Introduction
Gastric cancer remains a major cause of
cancer-associated mortality worldwide (1), and the morbidity and mortality from
this disease often rank in the top three in China (2). The seventh American Joint Committee on
Cancer states that the highest 5-year survival rate of early
gastric cancer (EGC) is 95.1% in three Eastern countries and
America, while that of late gastric cancer is only 58.4% (3). Therefore, it is important to identify
gastric cancer in the early stage. To date, the diagnosis of EGC
mostly depends on endoscopic screening, the Helicobacter
pylori test, pepsinogen analysis, long-term surveillance, and
follow-up for chronic atrophic gastritis. These methods may improve
the diagnostic rate of EGC in a proportion of patients with routine
physical examinations; however, the general population does not
routinely visit clinics unless they are experiencing some
discomfort. A retrospective study, which was undertaken by 10
hospitals and medical agencies in 2009, showed that the diagnostic
rate of EGC was <10% in China (4). However, these data exceeded 50% in
Korea and 80% in Japan at the same time (3,5). By
contrast, by 2017, this diagnostic rate was merely up to 20%
(6). Therefore, it is necessary to
identify a novel method for diagnosing gastric cancer in the early
stage to improve the diagnostic rate of EGC in regions with poor
rates of EGC. In recent years, the number of studies on hereditary
gastric cancer (HGC) has increased. Due to the strong specificity
and germline mutation, HGC may bring significant psychosomatic
pressure to family members of patients with, although the
percentage of HGC is low in the general population. However, the
management of patients with HGC is feasible because germline
mutations in these patients are carried throughout life. Therefore,
establishing management strategies for families with HGC may
improve the early-warning effect of EGC and increase the diagnostic
rate of EGC in the general population, which may be considered a
practical approach in the diagnosis of EGC.
Among all gastric cancers worldwide, ~90% are
sporadic, 10% are familial, and 3–5% have a genetic predisposition
(7–9). Generally, HGC is accompanied by
multiple cancers of different organs, including breast cancer and
colon cancer, which mostly represent hereditary cancer syndromes
(HCS) with their corresponding susceptibility genes (10). Compared with sporadic cancers, HCS
are usually characterized by an early age of cancer onset, which
may be a reason to perform screening for EGCs in the clinic. Jones
(11) reported that gastric cancer
occurred in a New Zealand Maori family for generations in 1964, and
the genetic factors were first presumed to serve a key role in the
occurrence of malignant disease. In 1998, Guilford et al
(12) demonstrated that hereditary
diffuse gastric cancer (HDGC) was associated with CDH1
(encoding E-cadherin) germline mutations for the first time after
analysing the family history of 25 gastric cancers in three
families. Since then, studies on the genetic susceptibility of
gastric cancer have been conducted.
The present review provides an overview of HCS and
its susceptibility genes and further proposes an idea for improving
the overall diagnostic rate (ODR) of EGC in the general population
by managing individuals with family histories of HCS and germline
mutations of susceptibility genes.
Hereditary cancer syndromes
This section describes HCSs that have been commonly
studied clinically to date. HDGC, characterized by autosomal
dominant inheritance, is a type of HCS with a large number of
studies and accounts for ~1–3% of gastric cancers (9). Furthermore, ~25–40% of HDGC family
members carry germline mutations of CDH1 (13–15).
