Introduction
Differentiated thyroid carcinoma (DTC) is a common
cancer among women with an increasing rate of incidence worldwide,
and the incidence rate of DTC is 2 to 8 per 100,000 women (1). Thyroid cancer is classified into
differentiated and undifferentiated types by histology, and DTC is
further classified as papillary thyroid cancer (PTC) or follicular
thyroid cancer (FTC) (2). The former
accounts for 75%, and the latter accounts for 16% of all thyroid
cancer cases (3). Although patients
with PTC usually have a favorable prognosis after standard
treatment, a small proportion (5–20%) of patients has a high risk
for disease recurrence and distant metastasis, resulting in
aggressive disease and lethal outcomes (4,5). Thus,
identification of a diagnostic and recurrence biomarker is
essential for optimal survival of patients with DTC.
Angiopoietin-like protein (ANGPTL) family members
participate in multiple biological processes such as lipid
metabolism (6–8), angiogenesis (9), hematopoietic stem cell expansion
(10), cancer progression (11) and inflammation (12). ANGPTL1 is the first discovered member
of the ANGPTL family and a potent regulator of angiogenesis
(13). As a key anti-angiogenic
protein, ANGPTL1 inhibits the proliferation, migration, tube
formation and adhesion of endothelial cells (14–16). Low
levels of ANGPTL1 expression in lung and breast cancer tissues have
been associated with an advanced stage of cancer, high tumor grade
and lymph node status, as well as a poor prognosis (17,18).
In vitro and in vivo studies have demonstrated that
ANGPTL1 has an effect on colorectal cancer cell migration,
invasion, proliferation and colony formation, although this effect
is limited (19). In hepatocellular
carcinoma, ANGPTL1 interacts with integrin
α1β1 to suppress angiogenesis and metastasis
by inhibiting janus kinase (JAK) 2/STAT3 signaling (20). Taken together, these findings suggest
that ANGPTL1 may be a novel tumor suppressor candidate for lung,
breast and colorectal cancer as well as hepatocellular carcinoma.
However, little is known about the effects of ANGPTL1 on thyroid
cancer malignancy or recurrence.
Thus, the present study aimed to investigate the
expression levels of ANGPTL1 in the tissues and plasma of patients
with benign thyroid nodules and DTC. Furthermore, the impact of
ANGPTL1 on DTC cell proliferation and metastasis were assessed to
investigate whether ANGPTL1 may be used as a novel predictive
biomarker for DTC diagnosis and recurrence.
Materials and methods
Datasets and thyroid cancer
samples
Serum samples were obtained from 54 individuals
(mean age, 49.48 years; age range, 31–66 years), including 26
patients with benign thyroid nodules and 28 patients with DTC at
Beijing Luhe Hospital (Beijing, China) between December 2016 and
December 2017. The present study was approved by the Research
Ethics Board of Beijing Luhe Hospital and was performed according
to the World Medical Association Declaration of Helsinki. All
individuals provided written informed consent. Serum samples were
stored at −80°C until further analysis.
mRNA expression data (RNA Seq v2) and clinical
information of patients in The Cancer Genome Atlas (TCGA) thyroid
cancer data set were downloaded from the Synapse (https://www.synapse.org) and cBioPortal (www.cbioportal.org) databases and used for
differential mRNA expression and prognosis analyses. 501 thyroid
cancer (492 cases of DTC and 9 cases of other histological type)
and 58 adjacent thyroid tissues were included in TCGA thyroid
cancer dataset. In TCGA dataset, ‘01’ representeda primary solid
tumor, and ‘11’ represented paracancerous tissue. The Gene
Expression Omnibus (GEO) datasets GSE3678 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE3678)
(21) and GSE3467 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE3467)
(22) were also used for ANGPTL1
expression analysis. The data from TCGA dataset were divided into
high (n=250) and low (n=251) ANGPTL1 expression groups based on the
median values of ANGPTL1 RNA-seq quantification results and further
analyzed using GSEA.
Cell culture and transfection
Cells were purchased from the China Infrastructure
of Cell Line Resources, Institute of Basic Medical Sciences,
Chinese Academy of Medical Sciences. TPC-1 DTC cells were cultured
in RPMI-1640 medium with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc.), in a 37°C/5% CO2 incubator. Cell transfection was
performed with Lipofectamine® 2000 (Invitrogen; Thermo
FisherScientific, Inc.) according to the manufacturer's
instructions. Small interfering RNA duplexes, siRNA-ANGPTL1 (cat.
no. sc-88171) and siRNA-negative control (cat. no. sc-37007) were
purchased from SantaCruz Biotechnology, Inc. Full-length cDNA
encoding human ANGPTL1 were cloned into the vector GV366 plasmid
(Shanghai Genechem Co.) with a HA-tag. And the vector GV366 plasmid
with a HA-tag was used as vector control (Shanghai GeneChem Co.,
Ltd.). The cells were treated with 10% FBS at 37°C for 48 h, then
the medium was removed, and the cells were harvested in sodium
dodecyl sulfate (SDS) sample buffer and analyzed by western
blotting.
