Introduction
Prostate cancer (PCa) is the second most common type
of malignancy among males worldwide; in 2018, the World Health
Organization estimated more than 1.3 million new prostate cancer
cases and over 359,000 mortalities due to this disease (1). In Mexico, PCa is the most common and
fatal cancer type among males, accounting for >10,000 new cases
and 5,000 mortalities during the same period of time (1). PCa is usually diagnosed in the fifth
decade of life, with a variable rate of progression that largely
depends on genetic and environmental factors, as well as the
patient's lifestyle (2).
Androgens are necessary for the correct development
and function of the prostate gland; however, they also serve a
critical role in driving the growth of early-stage PCa (3). Androgen action, mediated by the
androgen receptor (AR), leads to the activation of target genes
that stimulate the proliferation and inhibit the apoptosis of
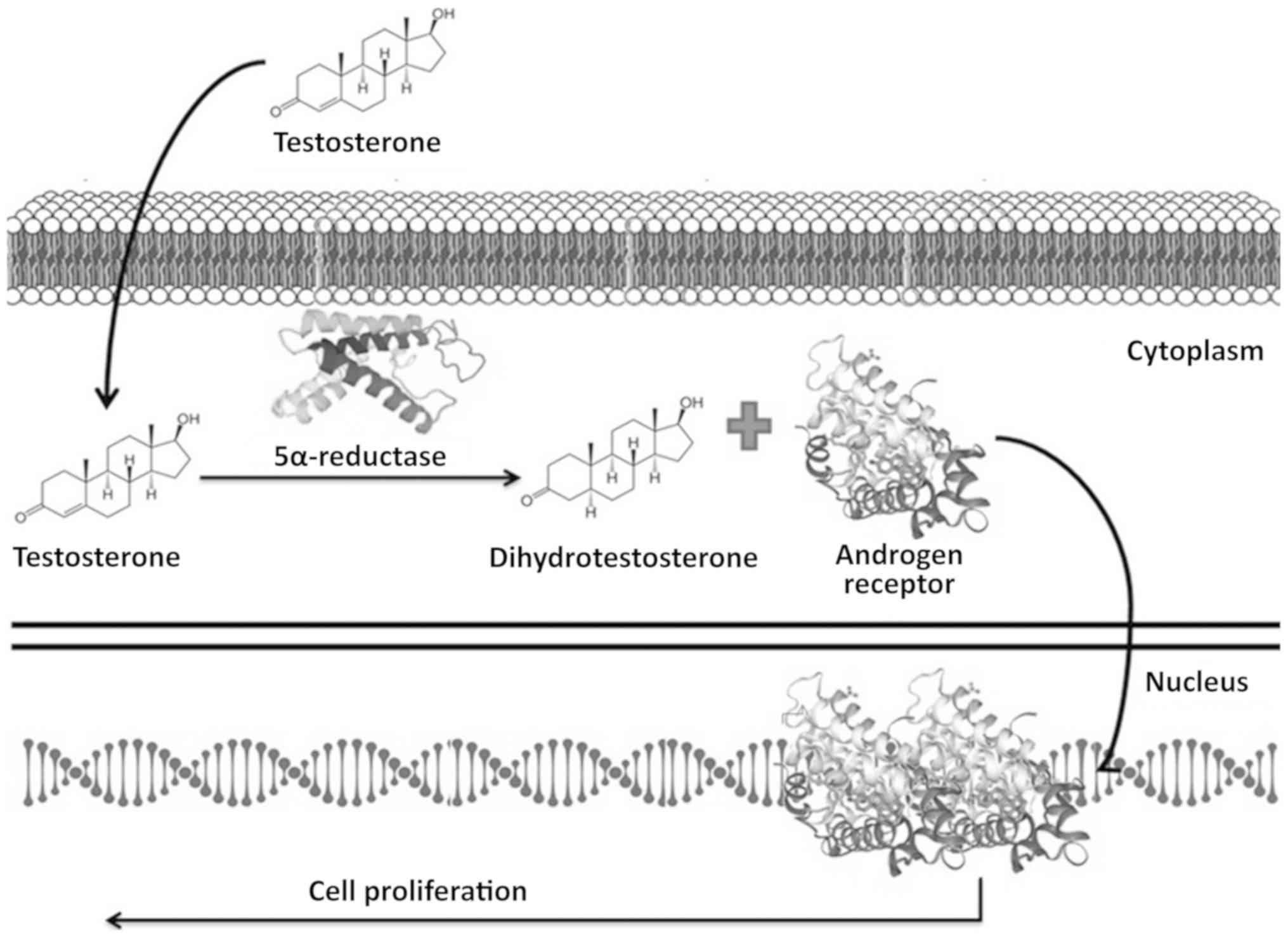
cancer cells (3,4). Free testosterone diffuses across the
membranes of target cells located within the prostatic tissue
acting as a substrate for the steroid 5 alpha-reductase 2 enzyme
(encoded by the SRD5A2 gene), which converts testosterone to
dihydrotestosterone (DHT), a more potent metabolite that activates
the AR (5). Once activated, the AR
is translocated to the nucleus where it dimerizes with another AR
and activates target genes that promote cell proliferation
(3,4,6,7) (Fig.
1).
Certain DNA variants in the genomic sequence of the
SRD5A2 gene alter the catalytic activity of steroid 5-alpha
reductase 2, which may increase the risk of PCa development
(8–11). In this context, certain variants have
been associated with an increased risk of developing PCa, including
TA dinucleotides in the 3′-UTR region (12,13),
rs9332964 (14,15), rs928258 (9) and rs523349 (16,17). The
rs928258 and rs523349 variants have been screened mainly across
different ethnic groups.
The rs9282858 (p.Ala49Thr or A49T) variant results
from a single nucleotide substitution, a G by an A (GCC/ACC),
causing an amino acid change from an alanine to a threonine. It has
been reported that this change increases the catalytic activity of
the enzyme by 5-fold (14,18). The ENSEMBL database reports this
variant as a) benign in ClinVar, b) deleterious in SIFT, c) benign
in PolyPhen, and d) likely benign in CADD (http://www.ensembl.org/Homo_sapiens/Variation/Mappings?db=core;r=2:31580256-31581256;v=rs9282858;vdb=variation;vf=57248637).
By contrast, the rs523349 (p.Val89Leu or V89L)
variant results from a single nucleotide substitution, a C by a G
(CTA/GTA), causing an amino acid change from a valine to a leucine.
It has been reported that this change reduces the enzyme activity
by 30% (14,16,19). The
ENSEMBL database reports this variant as a) benign in ClinVar, b)
tolerated substitution in SIFT, c) benign in PolyPhen, and d)
likely benign in CADD (http://www.ensembl.org/Homo_sapiens/Variation/Mappings?db=core;r=2:31580136-31581136;v=rs523349;vdb=variation;vf=54157055).
As in a number of other diseases, the molecular
findings on populations of European descent cannot be assumed for
all populations. To implement a precision medicine model, molecular
studies must be performed in diverse ethnic groups of interest.
Genomic and genetic data of populations of non-European descendants
remain under represented (20).
The Mexican population is genetically diverse
(21); therefore, a more detailed
study of the population structure alongside geographical data is
required to assess the frequency and prevalence of genetic diseases
in native and Mexican-mestizo populations (21–23). In
diseases, including prostate cancer, this information may aid in
diagnosis, prognosis, and treatment (21,22).
In Mexico, PCa is a national health problem, but
molecular studies regarding PCa in this population are limited.
Certain studies with regard to AR, VDR (24), VEGF (25), ATP6, and ND3 (26) genetic variants have been made.
However, to the best of our knowledge, there are no previous
reports that analyze the presence of the genetic variants, A49T and
V89L, of the steroid 5 alpha-reductase 2 enzyme in the
Mexican-mestizo population. Therefore, a molecular analysis of
these genetic variants and examination of relevant clinical data
was performed in the present study to identify a possible
association with the development of PCa on the Mexican-mestizo
population.
