Introduction
Topoisomerase 1 (Top1) poisons, including
camptothecin (CPT), irinotecan (CPT-11), and topotecan comprise a
major component of the conventional chemotherapy drugs that are
used for the treatment of cancer in developing countries due to low
costs and ease of access (1,2). However, the therapeutic efficacy of
conventional chemotherapy drugs is attenuated by the multidrug
resistance that malignant tumor cells develop against structurally
and mechanistically varied chemotherapeutic drugs upon exposure to
a single agent (3,4). In order to overcome multidrug
resistance or at least delay its occurrence, conventional
chemotherapy drugs are sequentially used or combined with other
forms of therapies, such as immunotherapy and radiotherapy, in
clinical practice (5,6). The combination of conventional agents
with a small molecule compound that essentially reverses multidrug
resistance or increases the chemosensitivity of tumor cells has
been proposed as a promising therapeutic approach (3,4). For
example, overexpression of adenosine triphosphate-binding cassette
(ABC) transporter proteins induced by tumor cells has been
validated as an important molecular mechanism of multidrug
resistance (7–9). Currently, molecules that target certain
ABC transporters, such as verapamil, biricodar (VX-710) and
elacridar (GF120918), are under investigation in an attempt to
discover candidates for combined therapy (10); however, these molecules are yet to
prove effective and safe enough for clinical application (9).
Gibberellins are commonly used to regulate plant
growth and are commercially available in large quantities (11,12).
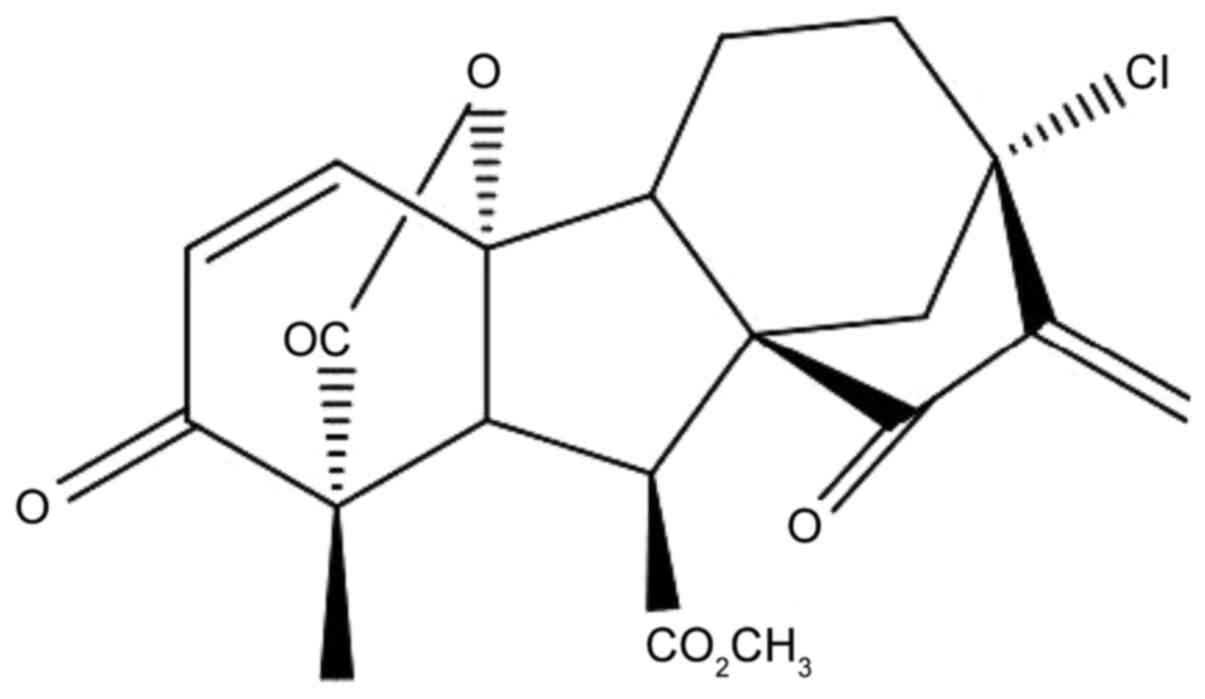
13-Chlorine-3,15-dioxy-gibberellic acid methyl ester (GA-13315) is
a gibberellin derivative that exhibits an antitumor effect in
vivo (13); its chemical
structure is presented in Fig.
1.
GA-13315 exhibits a stronger cytotoxicity to the
multidrug-resistant human breast carcinoma MCF-7/ADR cell line,
which overexpresses ABCB1 transporter proteins, than to the
sensitive parental MCF-7 cell line. GA-13315 is able to sensitize
the resistant MCF-7/ADR cells to conventional chemotherapy agents
when co-administered (14). Based on
these findings and in an attempt to determine the possibility of
using GA-13315 as adjuvant to conventional chemotherapy,
particularly Top1 agents, the present study aimed to investigate
whether long-term exposure to a subtoxic dose of GA-13315 would
confer resistance to sensitive tumor cell lines, or instead,
sensitize the cell line to conventional chemotherapy drugs. Thus, a
stepwise GA-13315 induction protocol was applied to human MCF-7 and
colon cancer HCT116 cell lines. The chemosensitivity of these cell
lines was assessed before and after exposure to GA-13315, and the
molecular mechanisms underlying the changes were investigated.
Materials and methods
Materials
GA-13315 was synthesized by Professor Hong-Bin Zhang
and Professor Jing-Bo Chen from the School of Chemical Science and
Technology, Yunnan University (Kunming, China). Irinotecan,
cisplatin and phenylmethylsulfonyl fluoride (PMSF) were purchased
from Sigma-Aldrich; Merck KGaA. TRIzol® reagent and the
M-MLV First Strand kit were purchased from Invitrogen; Thermo
Fisher Scientific, Inc. RPMI-1640 medium, McCoy's 5A medium, fetal
bovine serum (FBS), penicillin and streptomycin were purchased from
Gibco; Thermo Fisher Scientific, Inc. The Cell Counting Kit-8
(CCK-8) was purchased from Shanghai life lab Bio Technology Co.,
Ltd. (http://www.life-ilab.com). Low
melting-point agarose and DAPI were purchased from Thermo Fisher
Scientific, Inc. The TopoGEN Topoisomerase I Drug Screening kit was
purchased from TopoGEN. All other chemicals were of analytical
grade and were purchased from commercial sources.
Cell culture
MCF-7 and HCT116 cell lines were purchased from
Conservation Genetics CAS Kunming Cell Bank. MCF-7 cells were
cultured in RPMI-1640 medium, while HCT116 cells were maintained in
McCoy's 5A medium. All cells were supplemented with 10% FBS, 100
IU/ml penicillin and 100 IU/ml streptomycin, at 37°C in a
humidified atmosphere of 5% CO2.
Evaluation of cell viability
Cells were seeded into 96-well plates at a density
of 8×103 cells/well in a total volume of 180 µl and left
to attach overnight at 37°C. Cells were exposed to drugs (20 µl) at
different concentrations for 48 h at 37°C and cell viability was
subsequently analyzed via the CCK-8 assay at 450 and 630 nm using
SpectraMax Plus384 Molecular Devices (Molecular Devices, LLC),
according to the manufacturer's instructions. At least three
independent experiments were performed with duplicate
determinations. The half maximal inhibitory concentration
(IC50) values were calculated by non-linear regression,
using a sigmoidal dose-response equation (variable slope).
