Introduction
Nasopharyngeal carcinoma (NPC) is a form of head and
neck cancer caused by the cancerization of the nasopharynx
epithelium, which demonstrates high incidence rates in Southern
China, North Africa and Southeast Asia (1,2). NPC is
currently classified into three groups, including keratinizing
carcinoma (K-NPC), non-keratinizing carcinoma (NK-NPC) and basaloid
squamous carcinoma (BSCC) (3). K-NPC
has a solely keratinizing phenotype, characterized by variable
keratin formation and intercellular junctions (4); NK-NPC presents with a similar phenotype
to squamous metaplasia and exhibits ulceration of the overlying
epithelium and BSCC presents with a biphasic pattern and stromal
hyalinization (5). Significant
advances have been made in the treatment of NPC; however, the
5-year survival rate of NPC patients with advanced stage has not
significantly improved, and it remains at ~30.3–73.6% worldwide
since 2015 (6,7). Therefore, there is a need to determine
the important regulators and the underlying mechanisms of NPC
progression to improve the prognosis and survival rate of patients
with NPC.
Long non-coding RNAs (lncRNAs) are a class of RNA
transcripts of >200 nucleotides in length, which have no
protein-coding ability (8). The
dysregulation of lncRNAs is closely associated with the occurrence
and development of various types of cancer, for example the
increased expression levels of the lncRNA HULC predicted a poor
prognosis and accelerated tumor progression in prostate cancer
(9). In addition, lncRNA PICART1 was
reported to inhibit the progression of non-small cell lung cancer
cells through inactivation of the AKT1 signaling pathway (10), and lncRNA XIST is associated with a
poor prognosis and promotes a malignant phenotype in osteosarcoma
(11). Recently, KIF9-AS1 was also
reported to be a biomarker for inflammatory bowel disease (12). However, to the best of our knowledge,
there are currently no relevant studies regarding the role of
KIF9-AS1 in NPC.
MicroRNAs (miRNAs/miRs) are a family of small
non-coding RNAs of ~22 nucleotides in length, which regulate gene
expression by complementary binding or complex mechanisms (13–15).
miRNAs have been reported to be aberrantly expressed in NPC, where
they have been found to regulate NPC cell proliferation, invasion
and metastasis (16). Therefore,
investigations into cancer-associated miRNAs in NPC may help to
identify effective novel targets for NPC therapy.
The present study aimed to determine the potential
mechanisms of KIF9-AS1 in NPC. These findings may provide novel
insights into the progression of NPC and help to develop novel
therapeutics for the treatment of NPC.
Materials and methods
Patient studies
The present study was approved by the Ethics
Committee of Weifang Traditional Chinese Hospital (Weifang, China;
approval no. 2010A121005) and written informed consent was provided
by all patients. In total, 25 pairs of NPC and adjacent normal
tissues (2-cm adjacent to tumor) were collected from 25 patients
with NPC (19 males and 6 females) with a mean age of 41 years
(range, 28–63 years) between February 2011 and August 2012 in
Weifang Traditional Chinese Hospital (Weifang, China). The total
duration of follow-up was 6 years and follow-up was conducted over
the phone or in an outpatient clinic. The patients' clinical data
are presented in Table I. NPC stage
was classified according to the seventh edition of the AJCC staging
system (17).
 | Table I.Clinicopathological characteristics of
patients with nasopharyngeal carcinoma. |
Table I.
Clinicopathological characteristics of
patients with nasopharyngeal carcinoma.
| Clinicopathological
features | Value |
|---|
| Mean age (range),
years | 41 (28–63) |
| Sex (male/female),
n | 19/6 |
| Degree of
differentiation (undifferentiated/differentiated), n | 17/8 |
| Histology
(squamous/others), n | 25/0 |
| Lymph node metastasis
(+/−), n | 18/7 |
| Distal metastasis
(+/−), n | 0/25 |
| Clinical TNM stage
(I–II/III–IV), n | 9/16 |
Cell culture and transfection
NPC cells (SUNE1 and SUNE2), the human normal
nasopharyngeal epithelial cell line NP69 and HEK293T cells were
purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. The cells were cultured in DMEM
(Corning Inc.), supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), and maintained in an atmosphere of 5%
CO2 and constant humidity at 37°C.
For the transfection, short hairpin (sh)RNAs
targeting KIF9-AS1 (shKIF9-AS1, 5′-GGAAUGCAGCUGAAAGAUUGC-3′) or
scrambled negative control (shNC, 5′-AAUUCUCCGAACGUGUCACGU-3′) were
transfected into SUNE1 and SUNE2 cells. The miR-16 mimic
(5′-UAGCAGCACGUAAAUAUUGGUG-3′) and the scrambled negative control
(NC mimics, 5′-UACACCGAUCGAGUCAGGUUU-3′), and miR-16 inhibitor
(5′-CACCAAUAUUUACGUGCUGCUA-3′) and the scrambled negative control
(NC inhibitor; 5′-UCGAGACACGUACGCAGAAUU-3′) were synthesized by
Shanghai GeneChem Co., Ltd. Cell transfections were performed using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Subsequent experiments were performed 48 h post-transfection.
