Introduction
Hypopharyngeal cancer accounted for 3.0–5.0% of head
and neck cancer cases worldwide in 2009; however, it has one of the
poorest prognoses among all types of head and neck squamous cell
carcinoma (1). In recent years,
although numerous studies have been performed, and advances in
comprehensive treatment have been made that have increased the
organ preservation rate and local control rate, the 5-year survival
rate of patients with hypopharyngeal cancer has not improved
significantly; 30–40% compared with patients exhibiting the same
stage of laryngeal cancer (2,3). This
demonstrates that there is a considerable difference in survival
rates between these two types of cancer.
A previous study has demonstrated that the type of
treatment and degree of differentiation of head and neck squamous
cell carcinoma can have a significant impact on patient prognosis
(4). The rate of cervical lymph node
metastasis in poorly differentiated hypopharyngeal carcinoma was
significantly higher than that in the highly differentiated type,
and the survival rate in poorly differentiated hypopharyngeal
carcinoma was significantly lower than that in the highly
differentiated type (5). Due to the
location of hypopharyngeal cancer being well-concealed, a surgical
procedure is not the best option for the majority of patients.
According to the National Comprehensive Cancer Network clinical
guidelines for head and neck cancer (6), induction chemotherapy should be the
first line of treatment for patients with advanced hypopharyngeal
cancer, and subsequent treatment can be determined according to the
results of the induction chemotherapy. In a previous study
(7), it has been indicated that,
before and after induction chemotherapy, the change in vascular
classification, as determined using narrow-band imaging (NBI), is
associated with prediction of prognosis, and this may be associated
with different angiogenic factors serving their respective roles in
tumor angiogenesis. Additionally, a number of angiogenesis-related
factors, including interleukin (IL)-1, transforming growth factor
(TGF)-β and matrix metalloproteinase-9 (MMP-9), have been
demonstrated to be associated with tumor development in solid
tumors (8,9). For example, IL-1 is involved in tumor
angiogenesis and lymphangiogenesis together with vascular
endothelial growth factor (VEGF) and fibroblast growth factor (FGF)
(8). Expression of MMP-9 and other
angiogenesis-associated factors also serves an important role in
promoting tumor angiogenesis, invasion and metastasis (9). Therefore, the present study used a
microarray of angiogenesis-associated factors to screen the types
of vascular factors that are differentially expressed.
In the present study, the clinical characteristics
of hypopharyngeal carcinoma, the changes in blood vessel
classification according to NBI and the differential expression of
angiogenic factors were analyzed retrospectively to determine the
role of angiogenesis factors in hypopharyngeal cancer and their
predicted function in the prognosis of patients.
Materials and methods
Clinical data
Data from 60 patients with poorly differentiated
hypopharyngeal cancer diagnosed according to the World Health
Organization diagnosis standard (10) who were treated at Beijing Tongren
Hospital Affiliated to the Capital Medical University (Beijing,
China) between January 2012 and December 2016 were retrospectively
collected. The patients selected for analysis were required to meet
certain inclusion and exclusion criteria. The inclusion criteria
were: i) Stage III–IV hypopharyngeal cancer according to the
American Joint Committee on Cancer TNM stage (11); ii) primary cases; iii) age <80
years; and iv) first treatment was induction chemotherapy, with NBI
examination before and after treatment. The exclusion criteria
were: i) Other head and neck tumors, such as laryngeal cancer; ii)
stage I–II hypopharyngeal cancer; iii) first treatment was not
induction chemotherapy; iv) cancer recurrence and metastasis or
secondary cancer; v) patients who had already received surgery for
this cancer; vi) distant metastasis at admission; and vii) other
serious systemic diseases, such as other advanced tumors or
coronary heart disease, at admission. The present study screened
all patients who met the criteria in the allotted range of 5 years
to avoid bias. The present study was approved by the Ethics
Committee of Beijing Tongren Hospital (Beijing, China), and
patients provided written informed consent to participate in the
present study.
Relevant standards of the treatment
process
The diagnosis of hypopharyngeal cancer was based on
the pathology of the patient at the time of admission, and the
pathological diagnosis was performed by senior doctors. The
chemotherapy plan was jointly formulated by the deputy chief
physician of the Head and Neck Surgery and Oncology Department and
the senior doctors following consultation. The chemotherapy plans
included TPF (130 mg/m2 paclitaxel, D1; 30
mg/m2 cisplatin, D2-4; 500 mg/m2 5-FU, D2-6,
21–28 day repeat, 2 cycles) and TP (135 mg/m2
paclitaxel, D1; 70 mg/m2 cisplatin, D2-4; 21 day repeat,
2 cycles). After induction chemotherapy treatment, the senior
doctors in the imaging department used image examination and
stroboscopic laryngoscopy to jointly determine the efficacy of
treatment, including complete remission (CR), partial remission
(PR), stable disease (SD) and progressive disease, and formulated
the subsequent treatment plan. Before induction chemotherapy
treatment and 2 weeks after induction chemotherapy treatment, the
blood vessel classification according to NBI was determined by
senior doctors at the Head and Neck Surgery and Voice Center. In
the present study, an NBI electronic fiber laryngoscope (model,
Otv-s190) manufactured by Olympus Corporation was used. The image
system was OTV-S7PRO (Olympus Corporation) and the cold light