Chronic exposure of cell lines to
GA-13315
A reported drug exposure regimen was referred to and
followed, with modifications (15).
HCT116 cells were exposed to 1 µM GA-13315 for 84 days, which
yielded ≥90% cell viability. The medium was replaced every 2–3 days
with GA-13315 of the same concentration. Sensitivity to
chemotherapeutic drugs (GA-13315, irinotecan and cisplatin) was
monitored every 4 weeks, with an interval of 2 days during which
cells were cultured in the absence of GA-13315 before being plated
for sensitivity monitoring. In addition, confluent monolayer cells
in 10-cm diameter dishes were either washed twice with PBS and
stored at −80°C for protein expression analysis or lysed with
TRIzol® reagent and stored at −80°C for mRNA expression
analysis. MCF-7 cells were treated with the same procedure as
HCT116 cells for 4 weeks and then separated into 2 derivative
lines. The first line continued with the same regimen up to 12
weeks, while the second line was exposed to 2 µM GA-13315 for 4
weeks and subsequently exposed to 4 µM GA-13315 for another 4
weeks. In total, ≥90% of MCF-7 cells were viable at all the
aforementioned concentrations of GA-13315.
Western blotting
After chronic exposure to GA-13315 at 4, 8 and 12
weeks, cells were frozen at −80°C, respectively. Following
successful exposure, cells were thawed and lysed on ice using RIPA
lysis buffer (P0013C; Beyotime Institute of Biotechnology)
supplemented with 1 mM PMSF, 1 µg/ml aprotinin (Sigma-Aldrich,
Merck KGaA), and 0.5 µg/ml leupeptin (Sigma-Aldrich, Merck KGaA).
Total protein concentrations were measured using the Micro BCA™
Protein Assay kit (20151201; Nanjing Jiancheng Bioengineering
Institute), and bovine serum albumin was used as standard (16). The gel kit was purchased from Life
iLab China (www.life-ilab.com), which can detect
protein from 10–250 kDa. Equal amounts of cell lysate (20 µg of
protein) were separated via SDS-PAGE, transferred onto
polyvinylidene difluoride membranes and blocked with 5% skimmed
milk in TBST buffer (10 mM Tris-HCl, 150 mM NaCl and 0.1% Tween-20;
pH 8.0) for 1 h at room temperature. The membranes were incubated
with primary antibodies against Top1 (1:10,000; cat. no. ab109374;
Abcam), tyrosyl DNA phosphodiesterase 1 (Tdp1; 1:1,000; cat. no.
2360; Cell Signaling Technology, Inc.), Chk1 (cat. no. 59710; Cell
Signaling Technology, Inc.), Bax (1:10,000; cat. no. ab32503;
Abcam), Bcl-2 (1:1,000; cat. no. ab32124; Abcam) and β-actin
(1:5,000; cat. no. ab8227; Abcam) overnight at 4°C. Membranes were
washed three times with distilled water and subsequently incubated
with horseradish peroxidase-conjugated goat anti-rabbit IgG
secondary antibody (1:3,000; L3001; Signalway Antibody LLC) for 2 h
at room temperature. Protein bands were visualized using the
enhanced Phototope TM-HRP Detection kit (Cell Signaling Technology,
Inc.) and captured using a FluorChem E Imaging system
(ProteinSimple). Relative protein expression was measured by
densitometry using ImageJ 1.49v software (National Institutes of
Health).
Reverse transcription-quantitative
(RT-q)PCR
mRNA expression levels of Top1, Tdp1, Chk1, Bax and
Bcl-2 were analyzed via RT-qPCR analysis. Confluent monolayer cells
in 10-cm diameter dishes were extracted using TRIzol®
reagent. First strand cDNA was synthesized from 1 µg total RNA
using the M-MLV First Strand kit and the genes of interest were
amplified using the SYBR® Premix Select Master Mix kit
(Thermo Fisher Scientific, Inc.) and an ABI PRISM® 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The primer sequences used for qPCR are listed in Table I. The following thermocycling
conditions were used for qPCR: 50°C for 2 min, 95°C for 2 min,
followed by 40 cycles at 95°C for 15 sec, 56°C for 15 sec and 72°C
for 1 min. Specificity of each reaction was verified by the melt
curve stage, at 95°C for 15 sec, 55°C for 1 min and 95°C for 30
sec. Relative mRNA levels of the genes of interest in each group
were calculated using the 2−ΔΔCq method
[ΔCq=Cqtarget gene-Cqβ-actin;
-ΔΔCq=-(ΔCqsample-ΔCqcontrol)], as the mean
value of three independent samples determined in triplicates, and
normalized to the internal reference gene β-actin (17).
 | Table I.Sequences of oligos used for
quantitative PCR. |
Table I.
Sequences of oligos used for
quantitative PCR.
| Gene | Forward primer | Reverse primer |
|---|
| Top1 |
5′-TGACAGCCCCGGATGAGA-3′ |
5′-TGCAACAGCTCGATTGGC-3′ |
| TdpI |
5′-GCAGCAGCATCATTTTCGTGT-3′ |
5′-GCTTGTGCATGGTGATAAGCG-3′ |
| Chk1 |
5′-GGCTCTGGGGAATCCTGGTGAATATAGTGCTGC-3′ |
5′-GGCTCTGGGGAATCCTGGTGAATATAGTGCTGC-3′ |
| Bax |
5′-AGGATGCGTCCACCAAGAAG-3′ |
5′-TGAAGTTGCCGTCAGAAAACA-3′ |
| Bcl-2 |
5′-ATGTGTGTGGAGAGCGTCAACC-3′ |
5′-TGAGCAGAGTCTTCAGAGACAGCC-3′ |
| β-actin |
5′-CACCTTCTACAATGAGCTGCGTGTG-3′ |
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ |
DNA agarose gel electrophoresis
To assess DNA fragmentation, MCF-7 and HCT-116 cells
were treated with a compound (irinotecan, cisplatin or GA-13315) at
the concentration of IC50 for 48 h and subsequently
subjected to agarose gel electrophoresis, following the reported
protocol (18). Briefly, cells were
harvested and washed twice with ice-cold PBS. A total of
1×105 cells were suspended in 130 µl of 0.7% low
melting-point agarose and subsequently layered on a fully frosted
slide. The slides were pre-coated with 80 µl of 1% normal
melting-point agarose, which was set aside to solidify. The slides
were incubated in freshly prepared alkaline lysis buffer [2.5 mM
NaCl (20190104; Guangdong Guanghua Sci-Tech Co Ltd China), 100 mM
Na2-EDTA (Invitrogen; Thermo Fisher Scientific, Inc.),
10 mM Tris-HCl (20180919; Beijing Solarbio Science & Technology
Co., Ltd.), 1% sodium lauroylsarcosinate (P1293625; Adamas-Beta,
Ltd., 1% Triton X-100 (Beyotime Institute of Biotechnology) and 10%
DMSO (201508; Amresco, LLC; pH 10] for 1 h at 4°C in the dark.
Subsequently, the slides were immersed in electrophoresis buffer [1
mM Na2-EDTA (Invitrogen; Thero Fisher Scientific, Inc.)
and 300 mM NaOH (pH 13) (1304282; Xilong Scientific Co., Ltd.
(http://www.xlhg.com)] for 30 min at room
temperature and subjected to electrophoresis at 25 V for 30 min.
The slides were rinsed with 0.4 M Tris buffer (pH 7.5), stained
with DAPI (1 µg/ml) at room temperature for 30 sec and observed
under a fluorescence microscope (DMI300B; Leica Microsystems GmbH,
magnification, ×40).
