Introduction
The estimated number of new prostate cancer cases
was ~1.3 million with 359,000 associated deaths worldwide in 2018,
with prostate cancer ranking as the second most common cancer and
the fifth leading cause of cancer-associated mortality in males
(1). Although it is highly curable
if the tumor is locally confined, patients with advanced late-stage
disease have a much lower 5-year survival rate (~30%) (2). Androgen deprivation therapy (ADT) is
the standard-of-care for patients with advanced prostate cancer.
Although highly effective initially, tumors eventually progress and
transform themselves into castration-resistant prostate cancer
(CRPC) (3). Metastatic CRPC (mCRPC)
remains the main clinical challenge and a major cause of mortality
(4). In patients with mCRPC,
docetaxel currently serves as the standard first-line chemotherapy.
However, in addition to its long-term toxicity, the benefits of
docetaxel are generally restricted, and drug resistance occurs
inevitably in nearly all patients. Clinical data have demonstrated
that, in addition to the ~50% of patients who fail to respond to
docetaxel inherently, in most patients who initially respond to the
drug, disease progression will recur within 1 year from the start
of treatment, indicating the generation of acquired chemoresistance
(5).
In this regard, docetaxel resistance has become a
major clinical issue to overcome. Although extensive research has
been performed into docetaxel resistance, there is no mechanism to
explain in detail the clinical response to docetaxel therapy.
Mammalian target of rapamycin (mTOR) is an oncoprotein that is
generally dysregulated in human cancer (6). In prostate cancer, the mTOR pathway is
frequently hyperactivated (7). The
pivotal role of mTOR in prostate cancer makes it a potential target
for therapeutic interventions.
To completely understand drug resistance, cultured
cell lines resistant to anticancer drugs need to be primarily
established. In the present study, a docetaxel-resistant prostate
cancer cell line was established and the characteristics of
docetaxel resistance in this cell line were examined. Furthermore,
the mTOR signaling components were compared between the parental
cells and cells that developed resistance to docetaxel to analyze
the impact of mTOR signaling on the acquired docetaxel-resistant
phenotype in preclinical CRPC models.
Materials and methods
Anticancer agents
Docetaxel (Sanofi S.A.) was dissolved in 95% ethanol
to the stock concentration of 100 nm and stored at 4°C.
Drug-resistant cancer cell lines
Docetaxel-resistant clones of PC-3 cells were
developed by exposing cells to docetaxel at an intermittently
increasing concentration. In brief, PC-3 cells were seeded onto a
96-well plate and exposed to 0.1 nM docetaxel for 24 h. Next, the
medium was changed to normal culture medium (F-12 medium with 10%
fetal bovine serum), and 100 U/ml penicillin and streptomycin (all
purchased from (Thermo Fisher Scientific, Inc.). Following the PC-3
cells being cultured three times, the cells were continuously
incubated with a higher concentration of 0.2 nM docetaxel for 24 h,
prior to the medium being changed to normal cultured medium. This
step was repeated with 0.50, 0.75, 1.00, 1.50, 2.00, 5.00, 7.50,
10.00, 15.00, 20.00 and 30.00 nM docetaxel. PC-3 cells that were
resistant to 30 nM docetaxel were named PC-3/DTX cells and stored
for further investigation.
Cell culture
The human prostate cancer PC-3 cell line was
purchased from American Type Culture Collection, Manassas, VA, USA
(CRL-1435). In brief, PC-3 cells and docetaxel-resistance PC-3
cells (PC-3/DTX) were cultured in F-12 medium (Thermo Fisher
Scientific, Inc.), supplemented with 10% fetal bovine serum (Thermo
Fisher Scientific, Inc.), penicillin (100 U/ml; Thermo Fisher
Scientific, Inc.) and streptomycin (100 U/ml; Thermo Fisher
Scientific, Inc.). The cultures were maintained at 37°C in a 95%
humidified atmosphere with 5% CO2.
Growth curves of PC-3 and PC-3/DTX
cells detected by CCK8 assay
Cell proliferation were measured by Cell Counting
Kit-8 (CCK-8; cat. no. C0037, Beyotime Institute of Biotechnology).
To compare the viability of PC-3 and PC-3/DTX cells, cells were
seeded onto 96-wells plates at a density of 2.0×103
cells/well. After 24 h incubation at 37°C in a 95% humidified
atmosphere with 5% CO2, 10 µl CCK8 solution was added
into each well, and incubated at 37°C for 1 h. Next, the absorption
at 450 nm was measured using a microplate spectrophotometer (MD
I3X). This measurement was repeated each day until day 5, and
growth curves were drawn. Each experiment was repeated three times,
and the average values were taken. The cell doubling cycle was
calculated using the following equation: T=txlg2/lg
(ODt-OD0), where T is the population doubling
time, t is the time of continuous culture, ODt is the
final absorption of cells, and OD0 is the initial
absorption of cells.
