Introduction
Breast cancer (BC) is classified into five intrinsic
subtypes according to gene expression profiling obtained by DNA
microarrays (1,2). Since the 2011 St Gallen symposium, a
surrogate classification of intrinsic subtypes based on a four BC
immunohistochemical (IHC) biomarker panel, namely estrogen receptor
(ER), progesterone receptor (PR), human epidermal growth factor
receptor 2 (HER-2) receptor and Ki-67, has been in use (3). Therefore, BC has begun to enter the era
of precision treatment. Molecular phenotypes may not only determine
the BC treatment approach, but also predict the prognosis of
patients; therefore, the accurate detection of these four molecular
markers is necessary. It is widely accepted that the expression of
the hormone receptors, ER and PR, determine the endocrine therapy
approach in order to inhibit the recurrence and metastasis of BC.
Additionally, both molecules exhibit a good prognostic index
(4,5). In clinical practice, HER-2-positive
patients are prone to recurrence and metastasis; therefore,
anti-HER-2 targeted therapy is commonly applied according to the
National Comprehensive Cancer Network (NCCN) guidelines (6–8).
Additionally, Ki-67 index exhibits a prognostic value in BC.
Therefore, increased Ki-67 index value is associated with a poorer
prognosis. Ki-67 protein is expressed in cell nuclei during cell