Wound healing assay
The transfected SUNE1 and SUNE2 cells were plated
into 6-well plates and cultured until 70% confluence for the wound
healing assay. Subsequently, a 200-µl pipette tip was used to
generate a single wound in the cell monolayer. Cells were washed
twice with DMEM and incubated with DMEM supplemented with 1% FBS
for 24 h at 37°C. Images of the migrating cells were acquired at 0
and 24 h using a light microscope (magnification, ×200; Leica
DMI4000B; Leica Microsystems, Ltd.) and measured using ImageJ
software version 1.8 (National Institutes of Health).
Transwell assay
The migration and invasion of the NPC cells was
analyzed using Transwell chambers (8.0-µm pore size; EMD Millipore)
and Matrigel (Corning Inc.). The transfected SUNE1 and SUNE2 cells
were plated in the upper chambers of the Transwell plates in
serum-free medium. Transwell membranes were precoated with Matrigel
for 1 h at room temperature. A volume of 600 µl DMEM, supplemented
with 10% FBS, was plated in the lower chambers. Following
incubation for 48 h at 37°C, the non-invasive cells in the upper
chamber were removed and invasive cells in the lower chamber were
fixed in 4% paraformaldehyde and stained with 0.1% crystal violet
(Beyotime Institute of Biotechnology) both for 20 min at room
temperature. For the migration assay, the same assay was performed;
however, the Transwell membranes were not coated in Matrigel.
Stained cells were counted using a light microscope (magnification,
×200; Zeiss GmbH).
Cell Counting Kit-8 (CCK-8) assay
Cell viability was determined using the CCK-8 assay
kit (Dojindo Molecular Technologies, Inc.) according to the
manufacturer's instructions. Transfected SUNE1 and SUNE2 cells were
cultured for 48 h, harvested and plated into 96-well plates at a
density of 2×103 cells/well. Cells were incubated with
100 µl DMEM for 48 h at 37°C and 5% CO2. Following the
incubation, 10 µl CCK-8 solution was added/well and incubated at
37°C for 2 h. The absorbance of each well was measured at a
wavelength of 450 nm using a microplate reader.
Bioinformatic prediction and
dual-luciferase reporter assay
StarBase 2.0 (http://starbase.sysu.edu.cn) was used to predict the
downstream target of KIF9-AS1. The pmirGLO-KIF9-AS1-wild-type
(WT)/mutant (Mut) reporter was purchased from Shanghai GenePharma
Co., Ltd. 293T cells were used for the dual-luciferase reporter
assay due to the high transfection efficiency (18). Subsequently, 293T cells were
co-transfected with the miR-16 mimic and the
pmiRGLO-KIF9-AS1-WT/Mut reporter. Transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Following incubation for 48 h at 37°C, the
relative luciferase activity was analyzed using a dual-luciferase
reporter assay system (Promega Corporation) and normalized to
Renilla luciferase activity.
Reverse transcription-quantitative
PCR
Total RNA was extracted from SUNE1 and SUNE2 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was reverse transcribed into cDNA
using the Reverse Transcription kit (Takara Bio, Inc.), according
to the manufacturer's protocol. qPCR was subsequently performed
using the SYBR Select Master mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on the ABI 7300 system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following thermocycling
conditions were used for qPCR: Initial denaturation at 95°C for 3
min; 40 cycles of 95°C for 5 sec and 60°C for 30 sec. The following
primer sequences were used for the qPCR: KIF9-AS1, Forward:
5′-CAGCACTGACTACACTGGGA-3′ and reverse: 5′-GCCCTCTTCTTCCTCCACAT-3′;
miR-16, forward: 5′-TAGCAGCACGTAAATATTGGCG-3′ and reverse:
5′-TGCGTGTCGTGGAGTC-3′; GAPDH, forward: 5′-GAGTCAACGGATTTGGTCGT-3′
and reverse: 5′-TTGATTTTGGAGGGATCTCG-3′; U6, forward:
5′-CTCGCTTCGGCAGCACATATACTA-3′ and reverse:
5′-ACGAATTTGCGTGTCATCCTTGCG-3′. The relative expression levels of
the mRNAs were calculated using the 2−∆∆Cq method
(19) and normalized to GAPDH and
U6, the endogenous controls.
Xenograft experiment
Eight male BALB/c nude mice (6-weeks old; ~20 g)
were acquired from the Laboratory Animal Center of Nanjing Medical
University and randomly divided into two groups with four in each
group. The mice were maintained under specific pathogen-free
conditions, with free access to water and food, at room temperature
of 26–28°C and humidity of 60±10%, and under a 12 h light/dark
cycle. Each group of mice was injected subcutaneously into the
right flank of nude mice with SUNE1 cells (5×106)
transfected with shKIF9-AS1 or shNC. After 35 days, the mice were
sacrificed by cervical dislocation after deep anesthesia (confirmed
by normal rectal temperature, respiratory rate and sleeping state)
with 2% isoflurane (Baxter Healthcare Corporation). The length (L),
width (W) and weight of tumors were measured and tumor volume was
calculated by the following formula: V = ½ × L × W2.
Animal experimental protocols were approved by the Animal Welfare
Committee of Weifang Traditional Chinese Hospital (Weifang, China),
and all methods were conducted according to the guidelines
(20).
Statistical analysis
Statistical analysis was performed using SPSS
version 16.0 (SPSS, Inc.). Data are represented as the mean ±
standard deviation of three independent experimental repeats.