Evaluation of cell inhibition by MTT
assay
The MTT cell viability/cytotoxicity assay kit (cat.
no. C0009, Beyotime Institute of Biotechnology) was used for this
assay. PC-3 and PC-3/DTX cells were seeded onto 96-well plates at a
density of 1×104 cells/well at 37°C in a 95% humidified
atmosphere with 5% CO2 for 24 h. Following washing with
PBS, the following concentrations of docetaxel: 0.781, 1.562,
3.125, 6.250, 12.500, 25.000, 50.000 and 100.000 nM, were added to
the cells, which were then incubated for 72 h. After introducing 10
µl MTT solution for 4 h, formazan was dissolved using formazan
solvent in the kit and 100 µl formazan was added to each well. The
absorption at 570 nm was measured using a microplate
spectrophotometer (MD I3X). Each experiment was repeated three
times, and the average values were used for analyses. Resistance
indices (RIs) were calculated as the ratio of the 50% inhibitory
concentration (IC50) values of PC-3/DTX to that of PC-3
cells.
Cell-cycle detection by flow
cytometric analysis
For cell cycle analysis, the cells were processed
using the cell cycle and apoptosis analysis kit (cat. no. C1052,
Beyotime Institute of Biotechnology). PC-3 and PC-3/DTX cells were
harvested and resuspended at a density 2×105 cells/ml in
500 µl binding buffer. According to the instruction of the kit, 5
µl propidium iodide (PI) and RNase were added to the samples and
incubated at 4°C for 30 min. Next, the cells were resuspended in
500 µl PBS and analyzed using a flow cytometer (BD-FACS-Calibur; BD
Biosciences). The fluorescence of PI was monitored at 488 nm.
Fluorescence intensity was quantified by flow cytometry analysis.
Each plot represented 20,000 viable cells, and non-viable cells
were excluded from flow cytometric analysis by appropriate gating.
All data analyses were performed using FCS express V3.0 (BD
Biosciences).
Western blot analysis
Cells were collected in RIPA buffer (containing 0.2%
Triton X-100, 5 mmol/l EDTA, 1 mmol/l PMSF, 10 mg/ml leupeptin, 10
mg/ml aprotinin, added with 100 mmol/l NaF, and 2 mmol/
Na3VO4) and lysed for 30 min on ice. Protein
concentration was assayed using the Bio-Rad protein kit (Beyotime
Institute of Biotechnology, Haimen, China), and equal amounts of 10
µl sample were loaded per well on a sodium dodecyl
sulfate-polyacrylamide gel. Subsequently, proteins were transferred
onto 0.45 µm pore sized positively-charged nylon polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA) and
blocked with blotto (5% dry milk in PBS with 0.1% Tween-20) at room
temperature for 1 h. The cells were incubated with the following
primary antibodies: Anti-Rictor [1:1,000; Cell Signaling Technology
(CST), Inc.; cat. no. 2114], anti-Raptor (1:1,000; CST; cat. no.
2280T), anti-AKT (1:1,000; ProteinTech Group, Inc.; cat. no.
10176-2-AP), anti-pAKT (S473; 1:1,000; CST; cat. no. 9271T),
anti-p70 S6 kinase 1 (1:1,000; CST; cat. no. 2708), anti-p70S6k
(T389; 1:1,000; CST; cat. no. 9234T), anti-4EBP1 (1:1,000; CST;
cat. no. 9644T), anti-4EBP1-S65 (1:1,000; CST; cat. no. 9451T) and
anti-GAPDH (1:1,000, CST; cat. no. 5174T) overnight at 4°C,
followed by washing three times with PBST (0.1% Tween-20). They
were then incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG(H+L) secondary antibodies (Beyotime Institute of
Biotechnology; 1:1,000; cat. no. A0208) in TBS-T for 1 h at room
temperature. The blots were detected using an enhanced
chemiluminescent substrate (Beyotime Institute of
Biotechnology).
Statistical analysis
All data are presented as the mean ± standard
deviation for the indicated number of separate experiments. Three
independent experiments were performed for each study. Comparisons
of differences in the quantitative data among groups were performed
using an unpaired t-test. P<0.05 was considered to indicate a
statistically significant difference. SPSS 19 software (IBM Corp.)
was used for statistical analyses.