cycle phases G1, S and M. In clinical practice, usually only the
biomarkers for primary tumors are used to predict primary tumors
accompanied by synchronous axillary metastases (9). However, whether the expression of
theses biomarkers is in concordance between the BC primary tumor
and synchronous ALN metastases remains controversial.
Therefore, the present prospective study aimed to
investigate whether the expression of four BC biomarkers (ER, PR,
HER-2 and Ki-67) in the primary tumors was in accordance with that
in the synchronous ALN metastases, to provide a more accurate
indicator for the clinical treatment and prognosis of patients with
BC.
Patients and methods
Patients
A total of 60 female patients with BC, aged between
27 and 68 years old (median age, 45 years), admitted to the
People's Hospital of Ganzhou (Ganzhou, China) between June 2017 and
June 2018, were enrolled in the present study. The inclusion
criteria were as follows: i) pathological diagnosis of invasive
ductal carcinoma; ii) early BC with a single lesion in the primary
tumor; and iii) with at least one positive axillary lymph node
(ALN; >2.0 mm). The exclusion criteria for all study subjects
were as follows: i) postoperative specimens from palliative
surgery; ii) with neoadjuvant chemotherapy; iii) a primary tumor
with multifocal/multicenter lesions; and iv) accompanied by distant
metastases.
Immunohistochemistry (IHC)
The expression profiles of ER, PR, HER-2 and Ki-67
in the primary tumors and synchronous ALN metastases were
determined using immunohistochemistry (IHC). The tissues were fixed
in 10% neutral buffered formalin at room temperature for 24–48 h
and were embedded in paraffin. Tissue sections (4-µm-thick) were
deparaffinized in xylene at 37°C for 10 min and rehydrated in 100,
96 and 70% ethanol. Endogenous peroxidase was blocked by incubation
in 3% H2O2 in methanol at 37°C for 10 min,
followed by rinsing in PBS. Sections were then subjected to antigen
retrieval by immersion in citrate buffer (pH 6.0) preheated to 99°C
for 40 min. The sections were incubated with primary antibodies
(all ready to use) against ER (clone SP1; cat. no. 790-4325;
Ventana Medical Systems, Inc.), PR (clone 1E2; cat. no. 790-4296;
Ventana Medical Systems, Inc.), HER-2 (C-erbB-2; clone 4B5; cat.
no. 790-2991; Ventana Medical Systems, Inc.) and Ki-67 (cat. no.
MAB-0672; Fuzhou Maixin Biotech Co., Ltd.). Blots were developed
using the labeled streptavidin biotinylated antibody (ready to use;
cat no. 760-500; Roche Diagnostics) at 37°C for 8 min using a
Ventana Benchmark XT automated immunostaining system (Roche
Diagnostics) according to the manufacturer's protocol. IHC results