Western blotting
Cells were lysed in ice-cold RIPA buffer (150 mM
sodium chloride, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1%
SDS, 50 mM Tris, protease and phosphatase inhibitor cocktail; Roche
Diagnostics). Protein concentrations were measured using a BCA
assay kit (Thermo Fisher Scientific, Inc.). Cell lysates were
heated for 10 min at 95°C. Equal amount of protein lysates (30 µg)
were separated on 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (0.22 µm). The blots were blocked in 5%
non-fat dry milk in TBS + 0.05% Tween-20 (TBST) buffer for 1–2 h at
room temperature and then incubated with primary antibodies against
ANGPTL1 (1:1,000; cat. no. sc-365146, Santa Cruz Biotechnology),
hemagglutinin tag (HA) (1:5,000; cat. no. 561, MBL International
Co.) and GAPDH (1:2,000; cat. no. TA-08; OriGene Technologies,
Inc.) overnight at 4°C. The blots were washed four times for 5 min
with TBST buffer and incubated with a horseradish peroxidase
(HRP)-conjugated anti-mouse IgG or anti-rabbit IgG secondary
antibody (1:3,000; cat. nos. TA130001 and TA130015, OriGene
Technologies, Inc.) for 1 h at room temperature. The blots were
washed four times for 5 min with TBST buffer and visualized using
an electrochemiluminescence (ECL) kit (Applygen Technologies,
Inc.).
Proliferation assay
The Cell Counting Kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc.) was used to assess the cell
proliferation, according to the manufacturer's protocol. The cells
were seeded at a density of 2×103 cells/well in 96-well
plates and incubated for 0, 24, 48, 72, 96 and 120 h. Cell medium
with CCK-8 (10%; 100 ul) was added per well, then incubated for 1 h
in 37°C in 5% CO2. Viable cells were analyzed by CCK-8
assay using an Enspire microplate reader (PerkinElmer, Inc.) at 450
nm.
Wound healing assay
A total of 3×105 cells were seeded in
6-well plates and allowed to reach 90% confluence. Cell monolayers
were carefully scratched through the center of the well with a
sterile 200 µl pipette tip. The detached cells were removed by
washing twice with PBS, and the monolayers were maintained in
RPMI-1640 medium with 5% FBS and 1% penicillin/streptomycin for 6 h
at 37°C. Images of the wound were captured under a light microscope
(magnification, ×40). The width of wound surfaces for each group
was noted and measured using NIH Image 1.62 (National Institutes of
Health). The relative migration distance at the final time point
relative to the starting time point was quantified and analyzed
(n=4).
Transwell invasion assay
The cell invasion assay was performed using modified
Boyden chambers in 24-well plates with 8-mm pore inserts (BD
Biosciences) coated with 1 mg/ml Matrigel for 5 h at 37°C in 5%
CO2. A total of 5×104 cells were plated in
100 µl serum-free RPMI-1640 medium in the upper chamber. The lower
chamber contained 600 ml complete medium (RPMI-1640 medium with 5%
FBS and 1% penicillin/streptomycin). After 24-h incubation at 37°C,
the invaded cells were fixed with 4% paraformaldehyde for 15 min
and stained with 0.5% crystal violet for 15 min at room
temperature. For each experiment, the numbers of cells in five
randomly selected fields were counted under light microscopy at
×200 magnification.
Gene set enrichment analysis
ANGPTL1 expression levels were determined by Gene
Set Enrichment Analysis (GSEA) in gene sets were obtained from the
Molecular Signatures Database of the Broad Institute (http://software.broadinstitute.org/gsea/msigdb). Tests
were performed using the default settings with permutation numbers
set at 1,000. A false discovery rate (FDR) <0.25 was considered
to indicate a statistically significant difference.
Serum ANGPTL1 measurement
Serum ANGPTL1 levels were measured using a
commercially available human ELISA kit (cat. no. LS-F5677; Lifespan
BioSciences, Inc.) according to the manufacturer's instructions
with an intra-assay coefficient of variation (CV) ≤10% and an
inter-assay CV ≤12%.
Statistical analysis
Statistical analyses were performed using SPSS 18.0
(SPSS, Inc). Data are presented as the mean ± standard deviation.
Two-tailed unpaired student's t-test was used to compare
differences between two groups, while one-way analysis of variance
(ANOVA) followed by Tukey's post hoc test was used to compare
differences between multiple groups. χ2 test was used to
determine the association between ANGPTL1 expression and tumor
stages. One-way ANOVA followed by Sidak's multiple comparisons test
were used to assess the differences in cells proliferation.
Receiver operator characteristic (ROC) curve and area under the
curve (AUC) analyses were performed to assess the diagnostic
ability of serum ANGPTL1 to DTC. P<0.05 was considered to
indicate a statistically significant difference.