Top1 assay
Inhibition of Top1 was assessed using the TopoGEN
Topoisomerase I Drug Screening kit (cat. no. 18FB14, http://www.topogen.com), according to the
manufacturer's protocol. Briefly, 0.2 µg of the supercoiled plasmid
DNA (pHOT1) substrate was incubated with 4 units of Top1 enzymes,
in the presence or absence of GA-13315, in Top1 reaction buffer for
30 min at 37°C. Reactions were terminated by adding 10% SDS
(322R032; Beijing Solarbio Science & Technology Co., Ltd.)
followed by treatment with proteinase K (D00091408; Calbiochem,
Inc.). Samples were mixed with loading buffer, loaded onto a 1%
agarose gel and electrophoresis was performed at 25 V for 4 h in
TAE buffer (pH 8.0) (Sigma-Aldrich; Merck KGaA). Gels were stained
with 0.5 µg/ml ethidium bromide at room temperature for 30 min
(cat. no. 0492; Invitrogen; Thermo Fisher Scientific, Inc.) and
rinsed with distilled water. DNA was visualized under a UV lamp and
captured using a FluorChem E Imaging System (ProteinSimple). For
determination of Top1-mediated DNA cleavage, another set of samples
of similar reactions were loaded onto a 1% agarose gel containing
0.5 µg/ml ethidium bromide, and electrophoresed in order to resolve
nicked DNA from supercoiled or relaxed DNA (19). Camptothecin (0.1 mM) was used as the
positive control.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 5.0; GraphPad Software, Inc.). Data are
presented as the mean ± standard deviation. One-way ANOVA followed
by Tukey's post-hoc test was used to compare differences between
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sensitivity changes following exposure
to GA-13315
The sensitivity of MCF-7 and HCT116 cells to
GA-13315, irinotecan and cisplatin, before and after chronic
GA-13315 exposure was assessed (Fig.
S1 and Table II). The results
demonstrated that exposure to 1 µM GA-13315 for 4 weeks increased
the susceptibility of MCF-7 cells to GA-13315; however, this effect
waned overtime (P<0.01 at 4 weeks vs. P>0.05 at 8 and 12
weeks, respectively). Notably, exposure to higher concentrations of
GA-13315 (2 and 4 µM) did not alter the susceptibility of MCF-7
cells to this compound. However, the sensitivity of MCF-7 cells to
irinotecan enhanced in a time-dependent manner following chronic
GA-13315 exposure (1 µM), as the IC50 of irinotecan
decreased significantly from 4 weeks (P<0.001 vs. 0 weeks) and
decreased even more at 8 and 12 weeks, respectively (P<0.001 vs.
4 weeks). The increase in the concentration of GA-13315 did not
incur stronger cytotoxicity of irinotecan. The sensitivity of MCF-7
cells to cisplatin remained unchanged by GA-13315, regardless of
the exposure time and concentration.
 | Table II.Change in sensitivity of MCF-7 and
HCT116 cells following chronic GA-13315 exposure. |
Table II.
Change in sensitivity of MCF-7 and
HCT116 cells following chronic GA-13315 exposure.
| A, MCF-7 cells |
|---|
|
|---|
|
| IC50 ±
standard deviation, µM (sensitizing fold) |
|---|
|
|
|
|---|
| GA-13315
concentration | GA-13315 | Irinotecan | Cisplatin |
|---|
| 1 µM |
| Week
0 | 36.70±2.39 | 35.58±0.75 | 5.10±0.54 |
| Week
4 | 30.12±1.72
(1.2)a | 21.89±2.20
(1.6)b | 5.45±0.91
(0.9) |
| Week
8 | 40.27±0.43
(0.9)c | 16.63±1.50
(2.1)b,c | 4.41±0.30
(1.4) |
| Week
12 | 38.73±1.87
(0.9)c | 15.19±0.53
(2.3)b,c | 5.29±0.27
(1.0) |
| 2 µM |
| Week
12 | 35.78±1.40
(1.0) | 15.13±1.25
(2.4)b | 4.88±0.38
(1.0) |
| 4 µM |
| Week
12 | 37.25±4.35
(1.0) | 14.11±0.78
(2.5)b | 5.61±0.40
(0.9) |
|
| B, HCT116
cells |
|
|
| IC50
± standard deviation, µM (sensitizing fold) |
|
|
|
| GA-13315
concentration |
GA-13315 |
Irinotecan |
Cisplatin |
|
| 1 µM |
| Week
0 | 7.37±0.83 | 1.52±0.02 | 6.25±1.09 |
| Week
4 | 7.47±0.41
(1.0) | 2.29±0.24
(0.7) | 6.11±0.40
(1.0) |
| Week
8 | 6.57±0.34
(1.1) | 5.74±1.66
(0.3)a,c | 9.94±1.82
(0.6)c,d |
| Week
12 | 7.95±0.10
(0.9) | 4.54±0.68
(0.4)d | 12.58±0.73
(0.5)b,c,e |
Following chronic exposure to GA-13315, sensitivity
of HCT116 cells remained unchanged; however, compared with MCF-7
cells, HCT116 cells acquired resistance following long-term
exposure to irinotecan (8 weeks vs. 0 weeks, P<0.01; 12 weeks
vs. 0 weeks, P<0.05). GA-13315 exposure also conferred HCT116
resistance to cisplatin (P<0.05, 8 and 12 weeks vs. 0 weeks) and
the degree of resistance was associated with exposure time
(P<0.05, 8 and 12 weeks vs. 0 and 12 weeks vs. 8 weeks).
