Introduction
Lung cancer is divided into two types: Small-cell
lung cancer and NSCLC (1). As the
predominant form of lung cancer, NSCLC is the leading contributor
to lung cancer-associated deaths, accounting for ~83% of all lung
cancer cases (2). As NSCLC usually
presents as a locally advanced disease or with distant metastasis,
the majority of NSCLC cases are diagnosed at stages III or IV
(3). Therefore, there is an urgent
requirement to thoroughly investigate the underlying molecular
mechanisms of NSCLC to develop accurate and efficient therapeutic
methods for managing NSCLC.
Inosine 5′-monophosphate dehydrogenase (IMPDH) is a
rate-limiting enzyme, which catalyzes the nicotinamide adenine
dinucleotide+-dependent oxidation of inosine
monophosphate to xanthosine monophosphate during the de novo
biosynthesis of guanine nucleotides (4). In fact, IMPDH serves a crucial role in
DNA synthesis (5). Human IMPDH
exists in two ubiquitously expressed isoforms: IMPDH type I
(IMPDH1) and IMPDH type II (IMPDH2), which serve different roles
despite the 84% similarity in the amino acid sequence (6). For example, IMPDH1 is found
constitutively expressed in normal human leukocytes and
lymphocytes, whereas IMPDH2 is an inducible enzyme, which is
generally found upregulated in tumor tissues and proliferating
cells (7). Previous studies have
revealed that IMPDH2 was involved in multiple types of malignancy,
such as colorectal, prostate, kidney and bladder cancer (8–10).
However, to the best of our knowledge, it remains unknown whether
IMPDH2 may have an effect in NSCLC.
The aberrant expression levels of Wnt/B-catenin
signaling pathway components are involved in NSCLC invasion and
metastasis (11). In addition, the
pathway was identified to be critical for embryonic development and
promoting rapid cell division and migration (12). However, in one previous study, the
overactivation of the Wnt/β-catenin signaling pathway was reported
to promote the uncontrolled proliferation of cells, which promoted
tumorigenesis (13). Thus, the
present study aimed to investigate the relationship between IMPDH2
and NSCLC. The results indicated that IMPDH2 may promote NSCLC
progression by activating the Wnt/β-catenin signaling pathway,
therefore IMPDH2 may represent a novel therapeutic target for
patients with NSCLC.
Materials and methods
Patient studies
A total of 30 fresh primary NSCLC tissues and
matched adjacent noncancerous lung tissues were collected from
patients (including 18 males and 12 females 12 with age of 42±13
years) at The People's Hospital of Danyang (Danyang, China) between
February, 2018 and February, 2019. The tissues samples were
obtained from patients who had not received chemoradiotherapy. The
study protocol was approved by the Ethics Committee of The People's
Hospital of Danyang (Danyang, China). All patient diagnose of NSCLC
had been confirmed based on pathological assay, and none of the
patients received any relative cancer treatment. Informed consent
was written and provided by all patients.
Cell lines and cultures
The human lung adenocarcinoma epithelial cells A549,
the normal primary human bronchial epithelium cell line BEAS-2B was
purchased from the American Type Culture Collection. BEAS2B cells
were cultured in bronchial epithelial cell growth medium (BEGM;
Lonza Group, Ltd.). All cell lines were supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific.) and 1% penicillin/streptomycin,
and maintained at 37°C in an humidified atmosphere of 5%
CO2. Cells used for the experiments were harvested using
0.05% trypsin-EDTA upon reaching 80–90% confluence. All assays were
performed in three separate wells.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cultured cells and
tissues using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was reverse transcribed into cDNA
using a SuperScript Reverse Transcriptase kit (Thermo Fisher
Scientific, Inc.) at 37°C for 15 min. Two-step qPCR was
subsequently performed using SYBR Green (Takara Bio, Inc.) and a
CFX96™ Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.).
The thermocycling conditions using the following format: 40 cycles,
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec,
elongation at 95°C for 10 sec and final extension at 65 to 95°C for
5 sec. The following primer sequences were used for the qPCR:
IMPDH2 forward, 5′-GTTTCTGCGGTATCCCAATC-3′ and reverse,
5′-CGAGCAAGTCCAGCCTAT-3′; β-actin forward,
5′-TCATCACCATTGGCAATGAG-3′ and reverse, 5′-CACTGTGTTGGCGTACAGGT-3′.
β-actin was used as the internal control for normalization. Three
duplicated wells were set for each sample. The fold changes were
calculated by means of relative quantification (2−ΔΔCq
method) (14).
Western blotting
Total protein from cells were extracted using RIPA
buffer (Cell Signaling Technology, Inc.) and the protein
concentrations were determined using the BCA Protein Assay kit
(Cell Signaling Technology, Inc.). Proteins (1 mg) were loaded per
lane and onto a 10% gel and separated by SDS-PAGE. The separated
proteins were subsequently transferred onto a PVDF membrane and
blocked with 5% skimmed milk at room temperature for 1 h. The
membranes were incubated with the following primary antibodies:
Anti-IMPDH2 (1:1,000; cat. no. ab131158), anti-β-actin (1:1,000;
cat. no. ab8226), anti-E-cadherin (1:1,000; cat. no. ab1416),
anti-N-cadherin (1:1,000; cat. no. ab18203), anti-vimentin
(1:1,000; cat. no. ab92547), anti-p-GSK (1:1,000; cat. no.
ab75814), anti-GSK (1:1,000; cat. no. ab40870), anti-Wnt (1:1,000;
cat. no. ab219412) (all from Abcam), anti-β-catenin (1:1,000; cat.
no. C2206; Sigma Aldrich; Merck KGaA), anti-Snail (1:1,000; cat.
no. ab53519), anti-c-Myc (1:1,000; cat. no. ab32072) and anti-Twist
(1:1,000; ab50581) (all from Abcam) overnight at 4°C. Following the
primary antibody incubation, the membranes were incubated with
secondary antibodies (anti-IgG; 1:20,000; cat. no. ab205718; Abcam)
for 1 h at room temperature. Total protein was visualized using
enhanced chemiluminescence reagent (ECL; SW2030, Beijing Solarbio
Science & Technology Co., Ltd.) and the blots were analyzed
using ImageJ version 1.48u software (National Institutes of
Health).
Immunofluorescence assay
Cells (1×104) were fixed with 4%
paraformaldehyde at room temperature for 15 min and then washed 3
times with PBS. The slides were subsequently blocked for 1 h at
room temperature in 5% BSA (Beijing Solarbio Science &
Technology Co., Ltd.) diluted in 0.5% Triton X-100 in PBS (blocking
buffer). The slides were then incubated with the following primary
antibodies in blocking buffer overnight at 4°C: Anti-E-cadherin,
anti-N-cadherin and anti-vimentin (as aforementioned). Following
the primary antibody incubation, the slides were incubated with a
secondary DyLight488-conjugated anti-rabbit IgG antibody (1:2,000,
D9542, Sigma-Aldrich; Merck KGaA) at room temperature for 2 h. The
slides were subsequently stained with DAPI at room temperature for
5 min (Sigma-Aldrich; Merck KGaA) and visualized using an Olympus
FV1000 confocal laser-scanning microscope at 200× magnification
(Olympus Corporation).