Results
Low ANGPTL1 expression is associated
with DTC occurrence and development
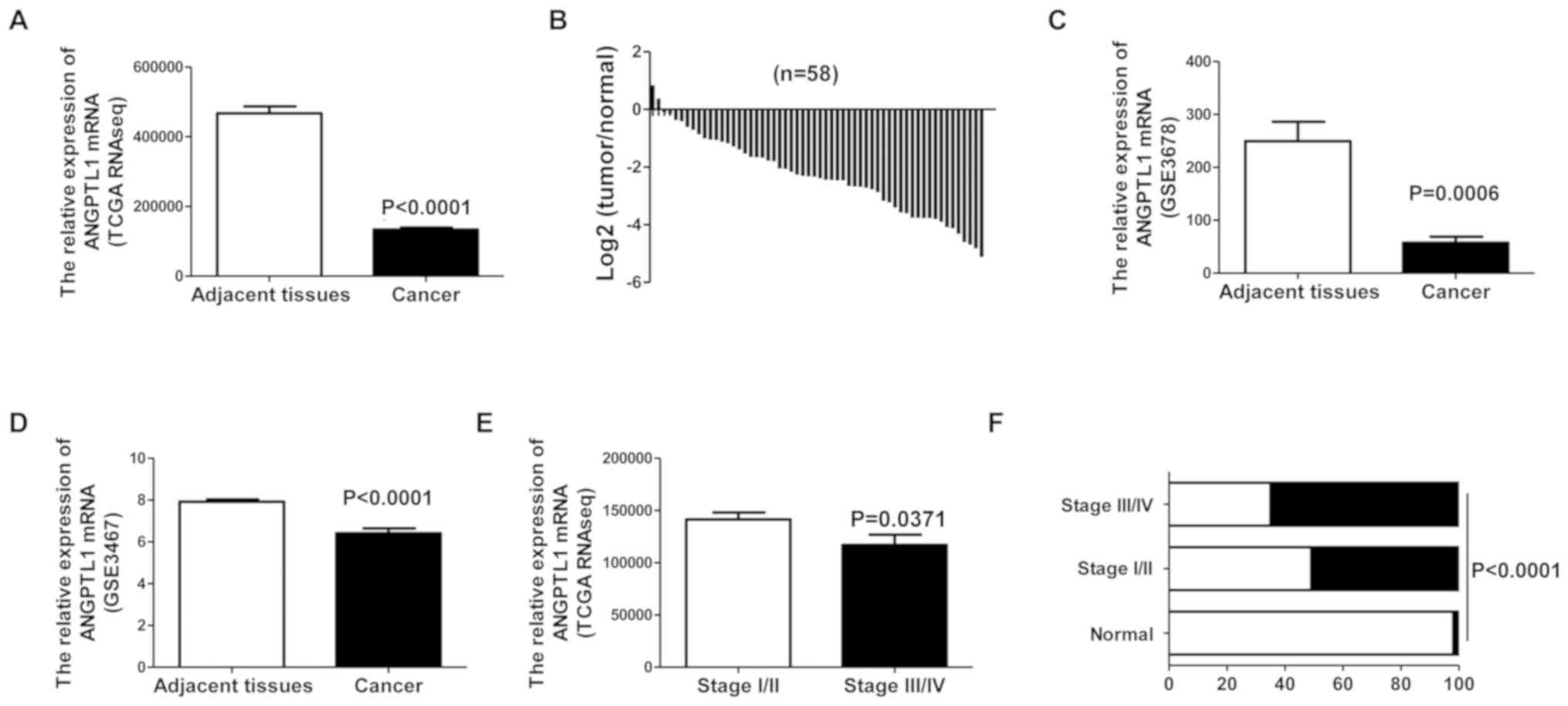
The mRNA expression of ANGPTL1 in DTC and adjacent
tissues was analyzed using TCGA RNA-seq data. ANGPTL1 expression
was downregulated in DTC tissue compared with that in adjacent
non-cancerous tissue (Fig. 1A and
B). To verify this result, ANGPTL1 expression levels were
assessed in the GEO thyroid cancer datasets. These results also
demonstrated that ANGPTL1 mRNA expression levels were downregulated
in DTC tissues compared with those in adjacent non-cancerous
thyroid tissues (Fig. 1C and D).
To explore the clinical relevance of ANGPTL1 in DTC,
the association between ANGPTL1 expression levels and tumor stage
was assessed in patients with different stages of DTC. Thyroid
cancer staging was determined using the 7th edition of the American
Joint Committee on Cancer Tumor-Node-Metastasis (AJCC-TNM) staging
system (23). The results
demonstrated that low ANGPTL1 expression was associated with
advanced tumor stages (Fig. 1E and
F).
ANGPTL1 decreases DTC cell
proliferation
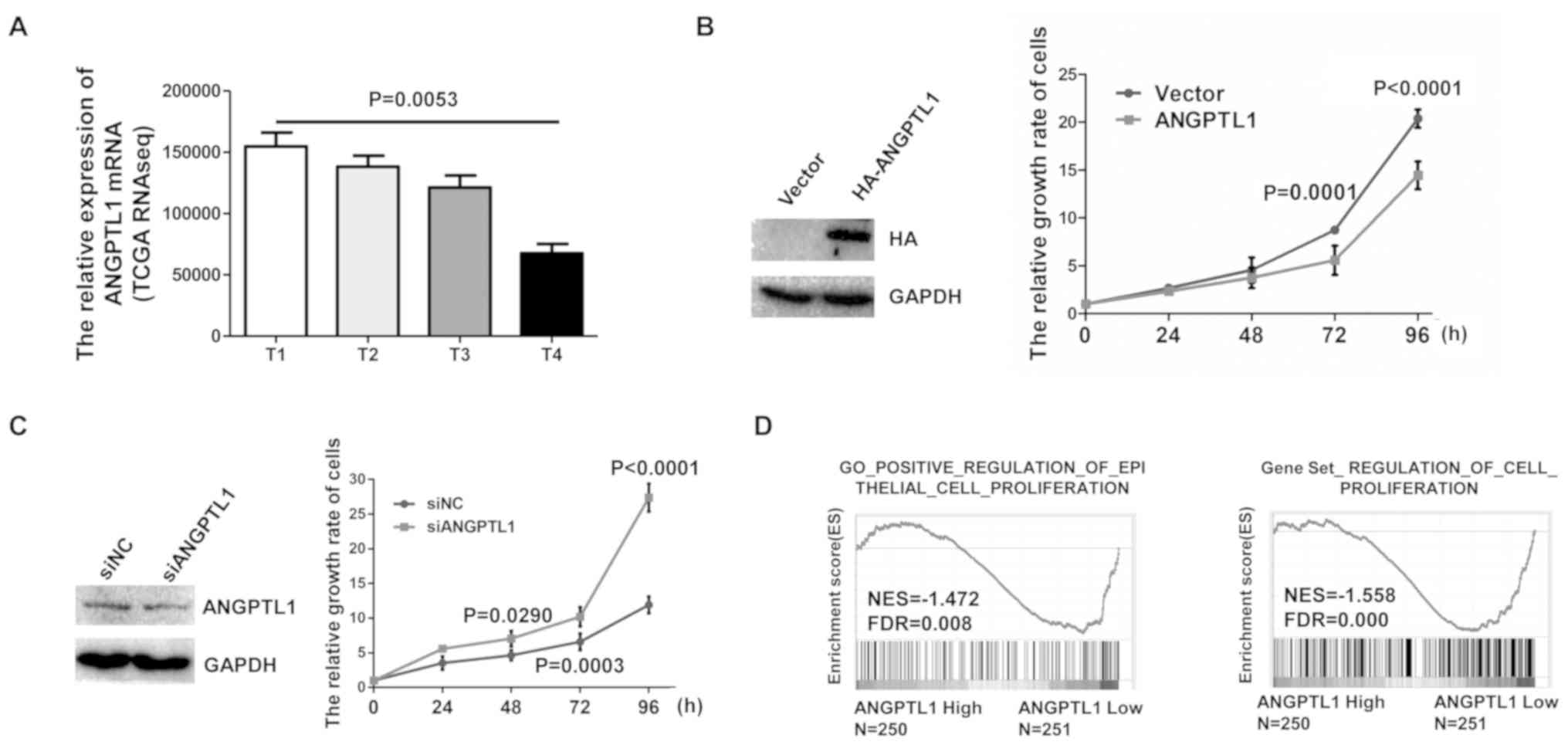
Tumor cell proliferation is a major representative
indicator of a malignant phenotype. RNA-seq data analytical results
demonstrated that ANGPTL1 mRNA levels were negatively associated
with the T stage of the primary tumor (Fig. 2A). The 7th edition of the TNM staging
system was used in the T stage of thyroid cancer (24). To determine the role of ANGPTL1 in
thyroid cancer cell proliferation, TPC-1 thyroid cancer cells were
transfected with the HA-ANGPTL1 plasmid. Overexpression of ANGPTL1
was confirmed by western blotting, and cell viability was
determined by CCK-8 assay. The results demonstrated that ANGPTL1
