Introduction
Lung cancer is one of the most common malignancies
and leads to the highest cancer-associated mortality rate in China
(1). Approximately 10–15% of
patients with lung cancer are diagnosed with small-cell lung cancer
(SCLC), and ~70% of them are diagnosed at advanced stages (2). SCLC is a highly aggressive disease and
has an increased tendency to metastasize. Patients with SCLC are
generally treated with platinum-based chemotherapy alone or in
combination with radiotherapy according to tumor stage. Although
60–80% of SCLC patients respond to first-line treatment, most of
them inevitably develop chemoresistance and relapse within a
relatively short time, leading to the spread of the disease
(3,4). Therapeutic efficacy is routinely
evaluated based on imaging outcomes. However, imaging-observed
changes in tumor volume are not notable in certain patients
receiving effective treatment, but imaging cannot be performed
prior to each chemotherapy cycle due to radiation exposure, and
this may result in a failure to detect recurrence and/or metastasis
in a timely manner. Therefore, there is an increasing requirement
for convenient tools to detect responsiveness to treatment and
predict prognosis in patients with SCLC in order to optimize
disease management.
Several tumor markers have been used in patients
with SCLC to improve diagnosis and treatment selection.
Progastrin-releasing peptide (ProGRP) and neuron-specific enolase
(NSE) are the most commonly used tumor markers in SCLC (5,6). NSE has
been the traditionally recommended tumor marker for SCLC. However,
several studies have demonstrated that the diagnostic proficiency
of ProGRP was higher than that of NSE in SCLC (7). McDonald et al first isolated
gastrin-releasing peptide (GRP) from gastric nerve fibers in 1978
(8). In 1988, immunohistochemical
studies confirmed the presence of GRP and its peptide precursor in
SCLC cell lines. Since then, several studies have investigated the
potential use of GRP as a biomarker in SCLC; however, this was
found to be challenging because GRP was unstable in plasma and
therefore, GRP levels were difficult to measure accurately. ProGRP
is the precursor of GRP and is more stable than GRP in plasma.
Circulating ProGRP has been demonstrated to be an effective
biomarker for discriminating SCLC from non-small cell lung cancer
and benign lung diseases with high sensitivity and specificity
(9,10). Numerous studies have focused on serum
ProGRP and NSE as tumor markers for diagnosing patients with SCLC.
However, few studies have prospectively evaluated the use of ProGRP
and NSE levels as therapeutic and prognostic indicators in patients
with SCLC (11), particularly in
patients with SCLC with higher ProGRP levels at diagnosis. In the
present study, the changes of the ProGRP levels in the responder
group and non-responder group were observed, and whether changes in
ProGRP levels may predict treatment response for SCLC was
investigated.
Materials and methods
Patients
Data were retrospectively collected regarding 285
patients with SCLC who were initially diagnosed at The First
Affiliated Hospital of the University of Science and Technology of
China between January 2015 and October 2018, and complete
information was available for 120 cases. A diagnosis of SCLC was
made pathologically using bronchoscopic biopsies, CT-guided needle
lung biopsies, or surgically resected specimens. The following
inclusion criteria were used: i) Diagnosis was confirmed
pathologically; ii) patients were first diagnosed prior to
receiving any treatment; iii) NSE and ProGRP levels were measured
prior to treatment; iv) NSE and ProGRP levels were measured at
several time points throughout the treatment period; v) the entire
chemotherapy process was performed in the hospital, with a 6-month
follow-up time; and vi) indicators associated with renal function,
including creatinine, creatinine clearance rate, and urine nitrogen
were at normal levels. Based on Response Evaluation Criteria in
Solid Tumors version 1.1, the treatment response was divided into
responder and non-responder groups (12). The responder group included patients
who had a partial response (PR) and those who had a complete
response (CR), and the non-responder group included patients who
had stable disease (SD) and those who had progressive disease (PD).
Chemoresistance was defined as progression or relapse following
first-line treatment in patients with SCLC. ProGRP and NSE levels,
and imaging outcomes were evaluated retrospectively at the time of
diagnosis following two cycles of chemotherapy and
post-chemoresistance. The present study was approved by the Ethics
Committee of The First Affiliated Hospital of the University of
Science and Technology of China. Due to the retrospective nature of
this study, written informed consent was not required.
ProGRP and NSE assay
Serum ProGRP and NSE levels were determined using an
electrochemiluminescence immunoassay (ECLIA) on a Cobas E601
Analyzer system (Roche Applied Science). All specimens were
processed within 6 h of collection using a centrifugal protocol
(2,200 × g for 10 min at room temperature). The serum ProGRP
sensitivity cut-off level was 75.3 pg/ml, which was the same as
that stated in the Roche reagent protocol (13). A high level of ProGRP was defined as
the level of ProGRP above the normal reference range, and a low
level of ProGRP was defined as a value within the normal reference
range.