Materials and methods
Study design
The protocol was approved by the Ethics and Research
Committee of the School of Medicine of the Universidad Autónoma de
Nuevo León (no. UR16-00007). This protocol was performed by the
Biochemistry and Molecular Medicine Department using convenience
sampling. Participants were enrolled between January 2018 and
December 2019 through the Urology and Oncology Services from the
‘Dr. José Eleuterio González’ University Hospital of the
Universidad Autónoma de Nuevo León, and each patient provided
written informed consent to participate in the present study.
Recruited participants were classified into three
groups according to subsequent analyses: PCa cases (n=101; median
age, 70 years; age range, 64.5–75.0 years) and non-PCa subjects
(n=100; median age, 58 years; age range, 48–67 years) composed of
males without prostate abnormalities (n=60) and subjects with
benign prostatic hyperplasia (BPH; n=40). The PCa cases were males
diagnosed with PCa regardless of the time elapsed since the
diagnosis or their treatment status.
Blood samples were collected in a 6 ml BD Vacutainer
tube with EDTA (Becton-Dickinson). Demographic data of clinical
importance were collected, and the database was prepared. PSA
levels were analyzed only for the PCa and BPH groups.
DNA extraction protocol
Each blood sample was centrifuged at 3,857 g for 10
min at room temperature. From the buffy coat, DNA extraction was
performed using a previously reported method with TSNT lysis buffer
(composed of 1% Triton, 1% sodium dodecyl sulfate, 100 mM NaCl, 10
mM Tris-HCl pH 8.0 and 1 mM EDTA) and followed by a
phenol-chloroform extraction step (27,28). The
DNA was precipitated from aqueous phase with ethanol and quantified
using NanoDrop 1000 (Thermo Fisher Scientific, Inc.) and its
quality was verified through absorbance ratios (260/280 and 260/230
nm) and agarose gel electrophoresis.
Genotyping
Genotyping assays were performed using the
commercial TaqMan SNP Genotyping Assays probes C__27532228_20 and
C___2362601_10 (Thermo Fisher Scientific, Inc.) for the A49T
(rs9282858) and V89L (rs523349) gene variants of the SRD5A2
gene (NM_000348.3), respectively.
DNA samples were processed using a StepOnePlus™
Real-Time PCR system (Thermo Fisher Scientific, Inc.). Each PCR
reaction was performed using 5 µl SensiFAST™ Hi-Rox Genotyping kit
(Bioline; Meridian Bioscience, Inc.), 0.5 µl probe, 5 µl
nuclease-free water, and 3 µl DNA (300 ng).
The amplification program used was the following:
Pre-PCR read 60°C/30 sec, holding stage 95°C/10 min, cycling stage:
i) 95°C/15 sec; ii) 60°C/1 min, and post-PCR read 60°C/30 sec. The
results were analyzed using the StepOne™ software v.2.2.2, (Thermo
Fisher Scientific, Inc.).
Statistical analysis
Gene analysis was conducted using the Golden Helix
SNP & Variation Suite 8.8.3 program (Golden Helix, Inc.). The
DNA variants were analyzed for deviation from the Hardy-Weinberg
Equilibrium (HWE) using the Fisher's exact test (P<0.05 was
considered to indicate a statistically significant difference and
HW disequilibrium). The genetic association study was performed
using the dominant and recessive gene models in order to assess
odds ratios (ORs), 95% confidence intervals (CIs), Bonferroni
P-values and false discovery rates (FDRs) (23). The dominant model considered the
analyzed phenotypes of Ala/Ala+Ala/Thr vs. Thr/Thr for the
rs9282858 and Val/Val+Val/Leu vs. Leu/Leu for the rs523349
variants. On the other hand, the recessive genetic model considered
the analyzed phenotypes Ala/Ala vs. Ala/Thr+Thr/Thr for the
rs9282858 and Val/Val vs. Val/Leu+Leu/Leu for the rs523349 variants
(29).
For the regression association study, a stepwise
linear regression model (qthelp://org.sphinx.svsmanual.8.8.3/doc/svsmanual/ftParts/logistic_regression.html)
with recoded genotypes with the additive gene model (DD=2, Dd=1,
dd=0) was used. False discovery rate correction (FDR) was
calculated to exclude spurious associations (qthelp://org.sphinx.svsmanual.8.8.3/doc/svsmanual/ftParts/general_statistics.html).
Results
Clinical characteristics of cases and
controls enrolled in the present study
The present study included 201 participants
classified as patients with PCa (n=101) and non-PCa subjects,
including males without prostate abnormalities (n=60) and subjects
with BPH (n=40). For patients with PCa, the median age (IQR) was 70
(range, 64.5–75) years. Thirty percent of patients had a history of
prostate pathology, while the median prostate-specific antigen
(PSA) value was 20.4 (IQR, 9.65–62.82); 20.8% had type 2 diabetes
mellitus (T2DM), and body mass index (BMI) calculations for this
group found that 33.7% were a normal weight, 40.8% were overweight,
and 22.4% had some degree of obesity. Gleason Grading was
calculated according to the recommendations of the International
Society of Urological Pathology (ISUP) (30). A total of 62% of patients were
classified as Gleason Grade Group 5, as they had tumors with
Gleason scores of 9 and 10. The majority of patients (67%) received
androgen deprivation therapy (ADT), predominantly bicalutamide
alone or in combination with other drugs, such as goserelin and
leuprolide. Table I shows the
detailed clinical variables of the patients with PCa.
 | Table I.Clinical characteristics of the
enrolled patients with prostate cancer. |
Table I.
Clinical characteristics of the
enrolled patients with prostate cancer.
| Clinical
characteristics | Prostate cancer
cases, n (%) |
|---|
| ISUP grade
group |
|
| Group
1 | 2 (2.0) |
| Group
2 | 13 (13.3) |
| Group
3 | 13 (13.3) |
| Group
4 | 9 (9.2) |
| Group
5 | 61 (62.2) |
| Extracapsular
invasion | 63 (62.4) |
| Neurovascular
invasion | 57 (56.4) |
| Recurrence | 7 (6.9) |
| Metastasis | 21 (20.8) |
|
Castration-resistance | 14 (13.9) |
| Androgen
deprivation therapy |
|
|
Bicalutamide | 22 (32.8) |
|
Bicalutamide + leuprolide | 9 (13.4) |
|
Bicalutamide + goserelin | 11 (16.4) |
|
Bicalutamide +
orchiecthomy | 12 (17.9) |
|
Orchiectomy | 6 (8.9) |
|
Otherb | 7 (10.4) |
For the non-PCa subjects, the median age (IQR) was
58.8 (range, 48–67) years; 42.4% had a history of prostate
pathology, while the median PSA value for subjects with BPH was
8.55 ng/ml (IQR, 5.12–17.71); 21% had T2DM, and the BMI
calculations for this group found that 23.1% were a normal weight,
44.9% were overweight, and the remaining 32.1% had some degree of
obesity.
Table II summarizes
the relevant data of the patients with PCa and non-PCa subjects
enrolled in the present study. Notably, an association was
identified between PCa and biomass exposure (P=0.012; OR=2.89;
CI=1.21–6.88) and tobacco use (P=0.028; OR=1.88; CI=1.07–3.31),
compared with controls.
 | Table II.Demographic characteristics of
patients with PCa (n=101) and control subjects (n=100). |
Table II.