Other susceptibility genes, including CTNNA1, DOT1L, FBXO24,
PRSS1, MAP3K6, MSR1 and INSR, which encode
alpha-E-catenin, histone methyltransferase, f-box protein,
trypsinogen, a serine/threonine protein kinase, the class A
macrophage scavenger receptors and receptor tyrosine kinase,
respectively (16), are rare but are
detected in HDGC in a minority of cases (9). Generally, patients with HDGC also have
colon cancer and breast cancer (17–19),
with a mean onset age of 38 years (20) and most cases occurring before the age
of 40 years, while the youngest age reported is 14 years (12). Chun and Ford (21) suggested that individuals in families
with HDGC who have CDH1 mutations should perform regular
surveillance for stomach and breast cancer from the age of 25
years. In addition, van der Post et al (22) recommended that colonoscopy
surveillance should be performed at the age of 40 years or earlier
for individuals with a positive familial history, and routine
surveillance for breast cancer should be performed at 30 years of
age for CDH1 germline mutation carriers. Secondly, the
susceptibility genes of Lynch syndrome (LS), also termed hereditary
nonpolyposis colorectal cancer, are MLH1, MSH2, MSH6, PMS2
and EPCAM (21,23). The patients diagnosed with this
syndrome usually have a mean age of onset of gastric cancer at 56
years, colon cancer at 44–61 years, and endometrial cancer at 48–62
years (21). Surveillance of
patients with this syndrome includes screening at 30–35 years of
age for stomach and 20–25 years of age for colon (24). Thirdly, juvenile polyposis syndrome
(JPS) presents with multiple gastric polyps in juveniles, and its
susceptibility genes are SMAD4 and BMPR1A (21,24).
Jasperson et al (24)
suggested that surveillance for this disease should begin when
symptoms occur or in the late teens if no symptoms occur. Fourthly,
Peutz-Jeghers syndrome (PJS) is characterized by a germline
mutation in STK11 in 30–80% of patients with this syndrome
(21,24). Previous studies have recommended that
surveillance for this syndrome be initiated from the age of 20, at
8 years for gastroscopy, 20 years for colonoscopy and 25 years for
breast screening (21,24). Furthermore, the susceptibility gene
for familial adenomatous polyposis is APC, and surveillance
should be performed at 10–12 years of age for colonoscopy and 20–25
years of age for gastroscopy for patients who meet the criteria
(21,24). Additionally, breast surveillance for
hereditary breast cancer (HBC) is recommended for patients who
fulfilled the criteria of HBC after the age of 35 years, and the
susceptibility genes of this syndrome are BRCA1 and
BRCA2 (25). Furthermore,
Li-Fraumeni syndrome (LFS) is a significant cancer syndrome with a
high risk of multiple cancer types, including gastric cancer, with
a frequency of up to 2.8% in LFS families, and the mean age of
diagnosing gastric cancer is 43 years (21) (Table
I).
 | Table I.The acronyms or aliases of genes and
their associated information. |
Table I.
The acronyms or aliases of genes and
their associated information.
| Gene | Corresponding
acronym or alias | Corresponding
protein | Corresponding
HCS | (Refs.) |
|---|
| CDH1 | Cadherin
1 | E-cadherin | Gastric cancer,
including HDGC | (9,13–17,19,21) |
| CTNNA1 | Catenin α
1 | α-E-catenin | HDGC, colorectal
cancer |
|
| INSR | Insulin
receptor | Receptor tyrosine
kinase | HDGC |
|
| FBXO24 | F-box protein
24 | F-box protein | HDGC |
|
| DOT1L | Disruptor of
telomeric silencing 1-like | Histone
methyltransferase | HDGC |
|
| MAP3K6 |
Mitogen-activated protein kinase kinase
kinase 6 | Serine/threonine
protein kinase | HDGC |
|
| PRSS1 | Serine protease
1 | Trypsinogen | HDGC |
|
| MSR1 | Macrophage
scavenger receptor 1 | Class A macrophage
scavenger receptor | HDGC |
|
| MLH1 | MutL homolog 1
gene | Mismatch repair
system component | LS | (21,23) |
| MSH2 | MutS homolog
2 | Mismatch repair
system component | LS |
|
| MSH6 | MutS homolog
6 | Mismatch repair
system component | LS |
|
| PMS2 | PMS1 homolog
2 | Mismatch repair
system component | LS |
|
| EPCAM | Epithelial cell
adhesion molecule |
Carcinoma-associated antigen | LS |
|
| SMAD4 | SMAD family
member 4 | Signal transduction
protein | JPS | (21,24) |
| BMPR1A | Bone
morphogenetic protein receptor type 1A | Transmembrane
serine/threonine kinase |
|
|
| STK11 | Serine/Threonine
Kinase 11 | Serine/threonine
kinase | PJS | (21,24) |
| APC | Adenomatous
polyposis coli | Tumor suppressor
protein | FAP | (21,24) |
| BRCA1,
BRCA2 | BRCA1/BRCA2 DNA
repair-associated | Corresponding
proteins encoded by these genes | HBC | (25) |
Generally, the age of onset of malignant diseases in
patients with a familial history of HCS and positive mutations is
earlier than that of sporadic cancers. The youngest age of onset of
gastric cancer in a family with HDGC is 14 years (21), and JPS and PJS were also found in
malignant diseases in clinical patients with juvenile onset.