overexpression in TPC-1 cells reduced cell viability compared with
that of the vector group (Fig. 2B).
In addition, knockdown of ANGPTL1 in TPC-1 cells transfected with
siRNA significantly decreased ANGPTL1 expression and cell
proliferation compared with that of cells transfected with si-NC
(Fig. 2C). To determine the
relationship between the level of ANGPTL1 and signaling pathways
associated with proliferation in DTC, data from TCGA dataset were
divided into high and low ANGPTL1 expression groups as described in
the method. The results demonstrated that gene signatures for
proliferation were enriched in patients with low levels of ANGPTL1,
which suggested a negative association between ANGPTL1 levels and
the activation of proliferation signaling (Fig. 2D). Taken together, these results
suggested that ANGPTL1 expression may inhibit DTC cell
proliferation.
ANGPTL1 inhibits DTC cell migration
and invasion
Cell migration and invasion are important factors in
tumor metastasis that are associated with the prognosis for
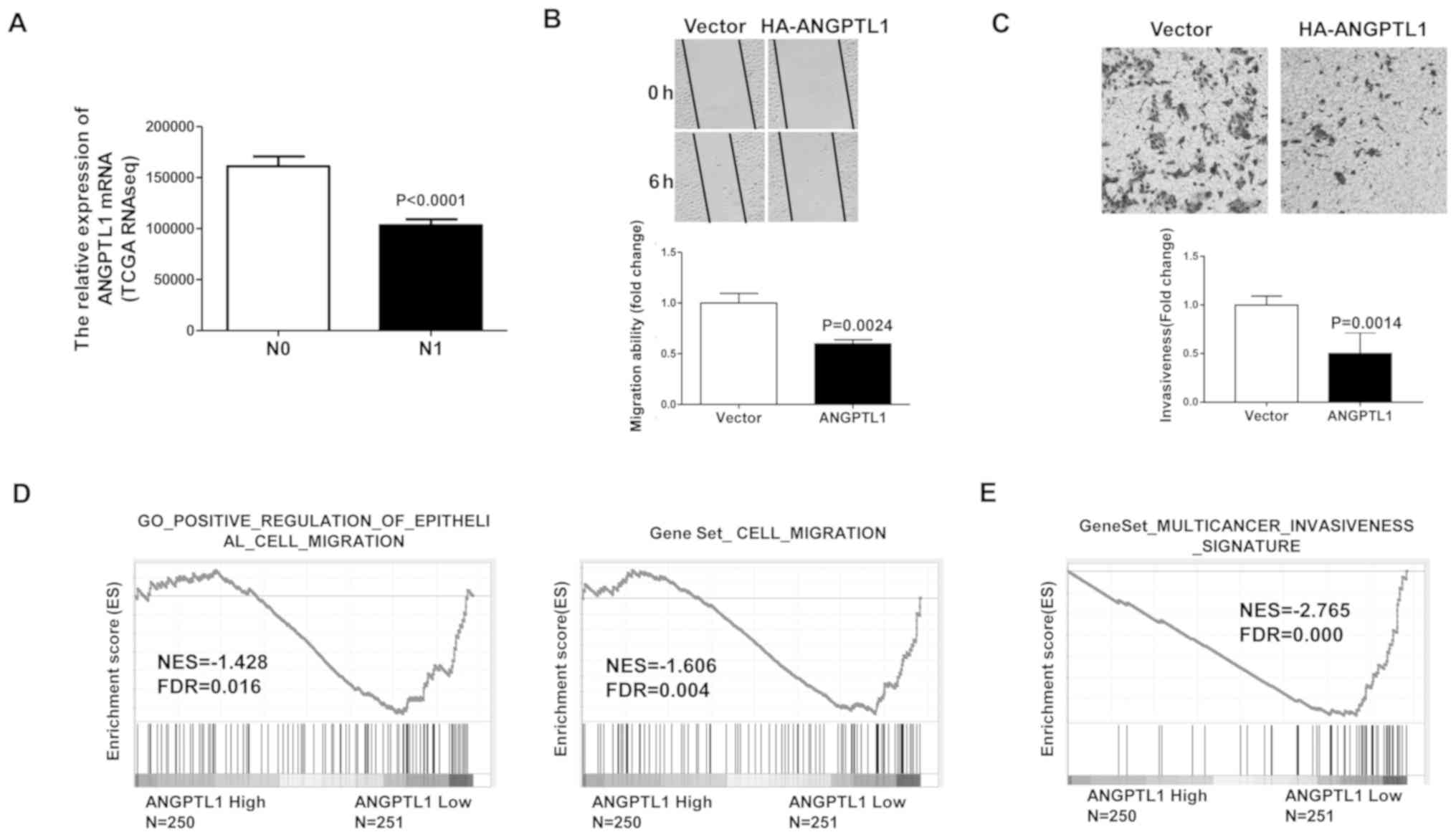
patients with thyroid cancer (25,26). The
ANGPTL1 mRNA levels were compared between DTC patients with N0 (no
lymphatic metastasis) and N1 (lymph node metastasis) stage using
TCGA data. The results demonstrated that the levels of ANGPTL1 mRNA
were downregulated in patients with N1 stage tumors compared with
those in patients with N0 stage tumors (Fig. 3A). DTC cell migration was evaluated
by wound healing assay to assess the role of ANGPTL2 in thyroid
cancer metastasis. The migration distance was significantly lower
in ANGPTL1-overexpressing cells compared with that in the vector
group (Fig. 3B). The effect of
