Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer in the world (1).
Although liver resection is one of the most effective treatments
for HCC, HCC has a high recurrence rate even after curative
resection (2). To improve the
prognosis of HCC, it is desirable to elucidate the mechanism of its
carcinogenesis and progression and to identify target molecules for
treatment.
The Wnt signaling pathway is classified into
canonical and noncanonical pathways. Inappropriate activation of
the former is associated with carcinogenesis via abnormal
accumulation of cytoplasmic β-catenin and its translocation to the
nucleus (3). However, the
association between the noncanonical pathway, which does not
involve activity of β-catenin, and carcinogenesis or tumor
progression is not well known. In the Wnt signaling pathway, its
ligands are the Wnt protein family, and there are 19 members in the
family in mammals (4). Wnt5a is
supposed to be the key ligand of the noncanonical pathway (5). The noncanonical pathway can be
subclassified mainly into the planar cell polarity and
Ca2+ pathways (6).
Through these pathways, Wnt5a signaling plays an important role in
regulating cell differentiation, proliferation, migration, adhesion
and polarity (5). Abnormal
activation or inhibition of Wnt5a signaling is associated with
cancer progression or suppression (7). Several studies have shown that the
function of Wnt5a differs depending on the type of cancer. For
example, activation of Wnt5a has a suppressive effect on thyroid,
colon and breast cancers (8–10), while it has a progressive effect on
prostate, gastric and non-small cell lung cancers, and malignant
melanoma (11–14). However, the effect of Wnt5a on HCC is
not well known. Recent research has provided evidence that the
WNT5A gene encodes two protein isoforms, termed Wnt5a-long
(Wnt5a-L) and Wnt5a-short (Wnt5a-S). The two isoforms appear to
have contrasting roles in cancer; that is, Wnt5a-L inhibits
proliferation and Wnt5a-S increases proliferation in breast cancer,
cervical cancer and neuroblastoma cell lines (15). In the present study, we investigated
the significance of expression of Wnt5a in HCC.
Materials and methods
Patients and tissue samples
We retrospectively screened 243 patients (200 male
and 43 female), with a median age of 63 years (range 35–82 years),
who underwent hepatic resection for HCC between January 1997 and
December 2006 at our institution. After surgery, patients were
followed up by monitoring dynamic computed tomography and/or
magnetic resonance imaging and tumor markers every 3 months on an
outpatient basis. Combined examination of tumor markers and imaging
studies was used for diagnosis of recurrence of HCC. We reviewed
the medical records of all patients for clinical information,
including sex, age, markers of hepatitis B virus and hepatitis C
virus (HCV), serum albumin, serum a-fetoprotein (AFP), and protein
induced by vitamin K absence or antagonist(PIVKA)II. We reviewed
tumor size, tumor number, vascular invasion, lymph node metastasis,
and the pathological findings of background liver. We used the
criteria of the Liver Cancer Study Group of Japan (6th edition) to
determine tumor-node-metastasis (TNM) stage of HCC (16). Informed consent of patients between
1997 and 2000 was obtained in the form of opt-out on the web site
of Hokkaido University Hospital and patients between 2001 and 2006
signed the written informed consent. This research was approved by
the Institutional Review Board of our institution (017–0237) and
performed in compliance with the Declaration of Helsinki.
Immunohistochemical staining
Four-micrometer-thick sections of formalin-fixed and
paraffin-embedded specimens were used for immunohistochemical
staining. They were deparaffinized using xylene and ethanol, and
antigen retrieval was performed using Target Retrieval Solution (pH
9.0; 415211; Nichirei Biosciences Inc., Tokyo, Japan), heated for
30 min at 95°C. The samples were incubated with Block Ace (UKB80;
KAC Co., Ltd.) for 5 min to block nonspecific antibody reactions
and incubated overnight at 4°C with anti-Wnt5a antibody (LS-C47384,
diluted 1:2,000; LifeSpan BioSciences Inc.). The samples were
incubated in Histofine Simple Stain MAX PO (MULTI; 724152; Nichirei
Biosciences) for 30 min at room temperature. Immunohistochemical
staining was visualized using 3,3′ diaminobenzidine, and sections
were counterstained with hematoxylin. Immunoreactivity was
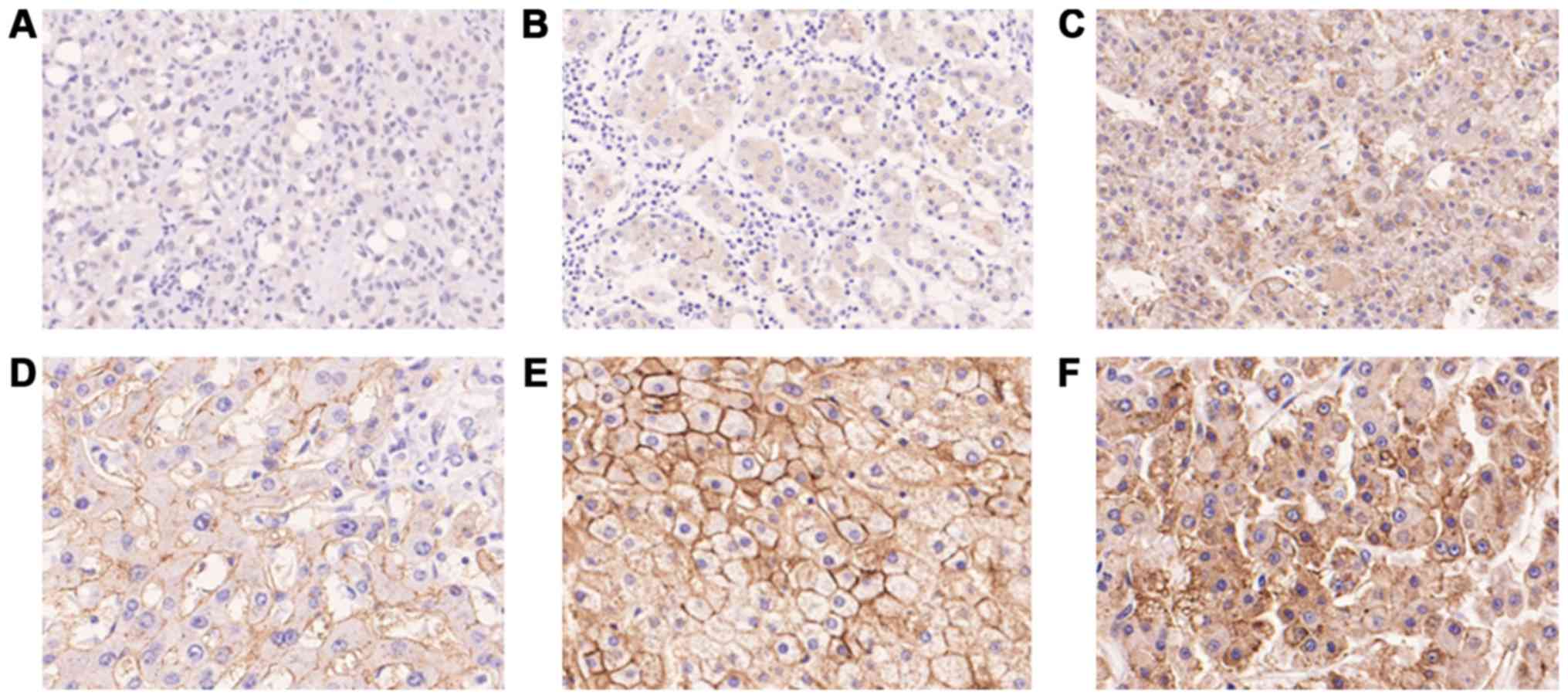
evaluated according to the distribution of positive cells.
