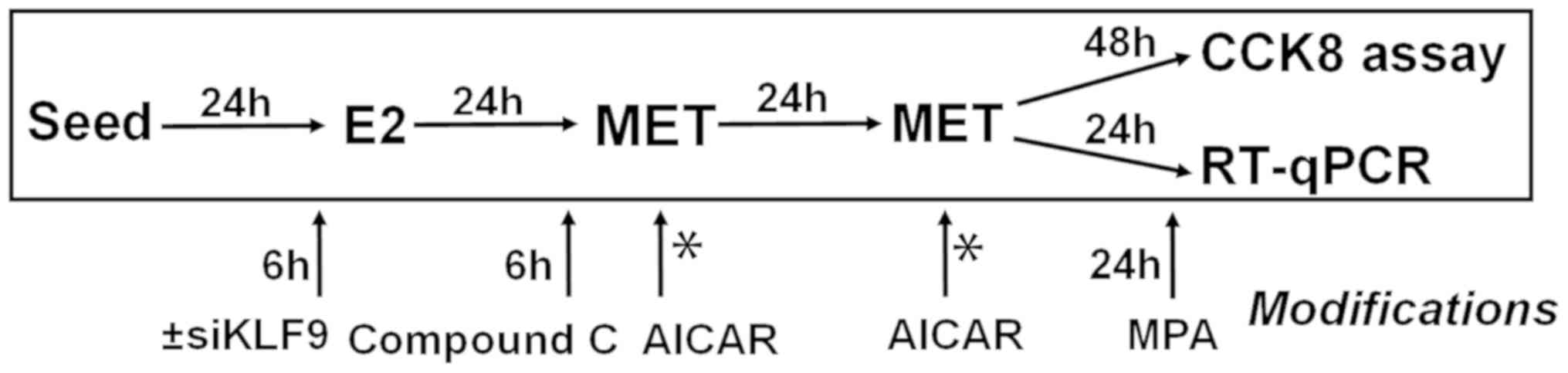
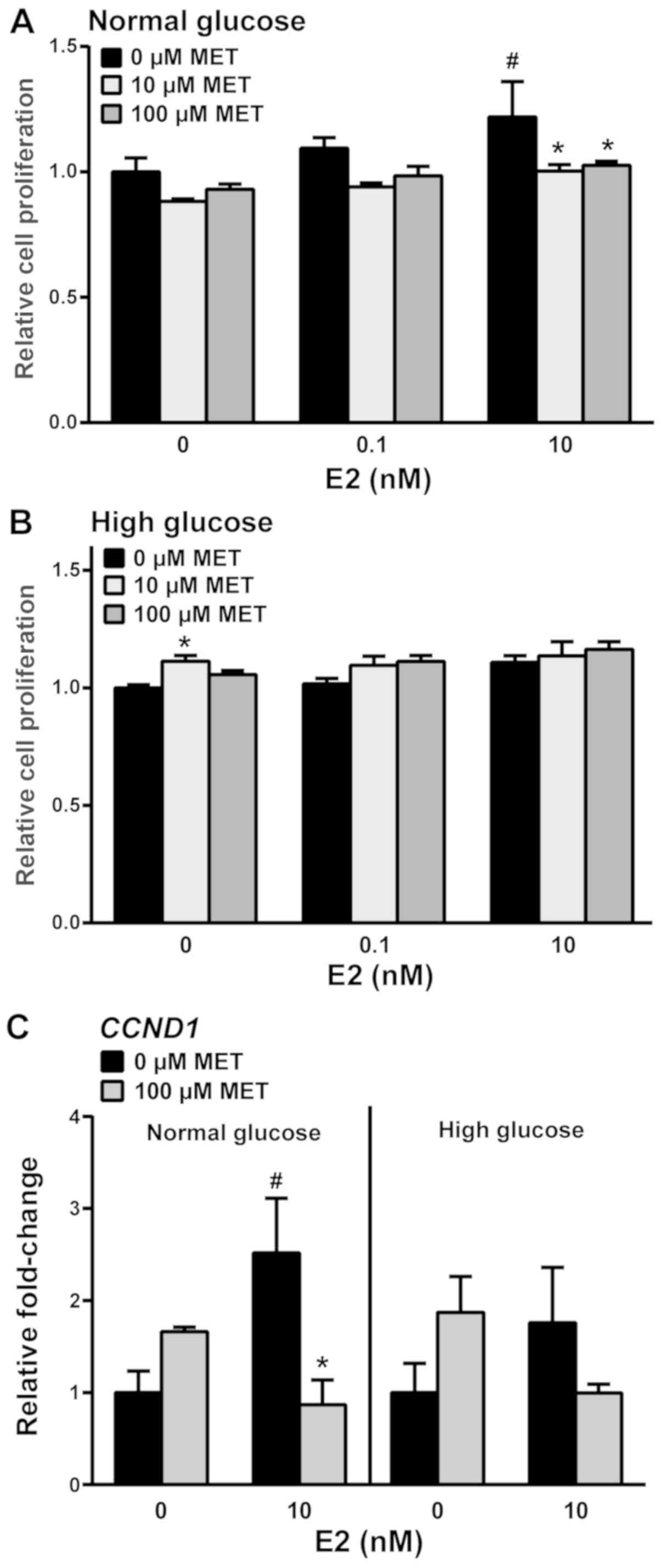
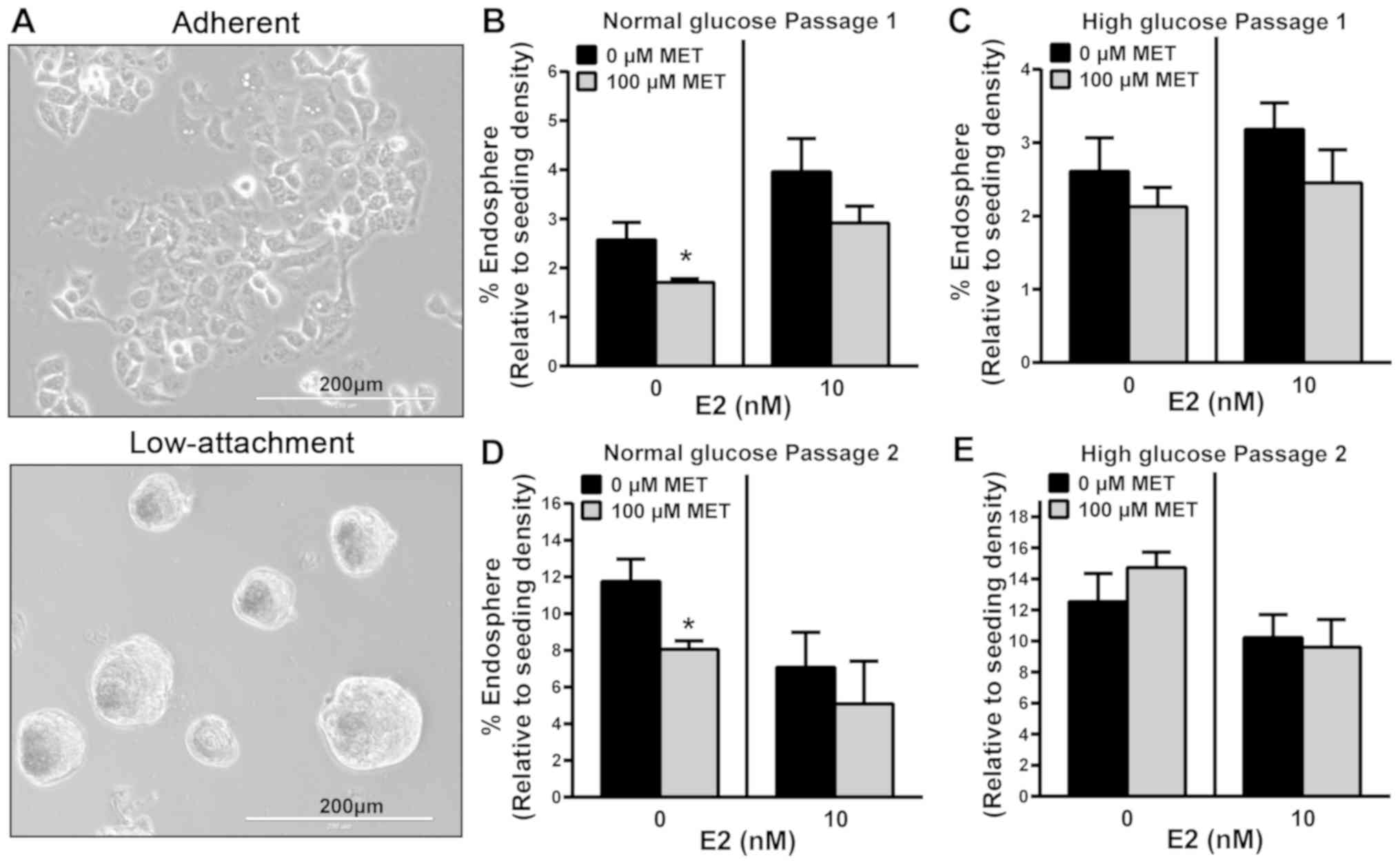
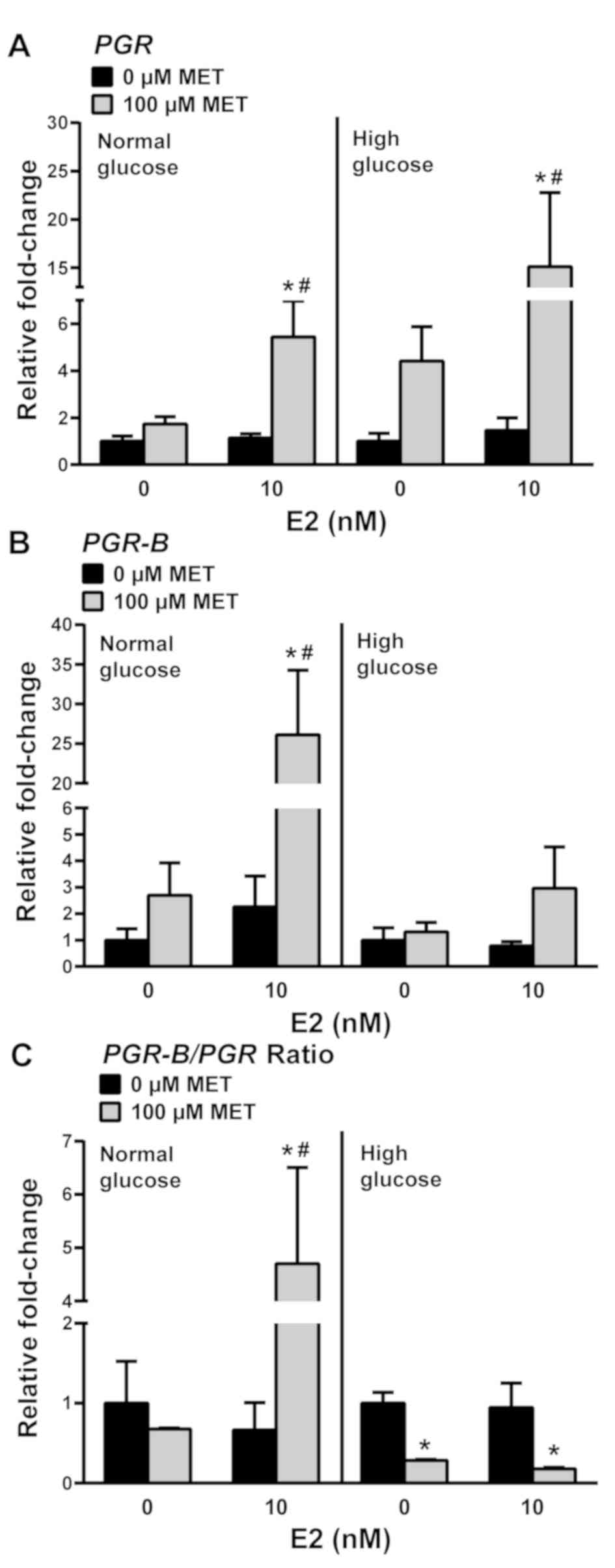
|
1
|
Howlader N, Noone AM, Krapcho M, Miller D,
Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, et al:
SEER Cancer Statistics Review, 1975–2017, based on November 2019
SEER data submission. National Cancer Institute; Bethesda, MD:
2020, https://seer.cancer.gov/csr/1975_2017/April
15–2020
|
|
2
|
Friberg E, Mantzoros CS and Wolk A:
Diabetes and risk of endometrial cancer: A population-based
prospective cohort study. Cancer Epidemiol Biomarkers Prev.