Results
Establishment of the
docetaxel-resistant PC-3/DTX cell line
PC-3 cells were exposed to increasing concentrations
of docetaxel intermittently for 12 months to establish a stable
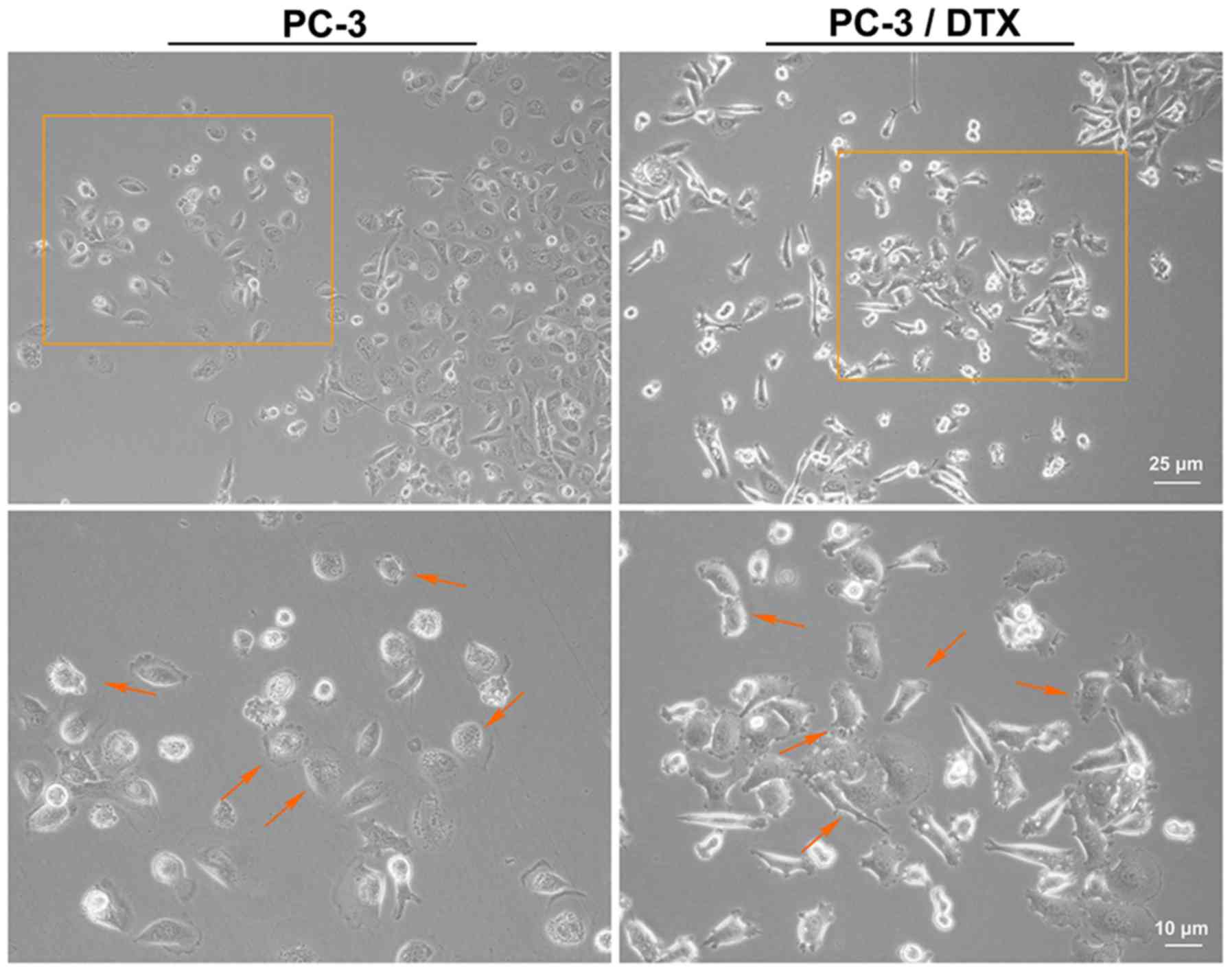
docetaxel-resistant cell line, PC-3/DTX. As shown in Fig. 1, the cell phenotype was captured
using an inverted microscope (magnifications, ×10 and ×20).
Compared to the PC-3 cells, the gap junction was increased and more
irregular cell margins were observed in PC-3/DTX cells. The cell
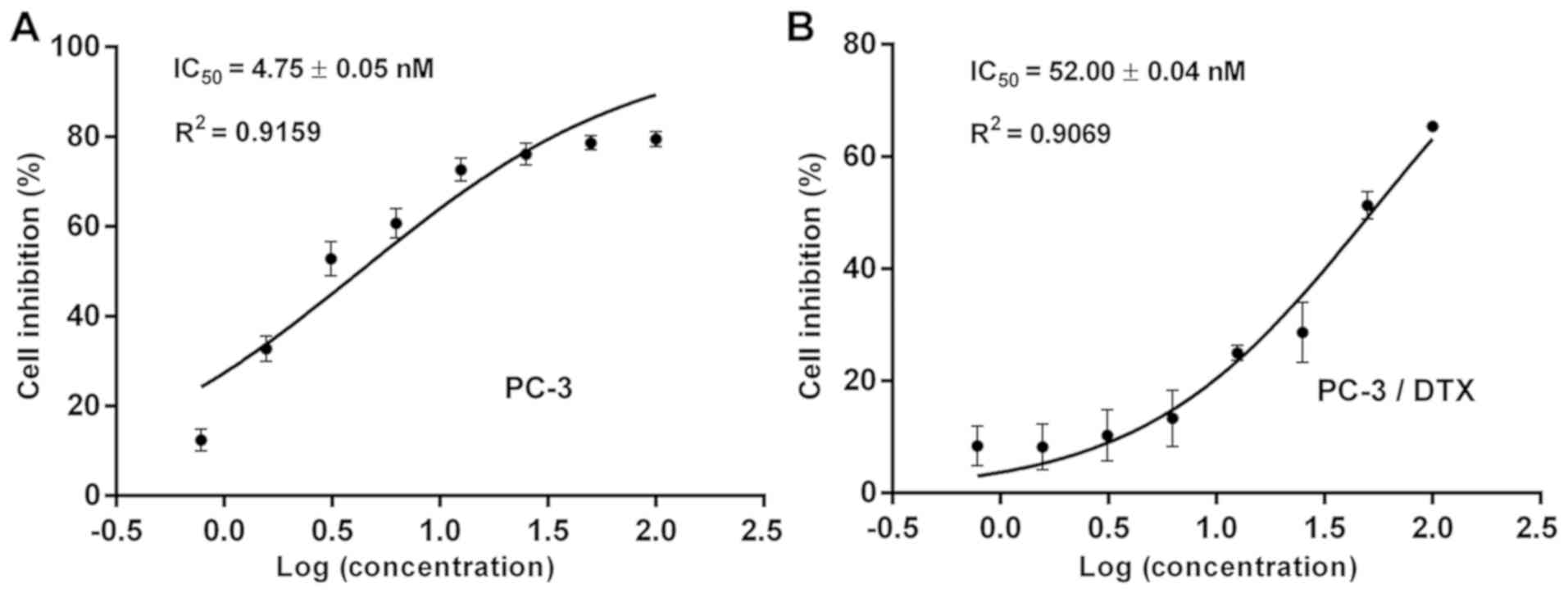
inhibition analysis demonstrated that the IC50 values
for PC-3 and PC-3/DTX cells were 4.75±0.05 and 52.00±0.04 nM,
respectively (Fig. 2). The
resistance index of PC-3/DTX cells was 10.9 times that of PC-3
cells, suggesting that PC-3/DTX cells exhibited a high resistance
to docetaxel.