Alterations in expression of proteins
involved in chemosensitivity following exposure to GA-13315
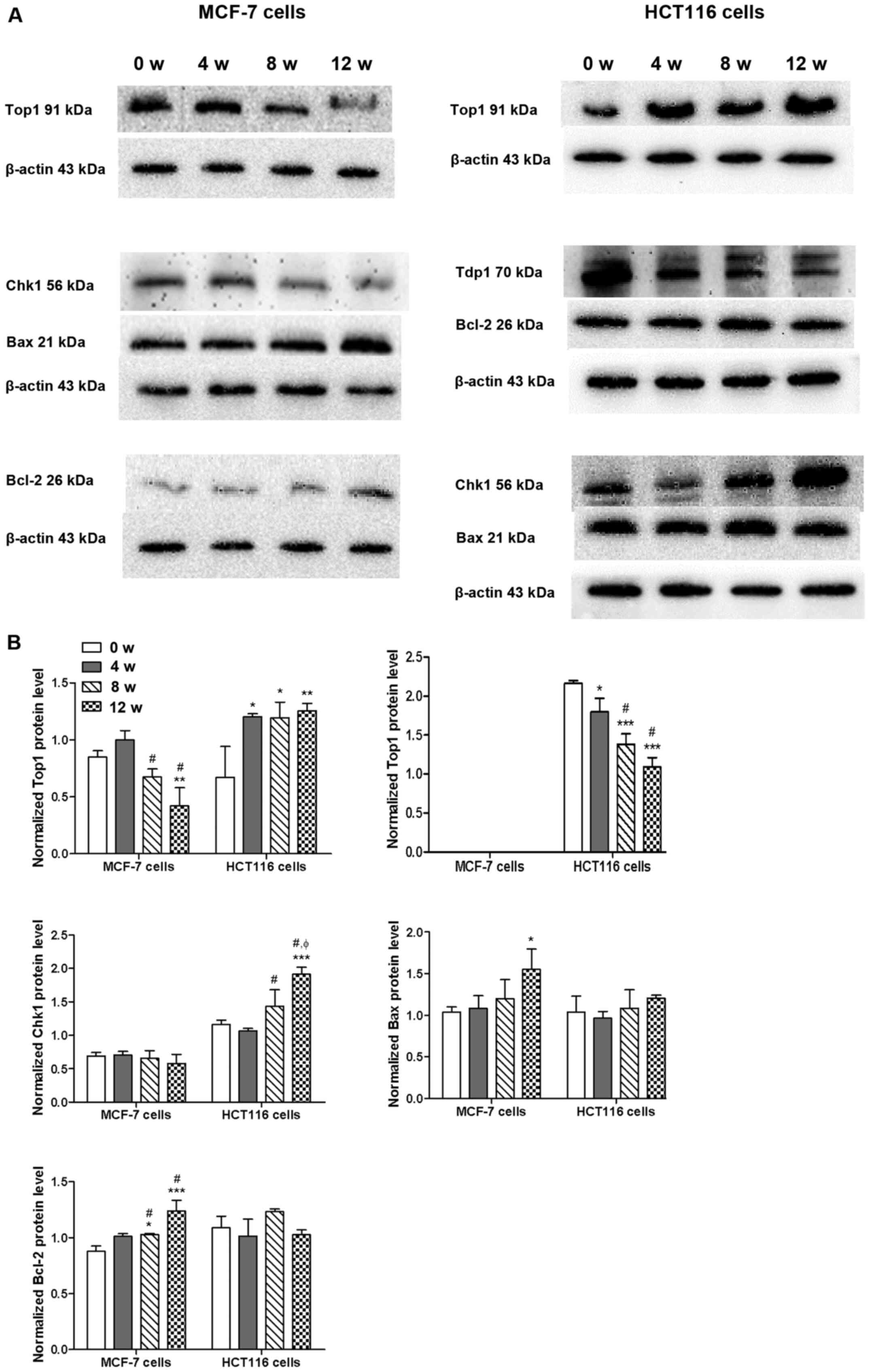
As presented in Fig.
2, western blot analysis demonstrated that chronic GA-13315
exposure caused a time-dependent decrease of Top1 protein
expression in MCF-7 cells, whereas the same regimen increased Top1
protein expression in HCT116 cells and downregulated Tdp1 protein
expression. However, Tdp1 protein expression was not observed in
MCF-7 cells. Chk1 expression did not change in MCF-7 cells
following exposure to GA-13315; however, Chk1 expression levels
increased in a time-dependent manner in HCT116 cells. Bax protein
expression increased in both MCF-7 and HCT116 cells; however,
different peak times were exhibited (12 weeks for MCF-7 cells vs. 8
weeks for HCT116 cells). Bcl-2 protein expression also increased in
a time-dependent manner in MCF-7 cells; however, Bcl-2 expression
remained unchanged in HCT-116 cells following chronic GA-13315
exposure.
mRNA expression of the genes involved
in chemosensitivity following exposure to GA-13315
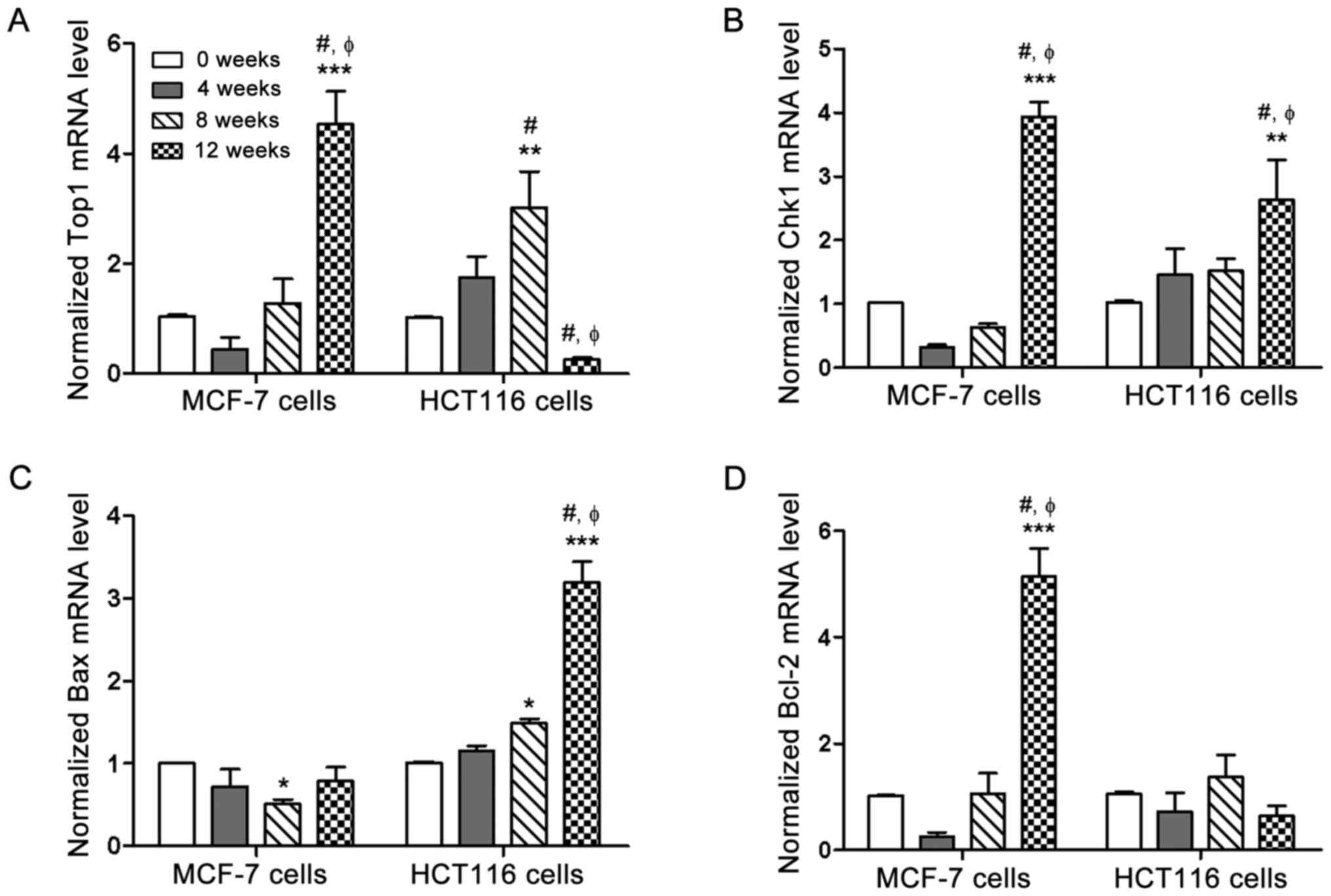
As presented in Fig.