16:276–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitson SJ, Evans DG and Crosbie EJ:
Identifying high-risk women for endometrial cancer prevention
strategies: Proposal of an endometrial cancer risk prediction
model. Cancer Prev Res (Phila). 10:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohkuma T, Peters SAE and Woodward M: Sex
differences in the association between diabetes and cancer: A
systematic review and meta-analysis of 121 cohorts including 20
million individuals and one million events. Diabetologia.
61:2140–2154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al IDF Diabetes Atlas Committee, : Global and
regional diabetes prevalence estimates for 2019 and projections for
2030 and 2045: Results from the International Diabetes Federation
Diabetes Atlas, 9th edition. Diabetes Res Clin Pract.
157:1078432019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abarca-Gómez L, Abdeen ZA, Hamid ZA,
Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, Adams RJ, Aekplakorn W,
Afsana K, Aguilar-Salinas CA, et al NCD Risk Factor Collaboration
(NCD-RisC), : Worldwide trends in body-mass index, underweight,
overweight, and obesity from 1975 to 2016: A pooled analysis of
2416 population-based measurement studies in 128·9 million
children, adolescents, and adults. Lancet. 390:2627–2642. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roshan MH, Shing YK and Pace NP: Metformin
as an adjuvant in breast cancer treatment. SAGE Open Med.
7:20503121198651142019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davies G, Lobanova L, Dawicki W, Groot G,
Gordon JR, Bowen M, Harkness T and Arnason T: Metformin inhibits
the development, and promotes the resensitization, of
treatment-resistant breast cancer. PLoS One. 12:e01871912017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi P, Liu W, Tala, Wang H, Li F, Zhang H,
Wu Y, Kong Y, Zhou Z, Wang C, et al: Metformin suppresses
triple-negative breast cancer stem cells by targeting KLF5 for
degradation. Cell Discov. 3:170102017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Varghese S, Samuel SM, Varghese E, Kubatka
P and Büsselberg D: High Glucose Represses the Anti-Proliferative
and Pro-Apoptotic Effect of Metformin in Triple Negative Breast
Cancer Cells. Biomolecules. 9:162019. View Article : Google Scholar
|
|
11
|
Ariaans G, Jalving M, Vries EG and Jong S:
Anti-tumor effects of everolimus and metformin are complementary
and glucose-dependent in breast cancer cells. BMC Cancer.
17:2322017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oliveras-Ferraros C, Vazquez-Martin A,
Cuyàs E, Corominas-Faja B, Rodríguez-Gallego E, Fernández-Arroyo S,
Martin-Castillo B, Joven J and Menendez JA: Acquired resistance to
metformin in breast cancer cells triggers transcriptome
reprogramming toward a degradome-related metastatic stem-like
profile. Cell Cycle. 13:1132–1144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pabona JMP, Burnett AF, Brown DM, Quick
CM, Simmen FA, Montales MTE, Liu SJ, Rose T, Alhallak I, Siegel ER,
et al: Metformin Promotes Anti-tumor Biomarkers in Human
Endometrial Cancer Cells. Reprod Sci. 27:267–277. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soliman PT, Zhang Q, Broaddus RR, Westin
SN, Iglesias D, Munsell MF, Schmandt R, Yates M, Ramondetta L and
Lu KH: Prospective evaluation of the molecular effects of metformin
on the endometrium in women with newly diagnosed endometrial
cancer: A window of opportunity study. Gynecol Oncol. 143:466–471.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laskov I, Drudi L, Beauchamp M-C, Yasmeen
A, Ferenczy A, Pollak M and Gotlieb WH: Anti-diabetic doses of
metformin decrease proliferation markers in tumors of patients with
endometrial cancer. Gynecol Oncol. 134:607–614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caillé G, Lacasse Y, Raymond M, Landriault
H, Perrotta M, Picirilli G, Thiffault J and Spénard J:
Bioavailability of metformin in tablet form using a new high
pressure liquid chromatography assay method. Biopharm Drug Dispos.
14:257–263. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wilcock C and Bailey CJ: Accumulation of
metformin by tissues of the normal and diabetic mouse. Xenobiotica.