Therefore, the family members with HCS with germline mutations have
a higher risk of early-onset cancer. Therefore, the individuals
with HCS with germline mutations begin routine surveillance at a
young age to detect malignant diseases, which is recommended in the
National Comprehensive Cancer Network guidelines and the
aforementioned studies (Table II).
Consequently, screening for a positive family history and germline
mutations in these patients will contribute toward confirming
malignant diseases. These diseases developed in the early stage,
which may aid the general population in identifying the onset of
EGC from this management system. Notably, the ODR in EGC may be
improved by managing the family members of HCS.
 | Table II.Susceptibility genes and recommended
starting ages for surveillance of hereditary cancer syndromes. |
Table II.
Susceptibility genes and recommended
starting ages for surveillance of hereditary cancer syndromes.
| Syndrome | Susceptibility
genes | Recommended age for
surveillance, years | (Refs.) |
|---|
| HDGC | CDH1 | 25 (stomach,
breast), 40 (colon) | (8,11–20) |
| LS | MLH1, MSH2,
MSH6, PMS2, EPCAM | 30–35 (stomach),
20–25 (colon) | (17,21,22) |
| JPS | SMAD4,
BMPR1A | Beginning with
symptoms or in late teens if no symptoms occur | (17,22) |
| PJS | STK11 | 20 or 8 (stomach),
20 (colon), 25 (breast) | (17,22) |
| FAP | APC | 10–12 (colon),
20–25 (stomach) | (17,22) |
| HBC |
BRCA1/BRCA2 | 35 (breast) | (23) |
Promoting the diagnostic rate of EGC by
managing the family members of patients with HCS
The diagnostic rate of EGC and the overall survival
rate of gastric cancer in the general population will be improved
by managing the family members of patients with HCS due to the
germline mutations being carried throughout the lives in every
patient with HCS. Therefore, individuals belonging to a family with
a history of HCS are recommended to undergo a susceptibility
genetic test to identify those who possess a positive family