Wnt5a-positive cells were defined according to the immunoreactivity
on the cell membrane, regardless of cytoplasmic immunoreactivity.
The immunohistochemical staining pattern of Wnt5a in HCC was
heterogeneous and Wnt5a positivity was recorded if the proportion
of Wnt5a-positive cells was >50% (Fig. 1). Two authors who were blinded to the
clinical and pathological parameters evaluated the results of
immunohistochemical staining.
Cell lines
Human liver cancer cell lines HLE, HLF, HepG2 and
Huh7 were obtained from the Japanese Collection of Research
Bioresources Cell Bank (Osaka, Japan). They were authenticated by
short tandem repeat profiling. They were cultured in Dulbecco's
modified Eagle's medium containing 10% fetal bovine serum (FBS).
The medium was replaced every second day and all cell cultures were
incubated at 37°C in 5% CO2.
Cell transfection
To establish Wnt5a knockdown cells, we used a
lentiviral short hairpin RNA (shRNA) targeting Wnt5a (TL320572;
OriGene). Lenti-X HTX Packaging System (631251; Takara Bio Inc.)
was used for transfection into cell lines. To create overexpressing
cells, we obtained the full-length cDNA encoding human Wnt5a from
cDNA of Huh7 cells by reverse transcription polymerase chain
reaction. The Wnt5a primers were: Forward,
5′-CAGTGTGGTGGAATTGCCACCATGAAGAAGTCC-3′ and reverse,
5′-GATATCTGCAGAATTCTACTTGCACACAAACTGG-3′. The cDNA was cloned into
pcDNA3.1 (V79020; Thermo Fisher Scientific, Tokyo, Japan). HLF
cells were seeded in cell culture dishes, and after reaching 70%
confluence, they were transfected with pcDNA3.1-Wnt5a
overexpression vector. Lipofectamine 2000 (Invitrogen; Life
Technologies) was used for cell transfection. The medium was
replaced after 48 h with G418-containing medium, and stably
transfected cells were selected using antibiotic resistance
preferentially.
Western blotting
Cells were cultured to reach 80% confluence and
harvested using lysis buffer on ice. A total of 10 µg of each
lysate was run on sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to polyvinylidene difluoride
membranes. After blocking with 3% bovine serum albumin, membranes
were immunoblotted using primary antibodies against Wnt5a
(ab174963, diluted 1:500; Abcam); glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; #3683, diluted 1:1,000; Cell Signaling
Technology); E-cadherin (#3195, diluted 1:1,000; Cell Signaling
Technology); ZO-1 (#8193, diluted 1:1,000; Cell Signaling
Technology); N-cadherin (ab76011, diluted 1:5,000; Abcam); and
vimentin (#5741, diluted 1:1,000; Cell Signaling Technology). The
blots were then reacted with secondary anti-rabbit antibodies
(#7074, diluted 1:5,000; Cell Signaling Technology), followed by
detection of the proteins with SuperSignal West Dura Extended
Duration Substrate (34076; Thermo Fisher Scientific).
Cell proliferation assay
Cells were seeded at 5,000/well in 96-well culture
plates. We determined the number of viable cells by a colorimetric
method using CellTiter 96 (G5430; Promega Corporation). The
measurement was performed on 0, 1, 2 and 3 days after seeding.
Cell invasion assay
Cell invasion assays were performed in Matrigel
invasion chambers (24 wells, 8 µm; 354480; Corning). Cells
(2.5×104) in FBS-free medium were seeded in the upper
chambers and medium containing 10% FBS was added to the lower
chambers. Cells above the membrane were wiped off using a cotton
swab after 24 h. Membranes were stained and average values were
obtained by counting five fields per membrane under a microscope
(×10).
DNA microarray
Purification of total RNA from HCC cell lines was
performed using RNeasy Mini Kit (74106; Qiagen). Comprehensive
analysis of gene expression was performed using SurePrint G3 Human
8×60 K version 3.0 (Agilent) at Hokkaido System Science Co., Ltd.
The fold changes in normalized signal values were used for
comparison of gene expression levels.
Statistical analysis
Statistical analysis was performed using EZR version
1.35 (Saitama Medical Center, Jichi Medical University, Saitama,
Japan) (17). Fisher's exact test
was used to examine the correlation between expression of Wnt5a and
clinical and pathological variables. The Kaplan-Meier method was
used to plot OS and relapse-free survival (RFS) curves and they
were compared using the log-rank test. The Cox proportional hazards
regression model was used to perform multivariate analyses. Values
in vitro were examined using Student's t-test. P<0.05 was
considered statistically significant.
Results
Clinical and pathological
characteristics and Wnt5a expression in HCC patients
Clinical and pathological characteristics are
summarized in Table I. Wnt5a
expression was positive in 63 patients (25.9%) and negative in 180
(74.1%).
 | Table I.Clinicopathological characteristics of
patients. |
Table I.