Growth curves and doubling time
(Td) of PC-3 and PC-3/DTX cells
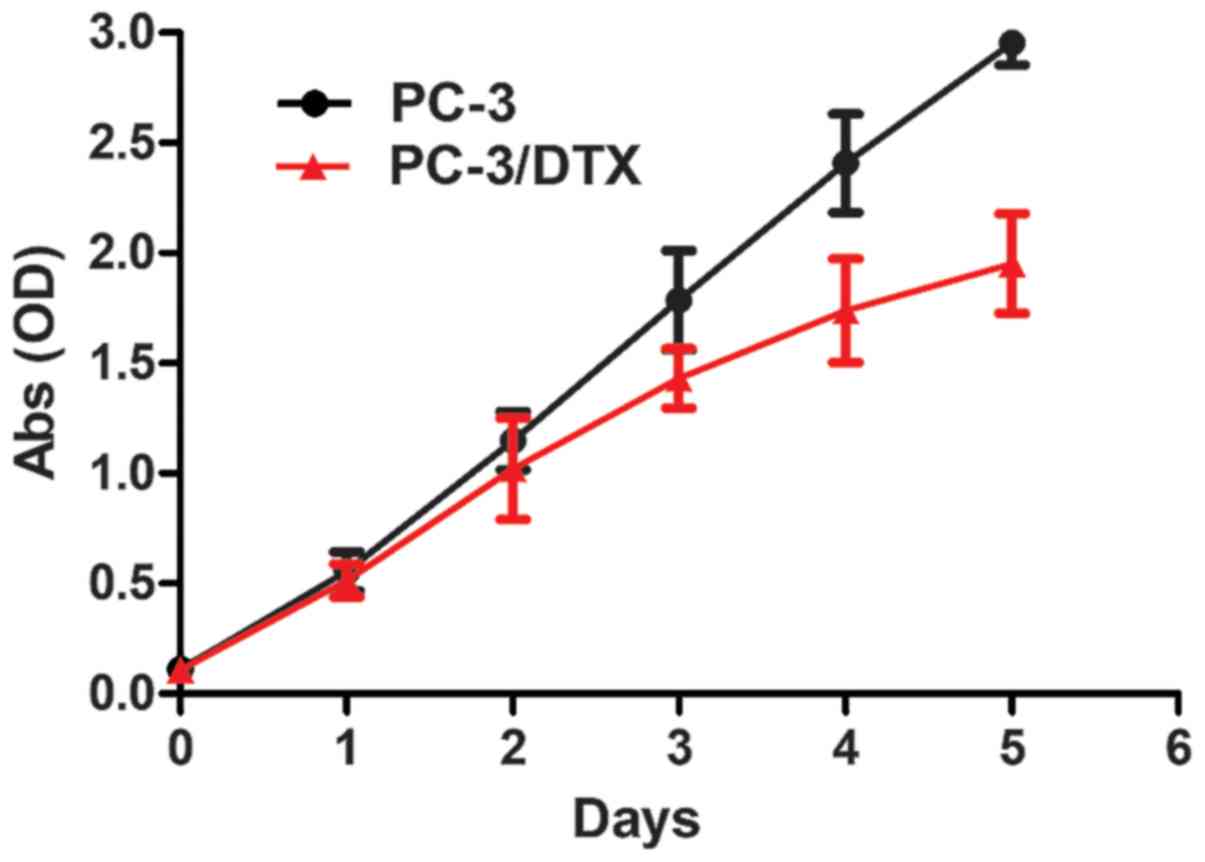
The cell growth curves are presented in Fig. 3. No significant differences were
observed between the growth curves of PC-3 and PC-3/DTX cells in 2
days. After 2 days, PC-3/DTX cells grew more slowly than PC-3
cells, as can be observed by the decrease in the growth curve. The
Td of PC-3 and PC-3/DTX cells was 25.34±0.02 and
28.87±0.75 h, respectively.
