories. Moreover, the results of the functional enrichment
analysis indicated that the DEGs were mostly associated with cell
parts, organelles, metabolic processes, cellular processes,
biological regulation, transporter activity and signal transducer
activity. A PPI network was constructed and eight hub genes were
selected for further investigation. The downregulation of ADIPOQ,
PPARG, FABP4, LPL and LEP was significantly associated with poor OS
in patients with DCIS.
ADIPOQ encodes an adipocytokine, adiponectin, which
is essential for metabolic and hormonal processes (27). A low level of plasma adiponectin has
been significantly associated with several types of cancer,
including colorectal and prostate cancer (28,29). It
was also reported that adiponectin may decrease cancer cell growth
by promoting the activity of autophagosomes and decreasing the
sequestosome 1/GAP-associated tyrosine phosphoprotein p62 signaling
pathway in BC (27). High ADIPOQ
expression may serve a protective role in BC (30), consistent with the results obtained
in the present study.
PPARG serves an important role in regulating
adipocyte differentiation by forming heterodimers with retinoid X
receptors (31). Several studies
have shown that PPARG serves an anti-inflammatory role and may
reduce the risk of breast cancer (32–34). The
expression level of PPARG was found to influence the susceptibility
to hepatocellular carcinoma (35)
and different types of cancer (36).
However, the association between PPARG and the risk of BC has not
been established (37,38). FABP4 is involved in lipoprotein
metabolism (39). A previous study
revealed that FABP4 increased cell apoptosis and decreased
proliferation (40). FABP4
overexpression has been reported to decrease hepatocellular
carcinoma cell growth through the snail family transcriptional
repressor 1/p-STAT3 signaling pathway in vitro and was
associated with tumor size and overall survival in hepatocellular
carcinoma (41).
LPL plays a critical role in lipid metabolism
(42). Studies have shown that LPL
may regulate metabolic pathways to provide additional energy for
cancer cells (43,44). Circulating LEP is usually secreted by
white adipose tissue and is involved in the regulation of
angiogenesis, energy balance, and immune and inflammatory responses
(45). Previous studies revealed
that LEP altered the cellular response to estrogens in BC and
served a role in mammary carcinogenesis (46,47).
Furthermore, LEP may promote cell cycle progression by upregulating
the levels of cyclin dependent kinase 2 and cyclin D1 (48). However, in the present study, LEP was
downregulated in DCIS tissues compared with normal tissues.
The remaining hub genes in the present study,
including LIPE, PNPLA2 and DGAT2, were also expressed at low levels
in DCIS tissues compared with normal breast tissues. However, these
genes were not associated with the prognosis of patients with DCIS.
It is worth noting that the GSE59248 dataset used in the present
study contained the gene expression profiles of only three normal
breast tissues. Therefore, future studies investigating the gene
expression profiles from multiple datasets are warranted.
The present study investigated the differences
between DCIS and normal breast tissues by integrating comprehensive
bioinformatics analyses. The top eight hub genes, namely LIPE,
PNPLA2, ADIPOQ, PPARG, FABP4, DGAT2, LPL and LEP, were identified
and considered to serve critical roles in the initiation of BC,
while only five of the hub genes, ADIPOQ, PPARG, FABP4, LPL and
LEP, were further found to be potential biomarkers for predicting
the prognosis of patients with DCIS. However, further research is
required to validate the results obtained in the present study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data that support the findings of this study are
available from GEO database, DAVID, STRING and GEPIA database, as
is mentioned in the Material and methods.
Author's contributions
MW and ZHM analyzed the data and wrote the
manuscript. ZHM supervised the study. Both authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was not necessary in this study
because public datasets were analyzed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith RA, Andrews KS, Brooks D, Fedewa SA,
Manassaram-Baptiste D, Saslow D, Brawley OW and Wender RC: Cancer
screening in the United States, 2018: A review of current american
cancer society guidelines and current issues in cancer screening.
CA Cancer J Clin. 68:297–316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurian AW, Lichtensztajn DY, Keegan TH,
Nelson DO, Clarke CA and Gomez SL: Use of and mortality after
bilateral mastectomy compared with other surgical treatments for
breast cancer in California, 1998–2011. JAMA. 312:902–914. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Veldt AA and Smit EF: Bevacizumab
in neoadjuvant treatment for breast cancer. N Engl J Med.
366:1637–1640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leal NF, Carrara HH, Vieira KF and
Ferreira CH: Physiotherapy treatments for breast cancer-related
lymphedema: A literature review. Rev Lat Am Enfermagem. 17:730–736.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeong J, Thike AA, Tan PH and Iqbal J:
Identifying progression predictors of breast ductal carcinoma in
situ. J Clin Pathol. 70:102–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ernster VL, Ballard-Barbash R, Barlow WE,
Zheng Y, Weaver DL, Cutter G, Yankaskas BC, Rosenberg R, Carney PA,
Kerlikowske K, et al: Detection of ductal carcinoma in situ in
women undergoing screening mammography. J Natl Cancer Inst.