Comparisons among multiple groups were performed using one-way
analysis of variance, followed by Tukey's post hoc test. Comparison
between NPC and adjacent normal tissue samples from patients with
NPC was performed using a paired Student's t-test, while
comparisons between the experimental and control groups was
performed using an unpaired Student's t-test. The correlation
between the mRNA expression levels was analyzed using Pearson's
correlation coefficient. The overall survival rates and the
survival curve were determined using the Kaplan-Meier method,
followed by the log-rank test. P<0.05 was considered to indicate
a statistically significant difference.
Results
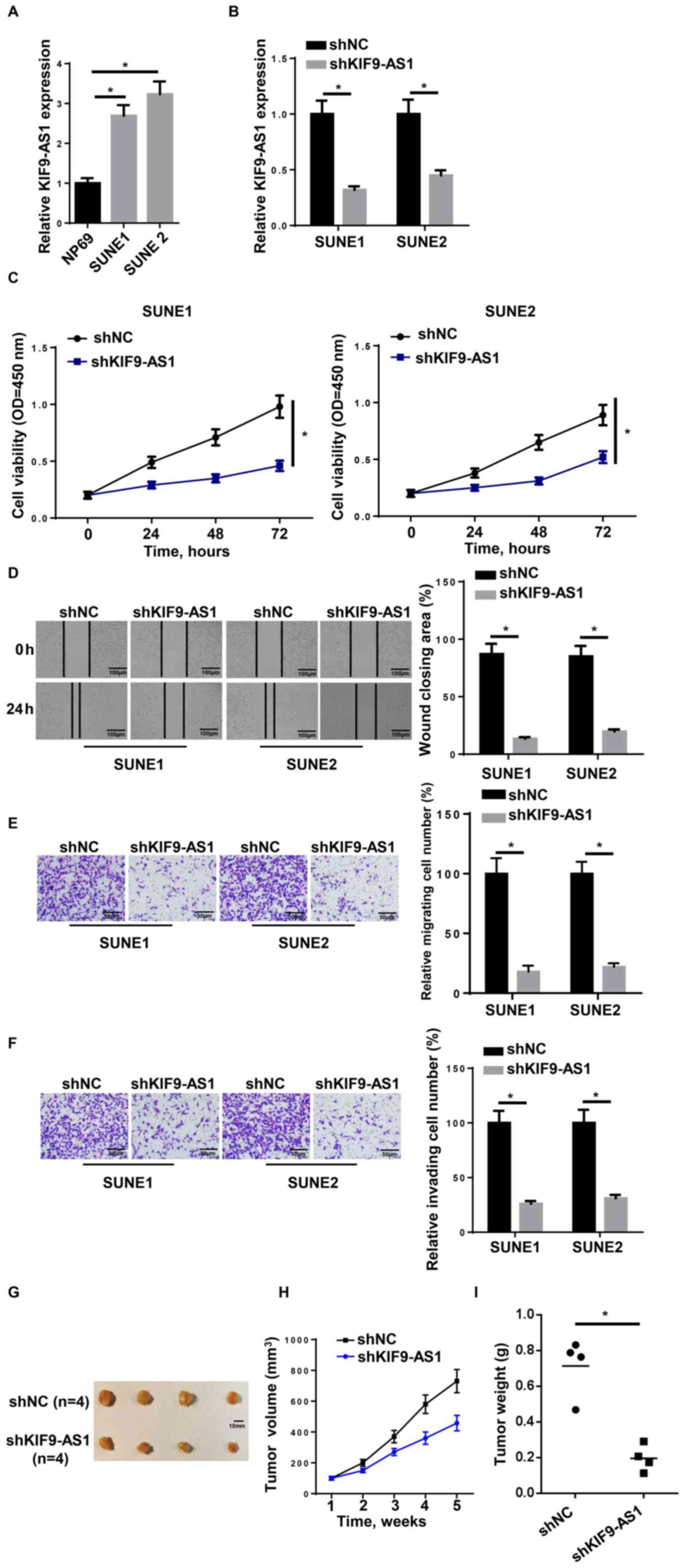
KIF9-AS1 expression levels are
increased in NPC cell lines, which facilitates NPC progression
To investigate the molecular mechanisms of KIF9-AS1
in NPC, KIF9-AS1 expression levels were analyzed using RT-qPCR. The
results indicated that the expression levels of KIF9-AS1 were
increased in the NPC cell lines (SUNE1 and SUNE2) compared with the
human normal nasopharyngeal epithelial cell line NP69 (Fig. 1A). RT-qPCR was subsequently used to
determine the transfection efficiency of the shRNA; KIF9-AS1
expression levels were markedly decreased in the shKIF9-AS1 group
compared with the shNC group (Fig.
1B). The CCK-8 assay revealed that SUNE1 and SUNE2 cell
viability was decreased following the suppression of KIF9-AS1
expression levels compared with shNC-treated cells at 72 h
(Fig. 1C). In addition, cell
migration and invasion were both attenuated by the knockdown of
KIF9-AS1 (Fig. 1D-F). Xenograft
tumor experiments were performed to determine the role of KIF9-AS1
in NPC in vivo, and results showed that the tumor volume and
weight were markedly decreased in the shKIF9-AS1 group compared
with the control (Fig. 1G-I). These
results suggested that KIF9-AS1 may serve as an oncogenic factor
during NPC progression.