3, Top1 and Chk1 mRNA expression levels in HCT116 cells were
consistent with the protein expression patterns; however, Top1 and
Chk1 mRNA expression levels both increased in a time-dependent
manner in MCF-7 cells following chronic GA-13315 exposure, which
was disassociated with the protein expression. As for Bax and
Bcl-2, the changes in mRNA expression levels were in accordance
with that of its protein expression patterns in HCT116 cells;
however, Bax mRNA expression decreased, while Bax protein
expression increased, and Bcl-2 mRNA expression increased, while
Bcl-2 protein expression remained unchanged in MCF-7 cells. The
chronic exposure of GA-13315 on MCF-7 and HCT116 cells was up to 12
weeks, and 4 time points (0, 4, 8 and 12 weeks) were monitored to
depict the changes of relevant mRNA and protein levels. Top1 mRNA
expression significantly decreased at 12 weeks compared with 0
week. Bax mRNA expression gradually increased and only
significantly changed at 12 weeks; however, this was not reflected
on protein expression level. The chronic exposure of GA-13315 on
MCF-7 and HCT116 cells did not significantly affect the apoptosis
pathway, but affected the translation process, as the mRNA and
protein expression levels were inconsistent.
Effect of GA-13315 on DNA integrity of
tumor cells
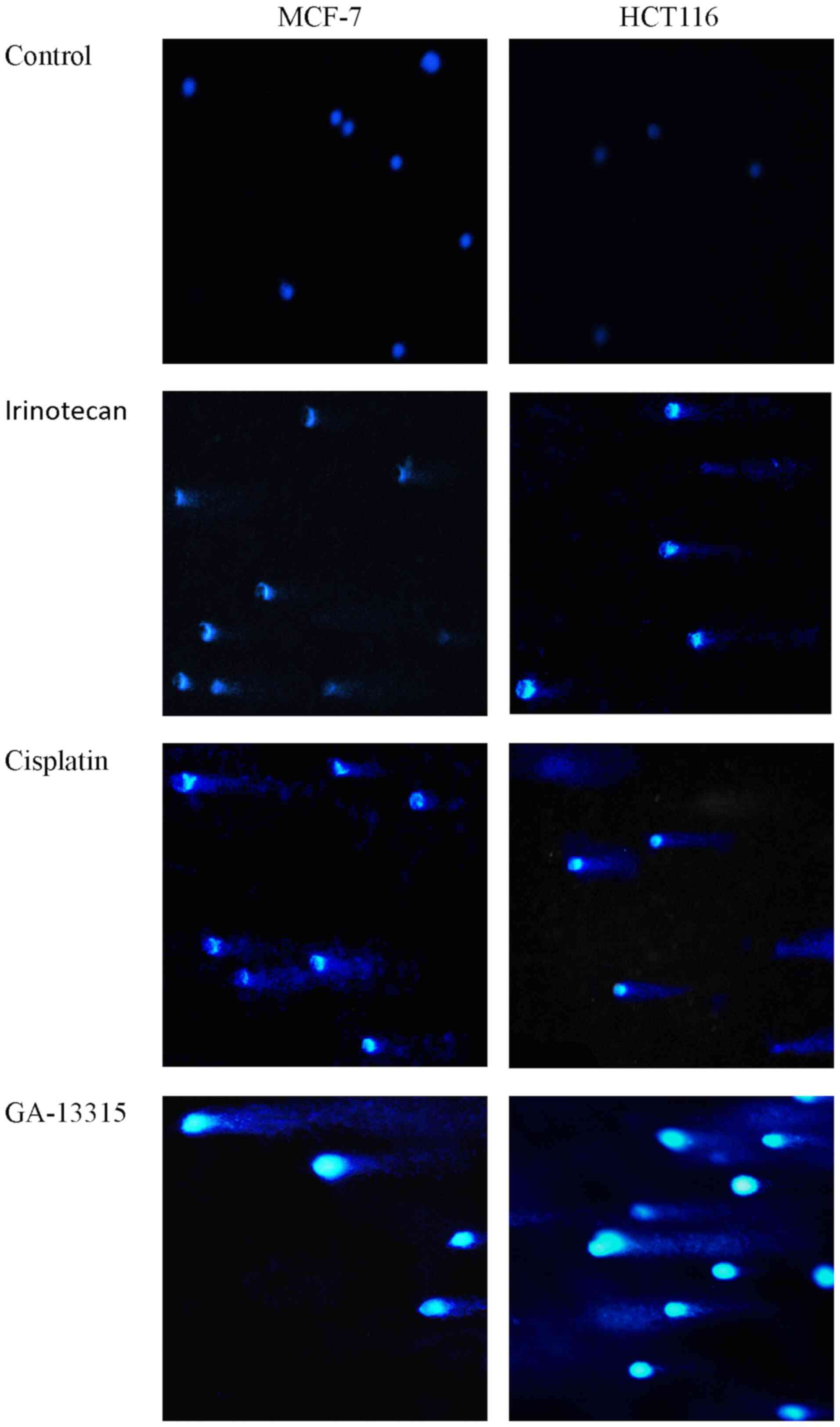
To determine the effect of GA-13315 on DNA
integrity, MCF-7 and HCT116 cells were treated with GA-13315 for 48
h and subsequently subjected to single-cell agarose gel
electrophoresis. As presented in Fig.
4, GA-13315 caused DNA fragmentation, as did the Top1 poison
irinotecan and the DNA alkylating agent cisplatin, in both MCF-7
and HCT116 cells, which was manifested by visible comet tail-like
features following electrophoresis.
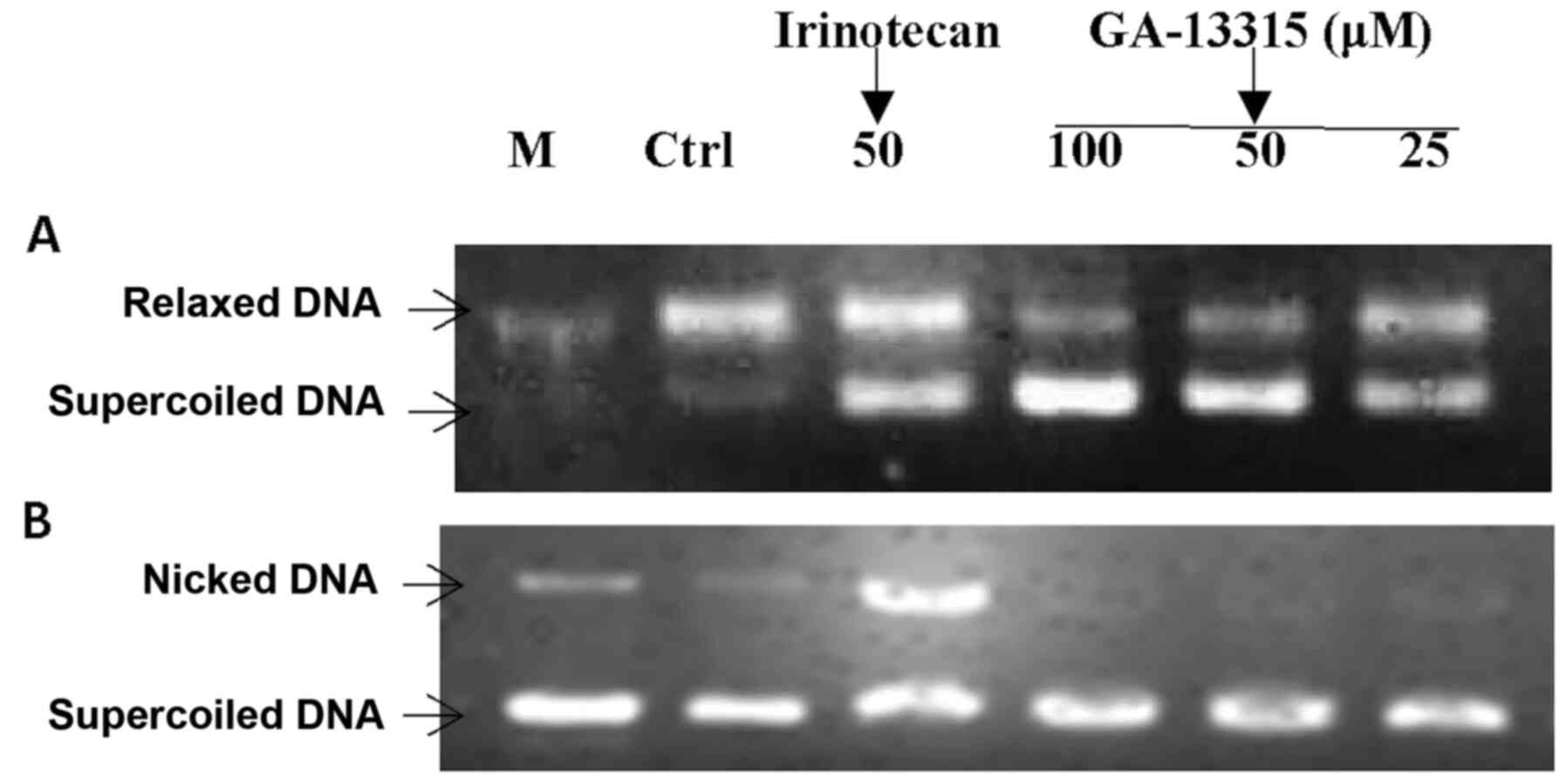
Effect of GA-13315 on Top1
activity
The effects of GA-13315 on the catalytic activity of
Top1 and Top1-mediated DNA cleavage were determined via the Top1
relaxation assay. As presented in Fig.