Statistical analysis
All the statistical analyses were performed using
GraphPad Prism version 8.5 (GraphPad Software, Inc.) and SPSS
version 19.0 (IBM Corp.). All data are presented as the median and
quartiles (Q, 25 and 75th percentiles), and statistical analysis
was performed using Friedman's test, followed by the Nemenyi
post-hoc test. Receiver operating characteristic (ROC)
curves were used to display the correlation between sensitivity and
specificity, and the therapeutic efficacy was assessed by
calculating the area under curve (AUC). Survival rate curves were
drawn using the Kaplan-Meier method, and the log-rank test was used
to compare the differences in the curves. Changes in ProGRP and NSE
levels following chemotherapy were performed using the Wilcoxon
signed-rank test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline clinicopathological features
of patients with SCLC
Of the total 285 patients with SCLC, 58 (20.4%) had
limited disease (LD) and 227 (79.6%) had extensive disease (ED),
and 26.6% (68/256) of patients had a low level of ProGRP and74.4%
(188/256) had a high level of ProGRP. A total of 80% (228/285) of
the total 285 patients with SCLC were male (Table SI). Among the 120 patients with
SCLC, the median age was 64 years (range, 43–85 years). Ninety-nine
(82.5%) patients were male and 21 (17.5%) were female. Seventy
patients were non-smokers and 50 were current or ex-smokers.
Twenty-three patients had LD at the time of diagnosis, and the
other 97 patients had ED. The median level of ProGRP in patients
with LD was 589 pg/ml (Q, 55.74-2,263.00 pg/ml), and the median
level of ProGRP in patients with ED was 1,742 pg/ml (Q,
137.2-4,006.5 pg/ml; Table I).
 | Table I.Baseline characteristics of patients
with small-cell lung cancer. |
Table I.
Baseline characteristics of patients
with small-cell lung cancer.
| Characteristic | n (%) | ProGRP, pg/ml (95%