ANGPTL1 on cell invasion was assessed by the Transwell invasion
assay. As displayed with staining of cells in Matrigel-coated
Boyden chambers, overexpression of ANGPTL1 significantly inhibited
the invasive ability of TCP-1 cells compared with that of the
control group (Fig. 3C). To
investigate the association between the level of ANGPTL1 mRNA and
activation of migration/invasion signaling in thyroid cancer
specimens, the high and low ANGPTL1 expression groups from TCGA
dataset were used. The results demonstrated that gene signatures
for migration/invasion were enriched in patients with low levels of
ANGPTL1, suggesting a negative association between ANGPTL1 levels
and the activation of migration/invasion signaling (Fig. 3D and E). These results suggested that
ANGPTL1 may inhibit the migration and invasion of DTC cells.
Low ANGPTL1 expression is associated
with DTC recurrence
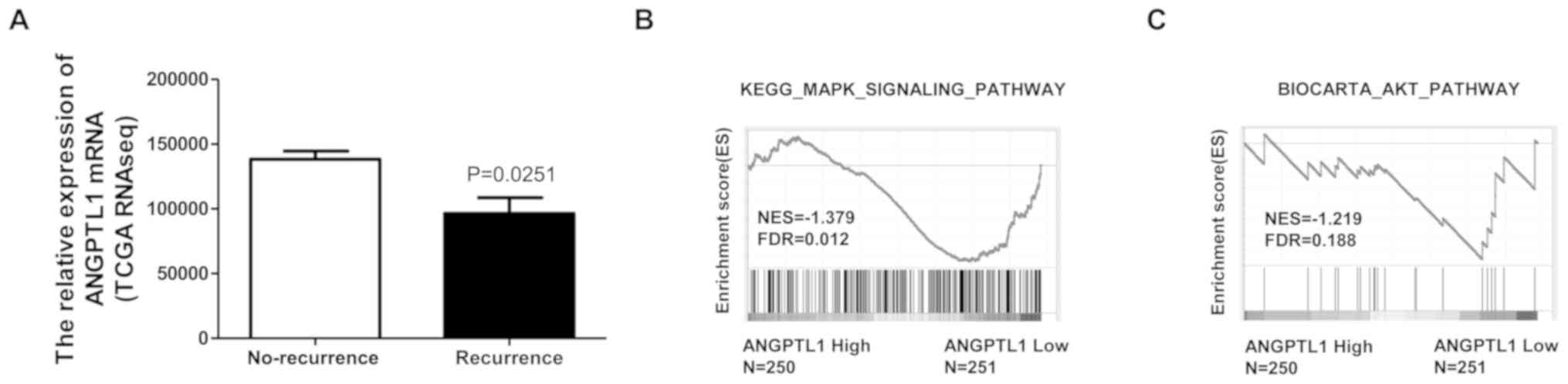
The association between ANGPTL1 expression and the
recurrence status of patients with DTC was examined using TCGA
dataset. Patients with recurrence exhibited significantly lower
ANGPTL1 expression compared with that in patients without
recurrence (Fig. 4A). The
mitogen-activated protein kinase (MAPK)/ERK1/2 and PI3K/Akt
signaling pathways have been reported to be associated with thyroid
cancer recurrence (27,28); thus, the relationship between ANGPLT1
levels and the activation of these signaling pathways was assessed
by GSEA. The results demonstrated that the gene signatures for the
MAPK/ERK1/2 and PI3K/Akt signaling pathways were enriched in
patients with low levels of ANGPTL1, suggesting a negative
association between ANGPTL1 levels and the activation of
MAPK/ERK1/2 and PI3K/AKT signaling (Fig.