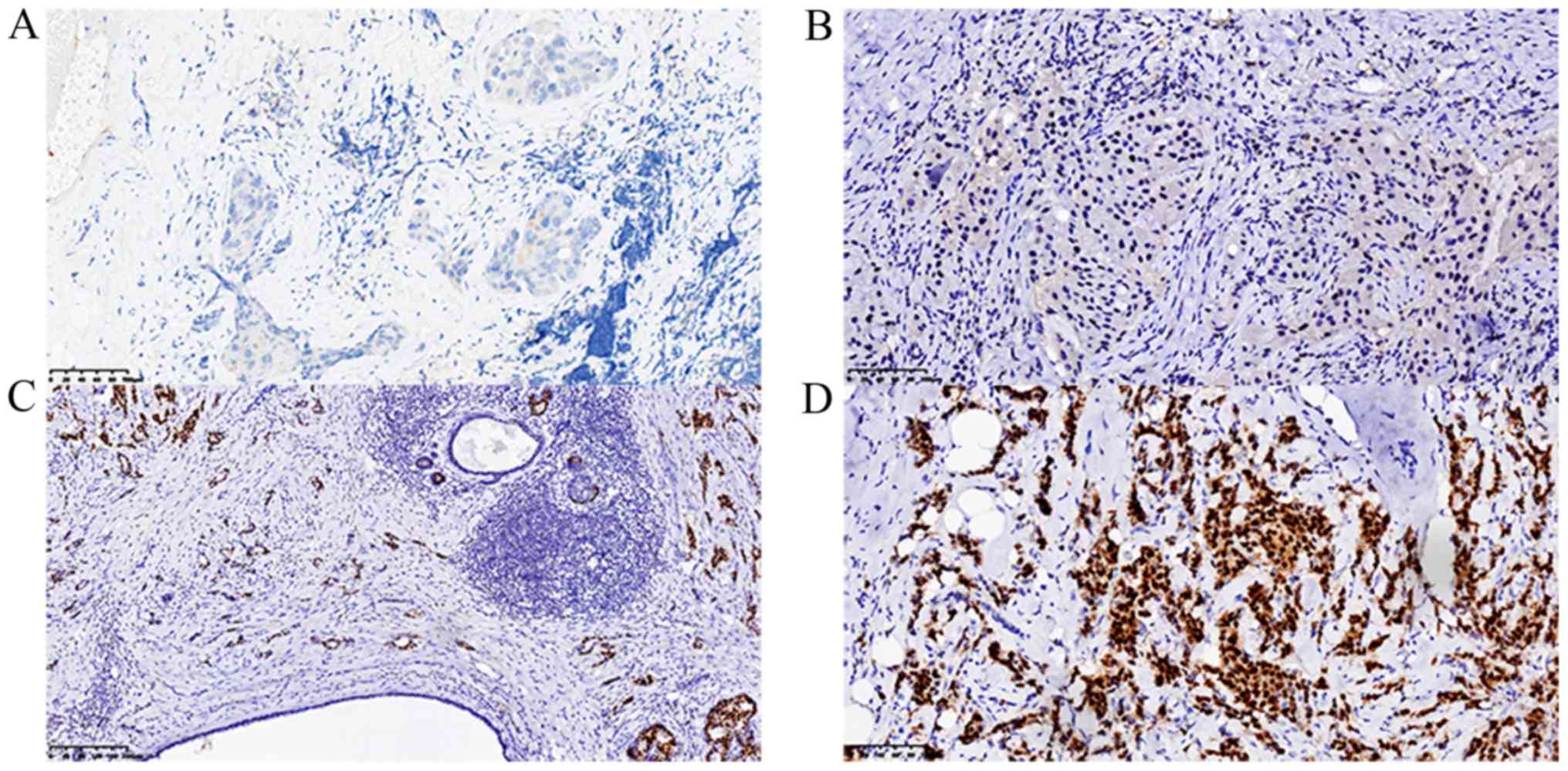
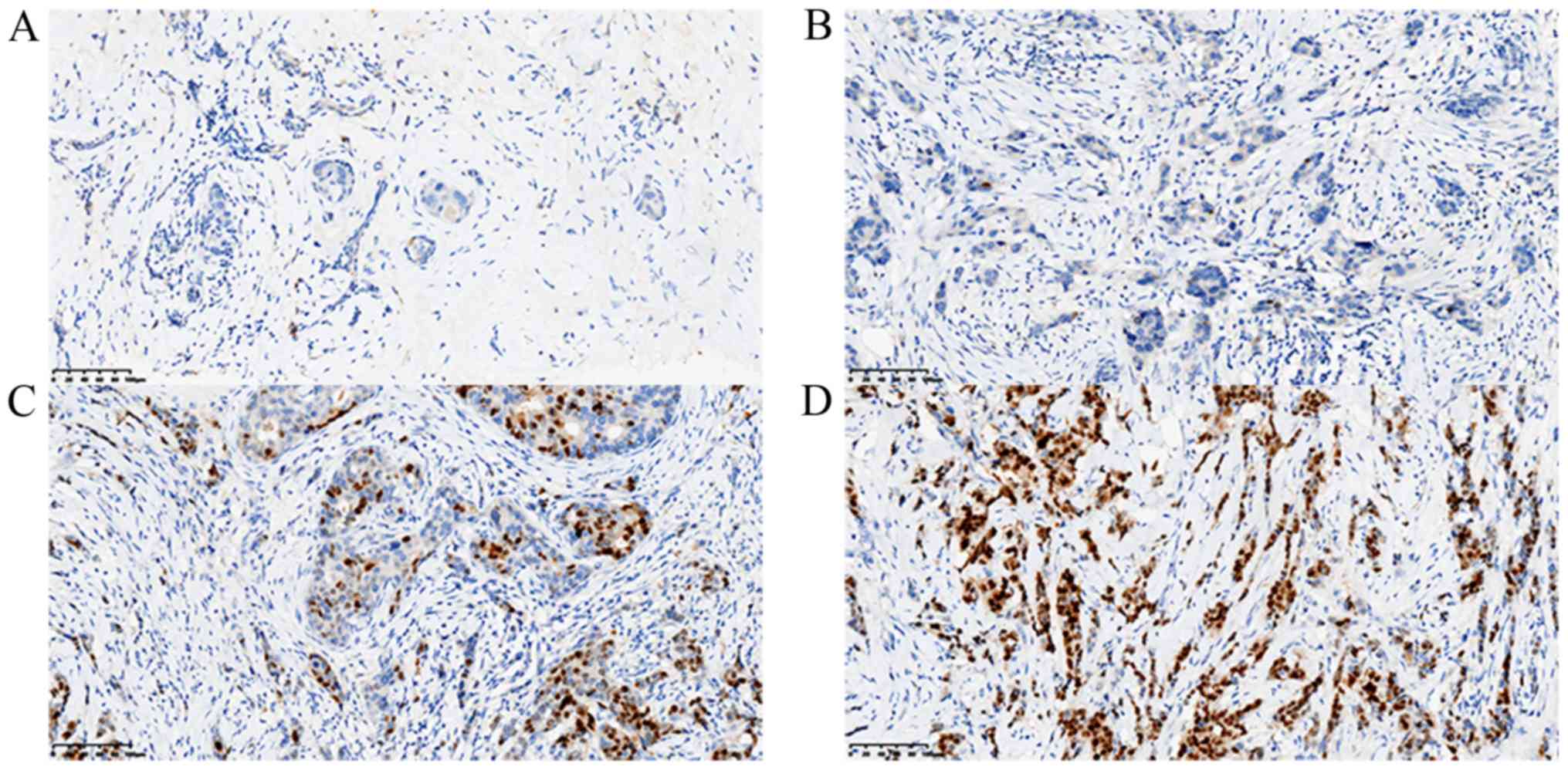
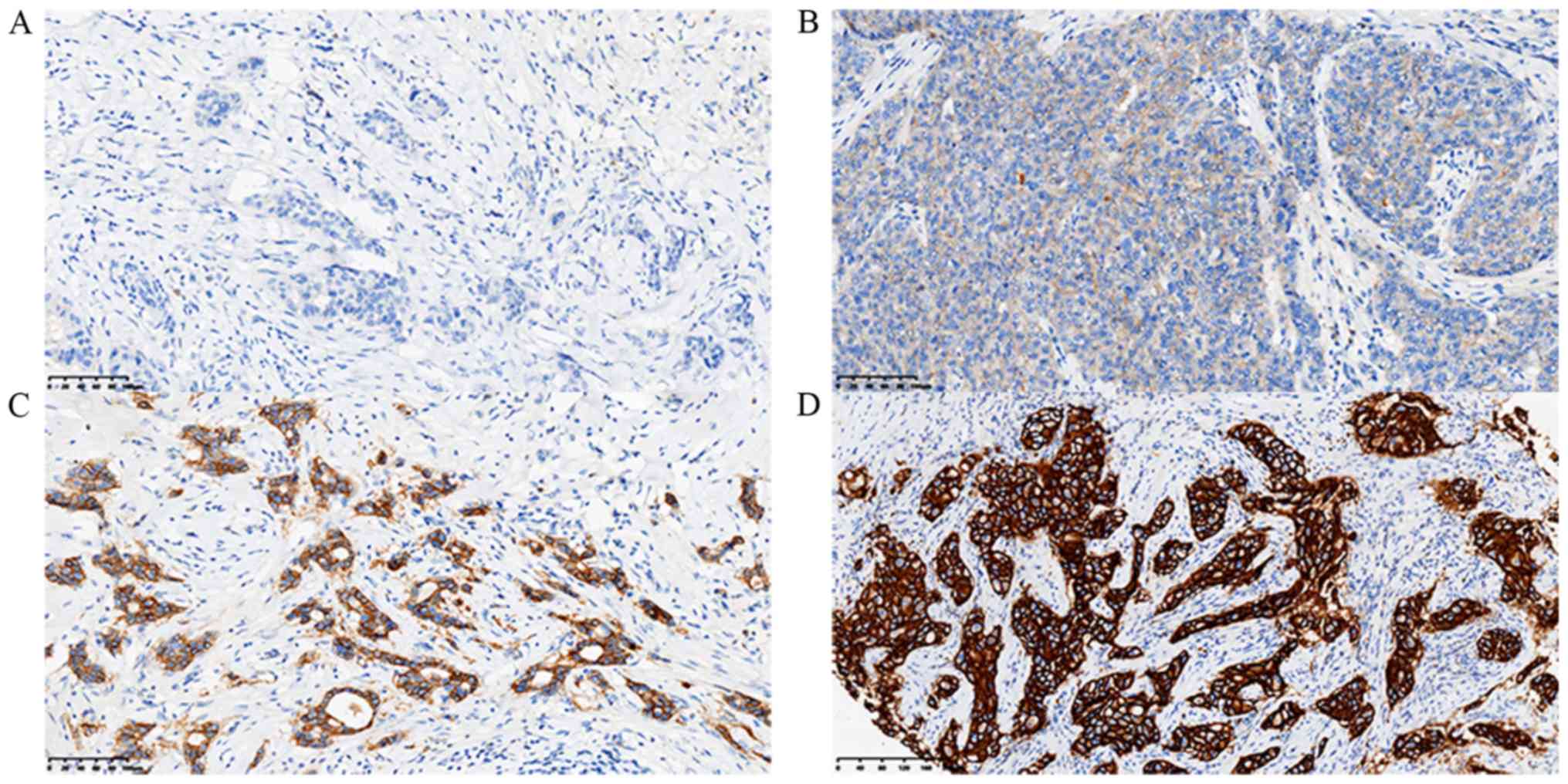
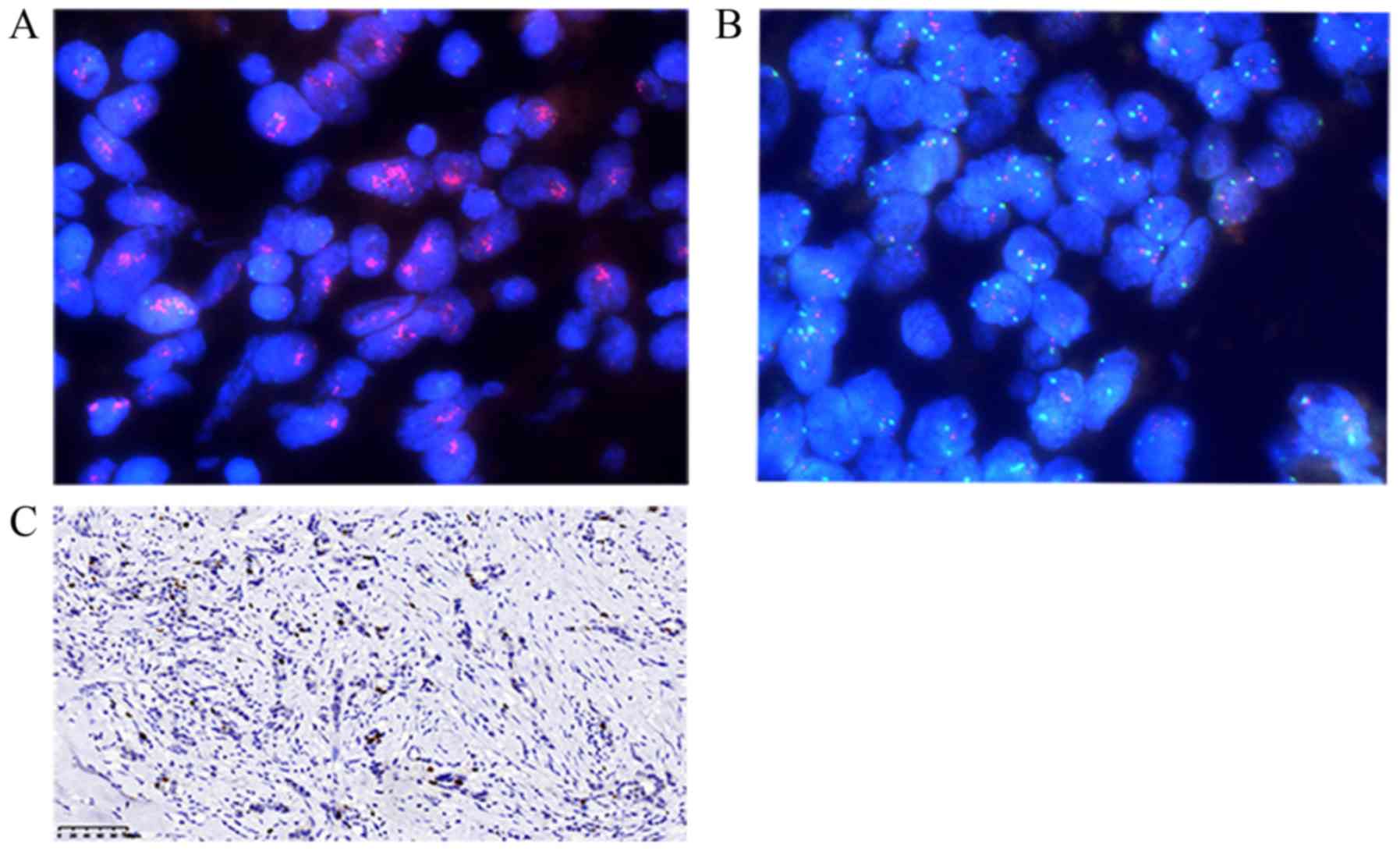
of ER (Fig. 1), PR (Fig. 2), HER-2 (Fig. 3) and Ki-67 (Fig. 4C) were visualized and imaged using
the ZEISS Axio Lab A1 light microscope at a magnification of
×200.
The cut-off level of ER and PR positively stained
cell nuclei was set to ≥10%. The expression intensity of ER and PR
was scored on a scale of ‘−’ to ‘+++’, where ‘−’, ‘+’, ‘++’ and
‘+++’ indicated 0, 0–25, 26–50 and >50% positive tumor cells,
respectively. HER-2 IHC staining was scored as 0, 1+, 2+ or 3+.
HER-2 scores of 0 and 1+ were considered negative, while a score of
3+ was considered positive. When a HER-2 score of 2+ was obtained,
the results were reassessed using fluorescence in situ
hybridization (FISH). FISH (FP-001; HER-2/CEP17 dual-color probe
mixture; Wuhan Healthcare Biotechnology Co., Ltd.) assay was
performed according to the American Society of Clinical Oncology
(ASCO) and the College of American Pathologists (CAP) guidelines
(10). FISH analysis of HER-2
(Fig. 4A and B) was visualized and
imaged using the LEICA DM2500 fluorescence microscope at a
magnification of ×600. The Ki-67 index values are expressed as the
percentage of tumor cells with positively stained nuclei and the
cut-off point was set to 14% (11).