CI) | P-value | NSE, ng/ml (95%
CI) | P-value |
|---|
| Sex |
|
|
|
|
|
| Male | 99 (82.5) | 1,428
(70.3-3568) | 0.346 | 41.37
(19.98-69.5) | 0.236 |
|
Female | 21 (17.5) | 2,583
(154.6-4901.5) |
| 43.92
(25.96-113.45) |
|
| Age |
|
|
|
|
|
|
<64 | 58 (48.3) | 2,064
(351.35-3580.75) | 0.168 | 45.46
(22.13-79.55) | 0.652 |
| ≥64 | 62 (51.7) | 775.3
(59.15-3870.25) |
| 35.8
(20.60-67.20) |
|
| Smoking |
|
|
|
|
|
|
Smoker | 50 (41.7) | 1,338.5
(95.67-3407) | 0.548 | 39.12
(18.93-67.20) | 0.259 |
|
Non-smoker | 70 (58.3) | 1,608
(67.28-4458.25) |
| 46.29
(23.16-80.10) |
|
| Stage |
|
|
|
|
|
|
Limited | 23 (19.2) | 589 (55.74-2263) | 0.092 | 27.95
(19.24-58.14) | 0.133 |
|
Extensive | 97 (80.8) | 1,742
(137.2-4006.5) |
| 46.44
(23.20-76.38) |
|
ProGRP as a therapeutic biomarker
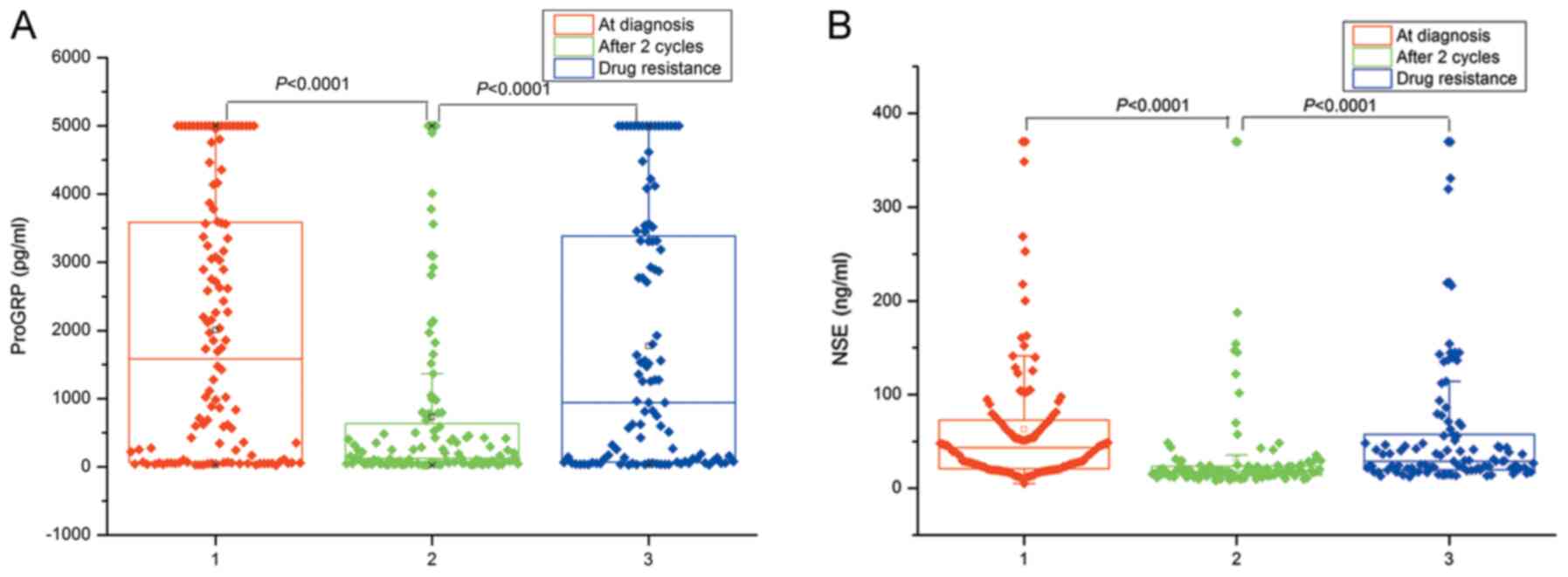
Changes in ProGRP and NSE levels in the 120 patients
with SCLC were analyzed, including at diagnosis, following two
cycles of chemotherapy and following the occurrence of drug
resistance, and levels of serum ProGRP and NSE were also measured
prior to the start of chemotherapy. The highest point of detection
of ProGRP is 5,000 pg/ml, and results >5,000 pg/ml were reported
as 5,000 pg/ml. Levels of serum ProGRP and NSE significantly
decreased following two cycles of chemotherapy (P<0.001) and
significantly increased following drug resistance (P<0.001;
Fig. 1). Figs. 2 and 3
are the breakdown data of Fig. 1.
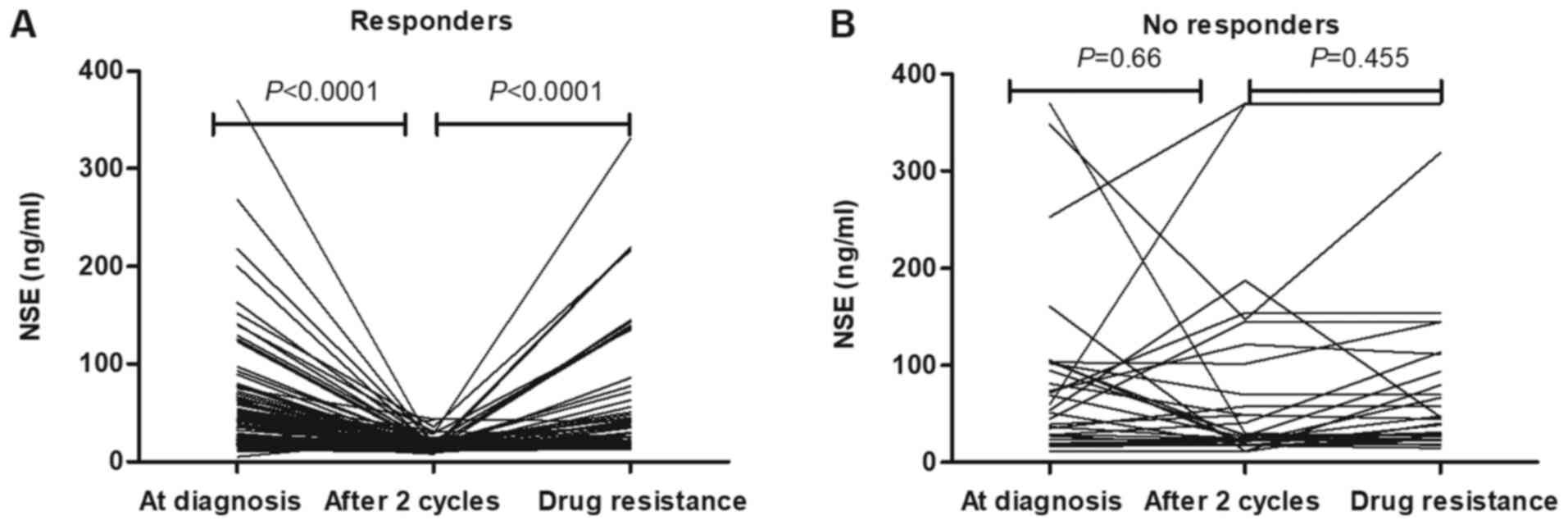
There were 88 responders and 32 non-responders. In the responders,
serum ProGRP levels following two cycles of chemotherapy were
significantly lower than baseline levels at diagnosis (P<0.001;
Fig. 2A). In non-responders,
following two cycles of chemotherapy, there were no significant
decreases in serum ProGRP concentrations (Fig. 2B; P=0.752). This was also the case
for the concentrations of NSE in patients with SCLC (Fig. 3).