history and germline mutations. Furthermore, individuals with both
indications, namely, a population with a high risk of cancer,
should undergo routine surveillance to confirm the risk level of
early cancer as well as to improve their prognosis. This process
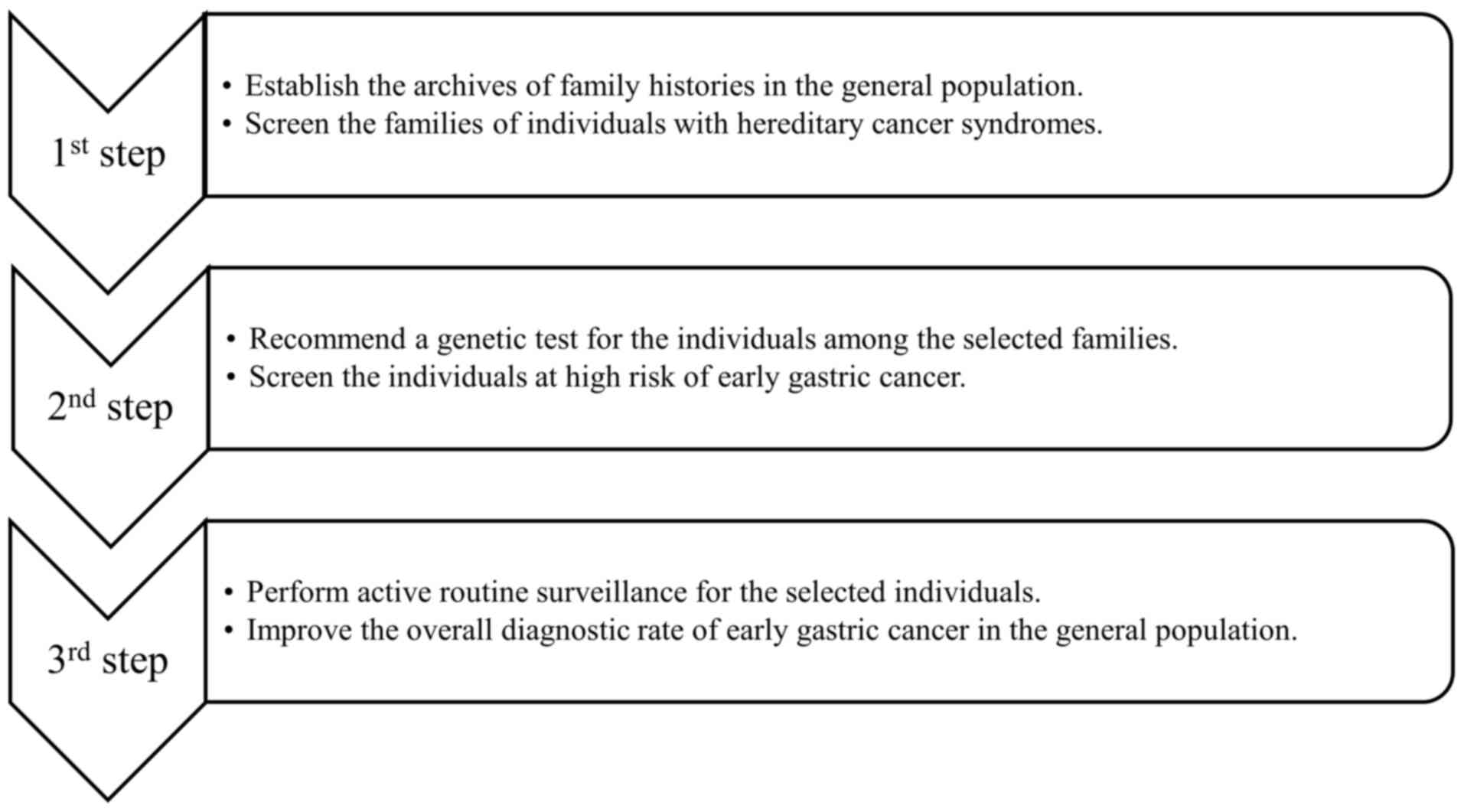
may be implemented as follows (Fig.
1).
To begin with, the archives of the family history of
the general population should be established in clinics and
hospitals/medical institutions to screen families for HCS. Monahan
and Hopkins (26) reported that the
cancer risk of individuals with first-degree relatives with gastric
cancer is increased by 1.3-3.5 times. Choi and Kim (27) also stated that first-degree relatives
in a family with a positive history have a strong and consistent
risk of being diagnosed with gastric cancer. Slavin et al
(28) reported that germline genetic
syndromes, similar to HDGC, PJS and LS, are associated with gastric
cancer predisposition. Therefore, a detailed family history is
important for the diagnosis of HCS. Karimi et al (29) reported that numerous factors may
serve a role in gastric cancer progression, including age, male
sex, tobacco smoking, physical activity, family history and
hereditary syndromes. The more detailed the family history is, the
better the outcomes. However, the most significant point that
should be emphasized is the impact on families with HCS,
particularly those with positive first-degree relatives (27,29,30).
Therefore, detailed family histories, including multiple
individuals in a family identified as having HCS, should be
established for statistical analysis in clinics and
hospitals/medical institutions, which may provide basic data for
screening the population with high-risk cancer types and may be
used to gradually establish a systematic database of hereditary
cancers. If the database were to be established across multiple
hospitals/medical institutions, more epidemiological and clinical
features of gastric cancer would be available for statistical
analysis.
