Introduction
Colorectal cancer (CRC) is a common malignancy in
males and females worldwide (1).
According to the latest GLOBOCAN data, CRC caused 551,269
mortalities, accounting for 5.8% of all cancer-associated
mortalities in 2018 (2). In the same
year, a total of 1,096,601 new cases of CRC were diagnosed,
accounting for 6.1% of all new cancer cases (2). Survival of patients with CRC is
significantly affected by cancer stages (3,4). More
than 90% of patients with localized CRC can live longer than 5
years after initial diagnosis, while this percentage may drop to
14% once distant tumor metastasis occurred (3,4).
Therefore, early diagnosis remains the key for the survival of
patients with CRC (5,6). However, sensitive early diagnostic
biomarkers for CRC are lacking and patient with CRC are mostly
diagnosed at advanced stages.
Molecular signals serve critical roles in the
occurrence and development of CRC (7,8).
Functional analyses of molecular pathways involved in CRC provide
guidance for the development of anti-CRC therapy with novel targets
(9). It has been well established
that long non-coding RNAs (lncRNAs, >200 nt) encode no proteins
but participate in human disease by regulating disease-related gene
expression (10,11). Therefore, regulating the expression
of certain lncRNAs may contribute toward the recovery of patients
with cancer (12). However, the
functions of most lncRNAs remain elusive. lncRNA UASR1 (UASR1) has
been characterized as an oncogenic lncRNA in breast cancer
(13). In a recent study, UASR1 was
reported to promote the proliferation of CRC cells by interacting
with the mTOR pathway (14).
However, the mechanism of the function of UASR1 in CRC is yet to be
fully elucidated. We hypothesized that UASR1 may interact with
miR-107, which serves tumor suppressive roles mainly by targeting
CDK8 (15). The present study was
therefore performed to investigate the interactions among UASR1,
miR-107 and CDK8 in CRC.
Materials and methods
CRC patients and tissue specimens
The present study was approved by the Ethics
Committee of the Second Hospital of Shandong University (no.
CRC201233255). A total of 62 patients with CRC, including 40 males
and 22 females (age range, 38–67 years; mean age, 57.2±7.6 years)
were enrolled at the aforementioned hospital between July 2012 and
July 2014. All patients were diagnosed with CRC by
histopathological examination. All patients were newly diagnosed
cases and other clinical disorders were excluded. Therapy was not
innititaed prior to this study. All patients provided written
informed consent. Prior to therapy, biopsy was performed on 62
patients with CRC to collect CRC tumor tissues and paired non-tumor
tissue samples.
Treatment and follow-up
The 62 patients were staged according to American
Joint Committee on Cancer staging criteria (16). There were 12, 17, 15 and 18 cases at
stage I, II, III and IV, respectively. Patients were treated with
chemotherapy, surgical resection, radiotherapy or immunotherapy
according to patients' clinical stages or health conditions. All
patients were followed up monthly for 5 years from the day of
admission to record patients survival. All 62 patients conpleted
the follow-up study.
CRC cells and transfections
Human CRC cell line CR4 (Sigma-Aldrich; Merck KGaA)
was used. Cell culture medium was composed of fetal bovine serum
(FBS; 10%; Sigma-Aldrich; Merck KGaA) and Eagle's minimal essential
medium (EMEM; 90%; Sigma-Aldrich; Merck KGaA). Cell culture
conditions were 95% humidity, 37°C and 5% CO2. Cells
were harvested at ~85% confluence to perform subsequent
experiments. With the pcDNA3.1 vector (Invitrogen; Thermo Fisher
Scientific, Inc.) as a backbone, expression vectors of UASR1 and
CDK8 were constructed. Negative control (NC) miRNA
(5′-UGUAACGUACGUUCGUACCGUGA-3′) and miR-107-mimic
(5′-AGCAGCAUUGUACAGGGCUAUCA-3′) were purchased from Sigma-Aldrich
(Merck KGaA). CR4 cells (107 cells in 10 ml medium) were
transfected with 10 nM vector and/or 40 nM miRNA using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). In
all transfections, untransfected cells were used as control (C)
cells and NC miRNA- or empty vector-transfected cells were used as
NC cells. Cells were collected at 48 h post-transfection prior to
subsequent experiments. In cases of co-transfection, cells were
simultaneously transfected with UASR1 expression vector and
miR-107.