Clinicopathological characteristics of
patients.
| Characteristics | Value |
|---|
| Sex, n (%) |
|
|
Male | 200 (82.3) |
|
Female | 43 (17.7) |
| Median
age, years (range) | 63 (35–82) |
| Viral infection, n
(%) |
|
|
HBV | 90 (37.0) |
|
HCV | 92 (37.9) |
|
HBV+HCV | 8 (3.3) |
|
NBNC | 53 (21.8) |
| Child-Pugh class, n
(%) |
|
| A | 237 (97.5) |
| B | 6 (2.5) |
| Albumin, n (%) |
|
| <4
g/dl | 96 (39.5) |
| ≥ 4
g/dl | 147 (60.5) |
| AFP, n (%) |
|
| ≤10
ng/ml | 127 (52.3) |
| >10
ng/ml | 114 (46.9) |
| PIVKAII, n (%) |
|
| ≤40
mAU/ml | 101 (43.2) |
| >40
mAU/ml | 136 (56.0) |
| Differentiation, n
(%) |
|
|
Well | 40 (16.5) |
|
Moderate | 156 (64.2) |
|
Poor | 47 (19.3) |
| Tumor number, n
(%) |
|
|
Solitary | 187 (77.0) |
|
Multiple | 56 (23.0) |
| Tumor size, n
(%) |
|
| ≤2
cm | 36 (14.8) |
| >2-5
cm | 129 (53.1) |
|
>5-10 cm | 59 (24.3) |
| >10
cm | 19 (7.8) |
| Vascular invasion,
n (%) |
|
|
Positive | 39 (16.1) |
|
Negative | 204 (83.9) |
| Lymph node
metastasis, n (%) |
|
|
Positive | 0 (0.0) |
|
Negative | 243 (100.0) |
| pStagea, n (%) |
|
| I | 24 (9.9) |
| II | 136 (56.0) |
|
III | 83 (34.2) |
|
IVA | 0 (0.0) |
|
IVB | 0 (0.0) |
| Non-cancerous
liver, n (%) |
|
|
Non-cirrhosis | 157 (64.6) |
|
Cirrhosis | 86 (35.4) |
| Wnt5a, n (%) |
|
|
Positive | 63 (25.9) |
|
Negative | 180 (74.1) |
Correlations between clinical and pathological
characteristics and Wnt5a expression are shown in Table II. Wnt5a negativity was
significantly associated with HCV (P=0.011), poor tumor
differentiation (P=0.001) and positive vascular invasion
(P=0.046).
 | Table II.Association between Wnt5a expression
and clinicopathological characteristics. |
Table II.
Association between Wnt5a expression
and clinicopathological characteristics.
|
| Wnt5a
expression |
|
|---|
|
|
|
|
|---|
|
Characteristics | Negative, n | Positive, n | P-value |
|---|
| Sex |
|
|
|
|
Male | 145 | 55 | 0.256 |
|
Female | 35 | 8 |
|
| Age, years |
|
|
|
|
≤60 | 73 | 29 | 0.462 |
|
>60 | 107 | 34 |
|
| HBV |
|
|
|
|
Negative | 110 | 35 | 0.459 |
|
Positive | 70 | 28 |
|
| HCV |
|
|
|
|
Negative | 97 | 46 | 0.011a |
|
Positive | 83 | 17 |
|
| Albumin, g/dl |
|
|
|
|
<4 | 68 | 28 | 0.372 |
| ≥4 | 112 | 35 |
|
| AFP, ng/ml |
|
|
|
|
≤10 | 87 | 40 | 0.056 |
|
>10 | 91 | 23 |
|
| PIVKAII,
mAU/ml |
|
|
|
|
≤40 | 77 | 24 | 0.652 |
|
>40 | 99 | 37 |
|
|
Differentiation |
|
|
|
|
Well-moderate | 135 | 59 | 0.001a |
|
Poor | 45 | 4 |
|
| Tumor number |
|
|
|
|
Solitary | 141 | 46 | 0.390 |
|
Multiple | 39 | 17 |
|
| Tumor size, cm |
|
|
|
| ≤5 | 125 | 40 | 0.434 |
|
>5 | 55 | 23 |
|
| Vascular
invasion |
|
|
|
|
Negative | 146 | 58 | 0.046a |
|
Positive | 34 | 5 |
|
| Non-cancerous
liver |
|
|
|
|
Non-cirrhosis | 118 | 39 | 0.647 |
|
Cirrhosis | 62 | 24 |
|
Survival analysis
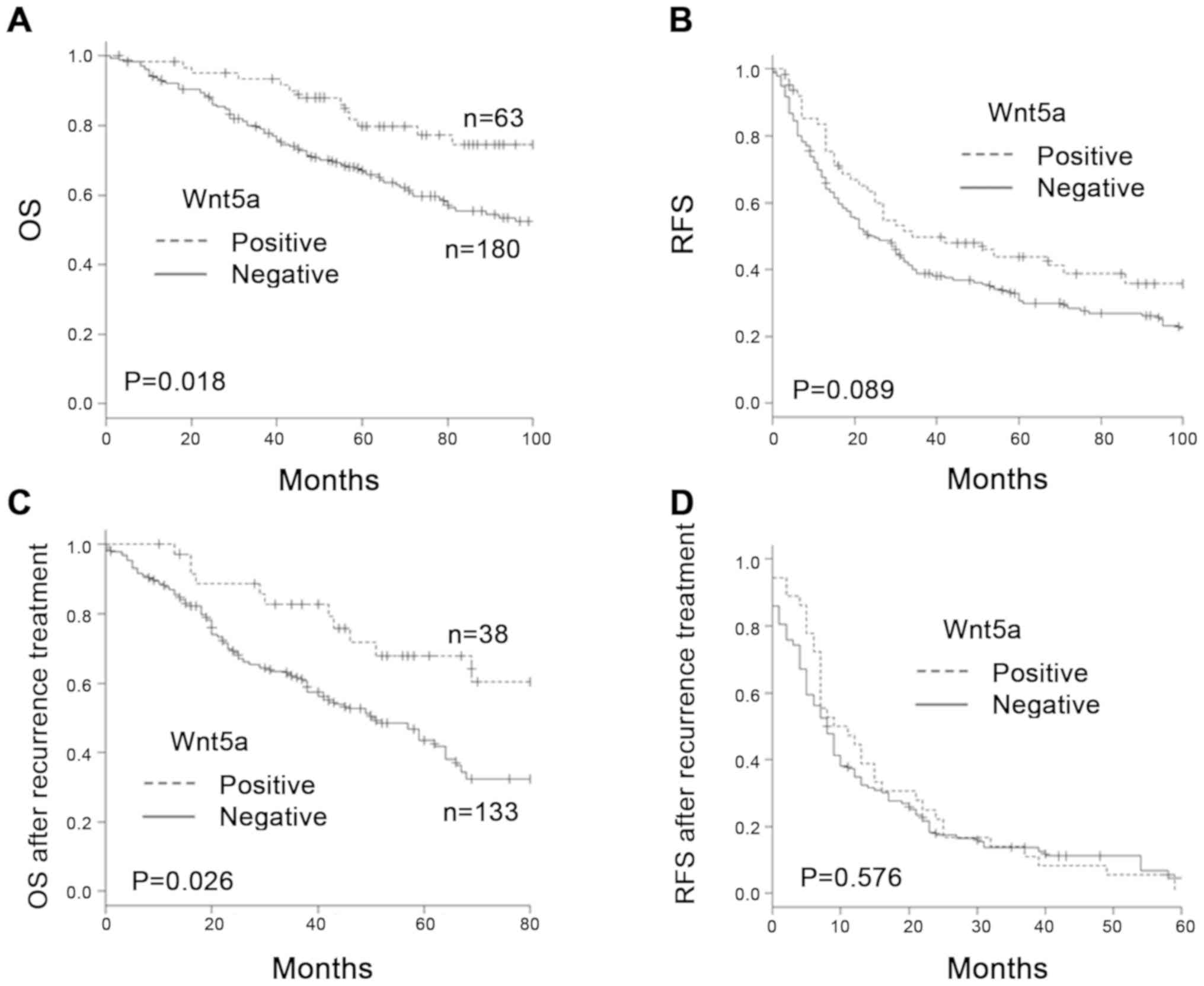
The 5-year OS rate in the Wnt5a-positive and
Wnt5a-negative groups was 79.7 and 66.6%, respectively. OS in the
Wnt5a-negative group was significantly poorer than in the
Wnt5a-positive group (P=0.018). The 5-year RFS rate in the
Wnt5a-positive and Wnt5a-negative groups was 43.7 and 30.7%,
respectively. RFS in the Wnt5a-negative group tended to be poorer
than in the Wnt5a-positive group, but there was no significant