Secondly, the individuals with a family history of
HCS should undergo genetic testing to screen for those who belong
to the high-risk cancer groups. Kim et al (31) reported that people with a family
history of HCS have a 3-fold higher risk of being diagnosed with
gastric cancer than those without. Individuals with a family
history of HCS and susceptibility gene mutations should be
considered high risk. Generally, each syndrome of HCS, including
HDGC, LS and JPS, has corresponding criteria for diagnosis and
genetic testing (32–34). For instance, the criteria for
diagnosis and genetic testing of HDGC had a significant application
in daily practice since they were implemented by the International
Gastric Cancer Linkage Consortium in 1999 (35) and updated in 2010 and 2015 (32). The approach for the diagnosis of LS
includes clinical criteria, computational models, and genetic
testing, through which ~95.1% of LS cases could be identified, as
reported by Bui et al (23).
Therefore, if a family history database were to be established
across medical institutions, particularly for HCS, methods to
diagnose HCS could be applied in daily clinical routines to ensure
that families with HCS are identified. All individuals in these
families who meet the criteria for HCS could be encouraged to
undergo testing for susceptibility genes (21,25,32),
which may reveal cancer risks earlier. In addition, Sun et
al (36) reported that the risk
of breast cancer in gene mutation carriers was significantly
different between non-carriers before the age of forty years in a
large Chinese cohort. As HCS is usually associated with an early
onset, genetic screening may disclose the cancer risk for
individuals and their children, which may contribute toward
improving the ODR of EGC. Furthermore, genetic testing has positive
influences on the treatment, prognosis, and recurrence risk of
gastric cancer.
Thirdly, routine surveillance for individuals with a
high risk of gastric cancer should be performed to promote the
diagnostic rate of EGC in the general population. The patients with
susceptibility gene mutations in families with gastric cancer
belong to the high-risk population and are encouraged to adopt
routine surveillance and/or prophylactic surgery to detect gastric
cancer at an early stage and/or treat it in a timely manner. The
patients with HDGC with CDH1 germline mutations were
recommended to undergo prophylactic gastrectomy or endoscopic
surveillance (37), and a
16-year-old female with several relatives with gastric cancer
accepted a prophylactic gastrectomy following the detection that
she was a positive carrier of a CDH1 germline mutation
(38). Furthermore, colectomy is
recommended for LS patients, but the choice of a partial colectomy
or total colectomy remains controversial due to its physical and
social impact (23). Due to the
young age at surgery and the irreversible process, prophylactic
surgery will bring certain social and psychological pressures, and
regular surveillance may be more easily accepted by patients. Mi
et al (39) reported that
routine surveillance may improve the participants' quality of life
and mental wellbeing, and they may also benefit from the support of
other medical researchers and continued counselling (39). Therefore, endoscopy surveillance
accompanied by routine inspection should be conducted routinely for
patients who are unwilling to accept prophylactic gastrectomy. More
targeted clinical examination, more thorough biopsy and more
frequent detection will contribute toward increasing the number of
identified diagnoses of EGC for susceptibility gene mutation
carriers and decreasing the number of missed cases. Routine
surveillance for individuals with a high risk of gastric cancer in
a family would aid in detecting the risk of gastric cancer in
multiple individuals in this family. Consequently, as the detection
efficiency of gastric cancer in the general population gradually
improved on a large scale, the ODR of EGC in the general population
may be increased.
Although individuals diagnosed with gastric cancer
occasionally have no records of a familial history of HCS, a
potential risk of cancer onset may be identified in other
individuals of the same family without any cancer signs. In
addition, the hereditary family history may be established for
families with no prior record of cancer-related history.
Consequently, their family history linked with HCS should be
collected to differentiate the individuals with positive family
history (i.e., HCS) from those with a negative family history
(i.e., non-HCS). Individuals with a positive family history should
undergo the flow process presented above early, which may
eventually promote the ODR of EGC in the general population;
however, individuals with a negative history may merely undergo
routine inspection when they feel it is warranted (Fig. 2).