24:49–57. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martin-Castillo B, Vazquez-Martin A,
Oliveras-Ferraros C and Menendez JA: Metformin and cancer: Doses,
mechanisms and the dandelion and hormetic phenomena. Cell Cycle.
9:1057–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pabona JM, Simmen FA, Nikiforov MA, Zhuang
D, Shankar K, Velarde MC, Zelenko Z, Giudice LC and Simmen RC:
Krüppel-like factor 9 and progesterone receptor coregulation of
decidualizing endometrial stromal cells: Implications for the
pathogenesis of endometriosis. J Clin Endocrinol Metab.
97:E376–E392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simmons CD, Pabona JM, Heard ME, Friedman
TM, Spataro MT, Godley AL, Simmen FA, Burnett AF and Simmen RC:
Krüppel-like factor 9 loss-of-expression in human endometrial
carcinoma links altered expression of growth-regulatory genes with
aberrant proliferative response to estrogen. Biol Reprod.
85:378–385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Qi S, Zhao X, Li M, Ding S, Lu J
and Zhang H: Metformin inhibits 17β-estradiol-induced
epithelial-to-mesenchymal transition via βKlotho-related ERK1/2
signaling and AMPKα signaling in endometrial adenocarcinoma cells.
Oncotarget. 7:21315–21331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Xu H, Zhou X, Li Y, Liu T, Yin X
and Zhang B: Role of metformin in inhibiting estrogen-induced
proliferation and regulating ERα and ERβ expression in human
endometrial cancer cells. Oncol Lett. 14:4949–4956. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deane JA, Cousins FL and Gargett CE:
Endometrial organoids: in vitro models for endometrial research and
personalized medicine. Biol Reprod. 97:781–783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kitson SJ, Rosser M, Fischer DP, Marshall
KM, Clarke RB and Crosbie EJ: Targeting endometrial cancer stem
cell activity with metformin is inhibited by patient-derived
adipocyte-secreted factors. Cancers (Basel). 11:6532019. View Article : Google Scholar
|
|
26
|
Hirsch HA, Iliopoulos D, Tsichlis PN and
Struhl K: Metformin selectively targets cancer stem cells, and acts
together with chemotherapy to block tumor growth and prolong
remission. Cancer Res. 69:7507–7511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Velarde MC, Zeng Z, McQuown JR, Simmen FA
and Simmen RCM: Kruppel-like factor 9 is a negative regulator of
ligand-dependent estrogen receptor α signaling in Ishikawa
endometrial adenocarcinoma cells. Mol Endocrinol. 21:2988–3001.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JJ and Chapman-Davis E: Role of
progesterone in endometrial cancer. Semin Reprod Med. 28:81–90.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Wang Y, Zhang Z, Park JY, Guo D,
Liao H, Yi X, Zheng Y, Zhang D, Chambers SK, et al: Mechanism of
progestin resistance in endometrial precancer/cancer through
Nrf2-AKR1C1 pathway. Oncotarget. 7:10363–10372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cantrell LA, Zhou C, Mendivil A, Malloy
KM, Gehrig PA and Bae-Jump VL: Metformin is a potent inhibitor of
endometrial cancer cell proliferation--implications for a novel
treatment strategy. Gynecol Oncol. 116:92–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanna RK, Zhou C, Malloy KM, Sun L, Zhong
Y, Gehrig PA and Bae-Jump VL: Metformin potentiates the effects of
paclitaxel in endometrial cancer cells through inhibition of cell
proliferation and modulation of the mTOR pathway. Gynecol Oncol.