source system was CLV-S40PRO (Olympus Corporation). The conversion
between white light mode and NBI mode could be completed by
pressing the down button. The method of classification was as
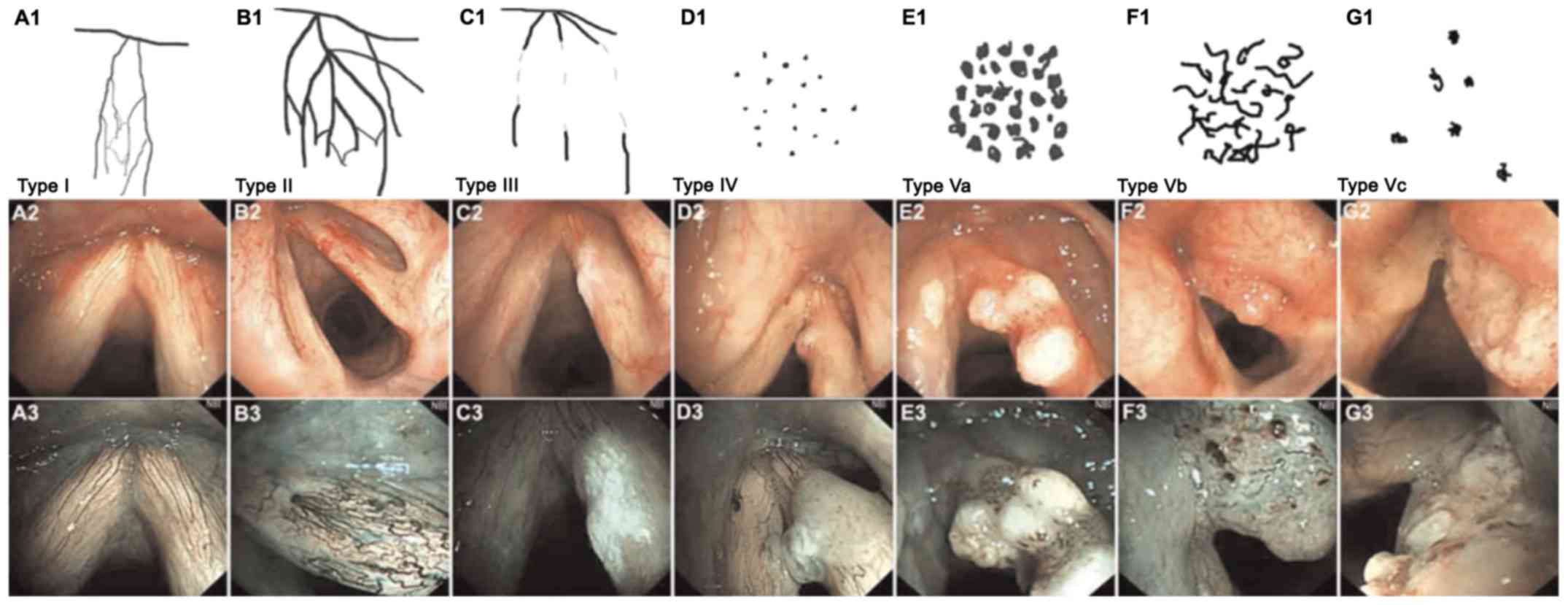
follows: Type I (poly P), hard to see oblique branches, such as
intrapapillary capillary loops (IPCL; A1-A3); type II (laryngitis),
enlarged, oblique branch-like blood vessels (B1-B3); type III
(simple hyperplasia and mild atypical hyperplasia), IPCL covered by
white mucosa (C1-C3); type IV (mild-to-moderate atypical
hyperplasia), spots clearly visible with a regular arrangement but
low density (D1-D3); type V (mild-to-severe atypical hyperplasia
and invasive cancer) was divided into three subtypes (type V A,
type V B and type V C), type V A exhibited bloated high density
spots with various shapes (E1-E3); type V B exhibited irregular
twisted earthworm-like IPCL (F1-F3); type V C exhibited irregular
twisted or spotted IPCL (G1-G3) on the surface of the tumor, of
which 100% of type V B and type V C indicated invasive cancer
(Fig. 1) (12). The more obvious the
neovascularization, the more likely the tumor is to be malignant
and the more serious the disease (12). During induction chemotherapy, the NBI
blood vessel classification changed from type V to type I–IV or
from type V B and V C to type V A, which was defined as a decrease
in blood vessel classification. In this group, 30 cases exhibited a
decrease in NBI blood vessel classification and 30 cases were
unchanged in NBI blood vessel classification.
Human angiogenesis antibody array
The RayBio® G-Series Human Angiogenesis
Antibody Array 1000 kit (RayBiotech, Inc.) was used. For tissue
lysis, tumor and peritumoral tissues were collected via resection
and frozen at −80°C until further use. The tissues were then cut
with surgical scissors into 1–3 mm3 cubes and
transferred to a 2-ml centrifuge tube. Cell lysate (150 µl) was
added, and the mixture was stored at a low temperature on ice and
homogenized for 5–15 min. The mixture was then centrifuged at room
temperature at 7.4 × g for 10 min and the supernatant was removed
for subsequent use.
To measure protein concentration and dilution,
following cell lysis, the protein concentration of the tissue
lysate was determined using a Pierce BCA protein assay kit (cat.
no. 23227; Pierce; Thermo Fisher Scientific, Inc.). A total of 100
µl 1X PBS as the sealing solution was added to each chip hole, and
the mixture was incubated in a shaker at room temperature for 30
min.
Subsequently, the sealing solution (PBS) was
removed, and 100 µl of the sample was added to each well and
incubated overnight at 4°C (the sample was centrifuged at room
temperature at 7.2 × g for 4 min; 0.5 mg/ml). A Wellwash Versa
Microplate Washer (Thermo Fisher Scientific, Inc.) was used to
clean the slides. A total of 70 µl biotin was added to each pore
and the samples were incubated at room temperature for 2 h.
Subsequently, 70 µl streptavidin fluorescent agent (dilution,
1,500) was added to each pore and samples were incubated for 1–2 h
at room temperature in the dark. A laser scanner (Axon GenePix
Microarray scanner; Molecular Devices, LLC) was used to detect the
signal, and a Cy3 channel (excitation wavelength, 532 nm) was used
to detect staining. Subsequently, the data of the relative
expression levels were obtained by over density quantitative
analysis and standardized calculation, and the Human Angiogenesis
Array G1 (AAH-ANG-G1) and Human Angiogenesis Array G2 (AAH-ANG-G2)
kits (RayBiotech, Inc.) were used for the data analysis according
to the manufacturer's protocol.
Immunohistochemistry
A total of 1 cm3 of tissue was taken and
fixed with 4% paraformaldehyde for 24 h at room temperature and
dehydrated in different ethanol concentrations (50, 70, 80 and 95%
for 2 h each, and 100% for 1 h at room temperature). Tissue samples
were treated with xylene, embedded in paraffin at 52°C and cut into
4-µm-thick sections. After paraffin-embedded sections were dewaxed
in xylene at room temperature and rehydrated in different ethanol
concentrations (100, 85 and 75%) at room temperature, the tissue
sections were placed in a repair box filled with EDTA antigen
repair buffer (pH 8.0), and then antigen was retrieved using a
microwave oven. The samples were heated at boiling temperature for
9 min, stopped for 7 min and then at medium heat for 7 min. After
natural cooling, the slides were placed in PBS (pH 7.4) and shaken
on the decolorizing shaker for 3 times (5 min each time). The
slides were incubated with 3% hydrogen peroxide solution for 25 min
at room temperature. The slides were placed in PBS (pH 7.4) and
washed 3 times on a decolorizing shaker for 5 min each time. Slides
were blocked with 3% BSA added into the histochemical circle to
cover the tissue homogeneously for 30 min at room temperature.
After shaking off the blocking solution gently, the slides were
incubated with primary antibodies (all diluted 1:200 in PBS)
against IL-1 (cat. no. ab2105; Abcam), TGF-β (cat. no. ab92486;
Abcam), MMP-9 (cat. no. ab58803; Abcam), angiopoietin-2 (Ang-2;
cat. no. ab56301; Abcam) and interferon-inducible T-cell α
chemoattractant (I-TAC; cat. no. 70R-IR053; Fitzgerald Industries)
overnight at 4°C. The slides were washed with PBS (pH 7.4) and were
then covered with HRP-labeled goat anti-rabbit (1:200; cat. no.
GB23303; Wuhan Servicebio Technology Co., Ltd.) or goat anti-mouse
(1:200; cat. no. GB23301; Wuhan Servicebio Technology Co., Ltd.)
secondary antibodies and incubated at room temperature for 50 min.
PBS replaced the primary antibody as the negative control, and the
company (Abcam) provided a positive reference to use as the
positive control. Each step was performed according to the
requirements of the 3,3′-diaminobenzidine staining kit (cat. no.
D7051; Beijing Solarbio Science & Technology, Co., Ltd.).