4B and C). These results suggested that low ANGPTL1 levels may
promote the recurrence of DTC.
Serum ANGPTL1 is a potential biomarker
for the diagnosis of DTC
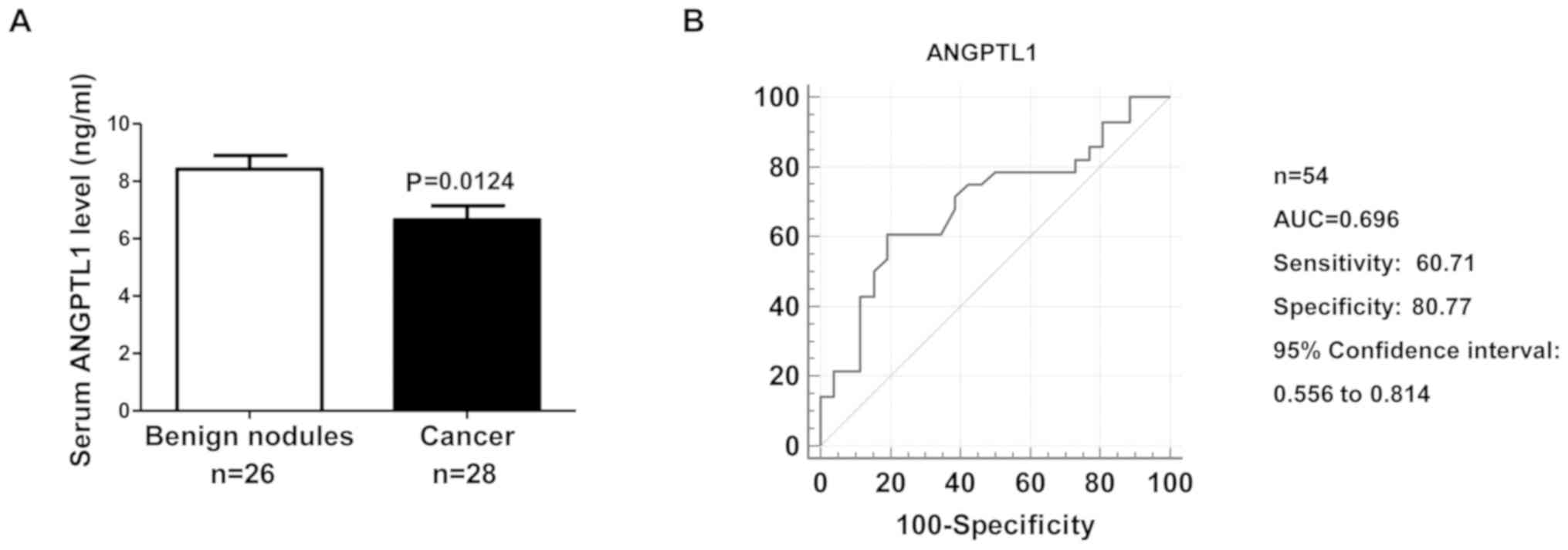
Serum ANGTPL1 levels were evaluated in patients with
DTC, the results demonstrated that serum ANGTPL1 was lower in
patients with DTC compared with that in patients with benign
thyroid nodules (Fig. 5A). The
results demonstrated that serum ANGPTL1 levels were a potential
biomarker for patients with DTC, with an AUC of 0.696 (95%
confidence interval: 0.556–0.814), a sensitivity value of 60.71%
and a specificity value of 80.77% (Fig.
5B).
Discussion
The incidence of thyroid cancer has risen sharply in
the past decade. Although the prognosis for most patients with DTC
is quite favorable after standard therapeutic approaches, the risk
of local recurrence and distant metastasis may be up to 20 and 10%,
respectively (29,30). At present, there are no specific and
sensitive molecular biomarkers for diagnosis or prognosis of DTC.
ANGPTL1 is involved in metastasis and invasion in breast, lung and
colorectal cancer, as well as hepatocellular carcinoma (17–20).
However, little is known about the effects of ANGPTL1 on the
malignant properties of thyroid cancer cells and whether ANGPTL1 is
involved in thyroid cancer recurrence.
A previous study has demonstrated that mRNA
expression of ANGPTL1 is significantly downregulated in prostate,
lung, kidney, thyroid and bladder cancer compared with that in
matched adjacent non-cancerous tissues (15). Smagur et al (16) reported an antitumor effect of
recombinant ANGPTL1 on murine melanoma cells. The results of the
present study demonstrated the mRNA levels of ANGPTL1 to be lower
in thyroid cancer compared with those in adjacent normal thyroid
tissue. In addition, ANGPTL1 levels further decreased with thyroid
tumor progression. These results suggested that ANGPTL1 may be
involved in the progression of thyroid cancer. In addition, the
present study examined ANGPTL1 concentrations in the serum of
patients with DTC and subjects with benign thyroid nodules; the
results demonstrated that serum ANGPTL1 levels were lower in
patients with DTC compared with those in individuals with benign
thyroid nodules. These results suggested that ANGPTL1 may be a
novel serum biomarker for the diagnosis of DTC.
Tumor cell angiogenesis, proliferation, migration
and invasion contribute factors to cancer progression (31). The results of the present study
demonstrated that ANGPTL1 overexpression in TPC-1 cells inhibited
cellular proliferation, while ANGPTL1 knockdown with small
interfering RNA increased cellular proliferation. ANGPTL1
overexpression in TPC-1 cells decreased cellular migration and
invasion, which was also reported in other types of tumor cells,
such as lung cancer, colorectal cancer and hepatocellular carcinoma
(17,19,20).