Genetic testing and genetic counselling have
gradually becoming popular among the general population in recent
years. To date, an increasing number of doctors have learned about
this subject. By improving medical practitioners' knowledge and
their familiarity with this subject, the individuals with HCS may
be more efficiently diagnosed and treated, and appropriate and
timely medical advice can be given to them. Family doctors who are
familiar with this subject may be more likely to identify these
asymptomatic individuals. In addition, as the patient information
recording has improved in medical institutions, these individuals
may be discovered timely through large-scale data analysis. Due to
the recommendations of medical practitioners and the increased
awareness of genetic diseases, the general asymptomatic population
has shown an increasing acceptance of routine medical surveillance.
Furthermore, people who accept regular medical monitoring tend to
have healthier lifestyle habits (31), which has a positive effect on
reducing the incidence of cancer in this group. As the cost
decreases and the awareness increases, genetic testing should have
a large space for expansion in general population. However, simple
genetic testing has limited effects on the detection rate of EGC.
It is necessary to combine the analysis of family history of HCS
and genetic testing to give reasonable medical surveillance
recommendations. This means that the individuals with gene
mutations and a family history of HCS belong to high-risk group
that requires intensive medical monitoring. Using this method, the
diagnostic rate of EGC within the overall population may be
improved.
The proportion of genetic diseases is very small,
but if one positive case is detected, it will serve a clinical
significance in the identification of HCS. Furthermore, it has a
potential effect on the detection rate of other asymptomatic
individuals in the family. Through improving patient record keeping
in medical institutions, a patient's information management system
is gradually formed and the family history of the general
population can be obtained easily. Chen et al (2) performed numerous studies and analysis
using the patient records collected by medical institutions. When
the scope of HCS research is gradually expanded, the patients with
genetic syndromes may be identified in a timely manner, and more
targeted suggestions may be made for individuals who require
genetic testing.
As this review recommends routine monitoring of
asymptomatic individuals who have no signs of cancer, the potential
cancer in these individuals may be detected as early as possible.
Studies have demonstrated that routine surveillance has a positive
effect on the detection of early gastric cancer (40). As the onset of HCS is often at an
earlier age, this has an early warning effect on other asymptomatic
individuals in the family, which may remind these people to
commence regular medical monitoring at an early age. However,
during the interval period of regular monitoring, an individual's
gastric cancer may develop from early to advanced stage, and, once
discovered, it is an advanced cancer. However, this method may
improve the detection rate of EGC in the overall population. The
method mentioned in this article is different from traditional
endoscopy monitoring, serum detection and other active detection
methods for EGC. This method is based on complete medical
information statistics and complete patient information
registration, including the existing medical foundation and
equipment; therefore, the recommendations often come from the
statistics and analysis of big data, which can serve a positive
role in the diagnosis of EGC.
Conclusions
The systematic and comprehensive archives of family
history for the general population required improvement.
Furthermore, the cost of genetic testing is expensive for certain
people, but certain effective attempts could be made to gradually
change this situation in certain areas. Due to the differences in
ethnic and regional populations, the social environment and dietary
habits, conducting large-sample and multi-centre studies is
necessary to establish management strategies for family members of
individuals with HCS and standards of genetic testing that will be
compatible with populations from different regions. In the future,
the diagnostic rate of EGC may benefit from the implementation of
these methods.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HZ conceived and designed the study, wrote and
edited the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EGC
|
early gastric cancer
|
|
HGC
|
hereditary gastric cancer
|
|
HCS
|
hereditary cancer syndromes
|
|
HDGC
|
hereditary diffuse gastric cancer
|
|
ODR
|
overall diagnostic rate
|
|
LS
|
Lynch syndrome
|
|
JPS
|
juvenile polyposis syndrome PJS,
Peutz-Jeghers syndrome
|
|
HBC
|
hereditary breast cancer
|
|
LFS
|
Li-Fraumeni syndrome
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suh YS and Yang HK: Screening and early
detection of gastric cancer: East versus west. Surg Clin North Am.