Positive expression of the yellow-brown reaction product was
indicated in the nucleus or the cytoplasm. Cells in a total of five
fields were randomly counted manually using a high power light
microscope (magnification, ×200). In total, 100 tumor cells were
counted in each field. A number of positively stained cells >10%
was considered to indicate a positive result. For the fixation of
samples, the present study used a fixed time of 24 h. If the
fixation time is too long, the antigenic determinants of cells will
be covered and lost, due to the cross-linking between formaldehyde
and tissue protein, and this will affect their expression activity
(13).
Follow-up
Patient follow-up was a combination of regular
outpatient reviews and telephone follow-up appointments. Up to the
follow-up time, no patient was lost, 46 patients survived and 14
died. The date for follow-up to death, or end of the study period,
was October 2019.
Statistical analysis
SPSS software version 22.0 (IBM Corp.) was used for
the statistical analysis. The χ2 test or unpaired
Student's t-test were used for categorical or continuous data,
respectively. and Cox regression analysis were used for single
factor analysis of clinical indicators. The Kaplan-Meier method and
the log-rank test were used to analyze the difference in
Kaplan-Meier curves between groups. The data are presented as the
averages. P<0.05 was considered to indicate a statistically
significant difference.
Results
Differential expression of angiogenic
antibody CHIP in tumors and peritumoral tissue
The log ratio of differentially expressed genes in
tumor tissue and para-cancerous tissue from each patient was
determined. The genes included epithelial-derived
neutrophil-activating protein 78, IL-1β, phosphatidylinositol
glycan anchor biosynthesis class F, urokinase-type plasminogen
activator, interferon-inducible T-cell α chemoattractant (I-TAC),
VEGF, MMP-1, monocyte chemoattractant protein 3, VEGF-D,
inflammatory cytokine I-309, IL-4, LEPTIN, TIMP metallopeptidase
inhibitor 1, insulin-like growth factor 1, angiopoietin (Ang-2),
epidermal growth factor and MMP-9. A total of five differentially
expressed genes, including IL-1β, TGF-β, MMP-9, Ang-2 and I-TAC,
were selected using the Angiogenesis Antibody Array in the 60
patients (Table I).
 | Table I.Expression levels of
tumor/peritumoral differential factors in 60 patients. |
Table I.
Expression levels of
tumor/peritumoral differential factors in 60 patients.
| Factor | Tumor (median) | Peri-tumor
(median) | Fold change | T | P-value | 95% CI |
|---|
| MMP-9 | 6,550.40 | 855.55 | 6.30 | 17.72 | <0.01 |
5,658.98–7,082.86 |
| TGF-β | 1,609.00 | 38.95 | 41.0 | 37.71 | <0.01 |
1,411.900–1,568.39 |
| IL-1β | 48,499.55 | 745.00 | 64.00 | 27.89 | <0.01 |
44,922.21–51,790.29 |
| Ang-2 | 19,049.50 | 5,490.00 | 3.30 | 37.96 | <0.01 |
13,145.55–14,592.52 |
| I-TAC | 3,510.50 | 360.50 | 10.00 | 232.69 | <0.01 |
28,938.80–3,317.82 |
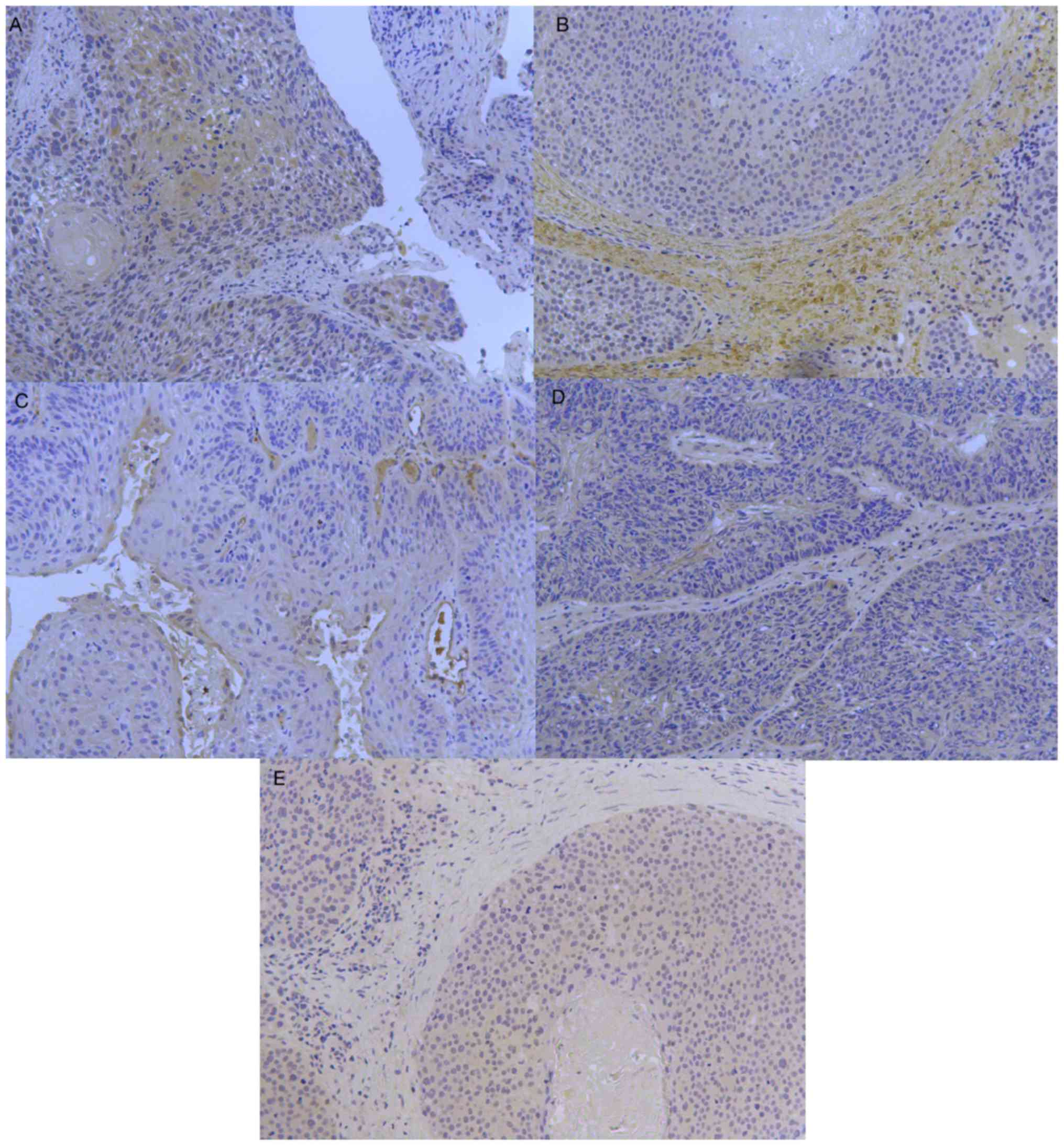
Immunohistochemistry results
The expression levels of IL-1β, TGF-β, MMP-9, Ang-2
and I-TAC in hypopharyngeal carcinoma were determined by
immunohistochemistry (Fig. 2). The
immunohistochemical results of each vascular factor were observed
under a microscope.