Demographic characteristics of
patients with PCa (n=101) and control subjects (n=100).
| Demographic
characteristic | PCa cases | Non-PCa cases | P-value | OR (95% CI) |
|---|
| Age, median years
(IQR) | 70 (64.5–75.0) | 58.8 (48–67) |
9.22×10−15 | 1.13
(1.08–1.17) |
| PSA, median ng/ml
(IQR) | 20.4
(9.65–62.82) | 8.55
(5.12–17.71) |
8.29×10−20 | 1.11
(1.07–1.16) |
| BMI, median
kg/m2 | 27.02 (±4.33) | 27.84 (±5.52) | 0.271 | NS |
| BMI <18.5 (%
underweight) |
3.1 |
0.0 | NA | NS |
| BMI 18.5–24.9,
% | 33.7 | 23.1 | 0.612 | NS |
| BMI 25–29.9, % | 40.8 | 44.9 | 0.226 | NS |
| BMI >30, % | 22.4 | 32.1 | 0.486 | NS |
| Type 2 diabetes
mellitus, % | 20.8 | 21.0 | 0.732 | NS |
| Arterial
hypertension, % | 38.6 | 33.0 | 0.694 | NS |
| Alcohol intake,
% | 57.4 | 64.0 | 0.182 | NS |
| Smoking habit,
% | 60.4 | 43.0 | 0.028 | 1.88
(1.07–3.31) |
| Biomass exposure,
% | 20.8 |
8.0 | 0.012 | 2.89
(1.21–6.88) |
| Family history of
prostate cancer, % | 18.8 | 12.0 | 0.224 | NS |
| Family history of
other cancers, % | 33.7 | 29.0 | 0.603 | NS |
Genotyping
Table III shows the
HWE analysis for PCa cases and controls, as well as the genotype
frequencies. The A49T variant was out of HWE equilibrium in the
population analyzed, unlike the V89L variant, which was maintained
in HWE.
 | Table III.Hardy-Weinberg equilibrium and
genotype frequencies of A49T (rs9282858) and V89L (rs523349)
variants in cases and controls. |
Table III.
Hardy-Weinberg equilibrium and
genotype frequencies of A49T (rs9282858) and V89L (rs523349)
variants in cases and controls.
|
|
| Fisher's HWE
P-value | Genotype
frequency |
|---|
|
|
|
|
|
|---|
| Variant | Reference
alleles | Cases | Controls | Cases (%) | Controls (%) |
|---|
| A49T
(rs9282858) | [C/T] | 1.90E-06 | 0.005 | T|T: 0.030 (3) | T|T: 0.010 (1) |
|
|
|
|
| C|C: 0.970
(98) | C|C: 0.990
(97) |
| V89L
(rs523349) | [C/G] | 0.678 | 0.999 | C|C: 0.376
(38) | C|C: 0.448
(44) |
|
|
|
|
| C|G: 0.455
(46) | C|G: 0.439
(43) |
|
|
|
|
| G|G: 0.168
(17) | G|G: 0.112
(11) |
Statistical analysis
The results of the association analysis were
categorized according to the method used. In the present study,
dominant and recessive gene models were used to perform analysis.
No association was identified in any of the conditions of the
models or in the development of PCa (A49T variant) or in conferring
a protective effect (V89L variant). Table IV presents the results obtained for
the variants A49T and V89L after performing a statistical analysis
using the dominance and recessiveness gene models, as well as the
OR and 95% CI range.
 | Table IV.Association analysis of A49T and V89L
variants. |
Table IV.
Association analysis of A49T and V89L
variants.
|
|
|
|
|
| Allele
frequency |
|---|
|
|
|
|
|
|
|
|---|
| Variant | Genetic model | χ2
FDR | OR (95% CI) |
| Cases | Controls |
|---|
| A49T
(rs9282858) | Dominant | 0.327 | T, 2.97
(0.30–29.05) | C, 0.34
(0.03–3.29) | T, 0.03 | T, 0.01 |
|
| Recessive | 0.327 | T, 2.97
(0.30–29.05) | C, 0.34
(0.03–3.29) | C, 0.97 | C, 0.99 |
| V89L
(rs523349) | Dominant | 0.594 | G, 1.35
(0.77–2.38) | C, 0.74
(0.42–1.30) | G, 0.396 | G, 0.332 |
|
| Recessive | 0.511 | G, 1.60
(0.71–3.62) | C, 0.62
(0.28–1.41) | C, 0.604 | C, 0.668 |
Clinical and genetic features
association
The analysis between clinical and genetic features
was conducted using V89L genotypes. No association was identified
between genotyping and clinical variables, including PSA, ISUP
Grade Group, or a history BPH. However, an association was
identified between rs523349 and biomass exposure (P=0.013; OR=3.17;
CI=1.23–8.17 for the G risk allele, and OR=0.32, CI=0.12–0.81 for
the C protective allele) using the dominant gene model (https://doi.org/10.5281/zenodo.3932702).
There was an association between V89L and patients with metastasis
(Val/Val vs. Leu/Leu+Val/Leu; P=0.048; OR=0.390; CI=0.142–1.073;
Table V).
 | Table V.Analysis of rs523349 (V89L) genotypes
and clinical features. |
Table V.
Analysis of rs523349 (V89L) genotypes
and clinical features.
|
| PSA | Grade Gleason Group
ISUP classificationa | Metastasis | Benign Prostatic
Hyperplasia |
|---|
|
|
|
|
|
|
|---|
| Genotype | Median ng/ml | P-value | ≤3 | ≥4 | P-value | % | P-value | % | P-value |
|---|
| VV | 48.46 | 0.684 | 14.28 | 23.47 | 0.071 | 3.96 | 0.492 | 20.60 | 0.282 |
| VL | 47.53 |
| 33.67 | 12.24 |
| 13.86 |
| 18.50 |
|
| LL | 53.22 |
| 14.28 | 2.04 |
| 2.97 |
| 3.30 |
|
| VV+VL | 51.07 | 0.655 | 26.50 | 57.10 | 0.125 | 17.82 | 0.766 | 39.10 | 0.3040 |
| LL | 47.53 |
| 2.04 | 14.30 |
| 2.97 |
| 3.30 |
|
| VV | 51.75 | 0.134 | 14.30 | 47.90 | 0.125 | 3.96 | 0.048 | 20.60 | 0.635 |
| LL+VL | 48.46 |
| 14.30 | 23.50 |
| 16.83 |
| 21.70 |
|
Discussion
Previous studies that have used the Mexican
population as a subject of study have reported an association
between certain variants in the SDR5A2 gene and diseases,
including pseudo hermaphroditism and hypospadias (31–34), but
not PCa. The effect of the two analyzed variants is different
according to the ethnic group of study. For example, homozygous
subjects possessing the V89L variant (Val/Val) may have a
protective effect if its origin is from Asia, but subjects with
this same phenotype may have an increased risk of developing PCa.
Cancer is a complex set of diseases in which different risk factors
serve a crucial role in its development, where the genetic
background is only one of them.
Logistic regression is an essential tool used in
many clinical applications, including in clinical prediction models
(35), patient screening (36), and for the developing and validation
of novel diagnostic models (37).
The clinical importance of genotyping the genetic variants analyzed
in the present study lies in predicting the behaviour of the
metabolic AR pathway. Logistic regression may then be applied in
clinical prediction models, to develop and validate novel
diagnostic models, and to assess and predict the success of steroid
5 alpha-reductase 2 inhibitors (38).
In the present study, the participants enrolled were
males with diagnosed PCa, males with other urological diseases, or
men without any apparent urological condition that serve as healthy
controls. PCa is more frequently diagnosed in the fifth decade of
life; therefore, the statistical difference between the ages of PCa
vs. non-PCa subjects in the present study was expected. The
analysis of specific clinical variables revealed that PCa was
associated with certain risk factors, including tobacco use and
biomass exposure. It was found that the median PSA value derived
from the PCa cases (20.4 ng/ml) was significantly higher than that
for the non-PCa subjects (8.5 ng/ml) included in the present study
(P<0.001), which is consistent with the results of previous
studies (39,40). It was reported that 125/973 (12.8%)
of participants had PSA values >4 ng/ml and 55 (44%) were
diagnosed with PCa in a previous screening study conducted by part
of our research group in the Northeast Mexican population (41).
A limitation of our work was the lack of
availability of PSA values in healthy controls. This limitation is
due to the fact that all participants were recruited as a
convenience sample by the Urology Department and that non-PCa cases
were men with BPH (n=40) or persons classified as men without
prostate abnormalities (n=60). The association of the gene variants
analyzed in this work was made by comparing PCa vs. total non-PCa
subjects.