difference between the groups (Fig. 2A
and B).
Prognostic factor analysis
Univariate analysis showed that the following were
predictive factors for lower OS in HCC patients: Albumin level
[hazard ratio (HR) 1.826, 95% confidence interval (CI) 1.204–2.770,
P=0.005]; AFP level (HR 1.668, 95% CI 1.090–2.553, P=0.018);
PIVKAII level (HR 1.570, 95% CI 1.002–2.459, P=0.049); tumor number
(HR 1.824, 95% CI 1.134–2.932, P=0.013); tumor size (HR 2.110, 95%
CI 1.384–3.218, P=0.001); vascular invasion (HR 2.630, 95% CI
1.645–4.205, P<0.001); cirrhosis (HR 1.721, 95% CI 1.130–2.623,
P=0.012); and Wnt5a negativity (HR 1.993, 95% CI 1.109–3.368,
P=0.020). Multivariate analysis showed that the following were
predictive factors for lower OS: Albumin level (HR 1.571, 95% CI
1.004–2.458, P=0.048); tumor number (HR 1.965, 95% CI 1.292–3.239,
P=0.008); tumor size (HR 1.829, 95% CI 1.100–3.041, P=0.020);
vascular invasion (HR 2.256, 95% CI 1.317–3.865, P=0.003);
cirrhosis (HR 1.675, 95% CI 1.075–2.610, P=0.023); and Wnt5a
negativity (HR 1.939, 95% CI 1.076–3.497, P=0.028; Table III).
 | Table III.Univariate and multivariate analysis
of prognostic factors for overall survival. |
Table III.
Univariate and multivariate analysis
of prognostic factors for overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 1.030 | 0.581–1.823 | 0.921 |
|
|
|
| Age (>60 vs. ≤60
years) | 1.426 | 0.926–2.196 | 0.107 |
|
|
|
| HBV (positive vs.
negative) | 0.873 | 0.569–1.339 | 0.533 |
|
|
|
| HCV (positive vs.
negative) | 1.132 | 0.743–1.724 | 0.565 |
|
|
|
| Albumin (<4 vs.
≤4 g/dl) | 1.826 | 1.204–2.770 | 0.005a | 1.571 | 1.004–2.458 | 0.048a |
| AFP (>10 vs. ≤10
ng/ml) | 1.668 | 1.090–2.553 | 0.018a | 1.483 | 0.953–2.307 | 0.081 |
| PIVKAII (>40 vs.
≤40 mAU/ml) | 1.570 | 1.002–2.459 | 0.049a | 1.468 | 0.883–2.439 | 0.139 |
| Tumor number
(multiple vs. solitary) | 1.824 | 1.134–2.932 | 0.013a | 1.965 | 1.292–3.239 | 0.008a |
| Tumor size (>5
vs. ≤5 cm) | 2.110 | 1.384–3.218 | 0.001a | 1.829 | 1.100–3.041 | 0.020a |
| Vascular invasion
(positive vs. negative) | 2.630 | 1.645–4.205 |
<0.001a | 2.256 | 1.317–3.865 | 0.003a |
| Differentiation
(poor vs. well + moderate) | 1.361 | 0.826–2.241 | 0.226 |
| Non-cancerous liver
(cirrhosis vs. non-cirrhosis) | 1.721 | 1.130–2.623 | 0.012a | 1.675 | 1.075–2.610 | 0.023a |
| Wnt5a (negative vs.
positive) | 1.933 | 1.109–3.368 | 0.020a | 1.939 | 1.076–3.497 | 0.028a |
Analysis of recurrence
At the time of our investigation, 171 of the 243
patients (70.4%) had experienced recurrence. This included 133 of
the 180 Wnt5a-negative patients (73.9%) and 38 of the 63
Wnt5a-positive patients (60.3%). The recurrence patterns and their
management are summarized for each group (Table SI). The recurrence location did not
differ significantly between the groups. There were also no
significant differences in tumor number and diameter. The
management strategies for recurrence varied, including
transcatheter arterial chemoembolization, radiofrequency ablation,
microwave coagulation therapy, percutaneous ethanol injection
therapy, liver resection, metastatic resection, chemotherapy and
radiotherapy. There was no significant difference in recurrence
management between the Wnt5a-positive and Wnt5a-negative
groups.