Statistical analyses
The consistency of the expression ER, PR and HER-2
biomarkers between the primary tumors and ALN metastases was
analyzed using χ2 test. The concordance of ER, PR (−, +,
++, +++) and HER-2 (negative, positive) expression intensity
between the primary tumors and ALN metastases was analyzed using
Kappa test. When the values for ER, PR and Ki-67 expression in the
two groups were not normally distributed, a non-parametric Wilcoxon
signed rank test was performed. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using the SPSS 20.0 software (IBM
Corp.).
Results
Concordance/discordance in the
expression of ER
The different BC subtypes in the primary tumors and
ALN metastases are presented in Table
I. The positive expression rate of ER in the primary tumors and
ALN metastases in Table II was 71.7
(43/60) and 68.3% (41/60), respectively. In addition, the negative
ER expression rate was 28.3% (17/60) in the primary tumors and
31.7% (19/60) in ALN metastases. The P-value obtained when
comparing ER expression between primary tumors and ALN metastases
was not significant (P=0.393; Table
III). The kappa value obtained when comparing ER expression
intensity in primary tumors with that in ALN metastases was 0.773,
thereby indicating a high degree of consistency in ER expression
(Table IV). Finally, as shown in
Table V, the concordance and
discordance rates for ER were 96.7 (58/60) and 3.33% (2/60),
respectively. In the two discordance cases, both samples were
positive for ER in the primary tumors and negative in the ALN
metastases.
 | Table I.Breast cancer subtypes in primary
tumors and paired ALN metastases. |
Table I.
Breast cancer subtypes in primary
tumors and paired ALN metastases.
| HER-2 status | Primary tumor | ALN metastases |
|---|
| HER-2-positive status
(n=22) |
|
|
|
ER+/PR+ | 14 | 14 |
|
ER+/PR- | 3 | 1 |
|
ER-/PR- | 5 | 7 |
|
ER-/PR+ | 0 | 0 |
| HER-2-negative status
(n=38) |
|
|
|
ER+/PR+ | 25 | 23 |
|
ER+/PR- | 1 | 3 |
|
ER-/PR- | 12 | 12 |
|
ER-/PR+ | 0 | 0 |
 | Table II.Expression status of biomarkers in the
primary tumors and ALN metastases. |
Table II.
Expression status of biomarkers in the
primary tumors and ALN metastases.
| Biomarker | Primary tumor | ALN metastases | P-value |
|---|
| ER |
|
| 0.69 |
| Positive
rate (%) | 43/60 (71.7) | 41/60 (68.3) |
|
| Negative
rate (%) | 17/60 (28.3) | 19/60 (31.7) |
|
| PR |
|
| 0.71 |
|
Positive rate (%) | 39/60 (65) | 37/60 (61.7) |
|
|
Negative rate (%) | 21/60 (35) | 23/60 (38.3) |
|
| HER-2 |
|
| 1.0 |
|
Positive rate (%) | 22/60 (36.7) | 22/60 (36.7) |
|
|
Negative rate (%) | 38/60 (63.3) | 38/60 (63.3) |
|
 | Table III.ER, PR and Ki-67 numerical expression
variables. |
Table III.
ER, PR and Ki-67 numerical expression
variables.
| Marker | Primary tumor | ALN metastases | Z | P-value |
|---|
| ER | 70.00
(0.00–90.00) | 62.50
(0.00–90.00) | −0.854 | 0.393 |
| PR | 20.00
(0.00–50.00) | 6.50
(0.00–50.00) | −0.842 | 0.400 |
| Ki-67 | 30.00
(0.00–40.00) | 30.00
(15.25–40.00) | −0.973 | 0.331 |
 | Table IV.Expression intensity and consistency
of ER and PR in primary tumors and ALN metastases (n=60). |
Table IV.
Expression intensity and consistency
of ER and PR in primary tumors and ALN metastases (n=60).
|
| ALN metastases |
|
|---|
|
|
|
|
|---|
| Primary tumor | − | + | ++ | +++ | Kappa |
|---|
| ER |
|
|
|
| 0.773 |
| − | 17 | 0 | 0 | 0 |
|
| + | 1 | 7 | 3 | 0 |
|
| ++ | 1 | 2 | 8 | 2 |
|
|
+++ | 0 | 0 | 1 | 18 |
|
| PR |
|
|
|
| 0.654 |
| − | 21 | 0 | 0 | 0 |
|
| + | 1 | 10 | 2 | 0 |
|
| ++ | 1 | 8 | 9 | 1 |
|
|
+++ | 0 | 1 | 1 | 5 |
|
| HER-2 |
|
|
|
| 0.785 |
| − | 35 | 3 |
|
|
|
| + | 3 | 19 |
|
|
|
 | Table V.Changes in the expression of the
biomarkers between primary BC tumors and ALN metastases. |
Table V.
Changes in the expression of the
biomarkers between primary BC tumors and ALN metastases.
|
| Primary BC | ALN metastases |
|---|
|
|
|
|
|---|
| Biomarker | Discordance rate
(%) | (+) → (−) | (−) → (+) |
|---|
| ER | 2/60 (3.33) | 2 | 0 |
| PR | 2/60 (3.33) | 2 | 0 |
| HER-2 | 6/60 (10) | 3 | 3 |
Concordance/discordance in the
expression of PR
The expression of PR was determined in the primary
tumors and ALN metastases using IHC. The PR-positive and -negative
rates in primary tumors were 65 (39/60) and 35% (21/60),
respectively. Consistent with that in the primary tumors, the
PR-positive rate in ALN metastases was 61.7% (37/60) and the
negative rate was 38.3% (23/60); (Table
II). Furthermore, the P-value obtained when comparing PR
expression between primary tumors and ALN metastases did not
indicate a statistically significant difference (P=0.400; Table III). As shown in Table IV, the kappa value of consistency
was 0.654 for PR expression in primary lesions and ALN metastases,
suggesting a high degree of consistency in its expression.