Decline in ProGRP levels predicts
objective response to treatment
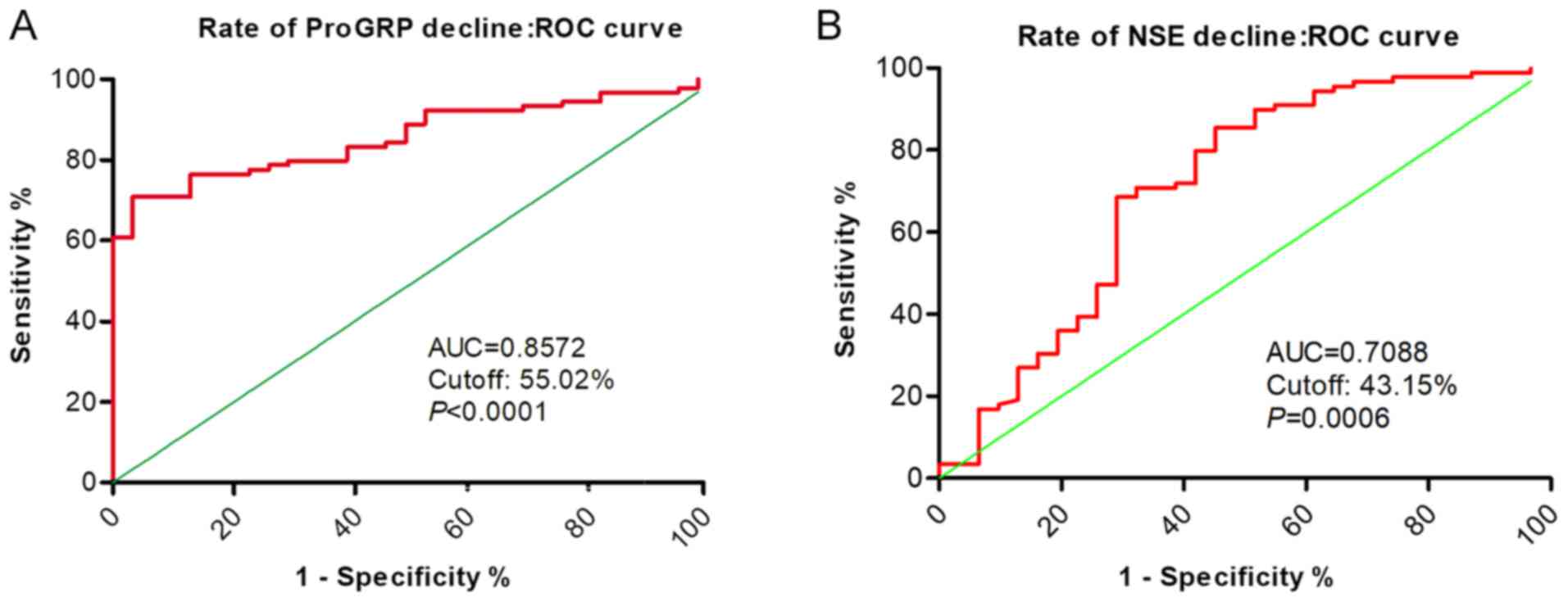
The association between decreases in serum ProGRP
and NSE levels and the effectiveness of SCLC chemotherapy are shown
in Fig. 4. Serum ProGRP levels were
associated with greater diagnostic accuracy and were predictive of
a patient's objective response to SCLC chemotherapy; corresponding
ROC curves showed a rate of ProGRP decline of 0.8572 (cut-off,
55.02%; P<0.001).
Notably, it was reported that following two cycles
of treatment or drug resistance, further changes in ProGRP levels
in patients with low ProGRP levels at the time of diagnosis were
not notable (Fig. 5), regardless of
whether patients were responders or non-responders. The cut-off
level of serum ProGRP was 75.3 pg/ml, as specified by the reagent
manufacturer. The AUC for the decline in higher ProGRP levels as a
biomarker for treatment monitoring of SCLC was 0.9643 (P<0.001).
A decline in ProGRP levels may be used as a good predictor of
objective response to SCLC chemotherapy, and the cut-off value also
was 55.02% (Fig. 6).