Prognosis after recurrence is shown in Fig. 2C and D. The 5-year OS after treatment
of recurrence was 67.9% in the Wnt5a-positive group and 43.5% in
the Wnt5a-negative group. OS after recurrence in the Wnt5a-negative
group was significantly poorer than in the Wnt5a-positive group
(P=0.026). Median RFS after treatment of recurrence was 10 months
in the Wnt5a-positive group and 8 months in the Wnt5a-negative
group. There was no significant difference in RFS after treatment
of recurrence (P=0.576).
Wnt5a expression in liver cancer cell
lines
Analysis using clinical samples revealed that Wnt5a
expression was related to tumor differentiation, vascular invasion,
and prognosis. Therefore, we used different cell lines to clarify
the mechanism. We evaluated expression of Wnt5a in liver cancer
cell lines using western blotting. We chose HLE and HLF as poorly
differentiated cell lines, Huh7 as a well-differentiated cell line,
and HepG2 as a hepatoma/liver cancer cell line. The intensity ratio
of expression to GAPDH was 20.2% in HLE cells, 23.5% in HLF cells,
40.2% in HepG2 cells and 85.5% in Huh7 cells (Fig. S1). Wnt5a expression was lower in HLE
and HLF cells than in HepG2 and Huh7 cells.
Effects of Wnt5a knockdown on Huh7
cells
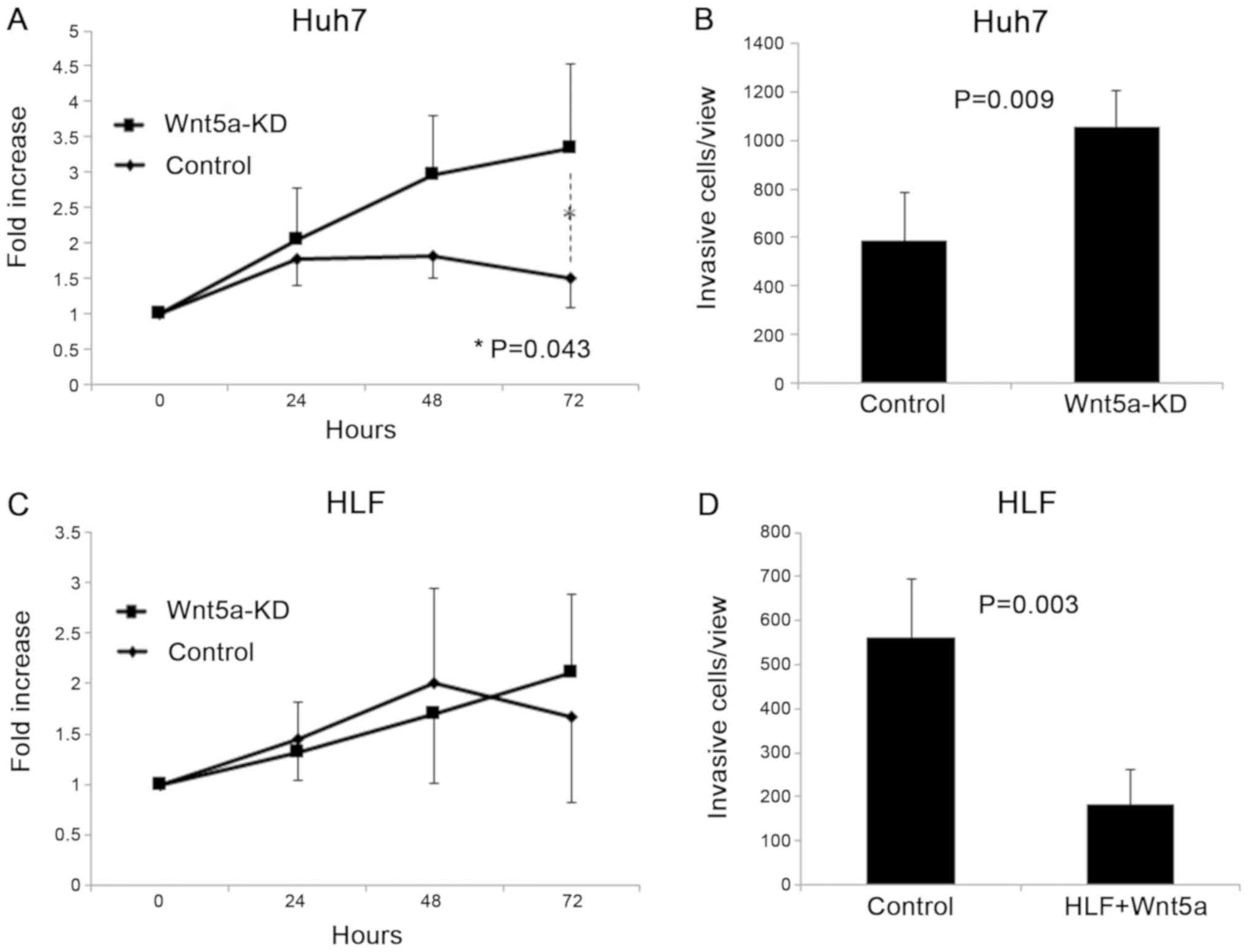
Huh7 cells had high expression of Wnt5a, and we
examined changes in proliferation and invasiveness of Huh7 cells by
knockdown of Wnt5a. The cell proliferation assay showed that
knockdown of Wnt5a significantly increased proliferation of Huh7
cells (P=0.043; Fig. 3A). The
invasion assay showed that knockdown of Wnt5a significantly
increased invasiveness of Huh7 cells (P=0.009; Fig. 3B).
Effects of Wnt5a overexpression on HLF
cells
We examined changes in proliferation and
invasiveness caused by overexpression of Wnt5a on HLF cells, which
have low expression of Wnt5a. Overexpression of Wnt5a did not
change proliferation of HLF cells (Fig.
3C) in the cell proliferation assay. Overexpression of Wnt5a
significantly decreased invasiveness of HLF cells in the cell
invasion assay (P=0.003; Fig.