Consistent with the ER IHC results, the concordance and discordance
rates for PR expression in Table V
were 96.7 (58/60) and 3.33% (2/60), respectively. In the two
discordance cases, all samples were positive for PR expression in
the primary tumor but negative in the ALN metastases.
Concordance/discordance in the
expression of HER-2
The expression of HER-2 was investigated. The
results revealed that the HER-2-negative rate in the primary tumors
and ALN metastases was 63.3% (38/60) in the two groups (Table II). The kappa value of consistency
for HER-2 was 0.785, indicating a high degree of consistency for
HER-2 expression between the primary tumors and ALN metastases
(Table IV). Finally, the
concordance rate for HER-2 was 90% (54/60) and the discordance rate
was 10% (6/60; Table V). Among the
six discordance cases, three were positive for HER-2 in the primary
tumor and negative in the ALN metastases, while three were negative
in the primary tumor and positive in ALN metastases.
Concordance/discordance in the
expression of Ki-67
Analysis of the P-values comparing Ki-67 expression
between primary tumors and ALN metastases (P=0.331), using a
non-parametric Wilcoxon signed rank test, suggested that the Ki-67
expression in the primary tumors was consistent with that in the
ALN metastases (Table III).
Discussion
ALN metastasis is very common in BC. Whether the
expression of the biomarkers in the primary tumors is in
concordance with that in the synchronous ALN metastases remains
controversial (12,13). It has been reported that the
discordance rates in the primary tumors and recurrent/metastatic
lesions were 10–30% for ER, 25–55% for PR and 10–15% for HER-2
(14–18). Other studies (19,20) have
investigated the discordance in biomarker expression between the
circulating tumor cells and the primary tumors. Furthermore,
several retrospective studies have revealed discrepancies in ER,
PR, Ki-67 and HER-2 expression between primary breast tumors and
synchronous ALN metastases (21–24).
Nedergaard et al (21)
investigated the ER expression status in 101 primary BC tumors and
the paired ALN metastases. The discordant rate for ER was 21%
between the primary tumors and the corresponding metastatic lesions
(21). Accordingly, Aitken et
al (22) identified the changes
in ER, PR and HER-2 expression between the primary tumors and
synchronous ALN metastases. The results revealed that the
discrepancy rates for ER, PR and HER-2 were 28.9 (55/190), 23.7
(45/190) and 8.9% (17/190), respectively (22). Additionally, Feng et al
(23) investigated the expression of
ER and HER-2 in primary tumors and ALN metastases in 80 patients
with invasive ductal BC. The discordance rates in the present study
were 8.8% for ER and 11.3% for HER-2 (23). Kinoe et al (24) reported that the discordance rates
between the primary tumors and the lymph node metastases were 28.8%
for ER (positive in primary tumors and negative in lymph nodes or
negative in primary tumors and positive in lymph nodes, 22.1/6.7%),
31.7% for PR (26.9/4.8%), 13.5% for HER-2 (12.5/1.0%) and 43.3% for
Ki-67 (high in primary tumors and low in lymph nodes or low in
primary tumors and high in lymph nodes, 12.5/30.8%). Overall, the
aforementioned studies revealed increased discordance rates in ER
and PR expression and decreased discordance in HER-2 expression.
Furthermore, the results of these studies suggested that the most
common changes in patients with breast cancer were hormone receptor
and HER-2 positive in primary tumors, but negative in synchronous
ALN metastases. Due to these discrepancies, researchers have
suggested that the determination of biomarker expression should be
performed simultaneously in the synchronous ALN metastases and
their corresponding primary tumors (25–27).
However, other studies have demonstrated that the
expression profile of the molecular markers was consistent between
the primary and metastatic lesions (9,19,28).
Consistent with the results of the present study, Zhao et al
(9) identified that the concordance
rates between the BC primary tumors and ALN metastases were 72.2%
(39/54) for ER, 88.9% (48/54) for PR and 90.7% (49/54) for HER-2.
Therefore, no statistically significant differences were observed
(9). However, in contrast to this
previous study, patients that had undergone neoadjuvant therapy
were not included in the present study.
Aktas et al (19) demonstrated that the primary tumors
and circulating tumor cells displayed a concordant HER-2, ER and PR
expression status in 59 (P=0.262), 39 (P=0.51) and 44% (P=0.62) of
cases, respectively (19).
Furthermore, D'Andrea et al (28) studied the expression of 10 biomarkers
in 90 pure invasive ductal carcinomas with 10 or more ALNs involved
and without evidence of distant metastasis. This study revealed a
close association between the primary tumors and the corresponding
metastatic nodes in terms of the expression of all 10 biomarkers
investigated (28). However, in the
aforementioned study, the authors did not investigate the
expression of ER, PR and HER-2 in order to compare their expression
values between the primary tumors and the paired metastatic
nodes.