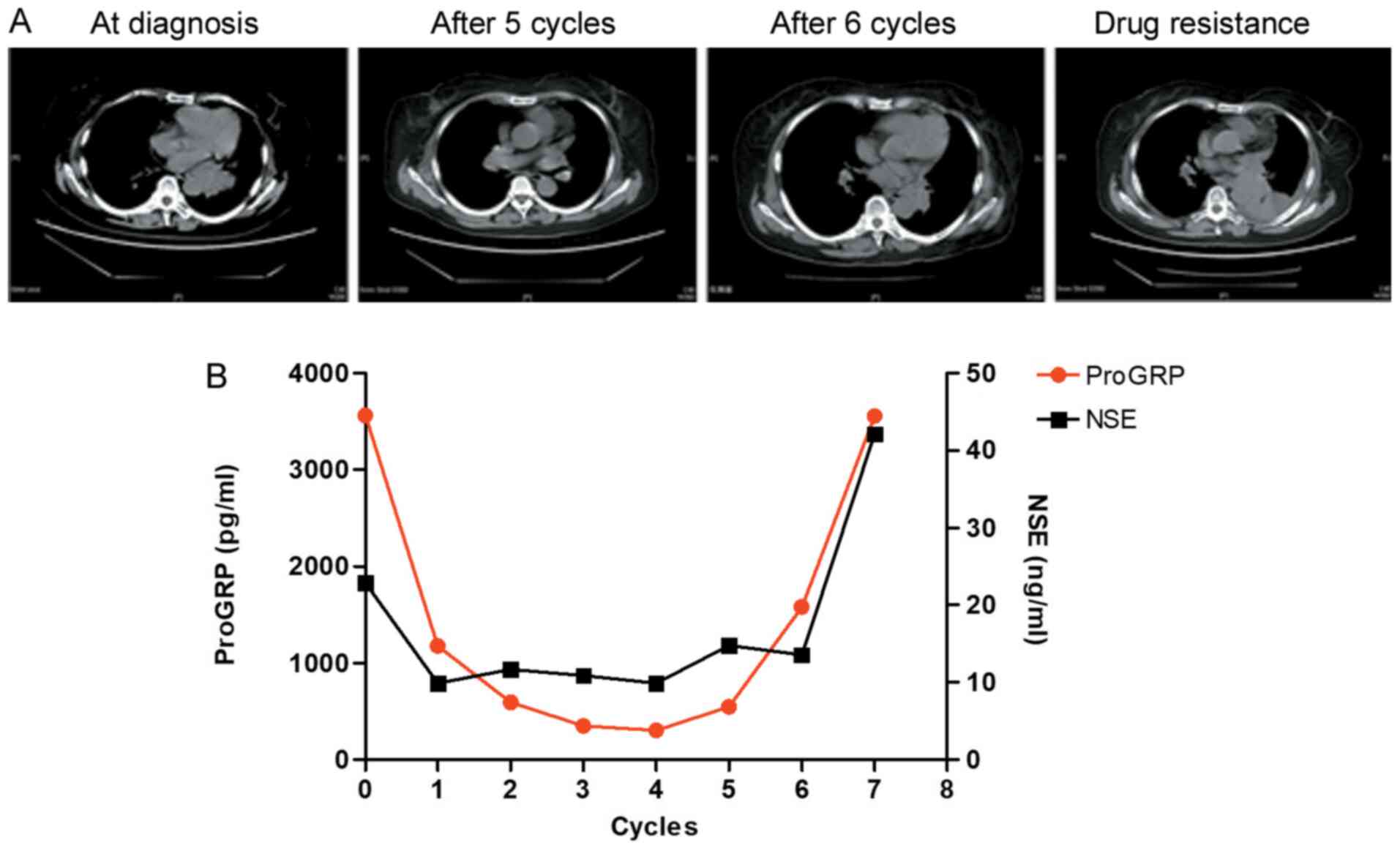
To verify whether changes in ProGRP levels may be
used to assess the efficacy of SCLC chemotherapy, the association
between expression levels of ProGRP and NSE, as well as imaging
characteristics of solid tumors, was investigated. Patients with
SCLC were diagnosed at an advanced disease stage and were treated
with cisplatin combined with etoposide. From the imaging data,
tumor shrinkage was observed and the treatment response was
assessed as PR. Levels of ProGRP declined steadily and
synchronously (Fig. 7); however,
tumor size began to increase again following six cycles of
chemotherapy. In line with the changes in tumor radiological
characteristics, ProGRP levels steadily decreased from the start of
treatment until five cycles of chemotherapy had been completed and
then increased following six cycles of chemotherapy. Although NSE
expression also significantly decreased from the start of treatment
until five cycles of chemotherapy, it did not increase after six
cycles of chemotherapy. These data indicated that serum ProGRP
maybe used as a potential biomarker for monitoring the therapeutic
response in patients with SCLC.