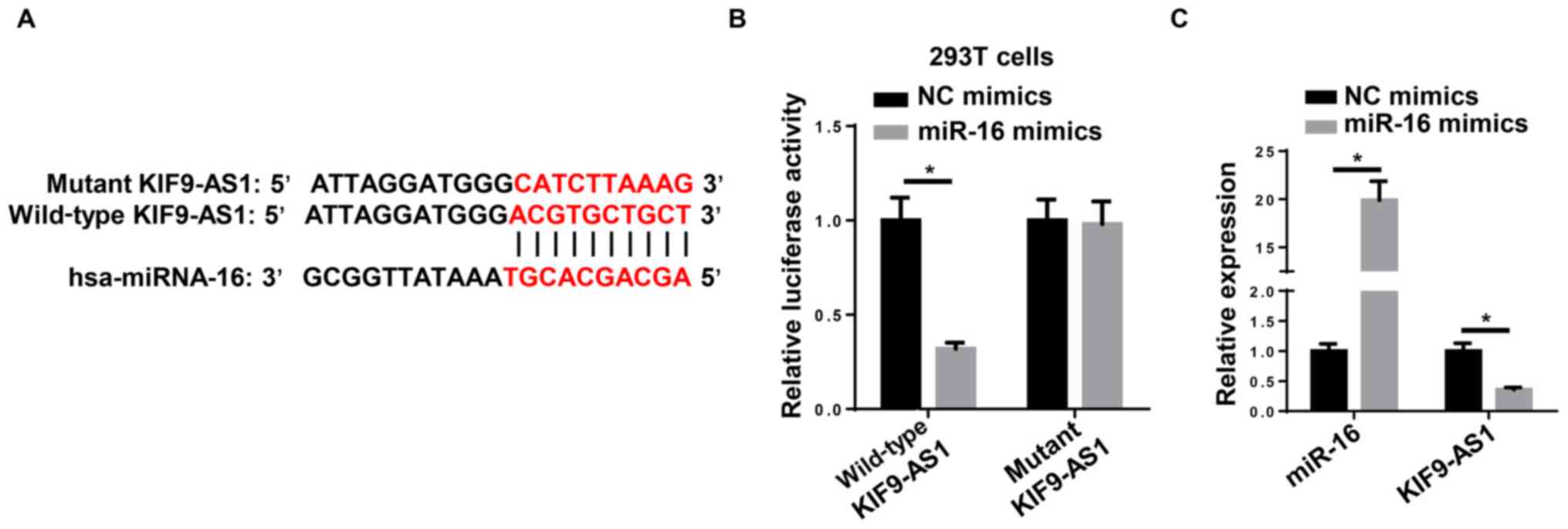
miR-16 is a direct target of KIF9-AS1
in NPC cells
Using the StarBase bioinformatics analysis software,
KIF9-AS1 was revealed to bind to miR-16 via complementary base
pairing (Fig. 2A). A dual-luciferase
reporter assay subsequently demonstrated that the miR-16 mimic
decreased the relative luciferase activity of 293T cells
transfected with the WT KIF9-AS1-fused luciferase gene, whereas no
significant differences were observed in the relative luciferase
activity in cells harboring Mut KIF9-AS1 (Fig. 2B). Furthermore, RT-qPCR revealed that
the miR-16 mimic significantly decreased the expression levels of
KIF9-AS1 compared with NC mimics (Fig.
2C). Taken together, these findings suggested that miR-16 may
inhibit the expression levels of KIF9-AS1 through direct
binding.
KIF9-AS1 regulates NPC progression via
miR-16
The expression levels of miR-16 were upregulated in
SUNE1 cells transfected with the miR-16 mimic (Fig. 3A). Moreover, the CCK-8, wound healing
and Transwell assays revealed that the miR-16 mimic inhibited the
cell viability, migration and invasion of NPC cells, respectively
(Fig. 3B-E). These findings
indicated that miR-16 inhibited the development and progression of
NPC cells. In addition, as miR-16 was observed to interact with
KIF9-AS1, the study aimed to determine whether miR-16 was involved
in KIF9-AS1-regulated NPC tumor progression. RT-qPCR results
indicated that miR-16 expression was decreased in SUNE1 cells
transfected with miR-16 inhibitor compared with NC inhibitor
(Fig. 3F). Subsequently, the miR-16
inhibitor was co-transfected into KIF9-AS1-knockdown cells and the
results indicated that the introduction of the miR-16 inhibitor was
able to decrease the expression levels of miR-16 in SUNE1 cells
transfected with shKIF9-AS1 (Fig.
3G). CCK-8 assays further revealed that the knockdown of
KIF9-AS1 decreased the cell viability of SUNE1 cells, which was
abolished by miR-16 inhibitor (Fig.
3H). In addition, the wound healing and Transwell assays
demonstrated that knockdown of KIF9-AS1 attenuated the migration
and invasion of SUNE1 cells compared with the cells transfected
with shNC; however, the miR-16 inhibitor was able to partially
rescue the shKIF9-AS1-induced NPC cell phenotype (Fig. 3I-K). Altogether, these data suggested
that miR-16 may be crucial for KIF9-AS1-mediated migration and
invasion of NPC cells.