No association was identified between T2DM and
prostate cancer development. However, the study subjects were only
classified as diabetic or not, without accounting for their glucose
levels or medications. A recent meta-analysis of 733 articles
identifying 17 cohort studies that included 274,677 male patients
suggested that diabetes may result in a poorer prognosis for males
with PCa, but was not associated with PCa development (42). Another independent study reported an
increased risk of mortality with PCa in diabetics, but not an
association between diabetes and the incidence of prostate cancer
(43). This may be explained by the
comorbidities associated with T2DM, including atherosclerosis and
renal failure, as patients with T2DM and other comorbidities may
have a poorer response to treatment. Two meta-analyses reported a
relative low risk of develop PCa in patients with T2DM (44,45).
Other studies have presented controversial results regarding this
comorbidity (46–48). Previously, our research team
identified an increased risk of developing PCa with Gleason Scores
>8 in patients with high glucose levels (49).
Additionally, a significant difference (P=0.028) was
reported between tobacco use in PCa patients and non-PCa subjects
(60.4% vs. 43%, respectively). Several previous studies have
reported tobacco use as a risk factor for developing PCa (50–52).
Smokers have an increased risk of developing certain types of
cancer, including lung cancer. However, it has been recognized that
tobacco use may contribute toward the development of urological
cancer, including prostate, bladder, ureters and kidney (50–53).
This could be because chemical compounds released when tobacco is
burned are distributed from the lung blood vessels to other tissues
of the body.
When tobacco is burned, it produces carcinogenic
compounds, mainly polycyclic aromatic hydrocarbons (PAHs) and
nicotine- derived nitrosamines, including N′-nitrosonornicotine
(NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). In
brief, tumorigenesis is caused by the formation of DNA adducts.
These adducts generate mutations in key cell maintenance genes; the
NNNs and NNKs may bind to acetylcholine receptors and promote
events, including proliferation, growth, survival and cell
migration (54). Furthermore, PAHs
may bind to aryl hydrocarbon receptors (AHRs), which activate
CYP1A1 and CYP1B1; the CYPs add an epoxide group to the PAHs, and
these PHAs-epoxide complexes may bind to DNA to form adducts, which
are crucial for tumorigenesis (55).
Notably, there was a significant difference between
biomass exposure in patients with PCa and that in non-PCa subjects
(20.8 vs. 8%; P=0.012; OR=2.89; CI=1.21–6.88). The Mexican
population (mainly its Northern population) has a tradition for
cooking grilled foods and performing other activities, such as
working for petrochemical, steel and construction industries, that
generate particles of sizes of <10 µm, or being exposed to
xenobiotic compounds, including polycyclic aromatic hydrocarbons
(PAHs) (56). In the present study,
the participants were surveyed to see with what frequency they cook
their foods using wood. A previous study demonstrated how the
exposure to this biomass is associated with the development of
different types of cancer, primarily lung cancer (57). The mechanism by which the biomass
compounds promote cancer development is unclear, but we hypothesize
that it is associated with PAHs, primarilybenzo(a)pyrene.
Benzo(a)pyrene serves a role in the formation of DNA adducts
(58), specifically in the mutations
involving the nucleotide change from G to T in the codons 157, 158,
248, and 273 of the TP53 gene, with catastrophic
consequences according to the IARC TP53 Database (R20, July
2019) (59). The two risk factors
identified in the present study associated with benzo(a)pyrene were
tobacco use and biomass exposure. The risk factor that was
statistically significant following FDR correction was biomass
exposure. Stepwise regression was a useful tool to identify this
risk factor.
There are few studies on biomass exposure and PCa
development, the most important of which is the Cancer Prevention
Studio-II (CPS-II) of the American Cancer Society (60); however, the authors assessed risk
factors for lung cancer, so the association with PCa was not
investigated.
The A49T variant is associated with an increase in
the activity of the5-alpha reductase 2 enzyme, which converts
testosterone to dihydrotestosterone (DHT). This more potent
metabolite binds to the AR and results in the subsequent activation
of target genes that promote cell proliferation, inhibition of
apoptotic signals, and PSA overexpression.
A meta-analysis published in 2011 by Li et al
(19) reported a significantly
higher risk of developing stage III/IV PCa in homozygous variant
subjects carrying the A49T variant (Thr/Thr allele), using a
recessive gene model (P=0.0001; OR=2.13; CI=1.44–3.15) (19). In the present study, no association
was identified between SRD5A2 gene variants and PCa. The
results demonstrated that 3% of subjects were carriers of the A49T
(Thr/Thr allele) variant and it was not associated with PCa
development (P=0.327). A limitation of the results of the present
study is the HW disequilibrium, possibly due to the low frequency
of this allele or the lack of heterozygous individuals in the
sampled population (61,62). Future studies should include an
increased number of analyzed samples to verify these results.
Other ethnic groups, Ecuadorian, African American
and Latin, had an association with the development of PCa and the
A49T variant (Thr/Thr allele) (16,63,64). By
contrast, no association between this allele and PCa was identified
in analyzes performed on Hispanic and Brazilian populations
(65,66). Table
VI shows these previously reported genotyping studies and their
association with PCa development.
 | Table VI.Clinical effect of A49T (rs9282858)
variant in other populations. |
Table VI.
Clinical effect of A49T (rs9282858)
variant in other populations.
| Authors, year | Population | Effect | Genotype(s) | Refs. |
|---|
| Makridakis et
al, 1999 |
African-American | Associated to
cancer development | Thr/Thr | (16) |
| Ribeiro et
al, 2002 | Brazilian | Not associated with
cancer development | Thr/Thr | (65) |
| Pearce et
al, 2008 |
Hispanic/African-American | Not associated with
cancer development | Ala/Thr | (66) |
| Paz-y-Miño et
al, 2009 | Ecuadorian | Associated risk of
prostate cancer | Thr/Thr vs.
Ala/Thr | (63) |
| Fang et al,
2017 | Latino | Associated with
cancer development | Thr/Thr | (64) |
Although patients harboring the A49T variant
(Thr/Thr allele) were expected to have high PSA levels, the
patients in the present study with the Thr/Thr genotype had PSA
values of 5.23, 8.00, and 19.98 ng/ml, possibly due to the tumor
stage at the time of diagnosis. A more significant number of
patients would be required to verify if there is any correlation
between these two variables and PCa development in the
Mexican-mestizo population.
As for the V89L variant, the homozygous allele
variant Leu/Leu causes a decrease in the catalytic activity of
steroid 5 alpha-reductase 2, while the homozygous wild-type Val/Val
genotype has been associated with PCa development and higher
Gleason stages.
In 1992, Batista et al (5) measured steroid 5-alphareductase 2
activity indirectly by quantifying testosterone metabolites in
Afro-American and Asian individuals, finding differences attributed
to enzyme activity levels; however, they did not take into account
the genotypes of the analyzed subjects (8). This is due to the Leu/Leu allele, and a
study in the Asian population reported this result with a
protective effect by decreasing the risk of developing PCa
(9).
In the present study, patients with this allele may
be developing PCa due to other independent metabolic AR pathways,
including damage repair DNA genes (67), PTEN (68) or TP53 (69). By contrast, the allele Leu/Leu was
associated with cancer development in genotyping studies of
European American (70) and Hispanic
populations (71). Notably, this
allele has been associated with PCa with Gleason scores >8 in
the French population (72) and
metastasis (73).
The present study identified a decreased statistical
trend with regards to metastatic PCa when analyzing carriers of
alleles Leu/Leu+Val/Leu vs. Val/Val (P=0.048; OR=0.390;
CI=0.142–1.073). To the best of our knowledge, there are no reports
that directly determined the cause of this association, but we
hypothesized that it may be due to the differential gene expression
associated with metastasis in PCa, including EGR2, EGR3, MTA1,
MYBL2 (74), SYNPO2, EGR3,
RDX, FOXM1 (75), KLF6,
MMP9 or WNT5A (76).