Cell-cycle analysis of PC-3 and
PC-3/DTX cells assessed by flow cytometry
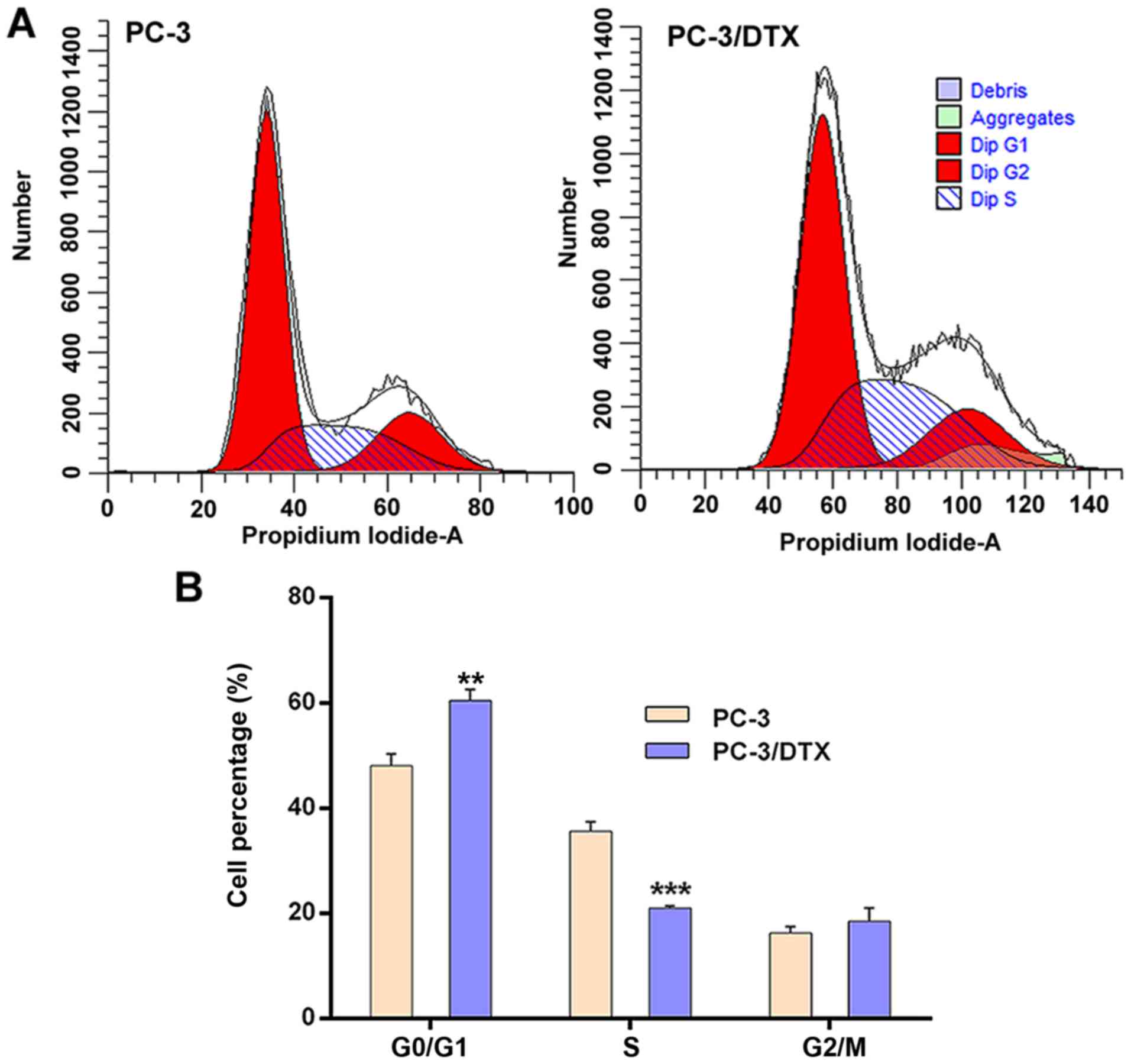
The cell cycle of PC-3 and PC-3/DTX cells was
assessed by flow cytometry. The results showed significant changes
in the G0/G1 phase and S phase between PC-3
and PC-3/DTX cells (Fig. 4). The
distributions of PC-3 and PC-3/DTX cells in the
G0/G1 phase were 48.09±2.20 and 60.48±2.10%
and those in S phase were 35.61±1.80 and 20.99±0.38%, respectively.
Compared with PC-3 cells, the number of PC-3/DTX cells in the
G0/G1 phase was 12.39% higher (P<0.05) and
14.62% lower in the S phase (P<0.01). There were no significant
differences between the two cell lines with respect to the
G2/M phase.
Protein changes in the mTORC2 signal
pathway in PC-3/DTX cells
The expression of Rictor and p-AKT(S473), which are
specific subunits or downstream substrates of mTORC2, were
significantly upregulated in PC-3/DTX cells (Fig. 5A and B). By contrast, the expression
of Raptor, p70S6K (T389) and 4EBP1-S65, which are specific subunits
or downstream substrates of mTORC1, exhibited no notable changes
(Fig. 5C and D).