Survival and prognosis according to
ProGRP and NSE
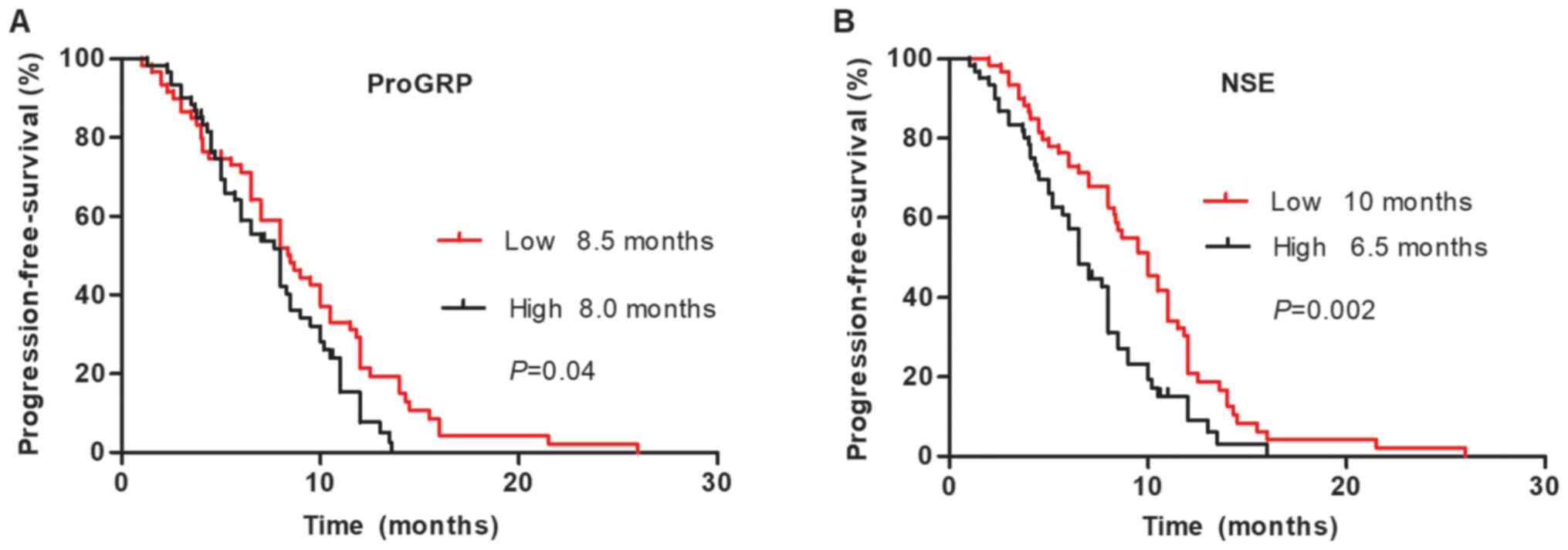
Among the 120 patients with SCLC, median
progression-free survival (PFS) times were significantly shorter in
patients with SCLC with ED (P=0.006) and higher ProGRP (P=0.048)
and NSE (P=0.001) levels. However, no significant differences in
PFS were identified when comparing male and female patients
(P=0.904), older and younger patients (P=0.276), or smokers and
non-smokers (P=0.669). In multivariate analysis, the level of NSE
[95% CI: 0.567 (0.384-0.837); P=0.004] and disease stage [95% CI:
0.543 (0.322-0.915); P=0.022] were prognostic factors of survival
in patients with SCLC (Table II).
The median PFS time was significantly longer in patients with lower
levels of baseline serum NSE, according to Kaplan-Meier survival
curves (Fig. 8).
 | Table II.Univariate and multivariate Cox
proportional hazard models for survival. |
Table II.
Univariate and multivariate Cox
proportional hazard models for survival.
| Variable | Univariate analysis
HR (95% CI) | P-value | Multivariate analysis
HR (95% CI) | P-value |
|---|
| Sex, male vs.
female | 0.967
(0.565-1.657) | 0.904 |
|
|
| Age, <64 vs.
≥64 | 0.806
(0.546-1.188) | 0.276 |
|
|
| Smoking, yes vs.
no | 0.918
(0.620-1.359) | 0.669 |
|
|
| Stage, LD vs. ED | 0.483
(0.287-0.815) | 0.006 | 0.543
(0.322-0.915) | 0.022 |
| ProGRP, high vs.
low | 0.664
(0.443-0.996) | 0.048 | 0.714
(0.475-1.073) | 0.105 |
| NSE, high vs.
low | 0.530
(0.360-0.781) | 0.001 | 0.567
(0.384-0.837) | 0.004 |
Discussion
One of the main treatment methods for SCLC is
chemotherapy, and first-line treatment for SCLC is cisplatin
combined with etoposide. The efficacy of chemotherapy is routinely
evaluated based on imaging following two cycles of chemotherapy.
Therefore, ProGRP and NSE serum concentrations and imaging outcome
data at the time of diagnosis, after two cycles of chemotherapy,
and following drug resistance, were obtained retrospectively.
Previous studies have reported that serum ProGRP and NSE levels may
provide useful diagnostic and prognosis value in SCLC (14,15).