Analysis of NBI, vascular factors and
survival
The average age of the 60 patients was 61.5 years
and the median follow-up time was 35 months (Table II). In October 2019, 46 patients
were still alive and 14 had died. A total of 38 patients had a
history of smoking, 40 had a history of drinking and all 60
patients were male. According to the American Joint Committee on
Cancer TNM stage (11), 20 patients
had stage III hypopharyngeal cancer and 40 patients had stage IV
hypopharyngeal cancer. The subsites of tumor location were:
Posterior area of cricoid cartilage (n=2); pyriform sinus (n=6);
posterior wall of laryngopharynx (n=0); and multiple subsites
(n=52). After induction chemotherapy, 8 patients had CR, 44 had PR
and 8 patients had SD. Until October 2019, a total of 8 patients
exhibited local recurrence, 36 exhibited lymph node metastasis and
20 exhibited distant metastasis (Table
III).
 | Table II.Summary of the association between
the decreased/unchanged NBI blood vessel classification and the
general situation of patients. |
Table II.
Summary of the association between
the decreased/unchanged NBI blood vessel classification and the
general situation of patients.
| Factor | Decreased NBI blood
vessel classification (n=30) | Unchanged NBI blood
vessel classification (n=30) |
χ2/T | P-value |
|---|
| Mean age ± SD,
years | 60.47±1.558 | 62.53±1.254 | 1.003 | 0.087 |
| Median follow up,
months | 35 | 35 | – | – |
| Sex |
|
| – | – |
|
Male | 30 | 30 |
|
|
|
Female | 0 | 0 |
|
|
|
Smokinga |
|
| 0.287 | 0.592 |
|
Yes | 20 | 18 |
|
|
| No | 10 | 12 |
|
|
|
Drinkingb |
|
| 1.200 | 0.273 |
|
Yes | 22 | 18 |
|
|
| No | 8 | 12 |
|
|
| TNM stage |
|
| 5.293 | 0.507 |
| Stage
III | 10 | 10 |
|
|
|
T2N1M0 | 0 | 2 |
|
|
|
T3N0M0 | 9 | 7 |
|
|
|
T3N1M0 | 1 | 1 |
|
|
| Stage
IV | 20 | 20 |
|
|
|
T2N2M0 | 6 | 2 |
|
|
|
T3N2M0 | 11 | 12 |
|
|
|
T4N0M0 | 2 | 4 |
|
|
|
T4N1M0 | 1 | 2 |
|
|
| Chemotherapy |
|
| 1.667 | 0.197 |
|
TPF | 22 | 26 |
|
|
| TP | 8 | 4 |
|
|
| Chemotherapy
response |
|
| 14.100 | <0.001 |
| CR | 8 | 0 |
|
|
| PR | 22 | 22 |
|
|
| SD | 0 | 8 |
|
|
| PD | 0 | 0 |
|
|
| Subsite |
|
| −0.652 | 0.517 |
|
Posterior area of cricoid
cartilage | 1 | 1 |
|
|
|
Pyriform sinus | 4 | 2 |
|
|
|
Posterior wall of
laryngopharynx | 0 | 0 |
|
|
|
Multiple subsites | 25 | 27 |
|
|
 | Table III.Analysis of single factors
influencing the prognosis of patients. |
Table III.
Analysis of single factors
influencing the prognosis of patients.
| Factor | Total, n | Event (death),
n | 95% CI |
χ2/T | P-value |
|---|
| Average age,
years | 61.50 | 61.48 | −3.743–5.793 | 20.229 | 0.210 |
| Sex |
|
| 39.325–48.609 | – | – |
|
Male | 60 | 14 |
|
|
|
|
Female | 0 | 0 |
|
|
|
|
Smokinga |
|
|
| 3.232 | 0.072 |
|
Yes | 38 | 6 | 38.654–47.452 |
|
|
| No | 22 | 8 | 29.467–47.076 |
|
|
|
Drinkingb |
|
|
| 0.334 | 0.563 |
|
Yes | 40 | 10 | 36.817–48.883 |
|
|
| No | 20 | 4 | 36.227–48.173 |
|
|
| TNM stage |
|
|
| 20.789 | 0.002 |
| Stage
III | 20 | 6 | 21.299–31.544 |
|
|
|
T2N1M0 | 2 | 0 |
|
|
|
|
T3N0M0 | 16 | 6 |
|
|
|
|
T3N1M0 | 2 | 0 |
|
|
|
| Stage
IV | 40 | 8 | 30.989–40.907 |
|
|
|
T2N2M0 | 8 | 0 |
|
|
|
|
T3N2M0 | 23 | 2 |
|
|
|
|
T4N0M0 | 6 | 4 |
|
|
|
|
T4N1M0 | 3 | 2 |
|
|
|
| Chemotherapy |
|
|
| 0.525 | 0.469 |
|
TPF | 48 | 12 | 37.569–48.431 |
|
|
| TP | 12 | 2 | 36.297–49.370 |
|
|
| Chemotherapy
response |
|
|
| 0.007 | 0.996 |
| CR | 8 | 2 | 30.048–47.452 |
|
|
| PR | 44 | 10 | 38.034–49.329 |
|
|
| SD | 8 | 2 | 27.799–42.201 |
|
|
| Therapy after
chemotherapy |
|
|
| 10.107 | 0.006 |
|
Surgery | 38 | 8 |
|
|
|
|
Radiotherapy | 10 | 6 |
|
|
|
| Surgery
+ radiotherapy | 12 | 0 |
|
|
|
| Local
recurrence |
|
|
| 4.547 | <0.001 |
|
Yes | 8 | 8 | 9.857–16.143 |
|
|
| No | 52 | 6 | 44.742–52.719 |
|
|
| Lymph node
metastasis |
|
|
| 8.729 | 0.003 |
|
Yes | 36 | 4 | 45.657–53.676 |
|
|
| No | 24 | 10 | 22.641–36.525 |
|
|
| Distant
metastasis |
|
|
| 34.071 | <0.001 |
|
Yes | 20 | 14 |
|
|
|
| No | 40 | 0 |
|
|
|
| Decreased NBI blood
vessel classification |
|
|
| 9.986 | 0.002 |
|
Yes | 30 | 2 | 48.231–54.836 |
|
|
| No | 30 | 12 | 26.054–39.546 |
|
|
The two groups with decreased or unchanged NBI
vascular classification were analyzed for differences in
clinicopathological characteristics, including age, sex, history of
tobacco and alcohol use, disease stage, induction chemotherapy
plan, and response to induction chemotherapy. A χ2 or
t-test was used for statistical analysis, and the results are
presented in Table II. The only
statistically significant difference between the two groups was the
change in NBI vascular classification before and after induction
chemotherapy (P<0.001). A t-test was performed to determine the
differences in the expression levels of five vascular factors
between the two groups, and the results indicated that the
expression levels of MMP-9, IL-1β and TGF-β were significantly
different between the two groups (P<0.001). The expression
levels were higher in the group with unchanged NBI vascular type
(Table IV).
 | Table IV.Association between
decreased/unchanged NBI blood vessel classification and vascular
factors. |
Table IV.