Immunohistochemistry
Immunohistochemical analysis was performed using a
standard two-step method. Briefly, paraffin-embedded tissues (4 µm)
were deparaffinized in 0.5% xylene at 65°C for 2 h, rehydrated
alcohol and blocked with 3% H2O2 for 10 min
at 37°C. Deparaffinized sections were incubated in 3% hydrogen
peroxide for 15 min at room temperature and boiled in citrate
antigen retrieval solution (pH 6.5) for 20 min at 95°C for antigen
retrieval. Subsequently, the slides were incubated with an
anti-IMPDH2 primary antibody (1:1,000) at 4°C overnight and then
incubated with IgG secondary peroxidase-conjugated antibody
(1:20,000; cat. no. ab205718; Abcam) for 1 h at room temperature.
After washing three times in PBS, the slides were stained with
3,3-diaminobenzidine (DAB) at room temperature for 10 min and
counterstained with Mayers hematoxylin at room temperature for 3
min. Stained cells were visualized in three randomly selected
fields using a light microscope (magnification, ×200).
Production of lentivirus for IMPDH2
overexpression and knockdown
The full-length open reading sequence of the human
IMPDH2 gene (NM_000884.3) was PCR amplified from pcDNA3.1-IMPDH2
(pc-IMPDH2) and subcloned into the self-inactivating lentiviral
vector pHIV-EGFP (both Addgene, Inc.). pcDNA3.1 empty vector was
used as the control. Lentiviral preparations were produced by
transient transfection of 293T cells (1×104) using
pHIV-EGFP-IMPDH2, pRSV-Rev, pMDLg/pRRE and pMD2.G, which was
purchased from Shanghai GenePharma Co., Ltd. at final concentration
of 10 uM for 48 h.
The corresponding oligo of short hairpin RNA
(shRNA/sh) targeting IMPDH2 (sh-IMPDH2), β-catenin (sh-β-catenin)
or scrambled sh-negative control (NC) was subcloned into the pLKO.1
vector (Addgene, Inc.). NC was empty plasmid and used as the
negative control. Lentiviral preparations were generated as above,
except for that the transfection cocktail was replaced with 6 µg
pLKO.1, 4.5 µg psPAX2 and 1.5 µg pMD2.G (Addgene, Inc.). The
transfections were carried out for 48 h using Lipofectamine 3000
Reagent (Thermo Fisher Scientific, Inc.).
Cell Counting Kit-8 (CCK-8) assay
A549 cells (1×104) were cultured in
96-well plates with DMEM medium at 37°C. Upon the cell confluence
reaching 80%, the cells were cultured for 24, 48 and 72 h at 37°C,
and 10 µl CCK-8 solution (Gibco; Thermo Fisher Scientific, Inc.)
was added to each well and incubated at 37°C for 2 h according to
the manufacturers protocol. The absorbance at 450 nm was measured
using a microplate autoreader (Bio-Rad Laboratories, Inc.).
Colony formation assay
A total of 200 A549 cells/well were seeded into
six-well plates and cultured at 37°C for 2 weeks. Following the
incubation, the cells were fixed with 4% paraformaldehyde at 37°C
for 30 min and stained with 1% crystal violet for 20 min at room
temperature. The number of colonies formed (>50 cells/colony)
were counted manually. Stained cells were visualized using a light
microscope (magnification, ×40).
Flow cytometric analysis of
apoptosis
A549 cells (1×104) were incubated for 72
h, collected and resuspended in PBS supplemented with 2% FBS,
centrifuged at 12,000 × g for 5 min at room temperature, and
resuspend with PBS. The cells were subsequently stained with
Annexin-V-FITC and propidium iodide at room temperature for 20 min
using the Annexin V-FITC Apoptosis Detection kit (Gibco; Thermo
Fisher Scientific, Inc.) according to the manufacturers protocol.
Apoptotic cells were analyzed including early and late apoptosis
using a flow cytometer (LSRFortessa X-20; Becton, Dickinson and
Company). Apoptotic rate (%)=percentage of early + percentage of
late apoptotic cells.
Wound healing assay
A549 cells were cultured in six-well plates until
they reached 100% confluence. Before making the wound, the DMEM
medium was replaced with fresh culture medium without FBS. Then,
wounds were scratched into the cell monolayer using a 10-µl pipette
tip and culture for 48 h at room temperature. The wound closure was
observed at 0 and 48 h using light microscope, after washing with
PBS, and imaged to evaluate the migratory rate of cells in each
well. The cell migratory ability was quantified by measuring the
width of the advancing margins of cells in three randomly selected
microscopic fields (magnification, ×100) at the two time
points.
Transwell migration assay
A total of 2×105 cells/well were plated
in serum-free DMEM in the upper compartments of 8-µm-pore Transwell
plates. DMEM, supplemented with 10% FBS, was plated in the lower
chambers. Following incubation at 37°C for 2 weeks, the migratory
cells in the lower chamber were stained with 0.5% crystal violet
for 15 min at room temperature. The invasion assay was performed as
described for the migration assay, except for the Transwell plates
were pre-coated with Matrigel (50 µg) at 37°C. Stained cells were
visualized in three randomly selected fields using a light
microscope (magnification, ×200). The experiment was independently
conducted three times.
Statistical analysis
All assays were conducted at least three times
independently. Statistical analysis was performed using SPSS 21.0
software (IBM Corp.) and all experimental data are presented as the
mean ± SD. A paired Students t-test was used to determine the
significant differences between two groups, and one-way analysis of
variance (ANOVA) followed Tukeys post host was used to test
differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
IMPDH2 expression levels are
upregulated in NSCLC
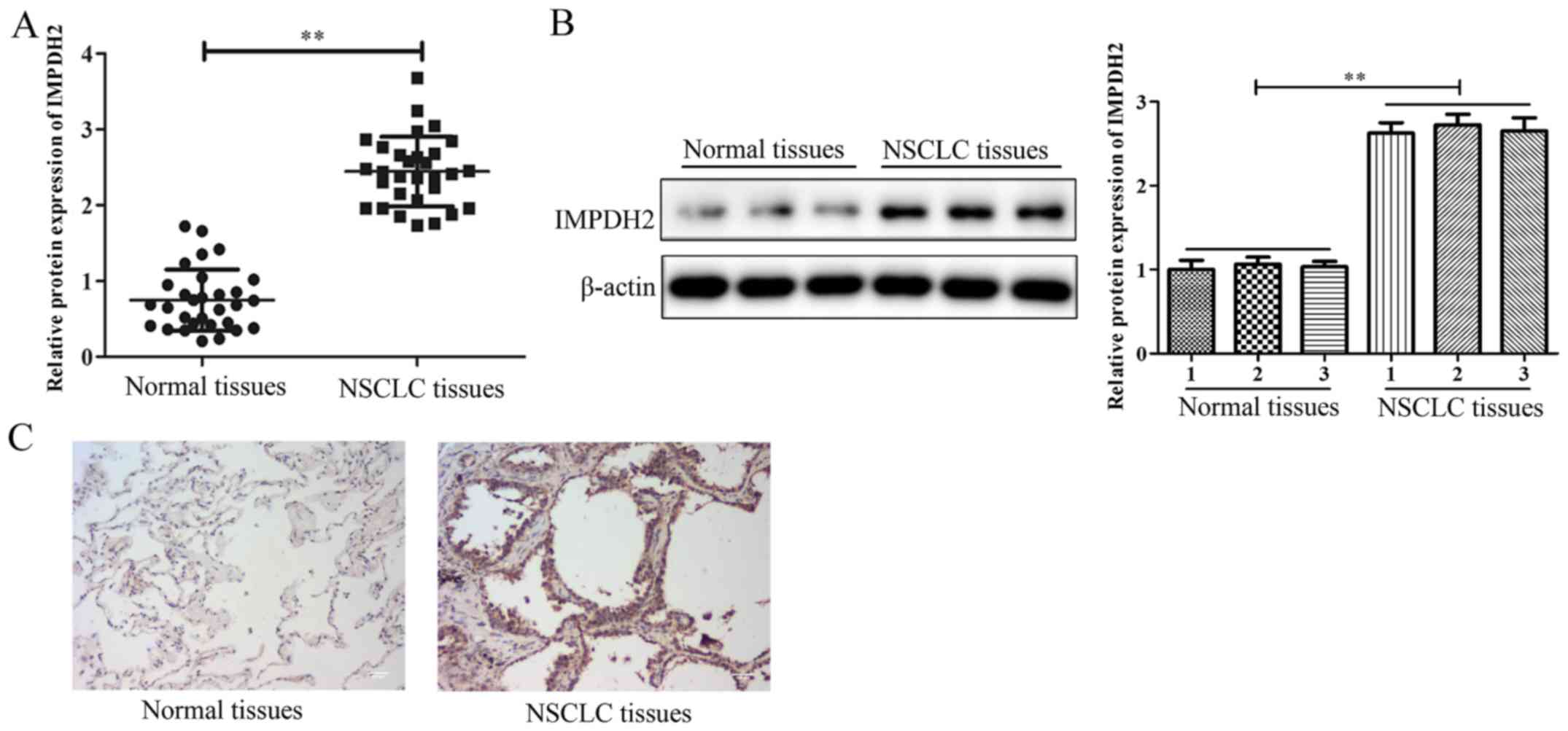
According to the RT-qPCR and western blotting
analysis, the IMPDH2 expression levels were discovered to be
significantly upregulated in NSCLC tissues compared with the
adjacent normal tissues and there was no significance between 3
normal tissues and NSCLC tissues (Fig.