95:1053–1066. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
SEGCCC, . Comparison on early-stage
gastric cancer screening in different levels of medical facilities
in Shanghai. Chin J Dig Endosc. 24:19–22. 2007.
|
|
5
|
Miki K, Fujishiro M, Kodashima S and
Yahagi N: Long-term results of gastric cancer screening using the
serum pepsinogen test method among an asymptomatic middle-aged
Japanese population. Dig Endosc. 21:78–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di L, Wu H, Zhu R, Li Y, Wu X, Xie R, Li
H, Wang H, Zhang H, Xiao H, et al: Multi-disciplinary team for
early gastric cancer diagnosis improves the detection rate of early
gastric cancer. BMC Gastroenterol. 17:1472017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palli D, Galli M, Caporaso NE, Cipriani F,
Decarli A, Saieva C, Fraumeni JF Jr and Buiatti E: Family history
and risk of stomach cancer in Italy. Cancer Epidemiol Biomarkers
Prev. 3:15–18. 1994.PubMed/NCBI
|
|
8
|
Zanghieri G, Di Gregorio C, Sacchetti C,
Fante R, Sassatelli R, Cannizzo G, Carriero A and Ponz de Leon M:
Familial occurrence of gastric cancer in the 2-year experience of a
population-based registry. Cancer. 66:2047–2051. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petrovchich I and Ford JM: Genetic
predisposition to gastric cancer. Semin Oncol. 43:554–559. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oliveira C, Pinheiro H, Figueiredo J,
Seruca R and Carneiro F: Familial gastric cancer: Genetic
susceptibility, pathology, and implications for management. Lancet
Oncol. 16:e60–e70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jones EG: Familial gastric cancer. N Z Med
J. 63:287–296. 1964.PubMed/NCBI
|
|
12
|
Guilford P, Hopkins J, Harraway J, McLeod
M, McLeod N, Harawira P, Taite H, Scoular R, Miller A and Reeve AE:
E-cadherin germline mutations in familial gastric cancer. Nature.
392:402–405. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hansford S, Kaurah P, Li-Chang H, Woo M,
Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K,
Zogopoulos G, et al: Hereditary diffuse gastric cancer syndrome:
CDH1 mutations and beyond. JAMA Oncol. 1:23–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Figueiredo J, Söderberg O, Simões-Correia
J, Grannas K, Suriano G and Seruca R: The importance of E-cadherin
binding partners to evaluate the pathogenicity of E-cadherin
missense mutations associated to HDGC. Eur J Hum Genet. 21:301–309.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lo W, Zhu B, Sabesan A, Wu HH, Powers A,
Sorber RA, Ravichandran S, Chen I, McDuffie LA, Quadri HS, et al:
Associations of CDH1 germline variant location and cancer phenotype
in families with hereditary diffuse gastric cancer (HDGC). J Med
Genet. 56:370–379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Feng M, Feng Y, Bu Z, Li Z, Jia S
and Ji J: Germline mutations in hereditary diffuse gastric cancer.
Chin J Cancer Res. 30:122–130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richards FM, McKee SA, Rajpar MH, Cole TR,
Evans DG, Jankowski JA, McKeown C, Sanders DS and Maher ER:
Germline E-cadherin gene (CDH1) mutations predispose to familial
gastric cancer and colorectal cancer. Hum Mol Genet. 8:607–610.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dossus L and Benusiglio PR: Lobular breast
cancer: Incidence and genetic and non-genetic risk factors. Breast
Cancer Res. 17:372015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaurah P and Huntsman DG: Hereditary
Diffuse Gastric Cancer. GeneReviews® [Internet].
University of Washington. (Seattle, WA). 2018.
|
|
20
|
Sugimoto S, Komatsu H, Morohoshi Y and
Kanai T: Recognition of and recent issues in hereditary diffuse
gastric cancer. J Gastroenterol. 50:831–843. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chun N and Ford JM: Genetic testing by
cancer site: Stomach. Cancer J. 18:355–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van der Post RS, Vogelaar IP, Carneiro F,
Guilford P, Huntsman D, Hoogerbrugge N, Caldas C, Schreiber KE,
Hardwick RH, Ausems MG, et al: Hereditary diffuse gastric cancer:
Updated clinical guidelines with an emphasis on germline CDH1
mutation carriers. J Med Genet. 52:361–374. 2015. View Article : Google Scholar
|
|
23
|
Bui QM, Lin D and Ho W: Approach to lynch
syndrome for the gastroenterologist. Dig Dis Sci. 62:299–304. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jasperson KW, Tuohy TM, Neklason DW and
Burt RW: Hereditary and familial colon cancer. Gastroenterology.