5, GA-13315 inhibited the catalytic activity of Top1 in a
manner similar to the reference drug irinotecan, as demonstrated by
the decreased amount of relaxed DNA and increased amount of
supercoiled DNA. Although irinotecan inhibited DNA cleavage
activity of Top1, which was indicated by the formation of nicked
DNA in the presence of 50 µM irinotecan, GA-13315 did not affect
DNA cleavage mediated by Top1 at the highest concentration (100
µM). This suggests that the mechanism of inhibition of GA-13315 on
Top1 differs from that of irinotecan.
Discussion
In our previous study, GA-13315, a cytotoxic
compound that exhibits selectivity to the ABCB1-overexpressing
multidrug-resistant MCF-7/ADR cells when administered alone
(14), was evidenced to reverse the
resistance of MCF-7/ADR cells when administered at subtoxic doses
in combination with several chemotherapeutic agents (14). Following this finding, the present
study aimed to investigate the potential of GA-13315 being used as
an auxiliary agent to conventional chemotherapy, and was designed
to evaluate the influence of long-term treatment with GA-13315 (at
low dose) on sensitive tumor cell lines. The results of the present
study demonstrated that chronic exposure to low-dose GA-13315 up to
12 weeks did not render either MCF-7 or HCT116 cells resistant to
GA-13315. Notably, MCF-7 cells became more susceptible to GA-13315
following 4 weeks of exposure; however, this sensitizing effect
receded in a time-dependent manner. Taken together, these results
suggest that GA-13315 may be used in antitumor therapy, as it is
not prone to induce resistance in tumor cells. However, considering
that the exposure time in the present study was only 12 weeks, the
potential of GA-13315 conferring resistance to tumor cells if the
exposure time extends beyond 12 weeks cannot be excluded.
The chronic exposure of GA-13315 on MCF-7 and HCT116
cells had different effects, as their sensitivities to irinotecan
and cisplatin were inconsistent. The results of the present study
demonstrated that GA-13315 did not alter the sensitivity of either
MCF-7 or HCT116 cells to cisplatin, but increased the sensitivity
of MCF-7 cells to the Top1 poison irinotecan, while rendering
HCT-116 cells more resistant to cisplatin. Collectively, these
results suggest that the alteration of the chemosensitivity of
tumor cells by GA-13315 may be associated with Top1
activity-mediated mechanisms. Top1 removes DNA supercoils during
transcription and replication by cutting a single strand of DNA to
allow relaxation of torsional stresses and subsequent reannealing
(20,21). An intermediate, known as the cleavage
complex, consisting of Top1 covalently attached to the cleaved DNA,
is formed transiently in this process (22–24).
This cleavage complex is stabilized by irinotecan (in the form of
active metabolite SN38) (25), which
collides with advancing replication forks, resulting in single
strand breaks in DNA, eventually leading to cell cycle arrest and
cell death (26,27). Top1 poison-mediated DNA damage can be
repaired by hydrolysis of the phosphodiester bond between Top1 and
the 3′-phosphate of DNA to disassemble the stalled cleavage
complex, and this process is catalyzed by Tdp1 (28,29).
Top1 poison also elicits activation of the Chk1 pathway, which is
efficiently coupled with DNA repair to prevent further
replication-dependent DNA damage (30–32).
Elevation of Top1 mRNA, protein and catalytic activity in human
tumors have been reported, with particularly high Top1 expression
in colorectal cancer, while Tdp1 exhibits significantly higher
expression levels in non-small cell lung cancer and breast cancer
tissues compared with normal tissues (26,33,34).
Upon Top1 poison exposure, Top1 expression decreases in tumor
cells, which induces drug resistance to Top1 poisons in human
glioblastoma cells (26).
Suppression of Tdp1 results in cellular defects in the repair of
Top1-mediated DNA breaks (28,29).
Tdp1-knockout mice are viable but hypersensitive to irinotecan
(35). Irinotecan also induces Chk1
degradation (36), while
downregulation of Chk1 potentiates the cytotoxicity of irinotecan
in tumor cells, such as A549 lung carcinoma cells, HeLa cells and
MCF-7 cells (30–32,36). The
present study assessed the expression levels of Top1 in both MCF-7
and HCT116 cells. The results demonstrated that the sensitivity of
GA-13315-exposed cells to irinotecan was associated with Top1. The
protein expression of Top1 was upregulated in MCF-7 cells as the
sensitivity of MCF-7 cells to irinotecan increased. Conversely,
Top1 protein expression was downregulated in HCT116 cells. Elevated
Top1 expression coincided with decreased Tdp1 expression in HCT116
cells; however, Tdp1 expression was not detected in MCF-7 cells,
both before or after chronic GA-13315 exposure. Chk1 expression
increased in HCT116 cells following GA-13315 exposure, which
coincided with the enhanced resistance of HCT116 cells to
irinotecan; however, these resistant effects were not observed in
MCF-7 cells. Taken together, these results suggest that the
sensitivity of tumor cells to irinotecan may be in inverse ratio to
Top1 expression in cell lines that express low levels of Tdp1 or
Chk1. Although GA-13315 was not able to sensitize HCT116 cells to
irinotecan, the cytotoxicity of GA-13315 itself to HCT116 cells and
the maintenance of sensitivity of HCT116 cells to GA-13315 upon
chronic exposure may be attributed to the downregulation of Tdp1
caused by GA-13315. In addition, as GA-13315 exposure resulted in
increased Top1 and Chk1 expression in HCT116 cells, GA-13315 may
function through a mechanism different from that of irinotecan,
which has been reported to decrease Top1 and Chk1 expression levels
in tumor cells (30,35).