1A and B). In addition, the protein expression levels of IMPDH2
in NSCLC tissues were investigated using immunohistochemistry; the
results demonstrated that the NSCLC tissues exhibited markedly
increased expression levels of IMPDH2 compared with the normal
tissues (Fig. 1C). These data
suggested that IMPDH2 may be upregulated in NSCLC.
IMPDH2 regulates the proliferation,
invasion, migration and apoptosis of NSCLC cells
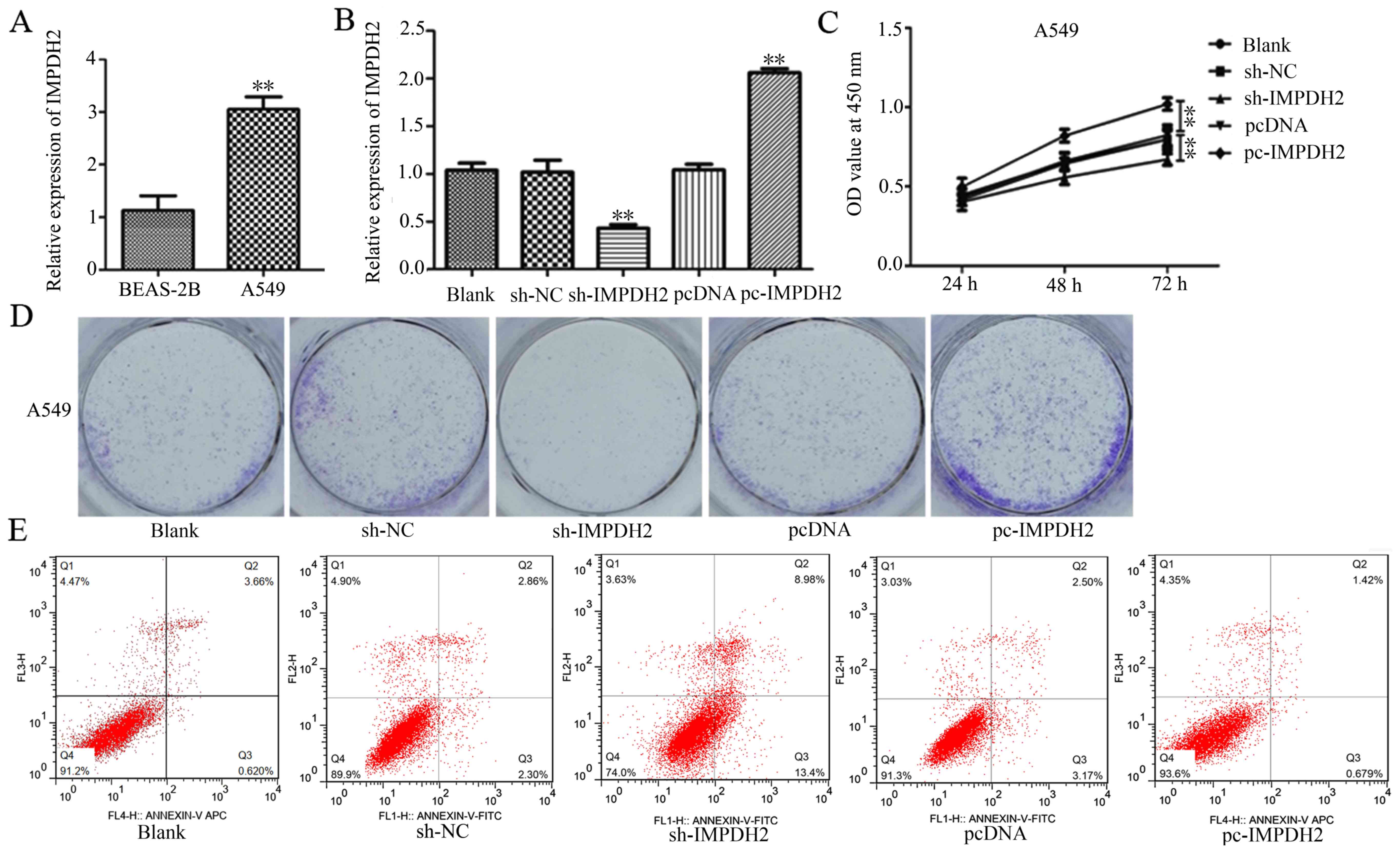
RT-qPCR analysis was performed to determine the
expression levels of IMPDH2 in cell lines and revealed that A549
and SPC-A1 cells had significantly upregulated expression levels of
IMPDH2 compared with the BEAS-2B cells, a normal primary human
bronchial epithelium cell line (Fig.
2A).
To investigate the possible functional value of
IMPDH2 in NSCLC progression, lentiviruses were constructed to
overexpress or knockdown IMPDH2. RT-qPCR analysis verified that the
transfections of the overexpression and knockdown IMPDH2
lentiviruses into A549 cells were successful (Fig. 2B).