Additionally, when a naïve PCa is treated with ADT, its evolution
to a metastatic state may be due to other transcriptional factors,
including GATA2 or FOXA1 (77), fusion mutation TMPRSS2-ERG
(78,79) or AR variants (AR-V7) (80,81).
SRD5A2, CYP17A1, and CYP19A1 are
involved in the steroid metabolic (GO:0008202) and steroid
biosynthetic processes (GO:0006694). In Mus musculus,
benzo(a)pyrene has been associated with the Protein-Protein
interaction Network oxidation-reduction process (GO:0055114;
Permanent link: http://bit.ly/2jIbOER) (82). The genes involved in this network
participate in the cytochrome P450-mediated oxidation (Cyp17a1,
Cyp19a1, Cyp1a2, Cyp21a1, Cyp3a11, Cyp3a13, Cyp3a16, Cyp3a25
and Cyp3a41a), and the reduction of oestrogens and androgens
(Srd5a2, Akr1d1, Hsd17b1 and Hsd3b4).
The Val/Val allele is more common in white
Hispanic/non-Hispanic and Ecuadorian populations and it has been
associated with PCa development; in the Mexican-mestizo population
investigated in the present study, this association was not found.
It has been seen that this allele may contribute toward worsening
clinical prognosis associated with the overall survival, as was
described in a previous study when comparing the different genetic
variants and lower AR activity (83).
Table VII
summarizes other genotyping studies of V89L and its potential
associations with PCa development, metastasis, Gleason score, or
protective effects against PCa (9,63,70–73,84).
 | Table VII.Clinical effect of V89L (rs523349)
variant in other populations. |
Table VII.
Clinical effect of V89L (rs523349)
variant in other populations.
| Author, year | Population | Effect | Genotype(s) | Refs. |
|---|
| Makridakis et
al, 1997 | Asian | Protective against
cancer | Leu/Leu | (9) |
| Sӧderstrӧm et
al, 2002 | Caucasian | Associated with
metastasis | Leu/Leu | (73) |
| Loukola et
al, 2004 |
European-Americans | Associated with
cancer development | Leu/Leu vs.
Val/Leua | (70) |
| Salam et al,
2005 | Hispanic | Associated with
cancer development | Leu/Leu | (71) |
| Cussenot et
al, 2007 | French | Gleason Scores
>8 | Leu/Leu | (72) |
| Torkko et
al, 2008 | Non-Hispanic
White | Associated with
cancer development |
Val/Valb | (84) |
|
| Hispanic White | Associated with
cancer development |
Val/Valc |
|
| Paz-y-Miño et
al, 2009 | Ecuadorian | Associated with
cancer development | Val/Val | (63) |
The analysis performed in the present study
demonstrated that there was no association between the
SRD5A2 rs523349 genotypes and Gleason scores ≥8; however,
there was a decreased tendency between patients with metastasis and
rs523349 genotypes (Val/Val vs. Leu/Leu+Val/Leu; P=0.048; OR=0.390;
CI=0.142–1.073).
The clinical prognosis of the cohort of patients
with PCa included in the present study was associated with 5-alpha
reductase 2 variants. However, there are two main limitations: i)
The present study included patients with localized and metastatic
disease without discriminating the time of evolution, and ii) the
type of ADT at the time of enrolment. The first-line treatment of
ADT in clinical practice is the administration of bicalutamide, as
the public medical care does not include new generation treatments
in Mexico, including enzalutamide or abiraterone acetate.
Finally, regarding benign prostate diseases, a
meta-analysis undertaken in 2017 by Zeng et al (85) found a risk of developing BPH in
individuals carrying the A49T variant (OR=2.75; CI=1.32–5.69), but
this was not statistically significance (P=0.373). By contrast, the
results of studies concerning the V89L variant and its association
with the development of BPH and PSA changes have been contradictory
(86,87). A previous study reported that the
Val/Leu+Leu/Leu genotype (P=0.047; OR=1.62; CI=1.00–2.61) was
associated with the development of this benign condition, but not
with the development of PCa (88).
However, in another study, none of these genotypes were associated
with the development of BPH (85).
In the future, a longitudinal analysis of Mexican patients with
this type of benign disease should be performed to describe the
frequency of the different genetic variants and to determine if
they have a role in the prognosis of PCa as potential biomarkers
for personalization of pharmacological treatments using 5
alpha-reductase enzyme inhibitors (87).
In conclusion, we identified the allelic frequencies
of both the A49T and V89L variants of the steroid 5 alpha-reductase
2 gene in the Mexican population. No association was identified
between either of the variants and the development of PCa, but no
increased risk of developing PCa was identified due to lifestyle
factors, including the exposure to biomass and tobacco use.
Furthermore, an association between V89L and biomass exposure was
identified using the dominant gene model. Additionally, there was
an association between V89L and patients with metastasis.
To the best of our knowledge, the present study was
the first to screen the Mexican population for these variants, and
one of the focussing on the Latino population. For these ethnic
groups, molecular characterization of PCa is required to improve
the understanding of this disease, and to determine if the results
of molecular characterization studies may serve a role in the
prognosis of PCa or as potential biomarkers for the personalization
of pharmacological treatments.
Acknowledgements
The authors would like to thank the Dr María Carmen
Barboza-Cerda (Laboratorio Nacional Biobanco, LANBIOBAN,
Universidad Autónoma de Nuevo León, Monterrey, Mexico), for
providing the equipment and facilities to perform certain
experiments. They would also like to acknowledge Mr. Eduardo
Coronado (Urology Service, ‘Dr. José Eleuterio González’ University
Hospital, Universidad Autónoma de Nuevo León, Monterrey, Mexico)
for his support in collecting the blood samples for the present
study, and Dr. Sergio Lozano-Rodriguez (Office of the Vice Dean of
Research, ‘Dr. José Eleuterio González’ University Hospital,
Universidad Autónoma de Nuevo León, Monterrey, Mexico) for his
review of the English language of this manuscript.
Funding
The present study was supported by the Program for
Scientific and Technological Research 2019 of the Universidad
Autónoma de Nuevo León, Mexico (PAICyT: SA763-19).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JRDB conducted the experiments, acquired, analyzed
and interpreted the data; and drafted the manuscript. JRDB, HLGB,
JFYL, LSGG and CNSD designed the study, conducted the experiments,
analyzed, interpreted the data, and critically revised the
manuscript. CNSD and AMRE made substantial contributions to the
conception of the study, and drafted, and critically revised the
manuscript. DHB, AMGB, GVM, MAOM and LSGG, as clinicians, selected
the patients, performed the biopsies to obtain the samples,
collected the clinical information from medical records, and
contributed toward the design of the study. DAH and BSG assisted in
technical support during the experimental work and were involved in
the acquisition, analysis and interpretation of data. RGG and MNM,
as pathologists, conducted the histopathological diagnoses of the
patients. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Ethics and Research
Committee of the School of Medicine (Universidad Autónoma de Nuevo
León, Mexico; UR16-00007). Prior to blood extraction, written
informed consent was obtained from the eligible participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SRD5A2 gene
|
steroid 5 alpha-reductase 2 gene
|
|
A49T
|
Alanine49Threonine
|
|
V89L
|
Valine89Leucine
|
|
A
|
adenine
|
|
G
|
guanine
|
|
C
|
cytosine
|
|
PCR
|
polymerase chain reaction
|
|
Ala
|
alanine
|
|
Thr
|
threonine
|
|
Val
|
valine
|
|
Leu
|
leucine
|
|
OR
|
Odds ratio
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
IQR
|
interquartile range
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giovannucci E, Liu Y, Platz EA, Stampfer
MJ and Willett WC: Risk factors for prostate cancer incidence and
progression in the health professionals follow-up study. Int J
Cancer. 121:1571–1578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chandrasekar T, Yang JC, Gao AC and Evans
CP: Mechanisms of resistance in castration-resistant prostate
cancer (CRPC). Transl Androl Urol. 4:365–380. 2015.PubMed/NCBI
|
|
5
|
Batista RL and Mendonca BB: Integrative
and analytical review of the 5-alpha-reductase type 2 deficiency
worldwide. Appl Clin Genet. 13:83–96. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kokal M, Mirzakhani K, Pungsrinont T and
Baniahmad A: Mechanisms of androgen receptor agonist- and
antagonist-mediated cellular senescence in prostate cancer. Cancers
(Basel). 12:18332020. View Article : Google Scholar
|
|
7
|
Kregel S, Bagamasbad P, He S, LaPensee E,
Raji Y, Brogley M, Chinnaiyan A, Cieslik M and Robins DM:
Differential modulation of the androgen receptor for prostate
cancer therapy depends on the DNA response element. Nucleic Acids
Res. 48:4741–4755. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ross RK, Bernstein L, Lobo RA, Shimizu H,
Stanczyk FZ, Pike MC and Henderson BE: 5-alpha-reductase activity
and risk of prostate cancer among Japanese and US white and black
males. Lancet. 339:887–889. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Makridakis N, Ross RK, Pike MC, Chang L,
Stanczyk FZ, Kolonel LN, Shi CY, Yu MC, Henderson BE and Reichardt
JK: A prevalent missense substitution that modulates activity of
prostatic steroid 5alpha-reductase. Cancer Res. 57:1020–1022.