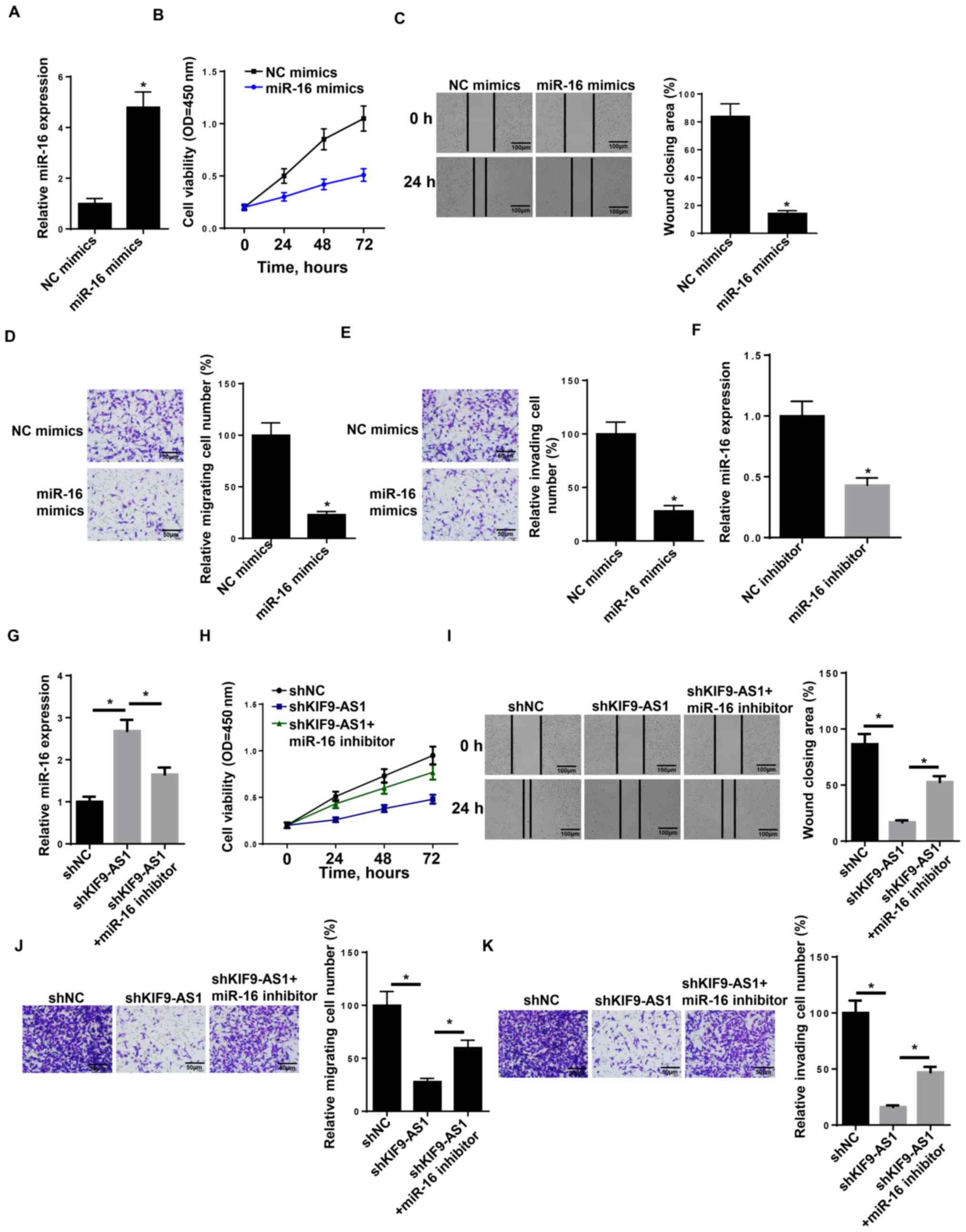 | Figure 3.KIF9-AS1 regulates nasopharyngeal
carcinoma progression via miR-16. (A) RT-qPCR showed the relative
miR-16 expression in SUNE1 cell lines transfected with miR-16
mimics. (B) CCK-8 assay showed the cell proliferation rate of SUNE1
cells transfected with miR-16 mimics at different time points of 0,
24, 48 and 72 h. (C) Wound healing assay of SUNE1 cells transfected
with miR-16 mimics. (D and E) Transwell assay of SUNE1 cells
transfected with miR-16 mimics. (F) RT-qPCR showed the relative
miR-16 expression in SUNE1 cell lines transfected with NC inhibitor
and miR-16 inhibitor. (G) RT-qPCR shows the relative miR-16
expression in SUNE1 cell lines transfected with shNC, shKIF9-AS1
and shKIF9-AS1+ miR-16 inhibitor. (H) CCK-8 assay showed the cell
proliferation rate of SUNE1 cells transfected with shNC, shKIF9-AS1
and shKIF9-AS1+ miR-16 inhibitor at different time points of 0, 24,
48, and 72 h. (I) Wound healing assay of SUNE1 cells transfected
with shNC, shKIF9-AS1 and shKIF9-AS1+ miR-16 inhibitor. (J and K)
Transwell assay of SUNE1 cells transfected with shNC, shKIF9-AS1
and shKIF9-AS1+ miR-16 inhibitor. The data were presented as mean ±
SD. *P<0.05. RT-q, reverse transcription-quantitative; miR,
microRNA; CCK-8, Cell Counting Kit-8; sh, short hairpin; NC,
negative control. |