Association between
decreased/unchanged NBI blood vessel classification and vascular
factors.
| Vascular
factor | Decreased NBI blood
vessel classification (average) | Unchanged NBI blood
vessel classification (average) | T | P-value | 95% CI |
|---|
| MMP-9 | 782.957 | 7,177.387 | 12.478 | <0.001 |
5,368.633–7,420.228 |
| TGF-β | 39.785 | 1,590.558 | 29.226 | <0.001 |
144.558–1,656.988 |
| IL-1β | 804.127 | 49,095.463 | 19.523 | <0.001 |
43,340.072–53,242.600 |
| Ang-2 | 6,630.833 | 8,109.133 | 1.179 | 0.243 |
−1,032.613–3,989.214 |
| I-TAC | 2567.676 | 2,408.063 | −0.265 | 0.792 |
−1,363.993–1,044.768 |
Single factor Kaplan-Meier analysis was performed
for factors that may affect the survival rate of the 60 patients,
including age, sex, smoking and alcohol history, disease stage,
induction chemotherapy plan, response to induction chemotherapy,
treatment plan after chemotherapy, local recurrence, lymph node
metastasis, distant metastasis, and decreased/unchanged NBI blood
vessel classification. The effects of the five vascular factors on
the survival and prognosis of patients were analyzed. As shown in
Tables III and V, the factors that may affect the prognosis
of patients were TNM stage, treatment plan after induction
chemotherapy, local recurrence, lymph node metastasis, distant
metastasis, decreased NBI blood vessel classification and
expression levels of vascular factors MMP-9, IL-1β and TGF-β.
 | Table V.Association between vascular factor
expression and survival. |
Table V.
Association between vascular factor
expression and survival.
| Factor | Survival
(average) | Death
(average) | χ2 | df | P-value |
|---|
| MMP-9 | 4,086.329 | 4,308.548 | 141.847 | 46 | <0.001 |
| TGF-β | 815.172 | 883.353 | 132.630 | 47 | <0.001 |
| IL-1β | 24,949.795 | 26,235.434 | 146.498 | 56 | <0.001 |
| Ang-2 | 7,969.983 | 7,974.060 | 116.868 | 58 | 0.737 |
| I-TAC | 2,387.869 | 2,399.797 | 102.252 | 53 | 0.837 |
Multivariate Cox regression analysis was also
performed to identify potential factors associated with prognosis
(Table VI). The results indicated
that decreased NBI blood vessel classification and distant
metastasis were factors that affected the prognosis of patients,
among which distant metastasis was a risk factor. Additionally,
decreased NBI vascular classification was a protective factor for
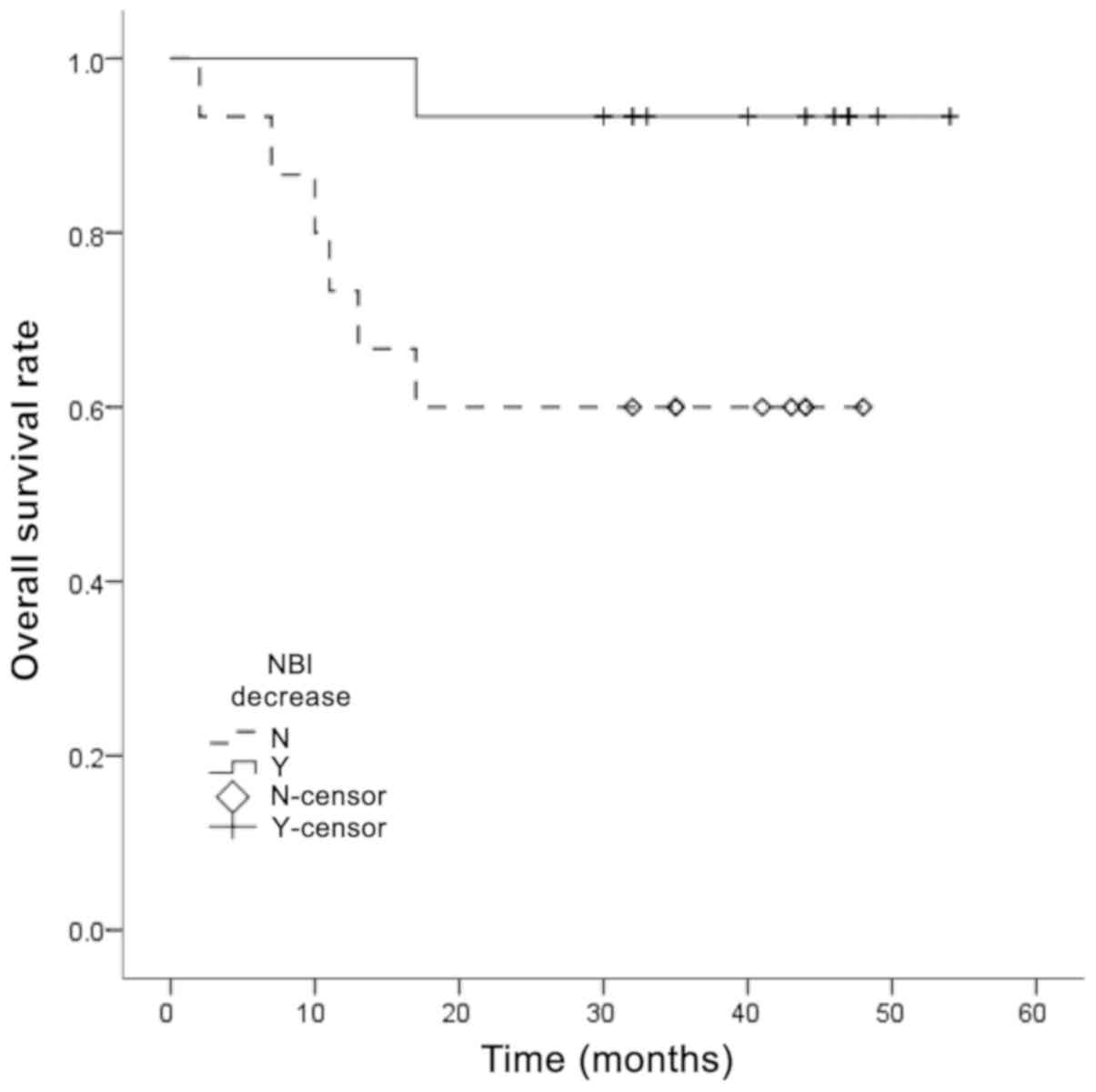
prognosis. The survival curve in Fig.
3 shows the association between decreased/unchanged NBI blood
vessel classification and overall survival rate, revealing that
unchanged NBI blood vessel classification was associated with a
worse overall survival rate.
 | Table VI.Multivariate Cox regression
analysis. |
Table VI.