However, due to the lack of large-scale clinical trial data, the
role of ProGRP and NSE as biomarkers of treatment efficacy remains
controversial (16). In the present
study, information was collected regarding 285 patients with SCLC
who were hospitalized in The First Affiliated Hospital of
University of Science and Technology of China, and 120 patients
with complete information were analyzed. The clinical
characteristics of the patients were consistent with those of
previous studies (17,18). The results of the present study
demonstrated that changes in serum ProGRP levels may be used as a
biomarker to monitor therapeutic efficacy in patients with SCLC.
Among the 120 patients with SCLC who were followed, levels of serum
ProGRP in responders decreased significantly following
chemotherapy, while there was no significant decrease in the
concentration of serum ProGRP in non-responders when comparing pre-
and post-treatment levels. Ono et al reported that a decline
in serum ProGRP levels was strongly correlated with tumor diameter
shrinkage prior to the third course of treatment (19). Changes in serum ProGRP levels showed
better correlation with overall tumor diameter shrinkage than did
changes in serum NSE levels. Therefore, the results demonstrated
that changes in ProGRP, compared with NSE, were more reliable for
monitoring treatment and predicting relapse in patients with
SCLC.
Notably, the present study reported that 26.6% of
patients with SCLC had a low level of ProGRP at diagnosis and had
smaller changes in ProGRP levels following chemotherapy or
recurrence, regardless of whether patients were responders or
non-responders, which may be associated with lower GRP expression
levels in these SCLC patients. Wojcik and Kulpa reported that ~30%
of patients with SCLC had low GRP expression levels (20), indicating that ProGRP levels would
not be suitable as a clinical biomarker for this group of patients.
Numerous studies have focused on serum proGRP as a diagnostic
marker for patients with SCLC. However, few studies have
systematically assessed the role of proGRP levels in monitoring
treatment efficacy in patients with SCLC (21,22). For
patients with SCLC with high ProGRP levels at diagnosis, a model
for predicting objective response to chemotherapy was established
based on chemotherapy-related decreased levels in serum ProGRP. The
AUC for the decline in higher ProGRP levels as a therapeutic
biomarker of SCLC was 0.9643, and the cut-off value was 55.02%.
Decreases in ProGRP levels may be a good predictor of the
therapeutic response to SCLC chemotherapy. This predictive model
may be used to replace imaging or be combined with imaging to
evaluate treatment efficacy in patients with SCLC.
In addition, SCLC patient survival and prognosis
were analyzed in the present study, and concentrations of NSE
showed a clear association with prognosis in patients with SCLCs,
which was weaker for ProGRP. We hypothesized that most of the SCLC
patients with ProGRP levels in the normal range had low GRP
expression levels, which may be a specific subtype of patients, who
may be more likely to develop resistance to chemotherapy.
Furthermore, in multivariate analysis that included simultaneous
evaluations of clinical parameters and concentrations of the two
markers, NSE remained an independent predictor of survival
(23).
In conclusion, changes in serum levels of ProGRP,
which are initially high at the time of diagnosis, may aid in
predicting the effectiveness of different chemotherapy regimens in
patients with SCLC. There are certain limitations to the present
study. The relatively small sample size may limit the
generalization of the conclusions made, which will require further
validation in larger cohorts. Further research will be required to
determine whether there is cellular heterogeneity in patients with
SCLC s who are either ProGRP-negative or -positive, and whether
ProGRP-negative patients should be considered a specific subtype of
SCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Fundamental
Research Funds for the Central University (grant no. WK9110000025),
the Natural Science Foundation of Anhui Province (grant no.
2008085MH288), the Science and Technology Major Project of Anhui
Province (grant no. 18030801140), the National Natural Science
Foundation of China (grant no. 81672647), the National Ministry of
Industry and Information Technology Science and Technology (grant
no. 2018MND102041).
Availability of data and materials
All data used and/or analyzed in the present study
are available from the corresponding author upon reasonable
request.
Authors' contributions
ML and WL contributed toward designing, drafting and
revising the manuscript. DH, YZ, DL and CD and LQ contributed
toward the collection and collation of clinical data. WW performed
the statistics on clinical data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of University of Science
and Technology of China. Due to the retrospective nature of this
study, written informed consent was not required.
Patient consent for publication
Not applicable.
Competing interests
All authors declare no competing interests regarding
the present study.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yue XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalemkerian GP, Akerley W, Bogner P,
Borghaei H, Chow LQ, Downey RJ, Gandhi L, Ganti AK, Govindan R,
Grecula JC, et al: Small cell lung cancer. J Natl Compr Canc Netw.
11:78–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Byers LA and Rudin CM: Small cell lung
cancer: Where do we go from here? Cancer. 121:664–672. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: Analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du J, Li Y, Wang L, Zhou Y, Shen Y, Xu F
and Chen Y: Selective application of neuroendocrine markers in the
diagnosis and treatment of small cell lung cancer. Clin Chim Acta.