Results from the CCK-8 assays indicated that the
cell proliferation rate was significantly attenuated in sh-IMPDH2
cells compared with the sh-NC cells, while enhanced in pc-IMPDH2
cells compared with the pcDNA cells (Fig. 2C). The colony formation assay, used
to investigate cancer cell proliferation in vitro, displayed
similar results; the number of colonies formed was markedly reduced
following IMPDH2 knockdown and increased following the
overexpression of IMPDH2 compared with their respective NCs
(Fig. 2D). Flow cytometric analysis
was subsequently used to determine whether IMPDH2 affected the
apoptotic rate of A549 cells. The results indicated that the
downregulation of IMPDH2 markedly increased the number of apoptotic
cells compared with the sh-NC-transfected cells (Fig. 2E). Conversely, the upregulation of
IMPDH2 markedly inhibited the rate of cell apoptosis compared with
pcDNA3.1-transfected cells. Thus, these findings suggested that
IMPDH2 may promote cell proliferation and inhibit apoptosis of
NSCLC cells.
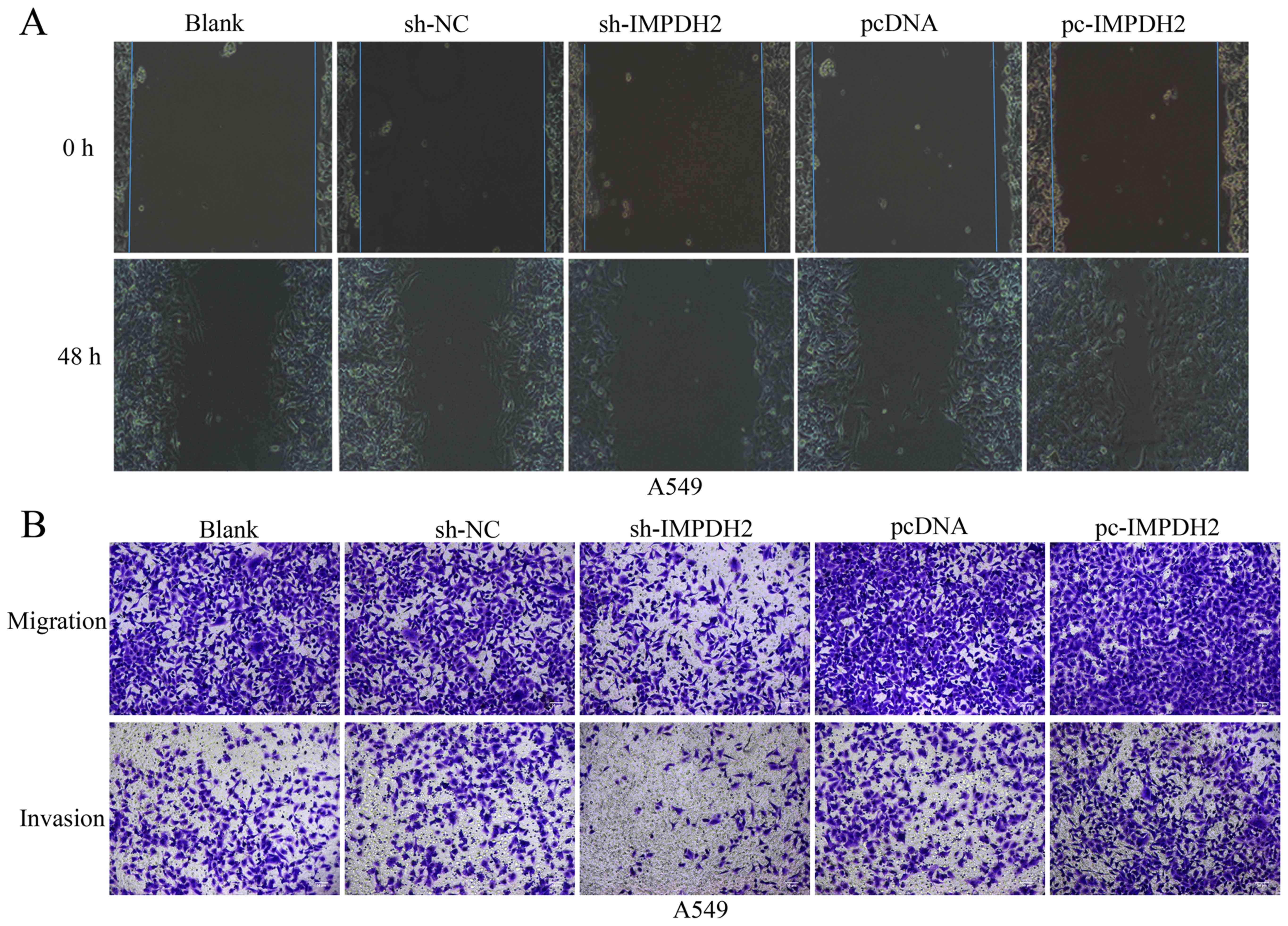
In addition to cell proliferation, one of hallmark
characteristics of cancer is the ability to migrate and lead to
metastasis (15). To investigate the
effect of IMPDH2 on cell migration, a wound healing assay was
performed. The cells transfected with pc-IMPDH2 demonstrated a
markedly increased migratory rate compared with the
pcDNA3.1-transfected cells after 48 h incubation (Fig. 3A). Conversely, the knockdown of
IMPDH2 inhibited the wound recovery, demonstrating a markedly
decreased migratory rate compared with the sh-NC-transfected cells
(Fig. 3A). Furthermore, the
Transwell assays revealed that the overexpression of IMPDH2 induced
cell invasion compared with the pcDNA3.1-transfected cells, whereas
the knockdown of IMPDH2 had the opposite effect compared with the
sh-NC-transfected cells (Fig. 3B).
These findings validated that knockdown of IMPDH2 had a suppressive
effect on NSCLC cell migration and invasion.
IMPDH2 promotes the invasion and
migration of NSCLC cells through epithelial-mesenchymal transition
(EMT)
IMPDH2 was previously reported to affect EMT in
colon cancer cells (5), thus the
present study investigated whether IMPDH2 may have the same
influence on NSCLC cells. Following the overexpression and
knockdown of IMPDH2 in A549 cells, the expression levels of the
epithelial markers, E-cadherin, and the mesenchymal markers,
vimentin and N-cadherin, were analyzed using western blotting. The
expression levels of E-cadherin were significantly downregulated,
whereas those of N-cadherin and vimentin were significantly
upregulated in pc-IMPDH2-transfected NSCLC cells compared with the
blank control cells (Fig. 4A). An
inverse trend was observed in the sh-IMPDH2-transfected cells; the
expression levels of E-cadherin were significantly upregulated and
the expression levels of vimentin and N-cadherin were significantly
downregulated compared with the sh-NC group. In contrast, The
E-cadherin expression level was decreased and vimentin and
N-cadherin expression levels were increased in pc-IMPDH2 cells
compared with their respective NCs. These findings were further
confirmed using an immunofluorescence assay and the data are
presented in Fig. 4B. With
downregulation of IMPDH2, the levels of N-cadherin and vimentin
were significantly downregulated (P<0.01), while the levels of
E-cadherin were markedly upregulated in A549 cells compared with
the sh-NC group (P<0.01). These results suggested that IMPDH2
may induce the invasion and migration of NSCLC cells via EMT
processes.