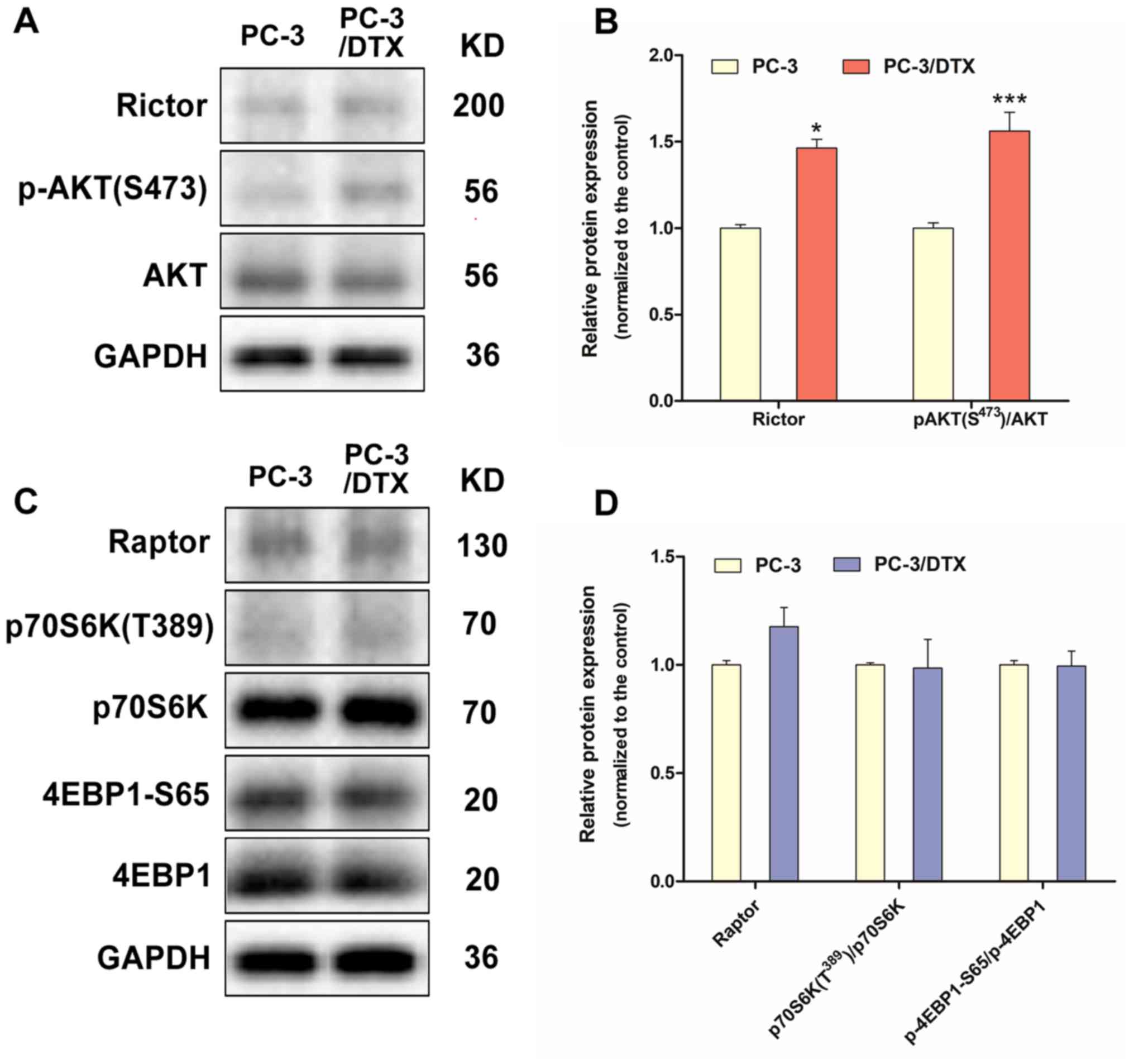 | Figure 5.Western blot analysis of protein
expression associated with the mTORC2 and mTORC1 signal pathway in
the docetaxel-resistant PC-3 cells. (A) The protein expression of
Rictor, p-Akt (S473) and Akt. (B) Quantification of protein
expression levels of Rictor, p-Akt (S473) and Akt. (C) The protein
expression of Raptor, p70 S6K1 (T389), p70 S6K1, 4E-BP1-S65 and
4E-BP1. (D) Quantification of the protein expression levels of
Raptor, p70 S6K1 (T389), p70 S6K1, 4E-BP1-S65 and 4E-BP1. Data are
presented as the mean ± standard deviation of independent
experiments performed in triplicate. *P<0.05, ***P<0.001 vs.