In order to determine the underlying molecular
mechanisms of GA-13315, the present study investigated the effect
of GA-13315 on DNA integrity by performing single-cell agarose gel
electrophoresis. Short-term (48-h) GA-13315 treatment at a
relatively high concentration (IC50) resulted in DNA
fragmentation, as did treatment with the reference drugs irinotecan
and cisplatin; however, the results failed to determine whether DNA
damage was the consequence of the direct interaction of GA-13315
with DNA or if it was mediated by Top1. The in vitro Top1
assay demonstrated that GA-13315 was not Top1 poison, but actually
inhibited the catalytic activity of Top1, which supported the
initial hypothesis that GA-13315 has different mechanisms of action
from irinotecan.
Given that the apoptotic pathway plays a substantial
role in the alterations of chemosensitivity of tumor cells, the
present study assessed the changes in expression levels of the
proapoptotic factor, Bax, and the antiapoptotic regulator, Bcl-2
(37–39). No significant alteration in the ratio
of Bax/Bcl-2 was observed, which indicates the activity of the
apoptotic pathway (40). This
implies that the mechanisms of action of chronic GA-13315 exposure
may be different from that of the apoptotic pathways. Notably, in
the MCF-7 cell line, which was sensitized to irinotecan by
GA-13315, the changes in mRNA expression levels were uncoupled with
that of the protein expression levels for Top1, Chk1 and Bax. This
imbalance between gene and protein expression levels may be caused
by the potential effects of GA-13315 on the mRNA (half-life)
stability (41–43). This phenomenon was not observed in
HCT116 cells, thus the sensitizing effect of GA-13315 was probably
associated with the disruption of the protein translation process;
however, this hypothesis requires further investigation.
In the present study, GA-13315 failed to induce drug
resistance in either MCF-7 or HCT116 cells when administered at a
subtoxic dose for 12 weeks. Chronic GA-13315 exposure was able to
potentiate the cytotoxicity of the Top1 poison irinotecan to MCF-7
cells, which barely express Tdp1; however, this effect was not
observed in HCT116 cells. The sensitivity of cells to irinotecan
following GA-13315 exposure was in direct ratio to Top1 expression,
while in inverse ratio to the expression levels of Tdp1 and Chk1.
Mechanistic analysis demonstrated that GA-13315 caused DNA damage,
but not via a Top1-dependent manner, as GA-13315 was not a Top1
poison despite inhibiting the catalytic activity of Top1. As the
responses of different cell lines upon chronic GA-13315 exposure to
chemotherapy drugs were inconsistent, GA-13315 may not be used
universally as an adjuvant to chemotherapy; however, the mechanism
of its influence on the sensitivity and resistance of tumor cell
lines merits further investigation in light of discovering new
pathways and strategies to overcome drug resistance in cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Yunnan Province, China (grant no. 2017FE468),
the National Natural Science Foundation of China (grant nos.
81460559 and 81160405) and the Ding Jian Academician Workstation
and Collaborative Innovation Center for Natural Products and
Biological Drugs of Yunnan of China (grant no. YSGZZ201310).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
CQ and JM designed the present study. XC, GW and YL
performed the experiments. JM and CQ analyzed the data. JM and XC
drafted the initial manuscript. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ABC
|
adenosine triphosphate-binding
cassette
|
|
Top1
|
topoisomerase I
|
|
Tdp1
|
tyrosyl DNA phosphodiesterase 1
|
|
Chk1
|
checkpoint kinase 1
|
References
|
1
|
Schoeffler AJ and Berger JM: DNA
topoisomerases: Harnessing and constraining energy to govern
chromosome topology. Q Rev Biophys. 41:41–101. 2008.PubMed/NCBI
|
|
2
|
Li TK and Liu LF: Tumor cell death induced
by topoisomerase-targeting drugs. Annu Rev Pharmacol Toxicol.
41:53–77. 2001.PubMed/NCBI
|
|
3
|
Wu CP, Calcagno AM and Ambudkar SV:
Reversal of ABC drug transporter-mediated multidrug resistance in
cancer cells: Evaluation of current strategies. Curr Mol Pharmacol.
1:93–105. 2008.PubMed/NCBI
|
|
4
|
Velasquez WS, Lew D, Grogan TM,
Spiridonidis CH, Balcerzak SP, Dakhil SR, Miller TP, Lanier KS,
Chapman RA and Fisher RI; Southwest Oncology Group, : Combination
of fludarabine and mitoxantrone in untreated stages III and IV
low-grade lymphoma: S9501. J Clin Oncol. 21:1996–2003.
2003.PubMed/NCBI
|
|
5
|
Gotwals P, Cameron S, Cipolletta D,
Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S,
Sabatos-Peyton C, Petruzzelli L, et al: Prospects for combining
targeted and conventional cancer therapy with immunotherapy. Nat
Rev Cancer. 17:286–301. 2017.PubMed/NCBI
|
|
6
|
Johnson BE: Concurrent approaches to
combined chemotherapy and chest radiotherapy for the treatment of
patients with limited stage small cell lung cancer. Lung Cancer. 10
(Suppl 1):S281–S287. 1994.PubMed/NCBI
|
|
7
|
Zhu Y, Liu C, Armstrong C, Lou W, Sandher
A and Gao AC: Antiandrogens inhibit ABCB1 Efflux and ATPase
activity and reverse docetaxel resistance in advanced prostate
cancer. Clin Cancer Res. 21:4133–4142. 2015.PubMed/NCBI
|
|
8
|
Palmeira A, Sousa E, Vasconcelos MH and
Pinto MM: Three decades of P-gp inhibitors: Skimming through
several generations and scaffolds. Curr Med Chem. 19:1946–2025.
2012.PubMed/NCBI
|
|
9
|
Chen Z, Shi T, Zhang L, Zhu P, Deng M,
Huang C, Hu T, Jiang L and Li J: Mammalian drug efflux transporters
of the ATP binding cassette (ABC) family in multidrug resistance: A
review of the past decade. Cancer Lett. 370:153–164.
2016.PubMed/NCBI
|
|
10
|
Yuan H, Li X, Wu J, Li J, Qu X, Xu W and
Tang W: Strategies to overcome or circumvent P-glycoprotein
mediated multidrug resistance. Curr Med Chem. 15:470–476.
2008.PubMed/NCBI
|
|
11
|
Bomke C and Tudzynski B: Diversity,
regulation, and evolution of the gibberellin biosynthetic pathway
in fungi compared to plants and bacteria. Phytochemistry.
70:1876–1893. 2009.PubMed/NCBI
|
|
12
|
Chen J, Sun Z, Zhang Y, Zeng X, Qing C,
Liu J, Li L and Zhang H: Synthesis of gibberellin derivatives with
anti-tumor bioactivities. Bioorg Med Chem Lett. 19:5496–5499.
2009.PubMed/NCBI
|
|
13
|
Zhang Y, Zhang H, Chen J, Zhao H, Zeng X,
Zhang H and Qing C: Antitumor and antiangiogenic effects of
GA-13315, a gibberellin derivative. Invest New Drugs. 30:8–16.
2012.PubMed/NCBI
|
|
14
|
Mo J, Kang M, Ye JX, Chen JB, Zhang HB and
Qing C: Gibberellin derivative GA-13315 sensitizes
multidrug-resistant cancer cells by antagonizing ABCB1 while
agonizes ABCC1. Cancer Chemother Pharmacol. 78:51–61.
2016.PubMed/NCBI
|
|
15
|
Das SG, Hermanson DL, Bleeker N, Lowman X,
Li Y, Kelekar A and Xing C: Ethyl
2-amino-6-(3,5-dimethoxyphenyl)-4-
(2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (CXL017): A novel
scaffold that resensitizes multidrug resistant leukemia cells to
chemotherapy. ACS Chem Biol. 8:327–335. 2013.PubMed/NCBI
|
|
16
|
Walker JM: The bicinchoninic acid (BCA)
assay for protein quantitation. Methods Mol Biol. 32:5–8.