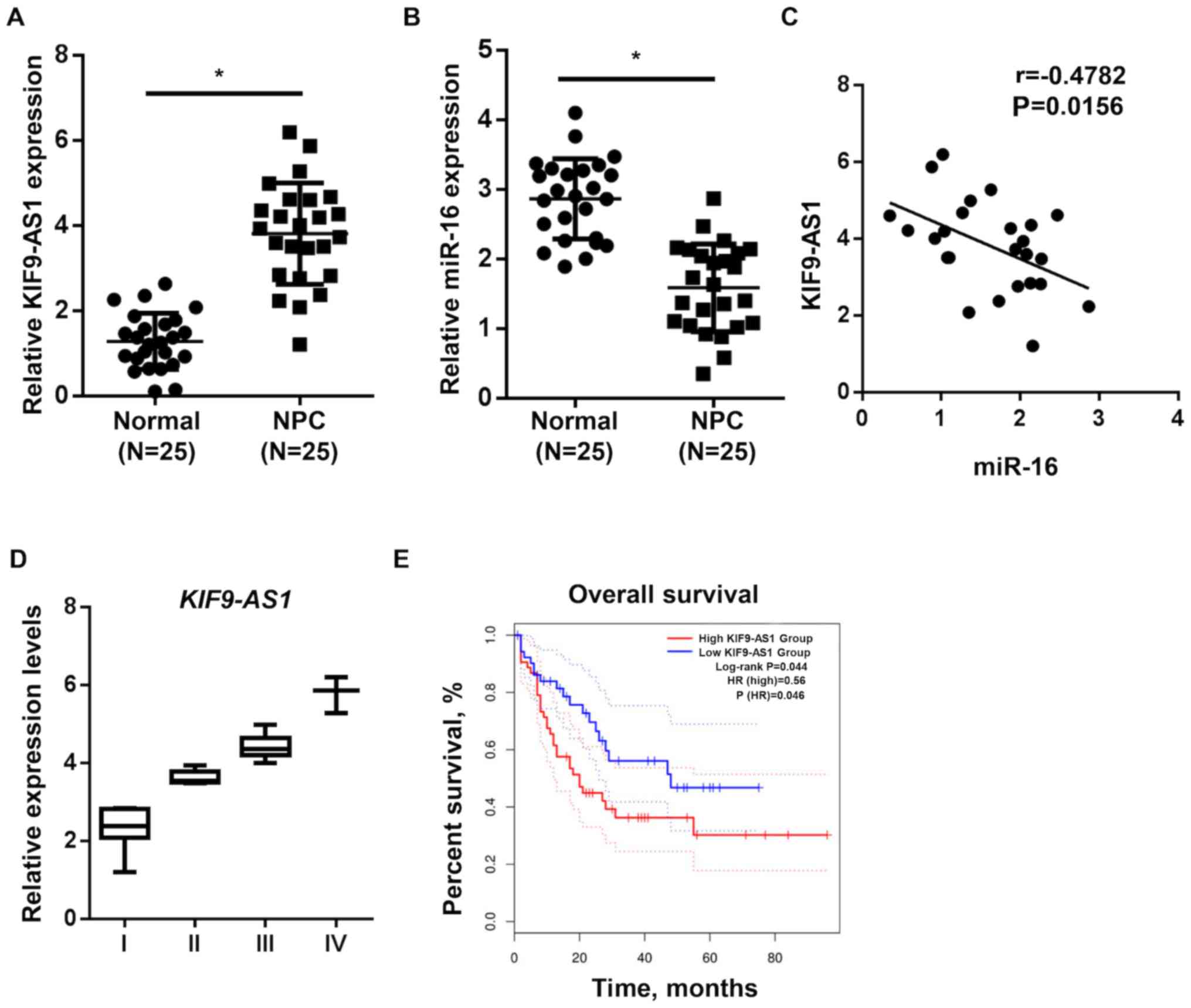
KIF9-AS1 and miR-16 expression levels
are dysregulated in patients with NPC
To confirm the roles of KIF9-AS1 and miR-16 in NPC
progression, the expression levels of KIF9-AS1 and miR-16 were
analyzed in patients with NPC. Patient characteristics are
presented in Table I. A total of
eight patients had differentiated NPC, 18 patients were documented
to have lymph node metastasis and 16 patients were in TNM stage
III–IV. The expression levels of KIF9-AS1 were increased in NPC
tumors compared with the adjacent normal tissues, whereas the
expression levels of miR-16 were decreased in the NPC tumor samples
(Fig. 4A and B). Moreover, a
negative correlation was identified between the expression levels
of KIF9-AS1 and miR-16 (Fig. 4C). In
addition, it was observed that the expression levels of KIF9-AS1
were increased in high-stage tumors (III–IV) compared with
low-stage tumors (I–II) (Fig. 4D).
Meanwhile, Kaplan-Meier analysis reported that patients with high
KIF9-AS1 expression levels were associated with a poorer prognosis
in NPC (hazard ratio, 0.234; 95% confidence interval,
0.06350–0.8655; Fig. 4E). These
results indicated that KIF9-AS1 expression levels may be
upregulated in high-stage tumors and associated with a less
favorable overall survival rate of patients with NPC.