In the present study, the consistency rate of ER and
PR expression was 96.7% (58/60). Only two cases with primary tumors
positive for ER and PR exhibited negative expression in the ALN
metastases, while only one case with primary tumors negative for ER
and PR expression exhibited positive expression in the ALN
metastases. In addition, the concordance rate for HER-2 was 90%
(54/60) and only six cases (10%) exhibited discordance. Among the
six cases, three were HER-2-negative in the primary tumors and
positive in the ALN metastases, while three were HER-2-positive in
the primary tumors but negative in the ALN metastases. Furthermore,
the present study compared the expression intensity of ER, PR and
HER-2, as well as the numeric expression variables of ER, PR and
Ki-67, between the primary tumors and the corresponding synchronous
ALN metastases. Therefore, the kappa values of consistency for ER,
PR and HER-2 expression intensity between the primary lesions and
the synchronous ALN metastases were 0.773, 0.654 and 0.785,
respectively, thereby demonstrating a high degree of consistency.
In addition, the P-values comparing ER, PR and Ki-67 expression
variables between the two groups were 0.393, 0.400 and 0.331,
respectively, as verified using a non-parametric Wilcoxon signed
rank test. Therefore, no statistically significant differences were
observed.
However, some studies have suggested that the
concordance between primary lesions and metastases is associated
with a poorer prognosis (16). Jung
et al (29) demonstrated that
the overall survival time was longer in patients with BC with
concordant hormone receptor expression between the primary breast
tumors and brain lesions, compared with patients exhibiting
discordant expression patterns. However, the difference was not
statistically significant (discordant vs. concordant median
survival time, 19.2 vs. 31.1 months; P=0.181) (29). Furthermore, other previous studies
reported that neoadjuvant/adjuvant chemotherapy, and endocrine and
targeted therapy may affect the expression of the biomarkers in the
primary and metastatic lesions (16,18). In
the present study, patients undergoing chemotherapy, endocrine
therapy or targeted therapy were excluded, and only early operable
patients were enrolled, which may explain the high consistency of
our findings. Sighoko et al (30) applied a Bayesian misclassification
correction method on data on hormone receptor expression status
from two primary BCs from the Surveillance, Epidemiology and End
Results (SEER) database between 1990 and 2010 and on data on
primary and paired recurrent or metastatic disease assembled from a
meta-analysis of 36 studies. The authors suggested a considerable
proportion of discordance in hormone receptor expression status
that should be attributed to misclassification in receptor
assessment (30).
In conclusion, the findings of the present study
indicated that the ER, PR, HER-2 and Ki 67 expression status was in
concordance between the primary breast tumors and synchronous ALN
metastases. Therefore, molecular biomarkers of the primary tumors
could be also used as biomarkers for synchronous axillary
metastases in some patients. However, the application of these
biomarkers in the lymph nodes should be further evaluated due to
the lack of high-quality, large-scale prospective studies or
high-quality meta-analysis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guiding
Science and Technology Program Project in Ganzhou City, Jiangxi,
China (grant no. GZ2017ZSF199).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this article.
Authors' contributions
XX and XWH conceived and designed the study. FLY was
responsible for the acquisition of data. JN performed the
statistical analysis. HZY and CH analysed and interpreted the data,
drafted the manuscript and revised it critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients for the use of their tissues for research purposes, and
the study protocol was approved by Ganzhou People's Hospital Ethics
Committee (Ganzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Prat A and Perou CM: Deconstructing the
molecular portraits of breast cancer. Mol Oncol. 5:5–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Genome Atlas Network, .
Comprehensive molecular portraits of human breast tumours. Nature.
490:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gándara-Cortes M, Vázquez-Boquete Á,
Fernández-Rodríguez B, Viaño P, Ínsua D, Seoane-Seoane A, Gude F,
Gallego R, Fraga M, Antúnez JR, et al: Breast cancer subtype
discrimination using standardized 4-IHC and digital image analysis.
Virchows Arch. 472:195–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Subik K, Lee JF, Baxter L, Strzepek T,
Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG and
Tang P: The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67
and AR by immunohistochemical analysis in breast cancer cell lines.
Breast Cancer (Auckl). 4:35–41. 2010.PubMed/NCBI
|
|
5
|
Voduc KD, Cheang MC, Tyldesley S, Gelmon
K, Nielsen TO and Kennecke H: Breast cancer subtypes and the risk
of local and regional relapse. J Clin Oncol. 28:1684–1691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Invasive breast cancer version 1.2016, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
14:324–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohsenifar J, Almassi-Aghdam M,
Mohammad-Taheri Z, Zare K, Jafari B, Atri M, Mortazavi SH, Azadeh
P, Bagherzadeh M, Azargashb E and Rahimi F: Prognostic values of
proliferative markers ki-67 and repp86 in breast cancer. Arch Iran
Med. 10:27–31. 2007.PubMed/NCBI
|
|
8
|
Kontzoglou K, Palla V, Karaolanis G,
Karaiskos I, Alexiou I, Pateras I, Konstantoudakis K and Stamatakos
M: Correlation between Ki67 and breast cancer prognosis. Oncology.
84:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao S, Xu L, Liu W, Lv C, Zhang K, Gao H,
Wang J and Ma R: Comparison of the expression of prognostic
biomarkers between primary tumor and axillary lymph node metastases
in breast cancer. Int J Clin Exp Pathol. 8:5744–5748.