3D).
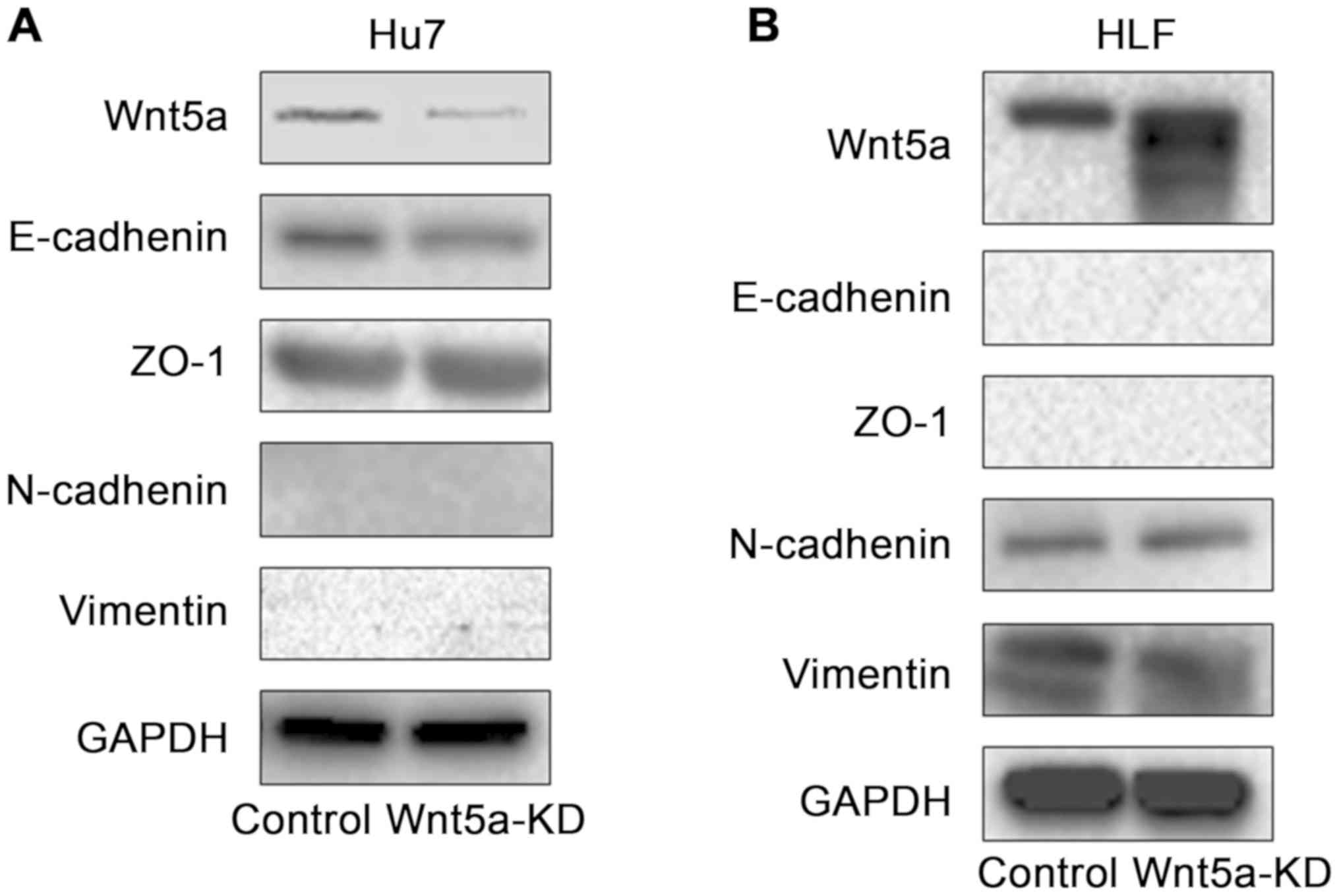
Changes in EMT-related molecules by
controlling Wnt5a expression
To elucidate the molecular mechanism involved in
changes in invasiveness induced by Wnt5a, changes in EMT-related
molecules were examined using western blotting (Fig. 4). We investigated changes in the
expression of E-cadherin and ZO-1 as epithelial markers and
N-cadherin and vimentin as mesenchymal markers. Knockdown of Wnt5a
in Huh7 cells with high expression of Wnt5a decreased E-cadherin
expression, and there was no change in ZO-1, N-cadherin, or
vimentin expression. Overexpression of Wnt5a in HLF cells with low
expression of Wnt5a decreased vimentin expression, and there was no
change in E-cadherin, ZO-1 or N-cadherin expression.
Changes in EMT-related genes by
overexpression of Wnt5a
To clarify the relationship between Wnt5a and EMT,
we investigated changes in EMT-related genes with Wnt5a
overexpression in HLF cells using DNA microarray analysis. The
changes in SNAIL, ZEB and TWIST families as EMT transcriptional
repressors are shown in Fig. S2.
Expression of SNAI2 and SNAI3 was decreased by overexpression of
Wnt5a. The changes in the matrix metalloproteinase (MMP) family are
shown in Fig. S3. Expression of
MMP2, MMP7, MMP9, MMP10 and MMP23B was decreased by overexpression
of Wnt5a.
Discussion
In this study, Wnt5a negativity was associated with
poor tumor differentiation and positive vascular invasion and was
an independent poor prognostic factor in HCC patients. Knockdown of
Wnt5a by shRNA increased the proliferation and invasiveness of Huh7
cells with high expression of Wnt5a, and decreased expression of
E-cadherin. In HLF cells with low expression of Wnt5a,
overexpression of Wnt5a inhibited invasiveness and decreased
expression of vimentin. From the above, Wnt5a may be a tumor
suppressor involved in EMT-mediated changes in invasiveness of HCC.
Previous studies have investigated the association of HCC and
Wnt5a. They found that Wnt5a positivity is a good prognostic factor
in HCC, and overexpression of Wnt5a in HCC cell lines suppresses
cell proliferation (9,18). These results may indicate that Wnt5a
is a tumor suppressor in HCC. We validated these findings with a
large sample in the present study.
Previous studies evaluated immunohistochemical
staining of Wnt5a by cytoplasmic staining (19–21). We
analyzed our data using the same methods as in previous studies
(Tables SII and SIII, and Fig.