Discussion
NPC is regarded as one of the most common head and
neck malignancies, with an incidence rate of 2.8/100,000
person-years in men and 1.9/100 000 person-years in women in China
(21). Despite significant advances
in the treatment of NPC, the incomplete understanding of the
pathogenesis of NPC has prevented the development of effective
targeted therapies.
An increasing number of studies have demonstrated
that the abnormal expression levels of lncRNAs serve as a
prognostic biomarker for different types of cancer, including NPC
(22–24). lncRNAs have been discovered to serve
roles in inhibiting or promoting the development of cancer by
influencing carcinogenic molecules to regulate cancer-related
progression. For example, lncRNA ZFAS1 was reported to accelerate
the proliferation of NPC cells via the activation of the PI3K/AKT
signaling pathway (25). Jia et
al (15) also demonstrated that
lncRNA PXN-AS1-L promoted the proliferation, migration and invasion
of NPC cells through upregulating the expression levels of
suppressor APC domain-containing protein 2. Therefore, determining
the involvement of cancer-specific lncRNAs may prove beneficial for
identifying novel targets for anticancer treatment. Wang et
al (11) suggested that lncRNA
KIF9-AS1 may belong to a novel group of non-coding RNAs, which are
associated with inflammation and tumorigenesis (12). Thus, the present study aimed to
determine the expression levels of KIF9-AS1 in NPC tissues and cell
lines. The results from the RT-qPCR analysis revealed that the
expression levels of KIF9-AS1 were significantly increased in NPC
tissues and cell lines. Furthermore, the clinical significance of
KIF9-AS1 was investigated in patients with NPC; the expression
levels of KIF9-AS1 in stage I–IV tumors demonstrated a gradually
upregulated trend, and Kaplan-Meier survival analysis indicated
that patients with NPC and high KIF9-AS1 expression levels had a
shorter OS time compared with those patients with low KIF9-AS1
expression levels. Thus, these results suggested that KIF9-AS1 may
be a potential target for the treatment of NPC.
Competing endogenous RNA (ceRNA) networks have
regulatory functions in numerous types of human malignancies
(26,27). For example, lncRNA CR749391 was
reported to promote the progression of gastric cancer by sponging
miR-181a (28); lncRNA HCG18
promotes the development of NPC by functioning as a ceRNA of miR-16
to upregulate G1/S-specific cyclin-D1 expression levels (29) and lncRNA AFAP1-AS1 was observed to
function as a ceRNA of miR-423-5p to promote NPC metastasis by
activating the Rho/Rac signaling pathway (30). Previous studies have also indicated
that miR-16 is associated with cancer regulation, including
cervical cancer, bladder cancer and neuroblastoma (31–33).
Consistent with these findings, the present study also observed an
interaction between KIF9-AS1 and miR-16 through a series of
experiments. In addition, the co-transfection with an miR-16
inhibitor partially reversed the shKIF9-AS1-mediated decrease in
the viability, migratory and invasive abilities of NPC cells. The
expression levels of miR-16 were also decreased in NPC tissues
compared with those in the corresponding normal nasopharyngeal
tissues. In addition, the expression levels of miR-16 were
negatively correlated with the expression levels of KIF9-AS1.
Altogether, these findings suggested that KIF9-AS1 may promote the
development and progression of NPC by inhibiting the expression
levels of miR-16.
In conclusion, the present study reported that
KIF9-AS1 promoted the progression of NPC by targeting miR-16. These
findings not only strengthen our understanding of NPC initiation
and progression, but they also inform the development of novel
biomarkers and therapeutic methods for NPC. However, the
limitations of the present study need to be addressed in future
studies. Firstly, other miRNAs or downstream effectors that are
crucial to KIF9-AS1-regulated phenotypes of NPC need to be
identified. Secondly, the present findings should be validated
using additional NPC cell lines to ensure a more in-depth analysis.
Thirdly, both SUNE1 and SUNE2 should be used to strengthen the
preciseness of the experiment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HY and SY designed the present study. HY, SW and SY
performed all the experiments, analyzed the data and prepared the
figures. HY and SY drafted the initial manuscript. SY reviewed and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weifang Traditional Chinese Hospital (Weifang, China;
approval no. 2010A121005) and written informed consent was provided
by all patients prior to the study start. Animal experimental
protocols were approved by the Animal Welfare Committee of Weifang
Traditional Chinese Hospital (Weifang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang LL, Chen WQ, Xue WQ, He YQ, Zheng RS,
Zeng YX and Jia WH: Global trends in incidence and mortality of
nasopharyngeal carcinoma. Cancer Lett. 374:22–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petersson F and Nasopharyngeal carcinoma:
A review. Semin Diagn Pathol. 32:54–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Mofty SK: Human papillomavirus-related
head and neck squamous cell carcinoma variants. Semin Diagn Pathol.
32:23–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lo KW, To KF and Huang DP: Focus on
nasopharyngeal carcinoma. Cancer Cell. 5:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee AW, Ma BB, Ng WT and Chan AT:
Management of nasopharyngeal carcinoma: Current practice and future
perspective. J Clin Oncol. 33:3356–3364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di W, Li Q, Shen W, Guo H and Zhao S: The
long non-coding RNA HOTAIR promotes thyroid cancer cell growth,
invasion and migration through the miR-1-CCND2 axis. Am J Cancer
Res. 7:1298–1309. 2017.PubMed/NCBI
|
|
9
|
Zheng P, Li H, Xu P, Wang X, Shi Z, Han Q
and Li Z: High lncRNA HULC expression is associated with poor
prognosis and promotes tumor progression by regulating
epithelial-mesenchymal transition in prostate cancer. Arch Med Sci.