1994.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI
|
|
18
|
Sabisz M, Wesierska-Gadek J and
Skladanowski A: Increased cytotoxicity of an unusual DNA
topoisomerase II inhibitor compound C-1305 toward HeLa cells with
downregulated PARP-1 activity results from re-activation of the p53
pathway and modulation of mitotic checkpoints. Biochem Pharmacol.
79:1387–1397. 2010.PubMed/NCBI
|
|
19
|
Patra N, De U, Kang JA, Kim JM, Ahn MY,
Lee J, Jung JH, Chung HY, Moon HR and Kim HS: A novel epoxypropoxy
flavonoid derivative and topoisomerase II inhibitor, MHY336,
induces apoptosis in prostate cancer cells. Eur J Pharmacol.
658:98–107. 2011.PubMed/NCBI
|
|
20
|
Champoux JJ: DNA topoisomerases:
Structure, function, and mechanism. Annu Rev Biochem. 70:369–413.
2001.PubMed/NCBI
|
|
21
|
Peterson KE, Cinelli MA, Morrell AE, Mehta
A, Dexheimer TS, Agama K, Antony S, Pommier Y and Cushman M:
Alcohol-, diol-, and carbohydrate-substituted indenoisoquinolines
as topoisomerase I inhibitors: Investigating the relationships
involving stereochemistry, hydrogen bonding, and biological
activity. J Med Chem. 54:4937–4953. 2011.PubMed/NCBI
|
|
22
|
Chen AY and Liu LF: DNA topoisomerases:
Essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol.
34:191–218. 1994.PubMed/NCBI
|
|
23
|
Hsiang YH, Lihou MG and Liu LF: Arrest of
replication forks by drug-stabilized topoisomerase I-DNA cleavable
complexes as a mechanism of cell killing by camptothecin. Cancer
Res. 49:5077–5082. 1989.PubMed/NCBI
|
|
24
|
Liu LF, Desai SD, Li TK, Mao Y, Sun M and
Sim SP: Mechanism of action of camptothecin. Ann NY Acad Sci.
922:1–10. 2000.PubMed/NCBI
|
|
25
|
Irinotecan, . Drugs and Lactation Database
(LactMed) National Library of Medicine (US). Bethesda, MD: 2006
|
|
26
|
Gilbert DC, Chalmers AJ and El-Khamisy SF:
Topoisomerase I inhibition in colorectal cancer: Biomarkers and
therapeutic targets. Br J Cancer. 106:18–24. 2012.PubMed/NCBI
|
|
27
|
Li F, Jiang T, Li Q and Ling X:
Camptothecin (CPT) and its derivatives are known to target
topoisomerase I (Top1) as their mechanism of action: Did we miss
something in CPT analogue molecular targets for treating human
disease such as cancer? Am J Cancer Res. 7:2350–2394.
2017.PubMed/NCBI
|
|
28
|
El-Khamisy SF, Hartsuiker E and Caldecott
KW: TDP1 facilitates repair of ionizing radiation-induced DNA
single-strand breaks. DNA Repair (Amst). 6:1485–1495.
2007.PubMed/NCBI
|
|
29
|
El-Khamisy SF, Saifi GM, Weinfeld M,
Johansson F, Helleday T, Lupski JR and Caldecott KW: Defective DNA
single-strand break repair in spinocerebellar ataxia with axonal
neuropathy-1. Nature. 434:108–113. 2005.PubMed/NCBI
|
|
30
|
Pommier Y, Barcelo JM, Rao VA, Sordet O,
Jobson AG, Thibaut L, Miao ZH, Seiler JA, Zhang H, Marchand C, et
al: Repair of topoisomerase I-mediated DNA damage. Prog Nucleic
Acid Res Mol Biol. 81:179–229. 2006.PubMed/NCBI
|
|
31
|
Feijoo C, Hall-Jackson C, Wu R, Jenkins D,
Leitch J, Gilbert DM and Smythe C: Activation of mammalian Chk1
during DNA replication arrest: A role for Chk1 in the intra-S phase
checkpoint monitoring replication origin firing. J Cell Biol.
154:913–923. 2001.PubMed/NCBI
|
|
32
|
Zachos G, Rainey M and Gillespie DA:
Lethal errors in checkpoint control-life without Chk1. Cell Cycle.
2:14–16. 2003.PubMed/NCBI
|
|
33
|
Liu C, Zhou S, Begum S, Sidransky D,
Westra WH, Brock M and Califano JA: Increased expression and
activity of repair genes TDP1 and XPF in non-small cell lung
cancer. Lung Cancer. 55:303–311. 2007.PubMed/NCBI
|
|
34
|
Dean RA, Fam HK, An J, Choi K, Shimizu Y,
Jones SJ, Boerkoel CF, Interthal H and Pfeifer TA: Identification
of a putative Tdp1 inhibitor (CD00509) by in vitro and cell-based
assays. J Biomol Screen. 19:1372–1382. 2014.PubMed/NCBI
|
|
35
|
Pommier Y and Cushman M: The
indenoisoquinoline noncamptothecin topoisomerase I inhibitors:
Update and perspectives. Mol Cancer Ther. 8:1008–1014.
2009.PubMed/NCBI
|
|
36
|
Zhang YW, Otterness DM, Chiang GG, Xie W,
Liu YC, Mercurio F and Abraham RT: Genotoxic stress targets human
Chk1 for degradation by the ubiquitin-proteasome pathway. Mol Cell.
19:607–618. 2005.PubMed/NCBI
|
|
37
|
Walensky LD: BCL-2 in the crosshairs:
Tipping the balance of life and death. Cell Death Differ.
13:1339–1350. 2006.PubMed/NCBI
|
|
38
|
Tsukahara S, Yamamoto S, Tin-Tin-Win-Shwe,
Ahmed S, Kunugita N, Arashidani K and Fujimaki H: Inhalation of
low-level formaldehyde increases the Bcl-2/Bax expression ratio in
the hippocampus of immunologically sensitized mice.
Neuroimmunomodulation. 13:63–68. 2006.PubMed/NCBI
|
|
39
|
Xu C, Xu W, Palmer AE and Reed JC: BI-1
regulates endoplasmic reticulum Ca2+ homeostasis
downstream of Bcl-2 family proteins. J Biol Chem. 283:11477–11484.
2008.PubMed/NCBI
|
|
40
|
Lo YL and Liu Y: Reversing multidrug
resistance in Caco-2 by silencing MDR1, MRP1, MRP2, and
BCL-2/BCL-xL using liposomal antisense oligonucleotides. PLoS One.
9:e901802014.PubMed/NCBI
|
|
41
|
Liaud N, Horlbeck MA, Gilbert LA, Gjoni K,
Weissman JS and Cate JHD: Cellular response to small molecules that
selectively stall protein synthesis by the ribosome. PLoS Genet.
15:e10080572019.PubMed/NCBI
|
|
42
|
Watters DJ: Ascidian toxins with potential
for drug development. Mar Drugs. 16:1622018.
|
|
43
|
Pavitt GD: Regulation of translation
initiation factor eIF2B at the hub of the integrated stress
response. Wiley Interdiscip Rev RNA. 9:e14912018.PubMed/NCBI
|