1997.PubMed/NCBI
|
|
10
|
Ntais C, Polycarpou A and Ioannidis JP:
SRD5A2 gene polymorphisms and the risk of prostate cancer: A
meta-analysis. Cancer Epidemiol Biomarkers Prev. 12:618–624.
2003.PubMed/NCBI
|
|
11
|
Reichardt JK, Makridakis N, Henderson BE,
Yu MC, Pike MC and Ross RK: Genetic variability of the human SRD5A2
gene: Implications for prostate cancer risk. Cancer Res.
55:3973–3975. 1995.PubMed/NCBI
|
|
12
|
Davis DL and Russell DW: Unusual length
polymorphism in human steroid 5 alpha-reductase type 2 gene
(SRD5A2). Hum Mol Genet. 2:8201993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruijter E, van de Kaa C, Miller G, Ruiter
D, Debruyne F and Schalken J: Molecular genetics and epidemiology
of prostate carcinoma. Endocr Rev. 20:22–45. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Makridakis NM, di Salle E and Reichardt
JK: Biochemical and pharmacogenetic dissection of human steroid 5
alpha-reductase type II. Pharmacogenetics. 10:407–413. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsing AW, Chen C, Chokkalingam AP, Gao YT,
Dightman DA, Nguyen HT, Deng J, Cheng J, Sesterhenn IA, Mostofi FK,
et al: Polymorphic markers in the SRD5A2 gene and prostate cancer
risk: A population-based case-control study. Cancer Epidemiol
Biomarkers Prev. 10:1077–1082. 2001.PubMed/NCBI
|
|
16
|
Makridakis NM, Ross RK, Pike MC, Crocitto
LE, Kolonel LN, Pearce CL, Henderson BE and Reichardt JK:
Association of mis-sense substitution in SRD5A2 gene with prostate
cancer in African-American and Hispanic men in Los Angeles, USA.
Lancet. 354:975–978. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neslund-Dudas C, Bock CH, Monaghan K, Nock
NL, Yang JJ, Rundle A, Tang D and Rybicki BA: SRD5A2 and HSD3B2
polymorphisms are associated with prostate cancer risk and
aggressiveness. Prostate. 67:1654–1663. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Russell DW and Wilson JD: Steroid 5
alpha-reductase: Two genes/two enzymes. Annu Rev Biochem. 63:25–61.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Huang Y, Fu X, Chen C, Zhang D, Yan
L, Xie Y, Mao Y and Li Y: Meta-analysis of three polymorphisms in
the steroid-5-alpha-reductase, alpha polypeptide 2 gene (SRD5A2)
and risk of prostate cancer. Mutagenesis. 26:371–383. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bentley AR, Callier S and Rotimi CN:
Diversity and inclusion in genomic research: Why the uneven
progress? J Community Genet. 8:255–266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moreno-Estrada A, Gignoux CR,
Fernández-López JC, Zakharia F, Sikora M, Contreras AV,
Acuña-Alonzo V, Sandoval K, Eng C, Romero-Hidalgo S, et al: Human
genetics. The genetics of Mexico recapitulates Native American
substructure and affects biomedical traits. Science. 344:1280–1285.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Silva-Zolezzi I, Hidalgo-Miranda A,
Estrada-Gil J, Fernandez-Lopez JC, Uribe-Figueroa L, Contreras A,
Balam-Ortiz E, del Bosque-Plata L, Velazquez-Fernandez D, Lara C,
et al: Analysis of genomic diversity in Mexican Mestizo populations
to develop genomic medicine in Mexico. Proc Natl Acad Sci USA.
106:8611–8616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Villarreal-Martínez A, Gallardo-Blanco H,
Cerda-Flores R, Torres-Muñoz I, Gómez-Flores M, Salas-Alanís J,
Ocampo-Candiani J and Martínez-Garza L: Candidate gene
polymorphisms and risk of psoriasis: A pilot study. Exp Ther Med.
11:1217–1222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patiño-García B, Arroyo C,
Rangel-Villalobos H, Soto-Vega E, Velarde-Félix JS, Gabilondo F,
Sandoval-Ramirez L and Figuera LE: Association between
polymorphisms of the androgen and vitamin D receptor genes with
prostate cancer risk in a Mexican population. Rev Invest Clin.
59:25–31. 2007.PubMed/NCBI
|
|
25
|
Martinez-Fierro ML, Garza-Veloz I,
Rojas-Martinez A, Ortiz-Lopez R, Castruita-de la Rosa C,
Ortiz-Castro Y, Lazalde- Ramos BP, Cervantes-Villagrana AR,
Castañeda-Lopez ME, Gomez-Guerra L, et al: Positive association
between vascular endothelial growth factor (VEGF) −2578 C/A variant
and prostate cancer. Cancer Biomark. 13:235–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Canto P, Benítez Granados J, Martínez
Ramírez MA, Reyes E, Feria-Bernal G, García-García E, Tejeda ME,
Zavala E, Tapia A, Rojano-Mejía D and Méndez JP: Genetic variants
in ATP6 and ND3 mitochondrial genes are not associated with
aggressive prostate cancer in Mexican-Mestizo men with overweight
or obesity. Aging Male. 19:187–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanchez-Dominguez CN, Reyes-Lopez MA,
Bustamante A, Cerda-Flores RM, Villalobos-Torres Mdel C, Gallardo-
Blanco HL, Rojas-Martinez A, Martinez-Rodriguez HG, Barrera-Saldaña
HA and Ortiz-Lopez R: The tumor necrosis factor alpha (−308 A/G)
polymorphism is associated with cystic fibrosis in Mexican
patients. PLoS One. 9:e909452014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jaramillo-Rangel G, Ortega-Martínez M,
Cerda-Flores RM and Barrera-Saldaña HA: C3435T polymorphism in the
MDR1 gene and breast cancer risk in northeastern Mexico. Int J Clin
Exp Pathol. 11:904–909. 2018.PubMed/NCBI
|
|
29
|
Zhao F, Song M, Wang Y and Wang W: Genetic
model. J Cell Mol Med. 20:7652016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Srigley JR, Delahunt B, Samaratunga H,
Billis A, Cheng L, Clouston D, Evans A, Furusato B, Kench J, Leite
K, et al: Controversial issues in Gleason and International Society
of Urological Pathology (ISUP) prostate cancer grading: Proposed
recommendations for international implementation. Pathology.