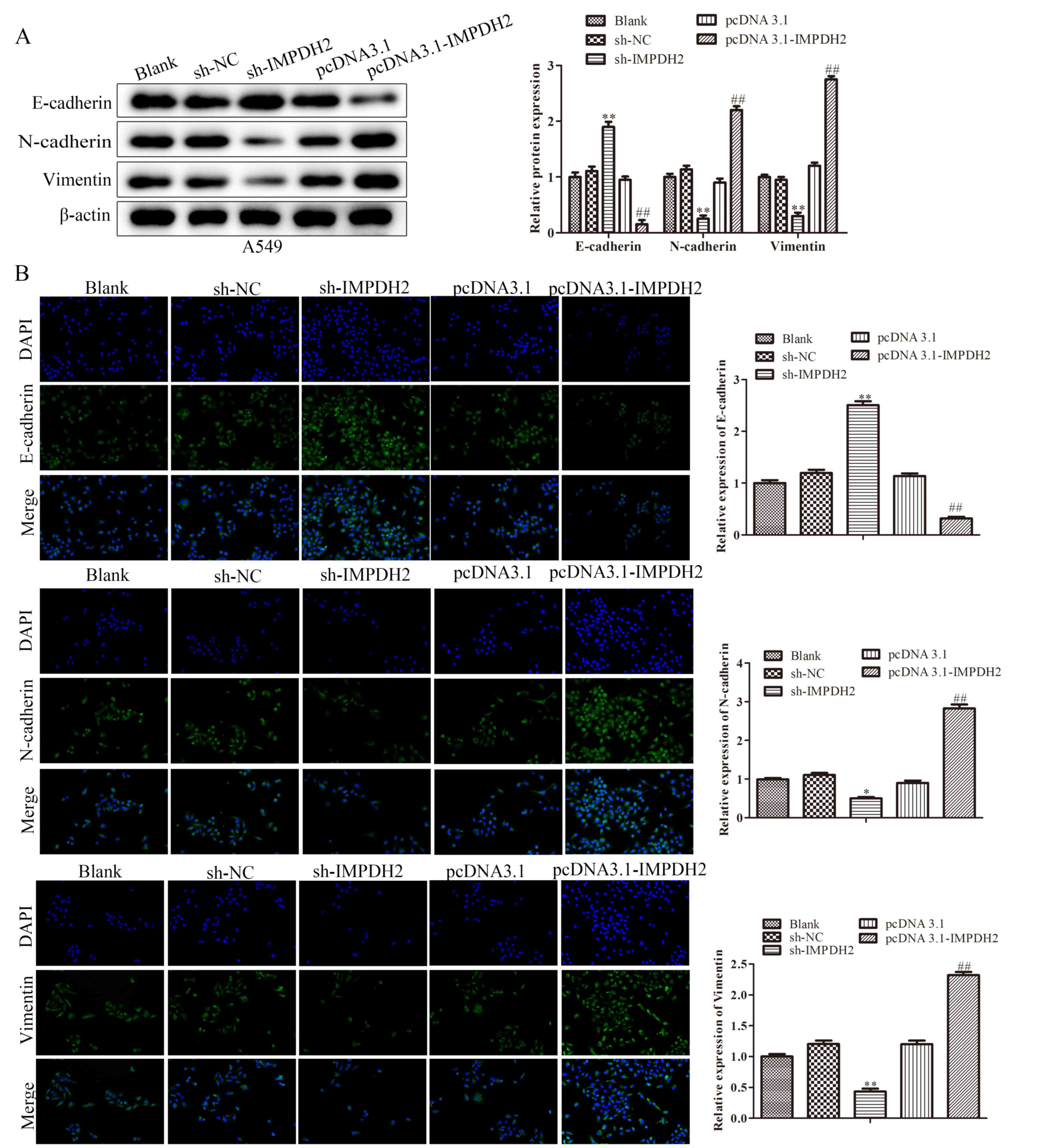 | Figure 4.Effect of IMPDH2 on EMT in non-small
cell lung cancer cells. (A) Western blotting was used to
investigate the effect of IMPDH2 overexpression or downregulation
on the expression levels of hallmark proteins of EMT, including
E-cadherin, N-cadherin and vimentin, in A549 cells. (B)
Immunofluorescence staining was used to investigate the expression
levels of E-cadherin, N-cadherin and vimentin in IMPDH2
overexpressed and knockdown A549 cells. Data are shown as mean ±
SD. n=3. Magnification, ×200. Scale bar, 100 µm. *P<0.05,
**P<0.01 vs. sh-NC, ##P<0.01 vs. pcDNA3.1. IMPDH2,
inosine 5′-monophosphate dehydrogenase type II; EMT,
epithelial-mesenchymal transition; sh, short hairpin RNA; NC,
negative control; pc, overexpression vector. |
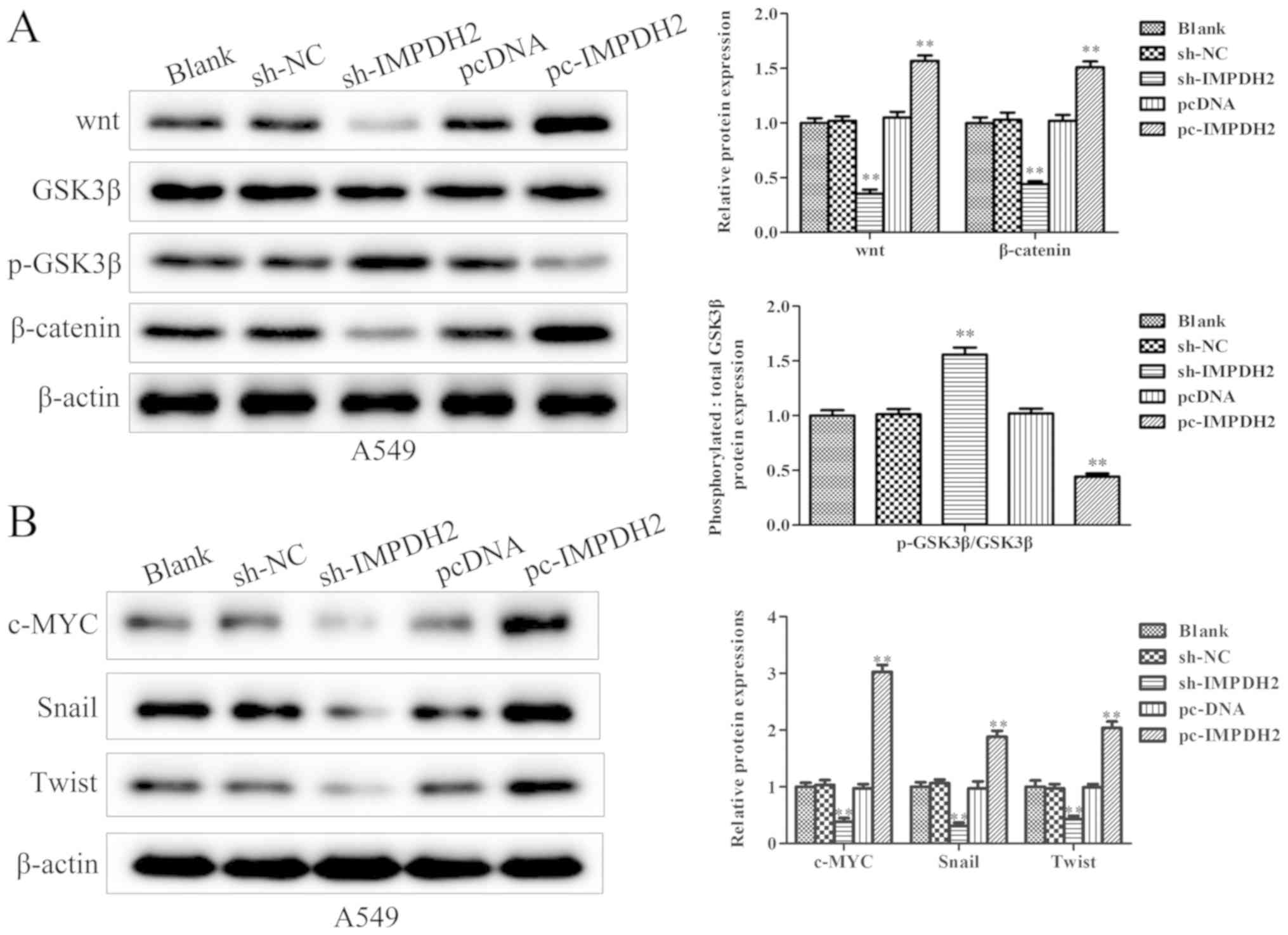
IMPDH2 promotes NSCLC progression
through the Wnt/β-catenin signaling pathway
After determining the impact of IMPDH2 on NSCLC
progression, the signaling pathways involved in this process were
investigated. It was identified that the overexpression or
knockdown of IMPDH2 affected the Wnt/β-catenin signaling pathway.