Dhanabal et al (15)
demonstrated that human HT1080 fibrosarcoma cells engineered to
ectopically overexpress ANGPTL1 (HT1080-ANGPTL1) exhibited lower
tumorigenicity following intravenous injection into nude mice, with
a reduction in the number and size of tumor nodules compared with
the control vector (HT1080-control). ANGPTL1 has been reported to
reduce lung cancer cell migration and invasion by regulating the
integrin α1β1/focal adhesion kinase
(FAK)/ERK/specificity protein 1/miR-630/zinc finger protein SNAI2
(Slug) signaling axis (17). In
addition, ANGPTL1 inhibits hepatocellular carcinoma sorafenib
resistance and stemness by repressing the epithelial-mesenchymal
transition through the inhibition of the Met
receptor-AKT/ERK-Egr-1-Slug signaling cascade (32). ANGPTL1 promotes apoptosis and
suppresses hepatocellular carcinoma cell angiogenesis and
metastasis in vitro and in vivo by regulating the
integrin α1β1/FAK-Src/JAK/STAT3 signaling
axis (20). These results suggest
that ANGPTL1 represses tumor-associated angiogenesis, tumor growth,
metastasis and invasion.
In conclusion, the results of the present study
demonstrated that ANGPTL1 inhibited DTC cell proliferation and was
associated with DTC metastasis. Additionally, the expression of
ANGPTL1 was demonstrated to be lower in patients with DTC with
recurrence compared with that in patients with DTC without
recurrence. Thus, ANGPTL1 may be a novel biomarker for DTC
diagnosis and prognosis.
Acknowledgements
The authors would like to thank Dr Junqi He (Capital
Medical University, Beijing, China) for his academic support.
Additionally, the authors would like to thank Mr. Jianduo An
(Department of Pathology, Beijing Luhe Hospital, Capital Medical
University, Beijing, China) for his technical assistance.
Funding
This study was supported by the Beijing Natural
Science Foundation in China (grant no. 7184222) and National
Science Funding in China (grant no. 81800768).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable request.
The Cancer Genome Atlas (TCGA) thyroid cancer datasets were
downloaded from Synapse (https://www.synapse.org) and cBioPortal (www.cbioportal.org). The Gene Expression Omnibus (GEO)
datasets were from https://www.ncbi.nlm.nih.gov/geo.
Authors' contributions
RS and YH performed the in vitro experiments.
LY participated in the design of the study, performed western
blotting, statistical analysis and drafted the initial manuscript.
YW, QZ and YZ collected and curated the clinical data. ZJ and DZ
designed the study and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study complied with the Helsinki Declaration for
investigation of human subjects. Ethical approval was obtained from
the Institutional Review Boards of the Luhe Hospital, Capital
Medical University (Beijing, China; approval no. 2019LH-KS-074).
All the participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Viola D, Valerio L, Molinaro E, Agate L,
Bottici V, Biagini A, Lorusso L, Cappagli V, Pieruzzi L, Giani C,
et al: Treatment of advanced thyroid cancer with targeted
therapies: Ten years of experience. Endocr Relat Cancer.
23:R185–R205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel UJ and May M: Lenvatinib in the
treatment of differentiated thyroid cancer and advanced renal cell
carcinoma. J Adv Pract Oncol. 8:757–64. 2017.PubMed/NCBI
|
|
4
|
Oyer SL, Fritsch VA and Lentsch EJ:
Comparison of survival rates between papillary and follicular
thyroid carcinomas among 36,725 patients. Ann Otol Rhinol Laryngol.
123:94–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toniato A, Boschin I, Casara D, Mazzarotto
R, Rubello D and Pelizzo M: Papillary thyroid carcinoma: Factors
influencing recurrence and survival. Ann Surg Oncol. 15:1518–1522.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carbone C, Piro G, Merz V, Simionato F,
Santoro R, Zecchetto C, Tortora G and Melisi D: Angiopoietin-like
proteins in angiogenesis, inflammation and cancer. Int J Mol Sci.
19:4312018. View Article : Google Scholar
|
|
7
|
Cinkajzlová A, Mráz M, Lacinová Z,
Kloučková J, Kaválková P, Kratochvílová H, Trachta P, Křížová J,
Haluzíková D, Škrha J, et al: Angiopoietin-like protein 3 and 4 in
obesity, type 2 diabetes mellitus, and malnutrition: The effect of
weight reduction and realimentation. Nutr Diabetes. 8:212018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geladari E, Tsamadia P and Vallianou NG:
ANGPTL3 inhibitors-their role in cardiovascular disease through
regulation of lipid metabolism. Circ J. 83:267–273. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hato T, Tabata M and Oike Y: The role of
angiopoietin-like proteins in angiogenesis and metabolism. Trends
Cardiovasc Med. 18:6–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug
C and Lodish HF: Angiopoietin-like proteins stimulate ex vivo
expansion of hematopoietic stem cells. Nat Med. 12:240–245. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC,
Pal M, Lam CR, Boukamp P, Pan JY, Tan SH, et al: Angiopoietin-like
4 protein elevates the prosurvival intracellular
O2(−):H2O2 ratio and confers
anoikis resistance to tumors. Cancer Cell. 19:401–415. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tabata M, Kadomatsu T, Fukuhara S, Miyata
K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, et al:
Angiopoietin-like protein 2 promotes chronic adipose tissue
inflammation and obesity-related systemic insulin resistance. Cell
Metab. 10:178–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim I, Kwak HJ, Ahn JE, So JN, Liu M, Koh
KN and Koh GY: Molecular cloning and characterization of a novel
angiopoietin family protein, angiopoietin-3. FEBS Lett.