Multivariate Cox regression
analysis.
| Variable | B | SE | Wald | P-value | HR | 95% CI |
|---|
| TNM stage | −1.457 | 1.006 | 2.097 | 0.148 | 0.233 | 0.032–1.674 |
| Decreased NBI blood
vessel classification | −18.105 | 8.526 | 4.509 | 0.034 | 0.000 | 0.000–0.248 |
| Lymph node
metastasis | −6.320 | 3.833 | 2.719 | 0.099 | 0.002 | 0.000–3.295 |
| Distant
metastasis | 27.078 | 12.414 | 4.758 | 0.029 | 3.163 | 0.660–15.164 |
| Local
recurrence | −4.363 | 3.009 | 2.102 | 0.147 | 0.013 | 0.000–4.642 |
| Therapy after
chemotherapy | −7.187 | 3.884 | 3.425 | 0.064 | 0.001 | 0.000–1.530 |
| MMP-9 | 0.000 | 0.000 | 0.053 | 0.817 | 1.000 | 1.000–1.001 |
| TGF | −0.001 | 0.002 | 0.071 | 0.790 | 0.999 | 0.995–1.004 |
| IL1 | 0.000 | 0.000 | 0.134 | 0.714 | 1.000 | 1.000–1.000 |
Discussion
Tumor angiogenesis refers to the process of tumor
cell-induced capillary growth and the establishment of tumor blood
circulation, which serves an important role in tumor growth,
invasion, diffusion and metastasis. This process is regulated by a
variety of cytokines, among which angiogenesis factor is an
important regulatory factor (14).
The abnormal secretion of angiogenic factors occurs
in the early stages of tumor occurrence, assists in the formation
of tumor neovascularization and promotes tumor occurrence (15). For example, Ang-2, which was
investigated in the present study, is an early angiogenic factor
(15). The Ang family is associated
with angiogenesis, and a large number of studies have confirmed
that it serves an important role in the occurrence and development
of human malignant tumors (15,16).
Ang-1 and Ang-2 are the most well-known members of the Ang family,
and Ang-2 belongs to the secretory proteins. In the pathological
state, Ang-2 is primarily expressed in areas of inflammation,
tissue damage and metastasis, and is expressed most abundantly in
the neovascular network (17). In
the early stages of malignant tumor formation, Ang-2 is expressed
in endothelial cells when new blood vessels begin to develop, which
destroys the stability of blood vessels and the original vascular
network around the new tumor body, and forms the vascular
co-selection area, leading to the reconstruction of
microvasculature.
To the best of our knowledge, no studies have
investigated Ang-2 in squamous cell carcinoma of the head and neck,
but it has been indicated that high expression of Ang-2 is an early
event in esophageal cancer (18).
Ang-2 expression has been demonstrated to not be significantly
increased in the middle or late stages of squamous cell carcinoma,
to not be an independent factor for the prognosis of esophageal
cancer (18) and to not be
associated with the prognosis of patients with this disease. These
results are consistent with the conclusions of the present study.
In addition, Ang-2 has been indicated to activate
infiltration-associated cytokines, including MMPs, and promote the
invasion and progression of tumor cells (19). Furthermore, Ang-2 has been
demonstrated to improve the responsiveness and sensitivity of tumor
cells and vascular endothelial cells to VEGF (19), and further participates in tumor
angiogenesis and growth.
When a tumor reaches a certain size, the original
blood vessels are not able to transport sufficient nutrients to the
distant tumor (20,21). The rapid growth and apoptosis rate of
the tumor are lower compared with normal tissue, which has a large
demand for oxygen, and can cause vasospasm and oxygen deficiency in
tissues; however, the tumor can mitigate this by regulating itself
and promoting angiogenesis (20–22).
During hypoxia, tumor cells can adapt to the hypoxic
microenvironment by expressing hypoxia inducible factor-1 (HIF-1)
(23). HIF-1 can promote the high
expression of TGF-β1 mRNA and the increased secretion of TGF-β1
protein in esophageal squamous cell carcinoma by regulating VEGF,
in order to regulate the neovascularization of the tumor and
control hypoxia (24). TGF-β is a
member of the TGF superfamily, which serves a role in the formation
of tumor blood vessels, and in tumor invasion and metastasis
(25).
TGF-β has been indicated to exhibit a two-way effect
on tumors, causing inhibition of cell canceration in early stage
tumors and promotion of invasion and metastasis in advanced stage
tumors (25). In the early stages of
tumor growth, TGF-β is primarily associated with the regulation of
the cell cycle and the induction of cell apoptosis to prevent cell
carcinogenesis (26). In the
advanced stages of tumor growth, TGF-β promotes tumor cell invasion
and metastasis by stimulating the proliferation of the
extracellular matrix, and promoting angiogenesis and
immunosuppression (26). This has
been demonstrated in a previous study that focused on esophageal
squamous cell carcinoma. In the early stages (stage I) of
esophageal cancer formation, TGF-β expression increases due to its
inhibitory effect in the early tumor, while in the late stage of
tumor invasion, TGF-β expression increases due to its promotive
effect on the advanced tumor, but decreases in the middle stage
(stage II) of tumor growth (18). In
the present study, the majority of patients with decreased NBI
vascular type were classified as V-type to IV-type, from the late
stage to the middle and late stages. Compared with the unchanged
NBI vascular type, the expression of TGF-β decreased in the
decreased NBI vascular type, which was statistically significant
and consistent with the conclusions drawn in a previous study
(27).
TGF-β has also been indicated to interact with a
variety of vascular factors. Dang et al (28) revealed that when TGF-β1 was added to
scc9 and myofibroblasts, the expression levels of MMP-3 and MMP-9
were increased. Additionally, the MMP family is an important factor
in tumor angiogenesis (29), since
it can degrade the extracellular matrix, destroy the structure of
the basement membrane and interact with basic FGF, TGF and other
cytokines in the process of vascular remodeling (30). This interaction means that the
front-end endothelium of neovascularization continues to
proliferate, whereas the back-end endothelium forms a close
connection and subsequently forms a complete lumen structure to
enable blood supply to tumor cells (30). MMP-9 is an important component of
MMPs, and as a gelatinase, and it mainly degrades collagen IV
(31). Furthermore, it has been
indicated that MMP-9 is an important angiogenic factor in promoting
the transformation of tumor epithelial stroma (32). According to experimental findings for
oral squamous cell carcinoma, TGF-β can regulate MMP-9 by enhancing
the expression of the transcription factor Snail/ETS proto-oncogene
1, transcription factor (33), and
thus promoting the development of oral cancer. Therefore, MMP-9 and
other angiogenesis-associated factors may be associated with the
transformation of the tumor epithelial stroma. Overexpression of
MMP-9 can lead to the exposure of hidden functional sites in the
normal state, which is associated with basement membrane
degradation, extracellular mechanism remodeling and cell migration,
and can accelerate the formation of blood vessels (31). In the present study, MMP-9 expression
decreased with decreased NBI blood vessel classification, which
also demonstrated that MMP-9 expression was higher in stages IV or
V according to the NBI vascular classification. In addition, MMP-9
has been revealed to be associated with the invasion and cervical
lymph node metastasis of tongue squamous cell carcinoma exhibiting
different invasion modes (34).