First, the western blotting analysis demonstrated that the
overexpression of IMPDH2 significantly upregulated the expression
levels of Wnt and β-catenin compared with the pcDNA3.1 group. In
contrast, IMPDH2 overexpression significantly downregulated the
expression levels of phosphorylated (p)-glycogen synthase kinase
(GSK)3β compared with the pcDNA3.1 group (Fig. 5A). Meanwhile, the knockdown of IMPDH2
expression levels presented the opposite trend; the expression
levels of Wnt and β-catenin were significantly downregulated, while
the expression levels of p-GSK3β were upregulated in
sh-IMPDH2-transfected cells compared with sh-NC group (Fig. 5A). Furthermore, the expression levels
of downstream transcription factors involved in the Wnt/β-catenin
signaling pathway were investigated by western blotting. It was
identified that the overexpression of IMPDH2 upregulated c-Myc,
Snail and Twist expression levels, while silencing IMPDH2
expression reduced the expression levels of c-Myc, Snail and Twist
compared with their respective NCs (Fig.
5B).
To further confirm that IMPDH2 influenced the
Wnt/β-catenin signaling pathway, shRNA targeting β-catenin was
used. Western blotting was used to verify that the knockdown of
β-catenin was successfully established in A549 cells. The result
shown that shβ-catenin transfection markedly downregulated Wnt and
β-catenin expression levels, while levels of p-GSK3β were increased
in A549 cell lines compared with sh-NC group (Fig. 6A). Subsequently, β-catenin shRNA was
transfected into pc-IMPDH2+shβ-catenin-transfected cells and it was
identified that the expression levels of c-Myc, Snail and Twist
were downregulated compared with pc-IMPDH2+sh-NC group (Fig. 6B). These findings indicated that the
effects of IMPDH2 in NSCLC cells may depend on β-catenin.
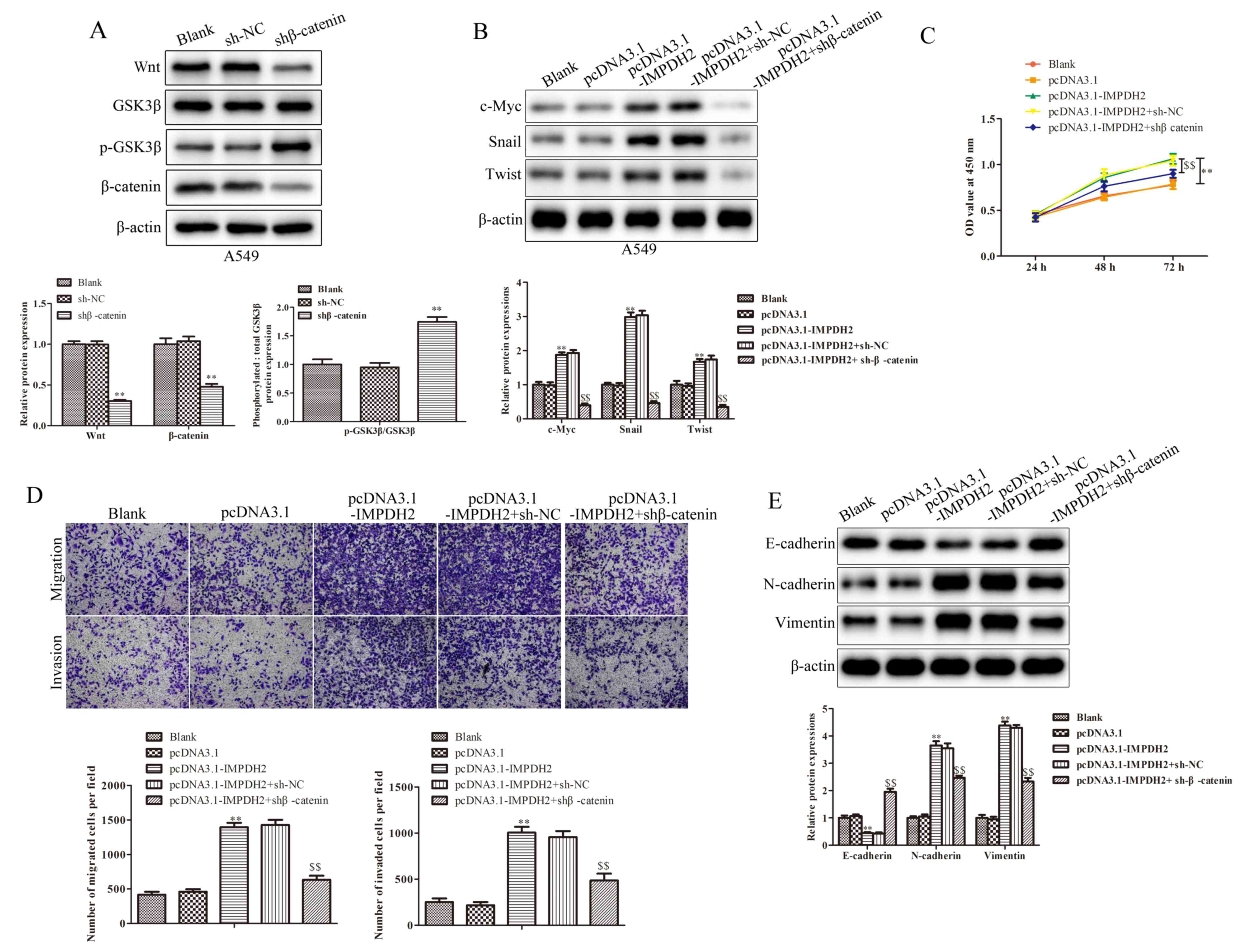 | Figure 6.IMPDH2 promotes non-small cell lung
cancer progression through the Wnt/β-catenin signaling pathway. (A)
The related protein expression levels of Wnt/β-catenin signaling
pathway were successfully inhibited in A549 cells using
sh-β-catenin. (B) Protein expression levels of c-Myc, Snail and
Twist were suppressed in pc-IMPDH2 cells following β-catenin
inhibition, as determined by western blotting. (C) Proliferative
ability of cells was determined using a Cell Counting Kit-8 assay.