control. PC-3/DTX, docetaxel-resistance PC-3 cells; mTOR, mammalian
target of rapamycin; Akt, protein kinase B; p-Akt, phosphorylated
Akt; 4E-BP1, eukaryotic initiation factor 4E binding protein 1; p70
S6K1, p70 S6 kinase 1. |
Discussion
Chemotherapy is the main method of treatment for
cancer. However, the occurrence of drug resistance is one of the
main reasons for the poor effect of chemotherapy in patients with
cancer. In patients with mCRPC, docetaxel serves as the standard
first-line chemotherapy following failure of ADT. Docetaxel is a
microtubule stabilizer that may inhibit microtubule disassembly,
thereby leading to G2/M cell-cycle arrest and several forms of cell
death, including apoptosis and mitotic catastrophe (5). However, various mechanisms have been
studied to interpret the existence of drug resistance that may be
attributable for the unsatisfactory clinical outcomes in patients,
for which the activation of compensatory pro-survival signaling
pathways independent of the AR is one potential mechanism (5). Several preclinical studies have
demonstrated that docetaxel may increase Akt phosphorylation
(p-Akt) at S473, a direct downstream target of mTORC2, in prostate
cancer cells (8). In line with this,
compared with drug-naïve cells, prostate cancer cells that have
gained resistance to docetaxel after an extremely long-term drug
exposure exhibit greater expression of p-Akt (S473) (8). These findings indicated the possibility
that the mTORC2 pathway, as indicated by increased p-Akt, may cause
CRPC cells to become refractory to docetaxel, a putative mechanism
that has not previously been investigated.
Few studies have focused on prostate cancer
drug-resistant cell lines, which has impeded research on the
mechanism of drug resistance in prostate cancer. The establishment
of drug-resistant cell lines is important to study the biological
characteristics, drug-resistant mechanism, and methods to overcome
drug resistance.
In the present study, a docetaxel-resistant prostate
cancer cell line was established in vitro as a model to
investigate chemotherapy resistance by intermittently exposing
prostate cancer parental cells to a high concentration of docetaxel
with time-stepwise increments. The drug concentrations were
increased to select cancer cell with drug resistance; this method
may accurately simulate the biological changes of tumor resistance.
Compared with the parental PC-3 cells, PC-3/DTX cells showed
10.9-times more resistance to docetaxel, indicating that PC-3/DTX
cells exhibited significant drug resistance to docetaxel. In the
present study, the growth of resistant cells and parental cells was
found to be similar, and the Td of PC-3/DTX and PC-3
cells did not exhibit a significant difference (28.87 vs. 25.34 h).
The difference in the cell proliferation rate was marked with 3–5
days, meaning that PC-3/DTX cells showed a delay in the initiation
of the logarithmic growth. More PC-3/DTX cells were in the
G0/G1 phase and fewer cells were in the S
phase. A comparison of the biological characteristics of the PC-3
and PC-3/DTX cells revealed that the growth of the drug-resistant
cells was relatively slower, and the cell cycle of PC-3/DTX cells
was altered.
mTOR is an oncoprotein that is generally
dysregulated in human cancer. In prostate cancer, the mTOR pathway
is frequently hyperactivated primarily due to the loss of function
of the upstream tumor suppressor, phosphatase and tensin homolog
(PTEN), which occurs in ~40% of primary tumors and ~70% of
metastatic lesions (6,7). Consequently, aberrant mTOR expression
has been revealed to be associated with disease progression and
poor clinical outcomes in prostate cancer (9). Notably, PTEN loss is also found in up
to 80% of mCRPC patients (10),
suggesting a high prevalence. This clinical observation is
supported by the preclinical findings demonstrating that loss of
PTEN is a driving force for developing castration resistance in
mice (11). In line with this,
cumulating evidence strongly indicates that Akt/mTOR signaling,
which is PTEN downstream, is activated in advanced prostate cancer,
particularly in CRPC (9).
It has been reported that the mTOR pathway serves a
crucial role in cancer; it regulates cell growth and cell survival.
Two distinct protein complexes, mTOR complex 1 (mTORC1) and mTOR
complex 2 (mTORC2), are involved in the regulation of cell
function. Therefore, the present study evaluated the protein
expression of the mTOR signaling pathway in docetaxel-resistant
PC-3/DTX cells. Functionally, mTOR serves a pleiotropic role in the
regulation of malignant phenotypes, including proliferation,
metabolism, angiogenesis and drug resistance. At the molecular
level, mTOR functions as two distinct complexes, mTORC1 and 2,
downstream of PI3K signaling. Raptor and Rictor are two key
proteins composing mTORC1 and 2, respectively. mTORC1
phosphorylates two downstream targets, p70 S6K1 and eukaryotic
initiation factor 4E binding protein 1 (4E-BP1), regulating protein
translation, cell size, cell proliferation, and survival. By
contrast, mTORC2 may directly phosphorylate AGC family proteins,
including Akt and PKCα, and regulates cell survival, cytoskeleton
rearrangement, and cell migration (6,12).
Preclinical studies have demonstrated that mTORC1 promotes tumor
growth, invasion, and angiogenesis in prostate cancer models
(13–16). Notably, mTORC2 is critical for the
prostate cancer tumorigenesis driven by PTEN loss, but is
dispensable for normal prostatic epithelial functions (17), supporting the selective role of
mTORC2 in prostate cancer. The results of the present study
demonstrated that PC-3/DTX cells exhibited stronger mTORC2 activity
and downstream signaling pathways. This new finding suggested that
the mTORC2 signaling pathway may serve an important role in the
regulation of the docetaxel resistance of PC-3 cells. In future
studies, the impact of mTORC2 signaling on the acquired docetaxel
resistance of CRPC cells and the potential of agents targeting
mTORC2 in reversing docetaxel resistance in vitro should be
investigated.