Tumor development is also affected by the tumor
microenvironment, which can change tumor angiogenesis by regulating
the tumor immune microenvironment and releasing cytokines. The
inflammatory microenvironment can increase the mutation rate of
cells and provide support for tumor initiation (24,35). It
has been indicated that the IL family can upregulate MMP-9
expression through the infiltration and recruitment of Th helper 17
cells to inflammatory cells, thus promoting tumor angiogenesis and
serving an important role in the process of tumor invasion and
metastasis (36). Other factors,
such as IL-6, are involved in the transformation of epithelial
stroma, and can influence the occurrence and development of tumors
by reacting to alterations in the tumor microenvironment (37). These factors are highly associated
with poor patient prognosis and the early recurrence of
oropharyngeal squamous cell carcinoma and esophageal squamous cell
carcinoma (38). Via the Janus
kinase/STAT3 signaling pathway, epithelial-to-mesenchymal
transition (EMT) is also mediated, and angiogenesis and
lymphangiogenesis are enhanced by VEGF, promoting tumor progression
(38). For the IL-1 that was used in
the present study, the relevant literature (8,39)
indicates that IL-1, together with VEGF and FGF, is associated with
tumor angiogenesis and lymphangiogenesis. In addition, studies by
Paik et al (40) and Cui
et al (41) have revealed
that the concentration of IL-6 secreted by fibroblasts is increased
13 times after 24 h of IL-1 stimulation. IL-1β can promote the
production of IL-6 in fibroblasts and promote tumor angiogenesis,
metastasis and invasion through EMT, which is induced by IL-6
(40,41). The results of the present study also
indicated that IL-1β expression decreased with decreased NBI
vascular classification, and increased in the later stage.
I-TAC was also used in the present study. In 1998,
the role of the chemokine I-TAC in the migration of chemotactic
cells was revealed for the first time (42). Cells were observed to migrate to the
source of the chemokine along the signal of increased chemokine
concentration (42). Subsequently,
it was demonstrated that chemokines, as key signaling molecules in
the tumor microenvironment, serve an important role in tumor
invasion and metastasis. In terms of angiogenesis, I-TAC can be
used as a factor of angiogenesis or anti-angiogenesis, and I-TAC
can also interact with VEGF (43).
Cancer cells can produce I-TAC directly, or release I-TAC to
regulate angiogenesis by regulating the surrounding inflammatory
cells (44). I-TAC is a novel tumor
microcirculation mode that is different from the classic tumor
angiogenesis and does not depend on endothelial cells; however, no
significant differential expression of I-TAC was observed in the
present study. Therefore, the role of chemokines in the
angiogenesis of head and neck squamous cell carcinoma requires
further study.
The present study revealed that in patients with
hypopharyngeal cancer, changes in NBI vascular classification
before and after induction chemotherapy reflected the therapeutic
effects of chemotherapy and were associated with the prognosis of
patients. IL-1β, TGF-β and MMP-9, as angiogenesis-associated
factors, were significantly different in patients with differing
NBI vascular classifications, although they were not independent
factors of survival prognosis. However, in terms of chemotherapy
and pre-treatment of patients, they may serve a role in guiding
patient treatment.
It was hypothesized that IL-1β, TGF-β and MMP-9 can
be used as predictors of the effect of induction chemotherapy on
poorly differentiated hypopharyngeal carcinoma. Therefore, when
patients with advanced hypopharyngeal cancer undergo induction
chemotherapy, NBI examination and screening for associated vascular
factors should be performed before and after chemotherapy, as
changes in vascular classification and abnormal expression of
vascular factors can be used as important reference factors. In
addition, the lack of quantitative immunohistochemistry analysis
may be a limitation of the present study. A more comprehensive
understanding of the mechanism of tumor angiogenesis will help to
inform the clinical application of anti-angiogenesis. Although a
variety of anti-angiogenic drugs combined with chemotherapy have
been introduced in clinical applications or have been entered into
clinical trials, they do not completely resolve tumor angiogenesis,
and the treatment therefore requires further improvement.
Acknowledgements
Not applicable.
Funding
Funding was received from the National Natural
Science Foundation of China (grant no. 81670946) and The Capital
Health Research and Development of Special (grant no.
CFH2018-1-2052).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GY and HD analyzed and interpreted the patient data.
WG and HL performed the experiments and WG was a major contributor
in writing the manuscript. ZH designed the study. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing Tongren Hospital (Beijing, China; approval no.
XMLX201507), and patients provided written informed consent to
participate in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cooper JS, Porter K, Mallin K, Hoffman HT,
Weber RS, Ang KK, Gay EG and Langer CJ: National cancer database
report on cancer of the head and neck: 10-year update. Head Neck.
31:748–758. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Newman JR, Timothy M Connolly, Illing EA,
Kilgore ML, Locher JL and Carroll WR: Survival trends in
hypopharyngeal cancer: A population-based review. Laryngoscope.
125:624–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuo P, Chen MM, Decker RH, Yarbrough WG
and Judson BJ: Hypopharyngeal cancer incidence, treatment, and
survival: Temporal trends in the United States. Laryngoscope.
124:2064–2069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen P, Yu W, Huang J, Xu H, Li G, Chen X
and Huang Z: Matched-pair analysis of survival in patients with
poorly differentiated versus well-differentiated glottic squamous
cell carcinoma. Oncotarget. 8:14770–14776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang CJ, Lin CY, Wang HM, Fan KH, Ng SH,
Lee LY, Chen IH, Huang SF, Liao CT and Yen TC: The number of
pathologically positive lymph nodes and pathological tumor depth
predicts prognosis in patients with poorly differentiated squamous
cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys.
81:e223–e230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pfister DG, Spencer S, Brizel DM, Burtness
B, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Dunphy F, Eisele
DW, et al: Head and neck cancers, Version 2. 2014. Clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
12:1454–1487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo W, Yin GF, Huang JW, Yang Z, Liu HF,
Zhang Y, Xu HB, Liu ZY and Huang ZG: Effect of vascular changes on
prognosis after induced chemotherapy for advanced hypopharyngeal
carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
54:591–596. 2019.(In Chinese). PubMed/NCBI
|
|
8
|
Kasza A: IL-1 and EGF regulate expression
of genes important in inflammation and cancer. Cytokine. 62:22–33.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
John A and Tuszynski G: The role of matrix
metalloproteinases in tumor angiogenesis and tumor metastasis.
Pathol Oncol Res. 7:14–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gale N, Poljak M and Zidar N: Update from
the 4th edition of the world health organization classification of
head and neck tumours: What is new in the 2017 WHO blue book for
tumours of the hypopharynx, larynx, trachea and parapharyngeal
space. Head Neck Pathol. 11:23–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amin MB: American Joint Committee on
Cancer. American Cancer Society. AJCC Cancer Staging Manual. 8th.
Springer; Chicago, IL: 2017
|
|
12
|
Ni XG, He S, Xu ZG, Gao L, Lu N, Yuan Z,
Lai SQ, Zhang YM, Yi JL, Wang XL, et al: Endoscopic diagnosis of
laryngeal cancer and precancerous lesions by narrow band imaging. J
Laryngol Otol. 125:288–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Engel KB and Moore HM: Effects of
preanalytical variables on the detection of proteins by
immunohistochemistry in formalin-fixed, paraffin-embedded tissue.