94:1546–1554. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanders ME, Schuyler PA, Dupont WD and
Page DL: The natural history of low-grade ductal carcinoma in situ
of the breast in women treated by biopsy only revealed over 30
years of long-term follow-up. Cancer. 103:2481–2484. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abba MC, Drake JA, Hawkins KA, Hu Y, Sun
H, Notcovich C, Gaddis S, Sahin A, Baggerly K and Aldaz CM:
Transcriptomic changes in human breast cancer progression as
determined by serial analysis of gene expression. Breast Cancer
Res. 6:R499–R513. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Castro NP, Osório CA, Torres C, Bastos EP,
Mourão-Neto M, Soares FA, Brentani HP and Carraro DM: Evidence that
molecular changes in cells occur before morphological alterations
during the progression of breast ductal carcinoma. Breast Cancer
Res. 10:R872008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schuetz CS, Michael B, Clare SE, Nieselt
K, Sotlar K, Walter M, Fehm T, Solomayer E, Riess O, Wallwiener D,
et al: Progression-specific genes identified by expression
profiling of matched ductal carcinomas in situ and invasive breast
tumors, combining laser capture microdissection and oligonucleotide
microarray analysis. Cancer Res. 66:5278–5286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi Y, Lee HJ, Jang MH, Gwak JM, Lee KS,
Kim EJ, Kim HJ, Lee HE and Park SY: Epithelial-mesenchymal
transition increases during the progression of in situ to invasive
basal-like breast cancer. Hum Pathol. 44:2581–2589. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shah S, Brock EJ, Jackson RM, Ji K,
Boerner JL, Sloane BF and Mattingly RR: Downregulation of Rap1Gap:
A switch from DCIS to invasive breast carcinoma via ERK/MAPK
activation. Neoplasia. 20:951–963. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakr RA, Weigelt B, Chandarlapaty S,
Andrade VP, Guerini-Rocco E, Giri D, Ng CK, Cowell CF, Rosen N,
Reis-Filho JS and King TA: PI3K pathway activation in high-grade
ductal carcinoma in situ-implications for progression to invasive
breast carcinoma. Clin Cancer Res. 20:2326–2337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hannemann J, Velds A, Halfwerk JB, Kreike
B, Peterse JL and van de Vijver MJ: Classification of ductal
carcinoma in situ by gene expression profiling. Breast Cancer Res.
8:R612006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mi H, Huang X, Muruganujan A, Tang H,
Mills C, Kang D and Thomas PD: PANTHER version 11: Expanded
annotation data from gene ontology and reactome pathways, and data
analysis tool enhancements. Nucleic Acids Res. 45:D183–D189. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lesurf R, Aure MR, Mørk HH, Vitelli V;
Oslo Breast Cancer Research Consortium (OSBREAC), ; Lundgren S,
Børresen-Dale AL, Kristensen V, Wärnberg F, Hallett M and Sørlie T:
Molecular features of subtype-specific progression from ductal
carcinoma in situ to invasive breast cancer. Cell Rep.
16:1166–1179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Green GH and Diggle PJ: On the operational
characteristics of the benjamini and hochberg false discovery rate
procedure. Stat Appl Genet Mol Biol. 6:Article272007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: CytoHubba: Identifying hub objects and sub-networks from
complex interactome. BMC Syst Biol. 8 (Suppl 4):S112014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meghanathan N: Correlation coefficient
analysis: Centrality vs. maximal clique size for complex real-world
network graphs. 2016. View Article : Google Scholar
|
|
24
|
Györffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan PH: Pathology of ductal carcinoma in
situ of the breast: A heterogeneous entity in need of greater
understanding. Ann The Acad Med Singap. 30:671–677. 2001.
|
|
26
|
Gorringe KL and Fox SB: Ductal carcinoma
in situ biology, biomarkers, and diagnosis. Front Oncol. 7:2482017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chung SJ, Nagaraju GP, Nagalingam A,
Muniraj N, Kuppusamy P, Walker A, Woo J, Győrffy B, Gabrielson E,
Saxena NK and Sharma D: ADIPOQ/adiponectin induces cytotoxic
autophagy in breast cancer cells through STK11/LKB1-mediated
activation of the AMPK-ULK1 axis. Autophagy. 13:1386–1403. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Divella R, Daniele A, Mazzocca A, Abbate
I, Casamassima P, Caliandro C, Ruggeri E, Naglieri E, Sabbà C and
De Luca R: ADIPOQ rs266729 G/C gene polymorphism and plasmatic
adipocytokines connect metabolic syndrome to colorectal cancer. J
Cancer. 8:1000–1008. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Canto P, Granados JB, Feria-Bernal G,
Coral-Vázquez RM, García-García E, Tejeda ME, Tapia A, Rojano-Mejía
D and Méndez JP: PPARGC1A and ADIPOQ polymorphisms are associated
with aggressive prostate cancer in Mexican-Mestizo men with
overweight or obesity. Cancer Biomark. 19:297–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Delort L, Jardé T, Dubois V, Vasson MP and
Caldefie-Chézet F: New insights into anticarcinogenic properties of
adiponectin: A potential therapeutic approach in breast cancer?