S4). The results were almost the same as above except that
Wnt5a was not significant in multivariate analysis. The appearance
of Wnt5a immunostaining varied, and we focused on its expression on
the cell membrane. Grouping by expression on the membrane made
Wnt5a significant in multivariate analysis and it was considered to
be a more dominant factor. It may be that expression of Wnt5a on
the cell membrane means that Wnt5a is bound to its receptors and
the non-canonical pathway is activated.
In this study, Wnt5a membrane negativity was
correlated with poor pathological features such as poor
differentiation, positive vascular invasion and poor prognosis.
These results indicate that Wnt5a is a tumor suppressor in HCC.
Previous studies supporting the tumor suppressor function of Wnt5a
have shown that expression of Wnt5a is a good prognostic marker for
long-term survival in colon cancer (22). Other research has shown that
reduction of Wnt5a expression is correlated with increased serum
AFP level and tumor stage, and that Wnt5a is an independent
prognostic factor for HCC (18,23). The
results of our investigation, with larger samples, support these
previous results.
OS was significantly poorer in Wnt5a-negative than
in Wnt5a-positive HCC patients. However, RFS was not significantly
different between the groups. These results for OS and RFS were
unexpected. To clarify the reason for this, the recurrence patterns
and treatment were examined, and showed no difference between the
two groups. HCC has a high frequency of recurrence caused by
multicentric carcinogenesis (24),
and this may cancel out the difference in recurrence. OS after
recurrence in the Wnt5a-negative group was significantly poorer
than in the Wnt5a-positive group despite receiving the same
treatment for recurrence. This indicated that the responsiveness to
treatment for recurrence was good because of the low malignancy of
Wnt5a-positive tumors. We considered that expression of Wnt5a and
malignancy of the tumor were related, and we used HCC cell lines to
clarify the mechanism.
We investigated the expression of Wnt5a protein in
liver cancer cell lines by western blotting. There are reports on
the expression of Wnt5a in liver cancer cell lines at the mRNA
level (25), but not at the protein
level. In a previous study, Wnt5a was highly expressed in poorly
differentiated cell lines at the mRNA level (25), but in our study, western blotting
showed high expression of Wnt5a protein in well-differentiated cell
lines. We also investigated mRNA expression (Fig. S5), but it was not correlated with
protein expression. The high expression of Wnt5a protein in
well-differentiated cell lines was consistent with the correlation
between tumor differentiation and Wnt5a expression in our clinical
study.
As a result of investigating the change in
malignancy by controlling expression of Wnt5a, Wnt5a knockdown
increased proliferation and invasiveness of Huh7 cells.
Additionally, overexpression of Wnt5a did not change proliferation,
but invasiveness was suppressed. Wnt5a is involved in the
cytoskeleton and cell motility through the non-canonical Wnt
pathway (5,7). In our clinical investigation, there was
a correlation between Wnt5a expression and vascular invasion, and
it was thought that expression of Wnt5a was particularly related to
changes in invasiveness. These results suggest that Wnt5a acts as a
tumor suppressor through suppression of invasiveness of HCC cell
lines.
In this study, we focused on EMT as a factor related
to invasiveness. EMT is a process by which epithelial cells lose
epithelial characteristics and acquire mesenchymal characteristics
(26). EMT enables cell migration,
which is a known mechanism of cancer invasion and metastasis
(26). EMT epithelial markers
include E-cadherin, ZO-1, claudins, occludin, cytokeratins and type
IV collagen, and mesenchymal markers include N-cadherin, vimentin,
FSP-1, a-SMA, fibronectin, and type I and III collagen (27). In our study, E-cadherin, ZO-1,
N-cadherin and vimentin were used as representative markers. The
association between Wnt5a and EMT has been reported in other cancer
cell lines, but not for HCC (22,28,29).
Cheng et al (22) showed that
overexpression of Wnt5a increased E-cadherin and decreased vimentin
in colon cancer cell lines. ZEB1 and TWIST, which are repressors of
E-cadherin, were decreased (22).
Additionally, it is reported that SNAI1 and SNAI2 as
transcriptional repressors promote EMT (30), and MMP family proteins such as MMP2
and MMP9 promote tumor cell invasion and metastasis through
degradation of extracellular matrix in cooperation with EMT
(31). In our study, knockdown of
Wnt5a in Huh7 cells with high expression of Wnt5a decreased
E-cadherin expression. Although overexpression of Wnt5a in HLF
cells with low expression of Wnt5a did not increase E-cadherin, it
decreased vimentin, SNAI2, MMP2 and MMP9, which are promoters of
EMT. These changes in EMT-related factors indicate that Wnt5a is
related to EMT and this is believed to be the first study to
indicate a relationship between Wnt5a and EMT in HCC.
This study had some limitations. It was a
retrospective single-center study. Further, prospective,
multicenter studies are therefore necessary to validate our
results. To clarify the relationship between Wnt5a and EMT in HCC,
it is necessary to examine the signal transduction pathways
involved. The Wnt5a isoforms Wnt5a-L and Wnt5a-S cannot be
distinguished by the antibody and transfection method used in this
study; therefore, it is not clear which one is significantly
expressed or acts.
In conclusion, Wnt5a negativity was associated with
poor tumor differentiation and positive vascular invasion, and it
was an independent poor prognostic factor in HCC patients. Wnt5a
may be a tumor suppressor involved in EMT-mediated changes of
invasiveness. Expression of Wnt5a may be useful as a prognostic
biomarker and affect the treatment after recurrence. Furthermore,
this study may lead to the development of treatment for HCC,
focusing on the tumor-suppressing effect of Wnt5a.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Cathel Kerr for
editing a draft of this manuscript. This abstract was presented at
the Gastrointestinal Cancer Symposium, January 23–25, 2020, San
Francisco, CA, USA, and was published as Abstract no. 573.