(D) Migratory and invasive abilities were markedly reduced in
pc-IMPDH2 cells following β-catenin inhibition. (E) Expression
levels of epithelial-mesenchymal transition-associated proteins,
E-cadherin was upregulated, while N-cadherin and vimentin, were
downregulated in pc-IMPDH2-transfected cells following β-catenin
knockdown. The data are presented as the mean ± SD. n=3.
Magnification, ×200. **P<0.01 vs. pcDNA3.1,
$$P<0.01 vs. pcDNA3.1-IMPDH2+sh-NC. IMPDH2, inosine
5′-monophosphate dehydrogenase type II; GSK3β, glycogen synthase
kinase 3β; p-, phosphorylated; sh, short hairpin RNA; NC, negative
control; pc, overexpression vector. |
To determine whether the knockdown of β-catenin
expression levels could suppress the function of IMPDH2, CCK-8 and
Transwell assays were performed. Following the knockdown of
β-catenin, the overexpression of IMPDH2 did not significantly
promote cell proliferation to a further extent (Fig. 6C). In addition, cell migration and
invasion were also determined, and the abilities in A549 cells were
repressed in pc-IMPDH2+shβ-catenin group compared with
pc-IMPDH2+sh-NC group (Fig. 6D).
Notably, the expression levels of E-cadherin were increased, while
N-cadherin and vimentin expression levels were decreased in
pc-IMPDH2+shβ-catenin-treated cells when compared with
pc-IMPDH2+sh-NC group (Fig. 6E). In
conclusion, these findings suggested that IMPDH2 may affect NSCLC
progression through regulating the Wnt/β-catenin signaling
pathway.
Discussion
IMPDH serves as a rate-limiting enzyme in the
synthesis of guanine nucleotides, thus it is an important regulator
for DNA synthesis (16,17). IMPDH has been associated with cell
proliferation and malignancy since 1975 (13). In particular, it has been previously
reported that IMPDH2 was the predominant isoform in neoplastic and
replicating cells (5). Accumulating
evidence has also suggested that IMPDH2 may be closely implicated
in different types of malignancy (18–21). In
addition, it was revealed that IMPDH2 may be a useful biomarker for
patient prognosis (20,21). In a previous study, IMPDH2 was also
discovered to be a target of efficient immunosuppressive agents
(22) and an IMPDH inhibitor has
been investigated and developed for tumor suppression (20,17,23–25). The
present study demonstrated that the expression levels of IMPDH2
were upregulated in NSCLC tissues, which prompted the
investigations into the potential role of IMPDH2 in NSCLC
progression. CCK-8, colony formation, wound healing and Transwell
assays were used in experiments involving the overexpression and
knockdown of IMPDH2 in A549 cells, which revealed that IMPDH2 may
positively affect cell proliferation, migration and invasion.
Therefore, these findings suggested that IMPDH2 may serve oncogenic
roles in NSCLC.
EMT is a hallmark of tumor invasion and metastasis,
which can alter the adhesion of epithelial cancer cells, promoting
them to invade and migrate to other distant sites (26–28).
During EMT, the repression of genes encoding epithelial cell
junction proteins is accompanied by the activation of genes
promoting mesenchymal adhesion (29), and the ‘Cadherin switch’ refers to
the process by which the downregulation of E-cadherin expression
levels are balanced by up-regulated N-cadherin expression levels
(30,31). In the current study, the analysis of
EMT-associated factors revealed that IMPDH2 suppressed E-cadherin
expression levels, while up-regulating N-cadherin and vimentin
expression levels. Therefore, IMPDH2 may induce EMT in NSCLC.
In addition, the present study identified the
signaling pathway through which IMPDH2 exerted oncogenic functions.
Western blotting analysis discovered that the expression levels of
Wnt3a and β-catenin were significantly down-regulated in the
sh-IMPDH2-transfected cells and up-regulated in the
pc-IMPDH2-transfected cells, suggesting that Wnt/β-catenin
signaling may be involved in the IMPDH2 oncogenic activity. In
addition, it was identified that the influence of IMPDH2 on tumor
growth and EMT processes may be attributed to the activation of the
Wnt/β-catenin signaling pathway in NSCLC. Several studies have
previous reported that the Wnt/β-catenin signaling pathway
participated in lung cancer, in addition to other types of cancer
(32,33). Under normal conditions, GSK3β, Axin,
serine/threonine-protein phosphatase (PP2A), adenomatous polyposis
coli protein and casein kinase I isoform α (CCKα) accumulate in the
cytoplasm to form degradation complexes (34). Upon the degradation of complex
β-catenin proteins, GSK3β promotes the phosphorylation of β-catenin
at Ser33 and Ser37, yielding ubiquitin-labeled β-catenin, which is
subsequently degraded by proteasomes (35); this regulatory mechanism is useful
for maintaining the stability of the Wnt/β-catenin signaling
pathway (36). However, upon the
phosphorylation of GSK3β, the kinase activity of GSK3β is
inactivated and can no longer promote the phosphorylation of
β-catenin, leading to the accumulation and functioning of
cytoplasmic β-catenin (37). The
results of the present study suggested that β-catenin may be
positively regulated by IMPDH2. β-catenin has been reported to
reflect the changes of the Wnt/β-catenin signaling pathway in cells
(9). The expression levels of c-Myc,
Snail and Twist, which are important transcriptional factors of the
Wnt signaling pathway (10), were
significantly upregulated following IMPDH2 overexpression. In
addition, Snail and Twist are also crucial transcriptional factors
in EMT and the Wnt/β-catenin signaling pathway can regulate EMT via
Snail and Twist (38,39). In addition, Mannava et al
(40) discovered that IMPDH2 may be
a direct target of c-Myc. Consistent with the findings of the
present study, it can be inferred that IMPDH2 and c-Myc may
influence each other. For instance, the current study suggested
that IMPDH2 may promote NSCLC progression through Wnt/β-catenin,
which subsequently promoted the upregulation of c-Myc.
To identify the critical role of the Wnt/β-catenin
signaling pathway in NSCLC, the present study subsequently
investigated the function of IMPDH2 during EMT after inhibiting
β-catenin. The results revealed that the functions of IMPDH2 were
suppressed following β-catenin inhibition; the cell proliferation
rate and the EMT-associated phenotype were not affected by IMPDH2
overexpression following the inhibition of β-catenin.
In conclusion, the findings of the present study
suggested that IMPDH2 may promote the cell proliferation and EMT of
NSCLC by activating the Wnt/β-catenin signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors contributions
HX designed the experiments. HM, LZ, QL, CX and LZ
performed the experiments. GY and HP contributed to data
acquisition. XF and XX were responsible for data analysis. GY, HP,
XF and XX were the major contributors in writing the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of The People's Hospital of Danyang (approval no.
JS-2018-04) and written, informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu R, Chen Y, Shou T, Hu J and Qing C:
miRNA-99b-5p targets FZD8 to inhibit non-small cell lung cancer
proliferation, migration and invasion. Onco Targets Ther.