443:353–356. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dhanabal M, Jeffers M, LaRochelle WJ and
Lichenstein HS: Angioarrestin: A unique angiopoietin-related
protein with anti-angiogenic properties. Biochem Biophys Res
Commun. 333:308–315. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dhanabal M, LaRochelle WJ, Jeffers M,
Herrmann J, Rastelli L, McDonald WF, Chillakuru RA, Yang M, Boldog
FL, Padigaru M, et al: Angioarrestin: An antiangiogenic protein
with tumor-inhibiting properties. Cancer Res. 62:3834–3841.
2002.PubMed/NCBI
|
|
16
|
Smagur A, Szary J and Szala S: Recombinant
angioarrestin secreted from mouse melanoma cells inhibits growth of
primary tumours. Acta Biochim Pol. 52:875–879. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo TC, Tan CT, Chang YW, Hong CC, Lee WJ,
Chen MW, Jeng YM, Chiou J, Yu P, Chen PS, et al: Angiopoietin-like
protein 1 suppresses SLUG to inhibit cancer cell motility. J Clin
Invest. 127:4202017. View
Article : Google Scholar
|
|
18
|
Sasaki H, Moriyama S, Sekimura A, Mizuno
K, Yukiue H, Konishi A, Yano M, Kaji M, Fukai I, Yamakawa Y and
Fujii Y: Angioarrestin mRNA expression in early-stage lung cancers.
Eur J Surg Oncol. 29:649–653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Xiao Q, Hu Y, Chen L, Jiang K,
Tang Y, Tan Y, Hu W, Wang Z, He J, et al: ANGPTL1 attenuates
colorectal cancer metastasis by up-regulating microRNA-138. J Exp
Clin Cancer Res. 36:782017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan Q, Jiang L, Liu M, Yu D, Zhang Y, Li
Y, Fang S, Li Y, Zhu YH, Yuan YF and Guan XY: ANGPTL1 Interacts
with Integrin alpha1beta1 to Suppress HCC Angiogenesis and
Metastasis by Inhibiting JAK2/STAT3 Signaling. Cancer Res.
77:5831–5845. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang C, Bo C, Guo L, Yu P, Miao S and Gu
X: BCL2 and hsa-miR-181a-5p are potential biomarkers associated
with papillary thyroid cancer based on bioinformatics analysis.
World J Surg Oncol. 17:2212019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Gao S, Jin Y, Yang Y, Tai J, Wang
S, ang H, Chu P, Han S and Lu J: Bioinformatics analysis to screen
key genes in papillary thyroid carcinoma. Oncol Lett. 19:195–204.
2020.PubMed/NCBI
|
|
23
|
Tuttle RM, Haugen B and Perrier ND:
Updated American Joint Committee on Cancer/Tumor-Node-Metastasis
staging system for differentiated and anaplastic thyroid cancer
(Eighth Edition): What changed and Why? Thyroid. 27:751–756. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adam MA, Thomas S, Roman SA, Hyslop T and
Sosa JA: Rethinking the Current American Joint Committee on Cancer
TNM staging system for medullary thyroid cancer. JAMA Surg.
152:869–876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang Z, Cai C, Han D, Gao Y, Li Q, Feng
L, Zhang W, Zheng J, Jin J, Zhang H and Wei Q: Anoctamin5 regulates
cell migration and invasion in thyroid cancer. Int J Oncol.
51:1311–1319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shang H, Wang S, Yao J, Guo C, Dong J and
Liao L: Salidroside inhibits migration and invasion of poorly
differentiated thyroid cancer cells. Thorac Cancer. 10:1469–1478.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen DL, Hu ZQ, Zheng XF, Wang XY, Xu YZ,
Li WQ, Fang HS, Kan L and Wang SY: EDAG-1 promotes proliferation
and invasion of human thyroid cancer cells by activating MAPK/Erk
and AKT signal pathways. Cancer Biol Ther. 17:414–421. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yarchoan M, Ma C, Troxel AB, Stopenski SJ,
Tang W, Cohen AB, Pappas-Paxinos M, Johnson BA III, Chen EY,
Feldman MD and Brose MS: pAKT expression and response to sorafenib
in differentiated thyroid cancer. Horm Cancer. 7:188–195. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Durante C, Haddy N, Baudin E, Leboulleux
S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De
Vathaire F and Schlumberger M: Long-term outcome of 444 patients
with distant metastases from papillary and follicular thyroid
carcinoma: Benefits and limits of radioiodine therapy. J Clin
Endocrinol Metab. 91:2892–2899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Liu Y, Lin Y and Liang J:
Radioactive Iodine-refractory differentiated thyroid cancer and
redifferentiation therapy. Endocrinol Metab (Seoul). 34:215–225.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang RH, Wang S, Zhang H, Lan JJ, Xu GB,
Zhao YL, Wang L, Li YJ, Wang YL, Zhou YH, et al: Discovery of
tetrandrine derivatives as tumor migration, invasion and
angiogenesis inhibitors. Bioorg Chem. 101:1040252020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen HA, Kuo TC, Tseng CF, Ma JT, Yang ST,
Yen CJ, Yang CY, Sung SY and Su JL: Angiopoietin-like protein 1
antagonizes MET receptor activity to repress sorafenib resistance
and cancer stemness in hepatocellular carcinoma. Hepatology.
64:1637–1651. 2016. View Article : Google Scholar : PubMed/NCBI
|