51:463–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Canto P, Vilchis F, Chávez B, Mutchinick
O, Imperato-McGinley J, Pérez-Palacios G, Ulloa-Aguirre A and
Méndez JP: Mutations of the 5 alpha-reductase type 2 gene in eight
Mexican patients from six different pedigrees with 5
alpha-reductase-2 deficiency. Clin Endocrinol (Oxf). 46:155–160.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chávez B, Valdez E and Vilchis F:
Uniparental disomy in steroid 5alpha-reductase 2 deficiency. J Clin
Endocrinol Metab. 85:3147–3150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vilchis F, Valdez E, Ramos L, García R,
Gómez R and Chávez B: Novel compound heterozygous mutations in the
SRD5A2 gene from 46, XY infants with ambiguous external genitalia.
J Hum Genet. 53:401–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shih EM and Graham JM Jr: Review of
genetic and environmental factors leading to hypospadias. Eur J Med
Genet. 57:453–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou ZR, Wang WW, Li Y, Jin KR, Wang XY,
Wang ZW, Chen YS, Wang SJ, Hu J, Zhang HN, et al: In-depth mining
of clinical data: The construction of clinical prediction model
with R. Ann Transl Med. 7:632019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong R, Jiang J, Zhang S, Shen Z, Chen G,
Huang Y, Zheng Y and Zheng S: Development and validation of novel
diagnostic models for biliary atresia in a large cohort of Chinese
patients. EBioMedicine. 34:223–230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun G, Nakayama Y, Dagdanpurev S, Abe S,
Nishimura H, Kirimoto T and Matsui T: Remote sensing of multiple
vital signs using a CMOS camera-equipped infrared thermography
system and its clinical application in rapidly screening patients
with suspected infectious diseases. Int J Infect Dis. 55:113–117.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shiota M, Fujimoto N, Yokomizo A, Takeuchi
A, Kashiwagi E, Dejima T, Kiyoshima K, Inokuchi J, Tatsugami K and
Eto M: The prognostic impact of serum testosterone during androgen-
deprivation therapy in patients with metastatic prostate cancer and
the SRD5A2 polymorphism. Prostate Cancer Prostatic Dis. 19:191–196.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kusuma Duarsa GW, Sari YA, Gde Oka AA,
Santosa KB, Yudiana IW, Wisnu Tirtayasa PM, Putra Pramana IB and
Kloping YP: Serum testosterone and prostate-specific antigen levels
are major risk factors for prostatic volume increase among benign
prostatic hyperplasia patients. Asian J Urol. Jun 7–2020.(Epub
ahead of print). doi: org/10.1016/j.ajur.2020.06.001. View Article : Google Scholar
|
|
40
|
Golchin-Rad K, Mogheiseh A, Nazifi S,
Ahrari Khafi MS, Derakhshandeh N and Abbaszadeh-Hasiri M: Changes
in the serum prostatic biomarkers during the treatment of benign
prostatic hyperplasia with a 5alpha-REDUCTASE inhibitor:
Finasteride. Top Companion Anim Med. 38:1004052020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gomez-Guerra LS, Martinez-Fierro ML,
Alcantara-Aragon V, Ortiz-Lopez R, Martinez-Villarreal RT,
Morales-Rodriguez IB, Garza-Guajardo R, Ponce-Camacho MA and
Rojas-Martinez A: Population based prostate cancer screening in
north Mexico reveals a high prevalence of aggressive tumors in
detected cases. BMC Cancer. 9:912009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee J, Giovannucci E and Jeon JY: Diabetes
and mortality in patients with prostate cancer: A meta-analysis.
Springerplus. 5:15482016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marrone MT, Selvin E, Barber JR, Platz EA
and Joshu CE: Hyperglycemia, classified with multiple biomarkers
simultaneously in men without diabetes, and risk of fatal prostate
cancer. Cancer Prev Res (Phila). 12:103–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bansal D, Bhansali A, Kapil G, Undela K
and Tiwari P: Type 2 diabetes and risk of prostate cancer: A
meta-analysis of observational studies. Prostate Cancer Prostatic
Dis. 16:151–158, S1. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin E, Garmo H, Van Hemelrijck M,
Adolfsson J, Stattin P, Zethelius B and Crawley D: Association of
type 2 diabetes mellitus and antidiabetic medication with risk of
prostate cancer: A population-based case-control study. BMC Cancer.
20:5512020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tseng CH: Metformin significantly reduces
incident prostate cancer risk in Taiwanese men with type 2 diabetes
mellitus. Eur J Cancer. 50:2831–2837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Azoulay L, Dell'Aniello S, Gagnon B,
Pollak M and Suissa S: Metformin and the incidence of prostate
cancer in patients with type 2 diabetes. Cancer Epidemiol
Biomarkers Prev. 20:337–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Häggström C, Van Hemelrijck M, Zethelius
B, Robinson D, Grundmark B, Holmberg L, Gudbjörnsdottir S, Garmo H
and Stattin P: Prospective study of Type 2 diabetes mellitus,
anti-diabetic drugs and risk of prostate cancer. International
journal of cancer Journal international du cancer. 140:611–617.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Garza-Guajardo R, Delgado-Enciso I,
Melo-De-La-Garza A, Rodriguez-Sanchez I, Laura GL, Martinez-Fierro
ML, Gómez-Guerra L, Gómez-Macías GS, Barboza-Quintana A,
Guzmán-Esquivel J, et al: High fasting glucose, but not metabolic
syndrome, is associated with elevated histologic aggressiveness in
Mexican prostate cancer patients. Int J Clin Exp Pathol.
9:11951–11957. 2016.
|
|
50
|
Plaskon LA, Penson DF, Vaughan TL and
Stanford JL: Cigarette smoking and risk of prostate cancer in
middle-aged men. Cancer Epidemiol Biomarkers Prev. 12:604–609.
2003.PubMed/NCBI
|
|
51
|
Zu K and Giovannucci E: Smoking and
aggressive prostate cancer: A review of the epidemiologic evidence.
Cancer Causes Control. 20:1799–1810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huncharek M, Haddock KS, Reid R and
Kupelnick B: Smoking as a risk factor for prostate cancer: A
meta-analysis of 24 prospective cohort studies. Am J Public Health.
100:693–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
How Tobacco Smoke Causes Disease, . The
Biology and Behavioral Basis for Smoking-Attributable Disease. A
Report of the Surgeon General. Centers for Disease Control and
Prevention; Atlanta, GA: 2010
|
|
54
|
Xue J, Yang S and Seng S: Mechanisms of
cancer induction by tobacco-specific NNK and NNN. Cancers (Basel).
6:1138–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Baird WM, Hooven LA and Mahadevan B:
Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and
mechanism of action. Environ Mol Mutagen. 45:106–114. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zelikoff JT, Chen LC, Cohen MD and
Schlesinger RB: The toxicology of inhaled woodsmoke. J Toxicol
Environ Health B Crit Rev. 5:269–282. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
IARC Working Group on the Evaluation of
Carcinogenic Risk to Humans. Household Use of Solid Fuels and
High-temperature Frying. Lyon (FR): International Agency for
Research on Cancer; 2010, (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, No. 95.) 2, Studies of Cancer in
Humans. https://www.ncbi.nlm.nih.gov/books/NBK385519/
|
|
58
|
Li N, Luo HD, Jia YZ, Zhou N and Li YQ:
Rapid determination of benzo(a)pyrene in processed meat and fish
samples by second-derivative constant-energy synchronous
fluorescence spectrometry. Food Addit Contam Part A Chem Anal
Control Expo Risk Assess. 28:235–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bouaoun L, Sonkin D, Ardin M, Hollstein M,
Byrnes G, Zavadil J and Olivier M: TP53 Variations in human
cancers: New lessons from the IARC TP53 database and genomics data.
Hum Mutat. 37:865–876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Stellman SD, Demers PA, Colin D and
Boffetta P: Cancer mortality and wood dust exposure among
participants in the American Cancer Society Cancer Prevention
Study-II (CPS-II). Am J Ind Med. 34:229–237. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen B, Cole JW and Grond-Ginsbach C:
Departure from Hardy Weinberg Equilibrium and genotyping error.