12:2615–2621. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zimmermann AG, Spychala J and Mitchell BS:
Characterization of the human inosine-5-momophosphate dehydrogenase
type II gene. J Biol Chem. 270:6808–6814. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dorn AR, Mountain LD, Ghoshal M, Hui RA,
Klinedinst LK, Rugaber JE, Schamerloh AF and Salamone SJ: The use
of inosine 5′-monophosphate dehydrogenase (IMPDH) in the
development of a new liquid homogeneous enzyme immunoassay
technology. Clin Chem. 47:1921–1922. 2001. View Article : Google Scholar
|
|
6
|
Carr SF, Papp E, Wu JC and Natsumeda Y:
Characterization of human type I and type II IMP dehydrogenases. J
Biol Chem. 266:27286–27290. 1993.
|
|
7
|
Colby TD, Vanderveen K, Strickler MD,
Markham GD and Goldstein BM: Crystal structure of human type II
inosine monophosphate dehydrogenase: Implications for ligand
binding and drug design. Proc Natl Acad Sci USA. 96:3531–3536.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duan S, Huang W, Liu X, Liu X, Chen N, Xu
Q, Hu Y, Song W and Zhou J: IMPDH2 promotes colorectal cancer
progression through activation of the PI3K/AKT/mTOR and
PI3K/AKT/FOXO1 signaling pathways. J Exp Clin Canc Res. 37:3042018.
View Article : Google Scholar
|
|
9
|
Zhou L, Xia D, Zhu J, Chen Y, Chen G, Mo
R, Zeng Y, Dai Q, He H, Liang Y, et al: Enhanced expression of
IMPDH2 promotes metastasis and advanced tumor progression in
patients with prostate cancer. Clin Transl Oncol. 16:906–913. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou J, Han Z, Zhou L, Cai C, Luo H, Huang
Y, Liang Y, He H, Jiang F, Wang C and Zhong W: Elevated expression
of IMPDH2 is associated with progression of kidney and bladder
cancer. Med Oncol. 32:3732015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kahlert UD, Nikkhah G and Maciaczyk J:
Epithelial-tomesenchymal(−like) transition as a relevant molecular
event in malignant gliomas. Cancer Lett. 331:131–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Ke X, Pu Q, Yuan Y, Yang W, Luo
X, Jiang Q, Hu X, Gong YI and Tang K: MicroRNA-410 acts as oncogene
in NSCLC through downregulating SLC34A2 via activating
Wnt/β-catenin pathway. Oncotarget. 7:14569–14585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) methods. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao C, Yao H, Liu H, Feng Y and Yang Z:
TM4SF1 is a potential target for anti-invasion and metastasis in
ovarian cancer. BMC Cancer. 19:2372019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kozhevnikova EN, van der Knaap JA,
Pindyurin AV, Ozgur Z, van Ijcken WF, Moshkin YM and Verrijzer CP:
Metabolic enzyme IMPDH is also a transcription factor regulated by
cellular state. Mol Cell. 47:133–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bairagya HR, Mukhopadhyay BP and Bera AK:
Role of salt bridge dynamics in inter domain recognition of human
IMPDH isoforms: An insight to inhibitor topology for isoform-II. J
Biomol Struct Dyn. 29:441–462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Wang WM, Zou LY, Li LI, Feng LU,
Pan MZ, Lv MY, Cao Y, Wang H, Kung HF, et al: Ubiquitin specific
peptidase 5 mediates Histidine-rich protein Hpn induced cell
apoptosis in hepatocellular carcinoma through P14-P53 signaling.
Proteomics. 17:2017. View Article : Google Scholar
|
|
19
|
Jackson RC, Weber G and Morris HP: IMP
dehydrogenase, an enzyme linked with proliferation and malignancy.
Nature. 256:331–333. 1975. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collart FR, Chubb CB, Mirkin BL and
Huberman E: Increased inosine-5-phosphate dehydrogenase gene
expression in solid tumor tissues and tumor cell lines. Cancer Res.
52:5826–5828. 1992.PubMed/NCBI
|
|
21
|
Nagai M, Natsumeda Y and Weber G:
Proliferation-linked regulation of type II IMP dehydrogenase gene
in human normal lymphocytes and HL-60 leukemic cells. Cancer Res.
52:258–261. 1992.PubMed/NCBI
|
|
22
|
Zhao Y, Yang Y, Dai J, Xing D and Dong Y:
IMPDH2 is highly expressed in breast cancer and predicts
unfavourable prognosis. Biomarkers. 1–9. Jun 30–2018.(Online ahead
of print).
|
|
23
|
Xu Y, Zheng Z, Gao Y, Duan S, Chen C, Rong
J, Wang K, Yun M, Weng H, Ye S and Zhang J: High expression of
IMPDH2 is associated with aggressive features and poor prognosis of
primary nasopharyngeal carcinoma. Sci Rep. 7:7452017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zimmermann AG, Wright KL, Ting JP and
Mitchell BS: Regulation of inosine-5′-monophosphate dehydrogenase
type II gene expression in human T cells. Role for a novel 5
palindromic octamer sequence. J Biol Chem. 272:22913–22923. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu TY, Peng Y, Pelleymounter LL, Moon I,
Eckloff BW, Wieben ED, Yee VC and Weinshilboum RM: Pharmacogenetics
of the mycophenolic acid targets inosine monophosphate
dehydrogenases IMPDH1 and IMPDH2: Gene sequence variation and
functional genomics. Br J Pharmacol. 161:1584–1598. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishitsuka K, Hideshima T, Hamasaki M, Raje
N, Kumar S, Podar K, Gouill SL, Shiraishi N, Yasui H, Roccaro AM,
et al: Novel inosine monophosphate dehydrogenase inhibitor VX-944
induces apoptosis in multiple myeloma cells primarily via
caspase-independent AIF/Endo G pathway. Oncogene. 24:5888–5896.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pua KH, Stiles DT, Sowa ME and Verdine GL:
IMPDH2 is an intracellular target of the cyclophilin A and
sanglifehrin A complex. Cell Rep. 18:432–442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo Q, Huang Y, Xu J, Wang W, Gao J, Su Y,
Gu Y and Yin X: Long non-coding RNA BANCR contributes to cervical
adenocarcinoma migration by affecting epithelial-mesenchymal
transition. Eur J Gynaecol Oncol. 40:408–412. 2019.
|
|
29
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gavert N and Ben-ZeEv A:
Epithelial-mesenchymal transition and the invasive potential of
tumors. Trends Mol Med. 14:199–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wheelock MJ, Shintani Y, Maeda M, Fukumoto
Y and Johnson KR: Cadherin switching. J Cell Sci. 121((Pt 6)):
727–735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rao TP and Kühl M: An updated overview on
Wnt signaling pathways: A prelude for more. Circ Res.
106:1798–1806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bienz M and Clevers H:
Armadillo/beta-catenin signals in the nucleus-proof beyond a
reasonable doubt. Nat Cell Biol. 5:179–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li K, Pan W, Ma Y, Xu X, Gao Y, He Y, Wei
L and Zhang J: A novel oncogene TRIM63 promotes cell proliferation
and migration via activating Wnt/β-catenin signaling pathway in
breast cancer. Pathol Res Pract. 215:1525732019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Segditsas S and Tomlinson I: Colorectal
cancer and genetic alterations in the Wnt pathway. Oncogene.
25:7531–7537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang J, Chen J, He J, Li J, Shi J, Cho WC
and Liu X: Wnt signaling as potential therapeutic target in lung
cancer. Expert Opin Ther Targets. 20:999–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mannava S, Grachtchouk V, Wheeler LJ, Im
M, Zhuang D, Slavina EG, Mathews CK, Shewach DS and Nikiforov MA:
Direct role of nucleotide metabolism in C-MYC-dependent
proliferation of melanoma cells. Cell Cycle. 7:2392–2400. 2008.
View Article : Google Scholar : PubMed/NCBI
|