138:2044–2058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Corso G, Intra M, Trentin C, Veronesi P
and Galimberti V: CDH1 germline mutations and hereditary lobular
breast cancer. Fam Cancer. 15:215–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monahan KJ and Hopkins L: Diagnosis and
management of hereditary gastric cancer. Recent Results Cancer Res.
205:45–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi YJ and Kim N: Gastric cancer and
family history. Korean J Intern Med. 31:1042–1053. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Slavin TP, Weitzel JN, Neuhausen SL,
Schrader KA, Oliveira C and Karam R: Genetics of gastric cancer:
What do we know about the genetic risks? Transl Gastroenterol
Hepatol. 4:552019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu KH, Wood ME, Daniels M, Burke C, Ford
J, Kauff ND, Kohlmann W, Lindor NM, Mulvey TM, Robinson L, et al:
American Society of Clinical Oncology Expert Statement: Collection
and use of a cancer family history for oncology providers. J Clin
Oncol. 32:833–840. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim HJ, Kwon M, Kim N, Lee JB and Won S:
The influence of family history on stage and survival of gastric
cancer according to the TGFB1 C-509T polymorphism in Korea. Gut
Liver. 14:79–88. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benusiglio PR, Colas C, Rouleau E,
Uhrhammer N, Romero P, Remenieras A, Moretta J, Wang Q, De Pauw A,
Buecher B, et al: Hereditary diffuse gastric cancer syndrome:
Improved performances of the 2015 testing criteria for the
identification of probands with a CDH1 germline mutation. J Med
Genet. 52:563–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balmaña J, Balaguer F, Cervantes A and
Arnold D; ESMO Guidelines Working Group, : Familial risk-colorectal
cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 24 (Suppl
6):vi73–vi80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haidle JL and Howe JR: Juvenile Polyposis
Syndrome. GeneReviews® [Internet]. University of
Washington. (Seattle, WA). 2017.
|
|
35
|
Caldas C, Carneiro F, Lynch HT, Yokota J,
Wiesner GL, Powell SM, Lewis FR, Huntsman DG, Pharoah PD, Jankowski
JA, et al: Familial gastric cancer: Overview and guidelines for
management. J Med Genet. 36:873–880. 1999.PubMed/NCBI
|
|
36
|
Sun J, Meng H, Yao L, Lv M, Bai J, Zhang
J, Wang L, Ouyang T, Li J, Wang T, et al: Germline mutations in
cancer susceptibility genes in a large series of unselected breast
cancer patients. Clin Cancer Res. 23:6113–6119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colvin H, Yamamoto K, Wada N and Mori M:
Hereditary gastric cancer syndromes. Surg Oncol Clin N Am.
24:765–777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wickremeratne T, Lee CH, Kirk J, Charlton
A, Thomas G and Gaskin KJ: Prophylactic gastrectomy in a
16-year-old. Eur J Gastroenterol Hepatol. 26:353–356. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mi EZ, Mi EZ, di Pietro M, O'Donovan M,
Hardwick RH, Richardson S, Ziauddeen H, Fletcher PC, Caldas C,
Tischkowitz M, et al: Comparative study of endoscopic surveillance
in hereditary diffuse gastric cancer according to CDH1 mutation
status. Gastrointest Endosc. 87:408–418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Syngal S, Brand RE, Church JM, Giardiello
FM, Hampel HL and Burt RW; American College of Gastroenterology, :
ACG clinical guideline: Genetic testing and management of
hereditary gastrointestinal cancer syndromes. Am J Gastroenterol.
110:223–262; quiz 263. 2015. View Article : Google Scholar : PubMed/NCBI
|