Vitam Horm. 90:397–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He W: PPARgamma2 polymorphism and human
health. PPAR Res. 2009:8495382009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Crusz SM and Balkwill FR: Inflammation and
cancer: Advances and new agents. Nat Rev Clin Oncol. 12:584–596.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robbins GT and Nie D: PPAR gamma,
bioactive lipids, and cancer progression. Front Biosci (Landmark
Ed). 17:1816–1834. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khandekar MJ, Cohen P and Spiegelman BM:
Molecular mechanisms of cancer development in obesity. Nat Rev
Cancer. 11:886–895. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang S, Jiang J, Chen Z, Wang Y, Tang W,
Chen Y and Liu L: Relationship of PPARG, PPARGC1A, and PPARGC1B
polymorphisms with susceptibility to hepatocellular carcinoma in an
eastern Chinese Han population. Onco Targets Ther. 11:4651–4660.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu W, Li Y, Wang X, Chen B, Liu S, Wang Y,
Zhao W and Wu J: PPARgamma polymorphisms and cancer risk: A
meta-analysis involving 32,138 subjects. Oncol Rep. 24:579–585.
2010.PubMed/NCBI
|
|
37
|
Gallicchio L, McSorley MA, Newschaffer CJ,
Huang HY, Thuita LW, Hoffman SC and Helzlsouer KJ: Body mass,
polymorphisms in obesity-related genes, and the risk of developing
breast cancer among women with benign breast disease. Cancer Detect
Prev. 31:95–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu MH, Chu CH, Chou YC, Chou WY, Yang T,
Hsu GC, Yu CP, Yu JC and Sun CA: Joint effect of peroxisome
proliferator-activated receptor gamma genetic polymorphisms and
estrogen-related risk factors on breast cancer risk: Results from a
case-control study in Taiwan. Breast Cancer Res Treat. 127:777–784.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Furuhashi M: Fatty acid-binding protein 4
in cardiovascular and metabolic diseases. J Atheroscler Thromb.
26:216–232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li H, Xiao Y, Tang L, Zhong F, Huang G, Xu
JM, Xu AM, Dai RP and Zhou ZG: Adipocyte fatty acid-binding protein
promotes palmitate-induced mitochondrial dysfunction and apoptosis
in macrophages. Front Immunol. 9:812018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhong CQ, Zhang XP, Ma N, Zhang EB, Li JJ,
Jiang YB, Gao YZ, Yuan YM, Lan SQ, Xie D and Cheng SQ: FABP4
suppresses proliferation and invasion of hepatocellular carcinoma
cells and predicts a poor prognosis for hepatocellular carcinoma.
Cancer Med. 7:2629–2640. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Davies BS, Beigneux AP, Fong LG and Young
SG: New wrinkles in lipoprotein lipase biology. Curr Opin Lipidol.
23:35–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Prieto D and Oppezzo P: Lipoprotein lipase
expression in chronic lymphocytic leukemia: New insights into
leukemic progression. Molecules. 22:20832017. View Article : Google Scholar
|
|
44
|
Heintel D, Kienle D, Shehata M, Kröber A,
Kroemer E, Schwarzinger I, Mitteregger D, Le T, Gleiss A,
Mannhalter C, et al: High expression of lipoprotein lipase in poor
risk B-cell chronic lymphocytic leukemia. Leukemia. 19:1216–1223.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Garofalo C and Surmacz E: Leptin and
cancer. J Cell Physiol. 207:12–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Garofalo C, Sisci D and Surmacz E: Leptin
interferes with the effects of the antiestrogen ICI 182,780 in
MCF-7 breast cancer cells. Clin Cancer Res. 10:6466–6475. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu X, Juneja SC, Maihle NJ and Cleary MP:
Leptin-a growth factor in normal and malignant breast cells and for
normal mammary gland development. J Natl Cancer Inst. 94:1704–1711.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Okumura M, Yamamoto M, Sakuma H, Kojima T,
Maruyama T, Jamali M, Cooper DR and Yasuda K: Leptin and high
glucose stimulate cell proliferation in MCF-7 human breast cancer
cells: Reciprocal involvement of PKC-alpha and PPAR expression.
Biochim Biophys Acta. 1592:107–116. 2002. View Article : Google Scholar : PubMed/NCBI
|