Front Genet. 8:1672017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
McQuillan R, Leutenegger AL, Abdel-Rahman
R, Franklin CS, Pericic M, Barac-Lauc L, Smolej-Narancic N,
Janicijevic B, Polasek O, Tenesa A, et al: Runs of homozygosity in
European populations. Am J Hum Genet. 83:359–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Paz-y-Miño C, Witte T, Robles P,
Llumipanta W, Diaz M and Arevalo M: Association among polymorphisms
in the steroid 5alpha-reductase type II (SRD5A2) gene, prostate
cancer risk, and pathologic characteristics of prostate tumors in
an Ecuadorian population. Cancer Genet Cytogenet. 189:71–76. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fang C, Guo ZQ, Chen XY, Liu TZ, Zeng XT
and Wang XH: Relationship between SRD5A2 rs9282858 polymorphism and
the susceptibility of prostate cancer: A meta-analysis based on 20
publications. Medicine (Baltimore). 96:e67912017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ribeiro ML, Santos A, Carvalho-Salles AB
and Hackel C: Allelic frequencies of six polymorphic markers for
risk of prostate cancer. Braz J Med Biol Res. 35:205–213. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pearce CL, Van Den Berg DJ, Makridakis N,
Reichardt JK, Ross RK, Pike MC, Kolonel LN and Henderson BE: No
association between the SRD5A2 gene A49T missense variant and
prostate cancer risk: Lessons learned. Hum Mol Genet. 17:2456–2461.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mateo J, Carreira S, Sandhu S, Miranda S,
Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A,
Tunariu N, et al: DNA-Repair Defects and Olaparib in Metastatic
Prostate Cancer. N Engl J Med. 373:1697–1708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ruscetti MA and Wu H: PTEN in prostate
cancer. Prostate Cancer. 87–137. 2013. View Article : Google Scholar
|
|
69
|
Ecke TH, Schlechte HH, Schiemenz K, Sachs
MD, Lenk SV, Rudolph BD and Loening SA: TP53 gene mutations in
prostate cancer progression. Anticancer Res. 30:1579–1586.
2010.PubMed/NCBI
|
|
70
|
Loukola A, Chadha M, Penn SG, Rank D,
Conti DV, Thompson D, Cicek M, Love B, Bivolarevic V, Yang Q, et
al: Comprehensive evaluation of the association between prostate
cancer and genotypes/haplotypes in CYP17A1, CYP3A4, and SRD5A2. Eur
J Hum Genet. 12:321–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Salam MT, Ursin G, Skinner EC, Dessissa T
and Reichardt JK: Associations between polymorphisms in the steroid
5-alpha reductase type II (SRD5A2) gene and benign prostatic
hyperplasia and prostate cancer. Urol Oncol. 23:246–253. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cussenot O, Azzouzi AR, Nicolaiew N,
Mangin P, Cormier L, Fournier G, Valeri A and Cancel-Tassin G:
Low-activity V89L variant in SRD5A2 is associated with aggressive
prostate cancer risk: An explanation for the adverse effects
observed in chemoprevention trials using 5-alpha-reductase
inhibitors. Eur Urol. 52:1082–1087. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sӧderstrӧm T, Wadelius M, Andersson SO,
Johansson JE, Johansson S, Granath F and Rane A: 5alpha-reductase 2
polymorphisms as risk factors in prostate cancer. Pharmacogenetics.
12:307–312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
LaTulippe E, Satagopan J, Smith A, Scher
H, Scardino P, Reuter V and Gerald WL: Comprehensive gene
expression analysis of prostate cancer reveals distinct
transcriptional programs associated with metastatic disease. Cancer
Res. 62:4499–4506. 2002.PubMed/NCBI
|
|
75
|
Chandran UR, Ma C, Dhir R, Bisceglia M,
Lyons-Weiler M, Liang W, Michalopoulos G, Becich M and Monzon FA:
Gene expression profiles of prostate cancer reveal involvement of
multiple molecular pathways in the metastatic process. BMC Cancer.
7:642007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Stanbrough M, Bubley GJ, Ross K, Golub TR,
Rubin MA, Penning TM, Febbo PG and Balk SP: Increased expression of
genes converting adrenal androgens to testosterone in
androgen-independent prostate cancer. Cancer Res. 66:2815–2825.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Obinata D, Takayama K, Takahashi S and
Inoue S: Crosstalk of the androgen receptor with transcriptional
collaborators: Potential therapeutic targets for
castration-resistant prostate cancer. Cancers (Basel). 9:222017.
View Article : Google Scholar
|
|
78
|
Squire JA: TMPRSS2-ERG and PTEN loss in
prostate cancer. Nat Genet. 41:509–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kiflemariam S, Mignardi M, Ali MA, Bergh
A, Nilsson M and Sjoblom T: In situ sequencing identifies
TMPRSS2-ERG fusion transcripts, somatic point mutations and gene
expression levels in prostate cancers. J Pathol. 234:253–261.
2014.PubMed/NCBI
|
|
80
|
Li H, Wang Z, Tang K, Zhou H, Liu H, Yan
L, Guan W, Chen K, Xu H and Ye Z: Prognostic value of androgen
receptor splice variant 7 in the treatment of castration-resistant
prostate cancer with next generation androgen receptor signal
inhibition: A systematic review and meta-analysis. Eur Urol Focus.
4:529–539. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Markowski MC, Silberstein JL, Eshleman JR,
Eisenberger MA, Luo J and Antonarakis ES: Clinical utility of
CLIA-Grade AR-V7 testing in patients with metastatic
castration-resistant prostate cancer. JCO Precis Oncol. Oct
10–2017.(Epub ahead of print). doi: 10.1200/PO.17.00127, 2017.
View Article : Google Scholar
|
|
82
|
Szklarczyk D, Santos A, von Mering C,
Jensen LJ, Bork P and Kuhn M: STITCH 5: Augmenting protein-chemical
interaction networks with tissue and affinity data. Nucleic Acids
Res. 44:D380–D384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shiota M, Fujimoto N, Yokomizo A, Takeuchi
A, Itsumi M, Inokuchi J, Tatsugami K, Uchiumi T and Naito S: SRD5A
gene polymorphism in Japanese men predicts prognosis of metastatic
prostate cancer with androgen-deprivation therapy. Eur J Cancer.
51:1962–1969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Torkko KC, van Bokhoven A, Mai P, Beuten
J, Balic I, Byers TE, Hokanson JE, Norris JM, Barón AE, Lucia MS,
et al: VDR and SRD5A2 polymorphisms combine to increase risk for
prostate cancer in both non-Hispanic White and Hispanic White men.
Clin Cancer Res. 14:3223–3229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zeng XT, Su XJ, Li S, Weng H, Liu TZ and
Wang XH: Association between SRD5A2 rs523349 and rs9282858
polymorphisms and risk of benign prostatic hyperplasia: A
meta-analysis. Front Physiol. 8:6882017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rył A, Rotter I, Grzywacz A, Małecka I,
Skonieczna-Żydecka K, Grzesiak K, Słojewski M, Szylińska A,
Sipak-Szmigiel O, Piasecka M, et al: Molecular analysis of the
SRD5A1 and SRD5A2 genes in patients with benign prostatic
hyperplasia with regard to metabolic parameters and selected
hormone levels. Int J Environ Res Public Health. 14:13182017.
View Article : Google Scholar
|
|
87
|
Gu X, Na R, Huang T, Wang L, Tao S, Tian
L, Chen Z, Jiao Y, Kang J, Zheng S, et al: SRD5A1 and SRD5A2 are
associated with treatment for benign prostatic hyperplasia with the
combination of 5α-reductase inhibitors and α-adrenergic receptor
antagonists. J Urol. 190:615–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Choubey VK, Sankhwar SN, Carlus SJ, Singh
AN, Dalela D, Thangaraj K and Rajender S: SRD5A2 gene polymorphisms
and the risk of benign prostatic hyperplasia but not prostate
cancer. Asian Pac J Cancer Prev. 16:1033–1036. 2015. View Article : Google Scholar : PubMed/NCBI
|