509:295–303. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wójcik E, Kulpa JK, Sas-Korczyńska B,
Korzeniowski S and Jakubowicz J: ProGRP and NSE in therapy
monitoring in patients with small cell lung cancer. Anticancer Res.
28((5B)): 3027–3033. 2008.PubMed/NCBI
|
|
7
|
Wu XY, Hu YB, Li HJ, Wan B, Zhang CX,
Zhang B, Hu H, Zhang Q, Lv TF, Zhan P and Song Y: Diagnostic and
therapeutic value of progastrin-releasing peptide on small-cell
lung cancer: A single-center experience in China. J Cell Mol Med.
22:4328–4334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McDonald TJ, Nilsson G, Vagne M, Ghatei M,
Bloom SR and Mutt V: A gastrin releasing peptide from porcine
nonantral gastric tissue. Gut. 19:767–774. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stieber P, Dienemann H, Schalhorn A,
Schmitt MU, Reinmiedl J, Hofmann K and Yamaguchi K:
Pro-gastrin-releasing peptide (ProGRP)-a useful marker in small
cell lung carcinomas. Anticancer Res. 19:2673–2678. 1999.PubMed/NCBI
|
|
10
|
Wang H and Qian J: Serum
pro-gastrin-releasing peptide in diagnosis of small cell lung
cancer: A meta-analysis. J Cancer Res Ther. 12 (Suppl)2:C260–C263.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Z, Xu D, Zhang F, Ying Y and Song L:
Pro-gastrin-releasing peptide and neuron-specific enolase: Useful
predictors of response to chemotherapy and survival in patients
with small cell lung cancer. Clin Transl Oncol. 18:1019–1025. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nordlund MS, Bjerner J, Warren DJ, Nustad
K and Paus E: Progastrin-releasing peptide: Stability in
plasma/serum and upper reference limit. Tumour Biol. 29:204–210.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang DW, Zhang Y, Hong QY, Hu J, Li C, Pan
BS, Wang Q, Ding FH, Ou JX, Liu FL, et al: Role of a serum-based
biomarker panel in the early diagnosis of lung cancer for a cohort
of high-risk patients. Cancer. 121 (Suppl 17):S3113–S3121. 2015.
View Article : Google Scholar
|
|
15
|
Lv ShP, Wang Y, Huang L, Wang F, Zhou JG
and Ma H: Meta-analysis of serum gastrin-releasing peptide
precursor as a biomarker for diagnosis of small cell lung cancer.
Asian Pac J Cancer Prev. 18:391–397. 2017.PubMed/NCBI
|
|
16
|
Cavalieri S, Morelli D, Martinetti A,
Galli G, Nichetti F, de Braud F and Platania M: Clinical
implications for pro-GRP in small cell lung cancer. A single center
experience. Int J Biol Markers. 33:55–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oh HJ, Park HY, Kim KH, Park CK, Shin HJ,
Lim JH, Kwon YS, Oh IJ, Kim YI, Lim SC, et al: Progastrin-releasing
peptide as a diagnostic and therapeutic biomarker of small cell
lung cancer. J Thorac Dis. 8:2530–2537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu D, Huang Y, Li L, Song J, Zhang L and
Li W: High neutrophil-to-lymphocyte ratios confer poor prognoses in
patients with small cell lung cancer. BMC Cancer. 17:8822017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ono A, Naito T, Ito I, Watanabe R, Shukuya
T, Kenmotsu H, Tsuya A, Nakamura Y, Murakami H, Kaira K, et al:
Correlations between serial pro-gastrin-releasing peptide and
neuron-specific enolase levels, and the radiological response to
treatment and survival of patients with small-cell lung cancer.
Lung Cancer. 76:439–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wojcik E and Kulpa JK:
Pro-gastrin-releasing peptide (ProGRP) as a biomarker in small-cell
lung cancer diagnosis, monitoring and evaluation of treatment
response. Lung Cancer (Auckl). 8:231–240. 2017.PubMed/NCBI
|
|
21
|
Holdenrieder S, von Pawel J, Dankelmann E,
Duell T, Faderl B, Markus A, Siakavara M, Wagner H, Feldmann K,
Hoffmann H, et al: Nucleosomes, ProGRP, NSE, CYFRA 21-1, and CEA in
monitoring first-line chemotherapy of small cell lung cancer. Clin
Cancer Res. 14:7813–7821. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nisman B, Biran H, Ramu N, Heching N,
Barak V and Peretz T: The diagnostic and prognostic value of ProGRP
in lung cancer. Anticancer Res. 29:4827–4832. 2009.PubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBI
|
|
23
|
Zhou M, Wang Z, Yao Y, Zhou H, Liu M and
Sun J: Neuron-specific enolase and response to initial therapy are
important prognostic factors in patients with small cell lung
cancer. Clin Trans Oncol. 19:865–873. 2017. View Article : Google Scholar
|