14:679–686. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang C, Su C, Song Q, Dong F, Yu S and
Huo J: lncRNA PICART1 suppressed non-small cell lung cancer cells
proliferation and invasion by targeting AKT1 signaling pathway. Am
J Transl Res. 10:4193–4201. 2018.PubMed/NCBI
|
|
11
|
Wang W, Shen H, Cao G and Huang J: Long
non-coding RNA XIST predicts poor prognosis and promotes malignant
phenotypes in osteosarcoma. Oncol Lett. 17:256–262. 2019.PubMed/NCBI
|
|
12
|
Wang S, Hou Y, Chen W, Wang J, Xie W,
Zhang X and Zeng L: KIF9AS1, LINC01272 and DIO3OS lncRNAs as novel
biomarkers for inflammatory bowel disease. Mol Med Rep.
17:2195–2202. 2018.PubMed/NCBI
|
|
13
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeh YM, Chuang CM, Chao KC and Wang LH:
MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis
by targeting SOX4 and HIF-1α. Int J Cancer. 133:867–878. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia XD, Niu P, Xie CC and Liu HJ: Long
noncoding RNA PXN-AS1-L promotes the malignancy of nasopharyngeal
carcinoma cells via upregulation of SAPCD2. Cancer Med.
8:4278–4291. 2019.PubMed/NCBI
|
|
16
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Backliwal G, Hildinger M, Hasija V and
Wurm FM: High-density transfection with HEK-293 cells allows
doubling of transient titers and removes need for a priori DNA
complex formation with PEI. Biotechnol Bioeng. 99:721–727. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Drummond GB, Paterson DJ and McGrath JC:
ARRIVE: New guidelines for reporting animal research. J Physiol.
588:25172010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao SM, Simons MJ and Qian CN: The
prevalence and prevention of nasopharyngeal carcinoma in China.
Chin J Cancer. 30:114–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang M, Ji YQ, Song ZB, Ma XX, Zou YY and
Li XS: Knockdown of lncRNA ZFAS1 inhibits progression of
nasopharyngeal carcinoma by sponging miR-135a. Neoplasma.
66:939–945. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Guo Q, Liu G, Zheng F, Chen J,
Huang D, Ding L, Yang X, Song E, Xiang Y and Yao H: NKILA represses
nasopharyngeal carcinoma carcinogenesis and metastasis by NF-kB
pathway inhibition. PLoS Genet. 15:e10083252019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kong YG, Cui M, Chen SM, Xu Y, Xu Y and
Tao ZZ: lncRNA-LINC00460 facilitates nasopharyngeal carcinoma
tumorigenesis through sponging miR-149-5p to up-regulate IL6. Gene.
639:77–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Jin Q, Wang X, Chen W and Cai Z:
lncRNA ZFAS1 promotes proliferation and migration and inhibits
apoptosis in nasopharyngeal carcinoma via the PI3K/AKT pathway in
vitro. Cancer Biomark. 26:171–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang LX, Wan C, Dong ZB, Wang BH, Liu HY
and Li Y: Integrative analysis of long noncoding RNA (lncRNA),
microRNA (miRNA) and mRNA expression and construction of a
competing endogenous RNA (ceRNA) network in metastatic melanoma.
Med Sci Monit. 25:2896–2907. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi S, Li D, Li Y, Feng Z, Du Y and Nie Y:
lncRNA CR749391 acts as a tumor suppressor to upregulate KLF6
expression via interacting with miR-181a in gastric cancer. Exp
Ther Med. 19:569–578. 2020.PubMed/NCBI
|
|
29
|
Li L, Ma TT, Ma YH and Jiang YF: lncRNA
HCG18 contributes to nasopharyngeal carcinoma development by
modulating miR-140/CCND1 and Hedgehog signaling pathway. Eur Rev
Med Pharmacol Sci. 23:10387–10399. 2019.PubMed/NCBI
|
|
30
|
Lian Y, Xiong F, Yang L, Bo H, Gong Z,
Wang Y, Wei F, Tang Y, Li X, Liao Q, et al: Long noncoding RNA
AFAP1-AS1 acts as a competing endogenous RNA of miR-423-5p to
facilitate nasopharyngeal carcinoma metastasis through regulating
the Rho/Rac pathway. J Exp Clin Cancer Res. 37:2532018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang S, Wang W, Wu X, Liu W and Ding F:
miR-16-5p modulates the radiosensitivity of cervical cancer cells
via regulating coactivator-associated arginine methyltransferase 1.
Pathol Int. 70:12–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu Q, Liu P, Han G, Xue X and Ma D:
CircRNA circPDSS1 promotes bladder cancer by downregulating miR-16.
Biosci Rep. 40:BSR201919612020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chava S, Reynolds CP, Pathania AS,
Gorantla S, Poluektova LY, Coulter DW, Gupta SC, Pandey MK and
Challagundla KB: miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit
tumor progression by directly targeting MYCN in neuroblastoma. Mol
Oncol. 14:180–196. 2020. View Article : Google Scholar : PubMed/NCBI
|