Funding
The present study was supported by Japan Agency for
Medical Research and Development (grant nos. JP19fk0310111 and
JP19fk0210041), and in part by the Academic Support Program of
Taiho Pharmaceutical Co., Ltd.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KWaki was involved in conceptualization, data
curation, experiments and writing the original draft. TK designed
the methodology, and was involved in supervision, and writing,
review and editing. KWaka curated the data, performed experiments
and supervised. TO, SS, AN and HK were involved in data curation
and performed experiments. HY and MF curated data, performed
experiments and supervised. NK was involved in data curation and
performed experiments. TM designed the methodology and
investigation. AT was involved in analysis and interpretation of
data, funding acquisition, conceptualization, supervision, writing,
review and editing. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This research was approved by the Institutional
Review Board of Hokkaido University Hospital (017–0237) and
performed in compliance with the Helsinki Declaration. Informed
consent of patients recruited between 1997 and 2000 was obtained in
the form of opt-out on the web site of Hokkaido University Hospital
and written informed consent was obtained from patients recruited
between 2001 and 2006.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Galle PR, Forner A, Llovet JM, Mazzaferro
V, Piscaglia F, Raoul JL, Schirmacher P and Vilgrain V: EASL
clinical practice guidelines: Management of hepatocellular
carcinoma. J Hepatol. 69:182–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamiyama T, Nakanishi K, Yokoo H, Kamachi
H, Tahara M, Suzuki T, Shimamura T, Furukawa H, Matsushita M and
Todo S: Recurrence patterns after hepatectomy of hepatocellular
carcinoma: Implication of Milan criteria utilization. Ann Surg
Oncol. 16:1560–1571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asem MS, Buechler S, Wates RB, Miller DL
and Stack MS: Wnt5a signaling in cancer. Cancers (Basel). 8:792016.
View Article : Google Scholar
|
|
6
|
van Amerongen R and Nusse R: Towards an
integrated view of Wnt signaling in development. Development.
136:3205–3214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Y, Kipps TJ and Zhang S: Wnt5a
signaling in normal and cancer stem cells. Stem Cells Int.
2017:52952862017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kremenevskaja N, Von Wasielewski R, Rao
AS, Schöfl C, Andersson T and Brabant G: Wnt-5a has tumor
suppressor activity in thyroid carcinoma. Oncogene. 24:2144–2154.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dejmek J, Dejmek A, Säfholm A, Sjölander A
and Andersson T: Wnt-5a protein expression in primary dukes B colon
cancers identifies a subgroup of patients with good prognosis.
Cancer Res. 65:9142–9146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prasad CP, Chaurasiya SK, Guilmain W and
Andersson T: WNT5A signaling impairs breast cancer cell migration
and invasion via mechanisms independent of the
epithelial-mesenchymal transition. J Exp Clin Cancer Res.
35:1442016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weeraratna AT, Jiang Y, Hostetter G,
Rosenblatt K, Duray P, Bittner M and Trent JM: Wnt5a signaling
directly affects cell motility and invasion of metastatic melanoma.
Cancer Cell. 1:279–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang B, Tang Z, Gong H, Zhu L and Liu X:
Wnt5a promotes epithelial-to-mesenchymal transition and metastasis
in non-small-cell lung cancer. Biosci Rep. 37:BSR201710922017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanzawa M, Semba S, Hara S, Itoh T and
Yokozaki H: WNT5A is a key regulator of the epithelial-mesenchymal
transition and cancer stem cell properties in human gastric
carcinoma cells. Pathobiology. 80:235–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin F, Qu X, Fan Q, Wang L, Tang T, Hao Y
and Dai K: Regulation of prostate cancer cell migration toward bone
marrow stromal cell-conditioned medium by Wnt5a signaling. Mol Med
Rep. 8:1486–1492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bauer M, Bénard J, Gaasterland T, Willert
K and Cappellen D: WNT5A encodes two isoforms with distinct
functions in cancers. PLoS One. 8:e805262013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
The Liver Cancer Study Group of Japan, .
The General Rules for the Clinical and Pathological Study of
Primary Liver Cancer. 6th. Kanehara & Co., Ltd.; Tokyo:
2015
|
|
17
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ
and Liu XH: Loss of Wnt5a and Ror2 protein in hepatocellular
carcinoma associated with poor prognosis. World J Gastroenterol.
18:1328–1338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prgomet Z, Andersson T and Lindberg P:
Higher expression of WNT5A protein in oral squamous cell carcinoma
compared with dysplasia and oral mucosa with a normal appearance.
Eur J Oral Sci. 125:237–246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong Z, Shan M, Wang J, Liu T, Shi Q and
Pang D: Decreased Wnt5a expression is a poor prognostic factor in
triple-negative breast cancer. Med Sci Monit. 22:1–7. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim Y, Hong M, Do IG, Ha SY, Lee D and Suh
YL: Wnt5a, Ryk and Ror2 expression in glioblastoma subgroups.
Pathol Res Pract. 211:963–972. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng R, Sun B, Liu Z, Zhao X, Qi L, Li Y
and Gu Q: Wnt5a suppresses colon cancer by inhibiting cell
proliferation and epithelial-mesenchymal transition. J Cell
Physiol. 229:1908–1917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu XH, Pan MH, Lu ZF, Wu B, Rao Q, Zhou
ZY and Zhou XJ: Expression of Wnt-5a and its clinicopathological
significance in hepatocellular carcinoma. Dig Liver Dis.
40:560–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang SL, Luo YY, Chen M, Zhou YP, Lu FR,
Deng DF and Wu YR: A systematic review and meta-analysis comparing
the prognosis of multicentric occurrence and vs intrahepatic
metastasis in patients with recurrent hepatocellular carcinoma
after hepatectomy. HPB (Oxford). 19:835–842. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuzugullu H, Benhaj K, Ozturk N, Senturk
S, Celik E, Toylu A, Tasdemir N, Yilmaz M, Erdal E, Akcali KC, et
al: Canonical Wnt signaling is antagonized by noncanonical Wnt5a in
hepatocellular carcinoma cells. Mol Cancer. 8:902009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xue Y, Zhang L, Zhu Y, Ke X, Wang Q and
Min H: Regulation of proliferation and epithelial-to-mesenchymal
transition (EMT) of gastric cancer by ZEB1 via modulating Wnt5a and
related mechanisms. Med Sci Monit. 25:1663–1670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin L, Yin YT, Zheng FJ, Peng LX, Yang CF,
Bao YN, Liang YY, Li XJ, Xiang YQ, Sun R, et al: WNT5A promotes
stemness characteristics in nasopharyngeal carcinoma cells leading
to metastasis and tumorigenesis. Oncotarget. 6:10239–10252. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018. View Article : Google Scholar : PubMed/NCBI
|