125:458–469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Viollet B, Guigas B, Sanz Garcia N,
Leclerc J, Foretz M and Andreelli F: Cellular and molecular
mechanisms of metformin: An overview. Clin Sci (Lond). 122:253–270.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou G, Myers R, Li Y, Chen Y, Shen X,
Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al: Role of
AMP-activated protein kinase in mechanism of metformin action. J
Clin Invest. 108:1167–1174. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Corton JM, Gillespie JG, Hawley SA and
Hardie DG: 5-aminoimidazole-4-carboxamide ribonucleoside. A
specific method for activating AMP-activated protein kinase in
intact cells? Eur J Biochem. 229:558–565. 1995.PubMed/NCBI
|
|
35
|
Carvalho MJ, Laranjo M, Abrantes AM,
Casalta-Lopes J, Sarmento-Santos D, Costa T, Serambeque B, Almeida
N, Gonçalves T, Mamede C, et al: Endometrial Cancer Spheres Show
Cancer Stem Cells Phenotype and Preference for Oxidative
Metabolism. Pathol Oncol Res. 25:1163–1174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Barros Machado A, Dos Reis V, Weber S,
Jauckus J, Brum IS, von Eye Corleta H, Strowitzki T, Capp E and
Germeyer A: Proliferation and metastatic potential of endometrial
cancer cells in response to metformin treatment in a high versus
normal glucose environment. Oncol Lett. 12:3626–3632. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu T, Chung YM, Guan M, Ma M, Ma J, Berek
JS and Hu MCT: Reprogramming ovarian and breast cancer cells into
non-cancerous cells by low-dose metformin or SN-38 through FOXO3
activation. Sci Rep. 4:58102014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Janzen DM, Cheng D, Schafenacker AM, Paik
DY, Goldstein AS, Witte ON, Jaroszewicz A, Pellegrini M and
Memarzadeh S: Estrogen and progesterone together expand murine
endometrial epithelial progenitor cells. Stem Cells. 31:808–822.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vegeto E, Shahbaz MM, Wen DX, Goldman ME,
O'Malley BW and McDonnell DP: Human progesterone receptor A form is
a cell- and promoter-specific repressor of human progesterone
receptor B function. Mol Endocrinol. 7:1244–1255. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y
and Liao QP: Metformin promotes progesterone receptor expression
via inhibition of mammalian target of rapamycin (mTOR) in
endometrial cancer cells. J Steroid Biochem Mol Biol. 126:113–120.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Graham JD, Yager ML, Hill HD, Byth K,
O'Neill GM and Clarke CL: Altered progesterone receptor isoform
expression remodels progestin responsiveness of breast cancer
cells. Mol Endocrinol. 19:2713–2735. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mouttet D, Laé M, Caly M, Gentien D,
Carpentier S, Peyro-Saint-Paul H, Vincent-Salomon A, Rouzier R,
Sigal-Zafrani B, Sastre-Garau X, et al: Estrogen-Receptor,
Progesterone-Receptor and HER2 Status Determination in Invasive
Breast Cancer. Concordance between Immuno-Histochemistry and
MapQuant™ Microarray Based Assay. PLoS One. 11:e01464742016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang D, Zhang XL, Michel FJ, Blum JL,
Simmen FA and Simmen RCM: Direct interaction of the Krüppel-like
family (KLF) member, BTEB1, and PR mediates progesterone-responsive
gene expression in endometrial epithelial cells. Endocrinology.
143:62–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang XL, Zhang D, Michel FJ, Blum JL,
Simmen FA and Simmen RCM: Selective interactions of Kruppel-like
factor 9/basic transcription element-binding protein with
progesterone receptor isoforms A and B determine transcriptional
activity of progesterone-responsive genes in endometrial epithelial
cells. J Biol Chem. 278:21474–21482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Smid-Koopman E, Blok LJ, Kühne LCM, Burger
CW, Helmerhorst TJM, Brinkmann AO and Huikeshoven FJ: Distinct
functional differences of human progesterone receptors A and B on
gene expression and growth regulation in two endometrial carcinoma
cell lines. J Soc Gynecol Investig. 10:49–57. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rao E, Zhang Y, Li Q, Hao J, Egilmez NK,
Suttles J and Li B: AMPK-dependent and independent effects of AICAR
and compound C on T-cell responses. Oncotarget. 7:33783–33795.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao X, Luo G, Cheng Y, Yu W, Chen R, Xiao
B, Xiang Y, Feng C, Fu W, Duan C, et al: Compound C induces
protective autophagy in human cholangiocarcinoma cells via
Akt/mTOR-independent pathway. J Cell Biochem. 119:5538–5550. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Philippe C, Pinson B, Dompierre J,
Pantesco V, Viollet B, Daignan-Fornier B and Moenner M: AICAR
Antiproliferative Properties Involve the AMPK-Independent
Activation of the Tumor Suppressors LATS 1 and 2. Neoplasia.
20:555–562. 2018. View Article : Google Scholar : PubMed/NCBI
|