2015.PubMed/NCBI
|
|
10
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American society of
clinical oncology/college of American pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao ZX, Lu LJ, Wang RJ, Jin LB, Liu SC, Li
HY, Ren GS, Wu KN, Wang DL and Kong LQ: Discordance and clinical
significance of ER, PR, and HER2 status between primary breast
cancer and synchronous axillary lymph node metastasis. Med Oncol.
31:7982014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song JL, Chen C, Yuan JP and Sun SR:
Progress in the clinical detection of heterogeneity in breast
cancer. Cancer Med. 5:3475–3488. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liedtke C, Broglio K, Moulder S, Hsu L,
Kau SW, Symmans WF, Albarracin C, Meric-Bernstam F, Woodward W,
Theriault RL, et al: Prognostic impact of discordance between
triple-receptor measurements in primary and recurrent breast
cancer. Ann Oncol. 20:1953–1958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sari E, Guler G, Hayran M, Altundag K,
Gullu I and Ozisik Y: Comparison of ER, PR, HER2 in primary and
paired relapsed/metastatic lesions of metastatic breast cancer
patients. J Clin Oncol. 27:10632009. View Article : Google Scholar
|
|
16
|
Niikura N, Liu J, Hayashi N, Mittendorf
EA, Gong Y, Palla SL, Tokuda Y, Gonzalez-Angulo AM, Hortobagyi GN
and Ueno NT: Loss of human epidermal growth factor receptor 2
(HER2) expression in metastatic sites of HER2-overexpressing
primary breast tumors. J Clin Oncol. 30:593–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dieci MV, Barbieri E, Piacentini F,
Ficarra G, Bettelli S, Dominici M, Conte PF and Guarneri V:
Discordance in receptor status between primary and recurrent breast
cancer has a prognostic impact: A single-institution analysis. Ann
Oncol. 24:101–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Curtit E, Nerich V, Mansi L, Chaigneau L,
Cals L, Villanueva C, Bazan F, Montcuquet P, Meneveau N, Perrin S,
et al: Discordances in estrogen receptor status, progesterone
receptor status, and HER2 status between primary breast cancer and
metastasis. Oncologist. 18:667–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aktas B, Kasimir-Bauer S, Müller V, Janni
W, Fehm T, Wallwiener D, Pantel K and Tewes M; DETECT Study Group,
: Comparison of the HER2, estrogen and progesterone receptor
expression profile of primary tumor, metastases and circulating
tumor cells in metastatic breast cancer patients. BMC Cancer.
16:5222016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Turner N, Pestrin M, Galardi F, De Luca F,
Malorni L and Di Leo A: Can biomarker assessment on circulating
tumor cells help direct therapy in metastatic breast cancer?
Cancers (Basel). 6:684–707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nedergaard L, Haerslev T and Jacobsen GK:
Immunohistochemical study of estrogen receptors in primary breast
carcinomas and their lymph node metastases including comparison of
two monoclonal antibodies. APMIS. 103:20–24. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aitken SJ, Thomas JS, Langdon SP, Harrison
DJ and Faratian D: Quantitative analysis of changes in ER, PR and
HER2 expression in primary breast cancer and paired nodal
metastases. Ann Oncol. 21:1254–1261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng T, Zhang J and Zhong L: Relationship
of ER and HER-2 expression in primary tumor and axillary lymph node
metastases of invasive ductal breast cancer. Chin J Gen Surg.
2012.
|
|
24
|
Kinoe H, Yamanouchi K, Kuba S, Morita M,
Sakimura C, Kanetaka K, Takatsuki M, Abe K, Yano H, Matsumoto M, et
al: Discordance of hormone receptor, human epidermal growth factor
receptor-2, and Ki-67 between primary breast cancer and synchronous
axillary lymph node metastasis. J BUON. 23:60–66. 2018.PubMed/NCBI
|
|
25
|
Santinelli A, Pisa E, Stramazzotti D and
Fabris G: HER-2 status discrepancy between primary breast cancer
and metastatic sites. Impact on target therapy. Int J Cancer.
122:999–1004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ataseven B, Gologan D, Gunesch A, Kehl V,
Hoegel B, Beer M and Eiermann W: HER2/neu, topoisomerase 2a,
estrogen and progesterone receptors: Discordance between primary
breast cancer and metastatic axillary lymph node in expression and
amplification characteristics. Breast Care (Basel). 7:465–470.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jensen JD, Knoop A, Ewertz M and Laenkholm
AV: ER, HER2, and TOP2A expression in primary tumor, synchronous
axillary nodes, and asynchronous metastases in breast cancer.
Breast Cancer Res Treat. 132:511–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Andrea MR, Limiti MR, Bari M,
Zambenedetti P, Montagutti A, Ricci F, Pappagallo GL, Sartori D,
Vinante O and Mingazzini PL: Correlation between genetic and
biological aspects in primary non-metastatic breast cancers and
corresponding synchronous axillary lymph node metastasis. Breast
Cancer Res Treat. 101:279–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung J, Lee SH, Park M, Youn JH, Shin SH,
Gwak HS and Yoo H: Discordances in ER, PR, and HER2 between primary
breast cancer and brain metastasis. J Neurooncol. 137:295–302.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sighoko D, Liu J, Hou N, Gustafson P and
Huo D: Discordance in hormone receptor status among primary,
metastatic, and second primary breast cancers: Biological
difference or misclassification? Oncologist. 19:592–601. 2014.
View Article : Google Scholar : PubMed/NCBI
|