In conclusion, a stable docetaxel-resistant PC-3
cell line was established. This cell line may serve as a useful
cell model to further study the molecular mechanisms of prostate
cancer drug resistance and may lead to the establishment of novel
therapeutic strategies for prostate cancer.
Acknowledgements
The authors would like to thank Ms Meijia Wu from
the Research Center for Clinical Pharmacy, Zhejiang Provincial Key
Laboratory for Drug Evaluation and Clinical Research, State Key
Laboratory for Diagnosis and Treatment of Infectious Disease, The
First Affiliated Hospital, Zhejiang University School of Medicine
(Hangzhou, China) for providing technical assistance.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
81702862).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JL was the principal investigator in the study and
was responsible for the literature search, study design, data
interpretation, and manuscript writing and revision. YH and DL were
the main executors of the study, contributed toward carrying out
the study, data analysis, and manuscript writing. YZ and XL
performed cell culture. YD participated in the design of the
present study and manuscript revision. DZ, LW and QZ participated
in the design of the present study and data interpretation. All
authors read and approved the final manuscript, and agreed to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the study were appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PC-3/DTX
|
docetaxel-resistant PC-3 cells
|
|
mTOR
|
mammalian target of rapamycin
|
|
Akt
|
protein kinase B
|
|
p-AKT
|
phosphorylated Akt
|
|
ADT
|
androgen deprivation therapy
|
|
CRPC
|
castration-resistant prostate
cancer
|
|
mCRPC
|
metastatic castration-resistant
prostate cancer
|
|
CCK8
|
Cell Counting kit-8
|
|
PI
|
propidium iodide
|
|
OD
|
optical density
|
|
IC50
|
50% inhibitory concentration
|
|
Td
|
growth curves and doubling time
|
|
PTEN
|
phosphatase and tensin homolog
|
|
mTORC1
|
mTOR complex 1
|
|
mTORC2
|
mTOR complex 2
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
p70 S6K1
|
p70 S6 kinase 1
|
|
4E-BP1
|
eukaryotic initiation factor 4E
binding protein 1
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pagliarulo V: Androgen deprivation therapy
for prostate cancer. Adv Exp Med Biol. 1096:1–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pienta KJ and Bradley D: Mechanisms
underlying the development of androgen-independent prostate cancer.
Clin Cancer Res. 12:1665–1671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seruga B, Ocana A and Tannock IF: Drug
resistance in metastatic castration-resistant prostate cancer. Nat
Rev Clin Oncol. 8:12–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taylor BS, Schultz N, Hieronymus H,
Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva
B, et al: Integrative genomic profiling of human prostate cancer.
Cancer Cell. 18:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kosaka T, Miyajima A, Shirotake S, Suzuki
E, Kikuchi E and Oya M: Long-term androgen ablation and docetaxel
up-regulate phosphorylated Akt in castration resistant prostate
cancer. J Urol. 185:2376–2381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morgan TM, Koreckij TD and Corey E:
Targeted therapy for advanced prostate cancer: Inhibition ofthe
PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 9:237–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lunardi A, Ala U, Epping MT, Salmena L,
Clohessy JG, Webster KA, Wang G, Mazzucchelli R, Bianconi M, Stack
EC, et al: A co-clinical approach identifies mechanisms
andpotential therapiesfor androgen deprivation resistance in
prostate cancer. Nat Genet. 45:747–755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mulholland DJ, Tran LM, Li Y, Cai H, Morim
A, Wang S, Plaisier S, Garraway IP, Huang J, Graeber TG and Wu H:
Cell autonomous role of PTEN in regulating castration-resistant
prostate cancer growth. Cancer Cell. 19:792–804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nardella C, Chen Z, Salmena L, Carracedo
A, Alimonti A, Egia A, Carver B, Gerald W, Cordon-Cardo C and
Pandolfi PP: Aberrant Rheb-mediated mTORC1 activation and Pten
haploinsufficiency are cooperative oncogenic events. Genes Dev.
22:2172–2177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clohessy JG, Reschke M and Pandolfi PP:
Found in translation of mTOR signaling. Cell Res. 22:1315–1318.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thoreen CC, Chantranupong L, Keys HR, Wang
T, Gray NS and Sabatini DM: Aunifying model for mTORC1-mediated
regulation of mRNA translation. Nature. 485:109–116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsieh AC, Liu Y, Edlind MP, Ingolia NT,
Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, et
al: The translationallandscape of mTOR signalling steers cancer
initiation and metastasis. Nature. 485:55–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guertin DA, Stevens DM, Saitoh M, Kinkel
S, Crosby K, Sheen JH, Mullholland DJ, Magnuson MA, Wu H and
Sabatini DM: mTOR complex 2 is required for the development
ofprostate cancer induced by Pten loss in mice. Cancer Cell.
15:148–159. 2009. View Article : Google Scholar : PubMed/NCBI
|