Arch Pathol Lab Med. 135:537–543. 2011.PubMed/NCBI
|
|
14
|
Jain RK and Carmeliet P: SnapShot: Tumor
angiogenesis. Cell. 149:1408 e12012. View Article : Google Scholar
|
|
15
|
Loges S, Clausen H, Reichelt U, Bubenheim
M, Erbersdobler A, Schurr P, Yekebas E, Schuch G, Izbicki J, Pantel
K, et al: Determination of microvessel density by quantitative
real-time PCR in esophageal cancer: Correlation with histologic
methods, angiogenic growth factor expression, and lymphnode
metastasis. Clin Cancer Res. 13:76–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bach F, Uddin FJ and Burke D:
Angiopoietins in malignancy. Eur J Surg Oncol. 33:7–15. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li LY, Barlow KD and Metheny-Barlow LJ:
Angiopoietins and Tie2 in health and disease. Pediatr Endocrinol
Rev. 2:399–408. 2005.PubMed/NCBI
|
|
18
|
Wu H: Morphological changes of capillary
loops in the epithelial papilla and correlation between vascular
growth factors, infiltration related cytokines and superficial
esophageal cancer [dissertation]. Shandong University. (Shandong).
2017.
|
|
19
|
Tsutsui S, Inoue H, Yasuda K, Suzuki K,
Takeuchi H, Nishizaki T, Higashi H, Era S and Mori M:
Angiopoietin-2 expression in invasive ductal carcinoma of the
breast: Its relationship to the VEGF expression and microvessel
density. Breast Cancer Res Treat. 98:261–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Viallard C and Larrivée B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Bock K, Cauwenberghs S and Carmeliet P:
Vessel abnormalization: Another hallmark of cancer? Molecular
mechanisms and therapeutic implications. Curr Opin Genet Dev.
21:73–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bikfalvi A: History and conceptual
developments in vascular biology and angiogenesis research: A
personal view. Angiogenesis. 20:463–478. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujisaka S, Usui I, Ikutani M, Aminuddin
A, Takikawa A, Tsuneyama K, Mahmood A, Goda N, Nagai Y, Takatsu K
and Tobe K: Adipose tissue hypoxia induces inflammatory M1 polarity
of macrophages in an HIF-1α-dependent and HIF-1α-independent manner
in obese mice. Diabetologia. 56:1403–1412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Falanga V, Zhou L and Yufit T: Low oxygen
tension stimulates collagen synthesis and COL1A1 transcription
through the action of TGF-beta1. J Cell Physiol. 191:42–50. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu SL, Reh D, Li AG, Woods J, Corless CL,
Kulesz-Martin M and Wang XJ: Overexpression of transforming growth
factor beta1 in head and neck epithelia results in inflammation,
angiogenesis and epithelial hyperproliferation. Cancer Res.
64:4405–4410. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biobe GC, Schiemann WP and Lodish HE: Role
of transforming growth factor beta in human disease. N Engl J Med.
343:1350–1358. 2000.PubMed/NCBI
|
|
27
|
Luo J, Chen XQ and Li P: The role of TGF-β
and its receptors in gastrointestinal cancers. Transl Oncol.
12:475–484. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dang D, Yang Y, Li X, Atakilit A, Regezi
J, Eisele D, Ellis D and Ramos DM: Matrix metalloproteinases and
TGFbeta1 modulate oral tumor cell matrix. Biochem Biophys Res
Commun. 316:937–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar S, Pan CC, Bloodworth JC, Nixon AB,
Theuer C, Hoyt DG and Lee NY: Antibody-directed coupling of
endoglin and MMP-14 is a key mechanism for endoglin shedding and
deregulation of TGF-β signaling. Oncogene. 33:3970–3979. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arriola BP, Scian R, Comerci DJ, Serantes
DR, Vanzulli S, Fossati CA, Giambartolomei GH and Delpino MV:
Brucella abortus induces collagen deposition and MMP-9 down
modulation in hepatic stellate cells via TGF-βl production. Am J
Pathol. 183:1918–1927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang JT, Sun W, Zhang WZ, Ge CY, Liu ZY,
Zhao ZM, Lu XS and Fan YZ: Norcantharidin inhibits tumor growth and
vasculogenic mimicry of human gallblad-der carcinomas by
suppression of the PI3-K/MMPs/Ln-5γ2 signaling pathway. BMC Cancer.
14:1932014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smith A, Teknos TN and Pan Q: Epithelial
to mesenchymal transition in head and neck squamous cell carcinoma.
Oral Oncol. 49:287–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun L, Diamond ME, Ottaviano AJ, Joseph
MJ, Ananthanarayan V and Munshi HG: Transforming growth
factor-beta1 promotes matrix metalloproteinase-9-mediated oral
cancer invasion through snail expression. Mol Cancer Res. 6:10–20.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji H, Gao Z and Feng Z: Expression of
matrix metalloproteinase-9 in squamous cell carcinoma of the tongue
with different pattern of invasion and clinical significance. J
Oral Sci Res. 28:488–490. 2012.
|
|
35
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu Y, Lu L, Qiu Z, Huang Q, Chen Y and
Chen L: Mechanical stretch aggravates aortic dissection by
regulating MAPK pathway and the expression of MMP-9 and
inflammation factors. Biomed Pharmacother. 108:1294–1302. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang HY, Zheng XZ, Wang XH, Xuan XY, Wang
F and Li SS: S100A4 mediated cell invasion and metastasis of
esophageal squamous cell carcinoma via the regulation of MMP-2 and
E-cadherin activity. Mol Biol Rep. 39:199–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang Q, Bournazou E, Sansone P, Berishaj
M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al:
The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and
metastasis. Neoplasia. 15:848–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Siveen KS, Prabhu K, Krishnankutty R,
Kuttikrishnan S, Tsakou M, Alali FQ, Dermime S, Mohammad RM and
Uddin S: Vascular endothelial growth factor (VEGF) signaling in
tumour vascularization: Potential and challenges. Curr Vasc
Pharmacol. 15:339–351. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paik JS, Cho WK, Oh EH, Lee SB and Yang
SW: Palmitate induced secretion of IL-6 and MCP-1 in orbital
fibroblasts derived from patients with thyroid-associated
ophthalmopathy. Mol Vis. 18:1467–1477. 2012.PubMed/NCBI
|
|
41
|
Cui G, Yuan A, Sun Z, Zheng W and Pang Z:
IL-1β/IL-6 network in the tumor microenvironment of human
colorectal cancer. Pathol Res Pract. 214:986–992. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cole KE, Strick CA, Paradis TJ, Ogborne
KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, et
al: Interferon-inducible T cell alpha chemoattractant (I-TAC): A
novel non-ELR CXC chemokine with potent activity on activated T
cells through selective high affinity binding to CXCR3. J Exp Med.
187:2009–2021. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Atanackovic D, Cao Y, Kim JW, Brandl S,
Thom I, Faltz C, Hildebrandt Y, Bartels K, de Weerth A,
Hegewisch-Becker S, et al: The local cytokine and chemokine milieu
within malignant effusions. Tumour Biol. 29:93–104. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vlahakis SR, Villasis-Keever A, Gomez TS,
Bren GD and Paya CV: Human immunodeficiency virus-induced apoptosis
of human hepatocytes via CXCR4. J Infect Dis. 188:1455–1460. 2003.
View Article : Google Scholar : PubMed/NCBI
|