Dual luciferase activity assay
With the pGL3 vector (Promega Corporation) as the
backbone, a luciferase vector of UASR1 was constructed. The
interaction between UASR1 and miR-107 was investigated by
performing a dual luciferase activity assay, which was performed by
co-transfecting CR4 cells with UASR1 luciferase vector + miR-107
mimic (miR-107 group) or UASR1 luciferase vector + NC miRNA (NC
group). Dual-luciferase reporter assay system (Promega Corporation)
was used for the determination of relative luciferase activities at
48 h post-transfection. Firefly luciferase activity was normalized
to Renilla luciferase activity. The value of the NC group was set
to ‘1’, and value of miR-107 group was normalized to NC group.
RNA preparations
Total RNA was isolated from CR4 cells and paired
tissue samples using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). To harvest miRNAs, 85% ethanol was used to
precipitate and wash RNA samples. Genomic DNAs in RNA samples were
removed by digesting with gDNA eraser (Takara Biotechnology Co.,
Ltd.).
Reverse transcription-quantitative
polymerase reaction (RT-qPCR)
Reverse transcription (55°C for 20 min and 85°C for
10 min) was performed to synthesize cDNA using SuperScript IV
Reverse Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.).
With cDNA samples as the template, qPCR reactions were prepared
using QuantiTect SYBR Green PCR kit (Qiagen GmbH) to determine the
expression of UASR1 and CDK8 mRNA with GAPDH as the internal
control. Poly (A) addition, mature miRNA reverse transcription and
qPCR reactions were performed using an All-in-One™ miRNA RT-qPCR
Detection kit (GeneCopoeia, Inc.) to measure the expression levels
of mature miR-107. Three replicate reactions were included in each
experiment and data were normalized using 2−ΔΔCq method
(17). The sample with the highest
ΔCq value was set to ‘1’, and all other samples were normalized to
this group. Primer sequences were: UASR1 forward,
5′-GCGGATCGCAGACCCTAA-3′ and reverse, 5′-AGAACACTTTGCGGAAGGC-3′;
CDK8 forward, 5′-GAATTTCTATGTCGGCATGC-3′ and reverse,
5′-ATAGTCAAAGAGAAGCCATACTTT-3′; and GAPDH forward,
5′-GTCTCCTCTGACTTCAACAGC-3′ and reverse,
5′-CCACCCTGTTGCTGTAGCCAA-3′. The forward primer for miR-107 was
5′-AGCAGCATTGTACAGGGCTATCA-3′. The universal reverse primer and U6
forward primer were from the kit. PCR reaction cycles were as
follows: 95°C for 1 min, followed by 40 cycles of 95°C for 10 sec
and 58°C for 40 sec.
Western-blot analysis
Total protein was isolated from CR4 cells using RIPA
buffer (Invitrogen; Thermo Fisher Scientific, Inc.). After that,
BCA assay (Invitrogen; Thermo Fisher Scientific, Inc.) was used to
determine protein concentration, followed by denaturation in
boiling water for 10 min. Denatured protein samples (30 µg per
lane) were separated using 8% SDS-PAGE gel. Polyvinylidene
difluoride membranes were used for gel transfer. Following blocking
in PBS (5% skimmed milk) at room temperature for 20 min, membranes
were first incubated with rabbit primary antibodies against CDK8
(1:1,000; cat. no. ab115155; Abcam) and GAPDH (1:1,000; cat. no.
ab9485; Abcam) at 4°C for 12 h, followed by incubation with an
immunoglobulin G horseradish peroxidase-conjugated secondary
antibody (1:1,000; cat. no. ab6721; Abcam) at room temperature for
2 h. Signals were developed using enhanced chemiluminescent
(Invitrogen; Thermo Fisher Scientific, Inc.). Image J 1.48 software
(National Institutes of Health) was used to normalize signals. The
value of C group was set to ‘1’, and values of other groups were
normalized to C group.
Cell proliferation assay
CR4 cells were harvested at 48 h post-transfection
to perform a cell proliferation assay. In brief, 6,000 cells in 0.1
ml medium were transferred to each well of a 96-well plate. Cells
were cultivated at 37°C, followed by measurement of OD values at
450 nm every 24 h for a total of 96 h. CCK solution (Sigma-Aldrich;
Merck KGaA) was added into each well at 4 h before the measurement
of OD values. The value of the C group at 96 h was set to ‘100’,
and all other groups were normalized to the C group.
Statistical analyses
Each experiment was performed in three biological
replicates. Data are expressed as the mean values. Comparison
between paired tissues was performed using a paired t-test. An
unpaired t-test was used to compare two independent groups.
Analysis of variance, followed by Tukey's post hoc test was used to
compare differences among multiple groups. The 62 patients with CRC
were divided into high and low UASR1 level groups (n=31) with the
median level of UASR1 in CRC tissues as the cut-off value. Survival
curves were plotted for the two groups based on follow-up data.
Survival curves were compared using a log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
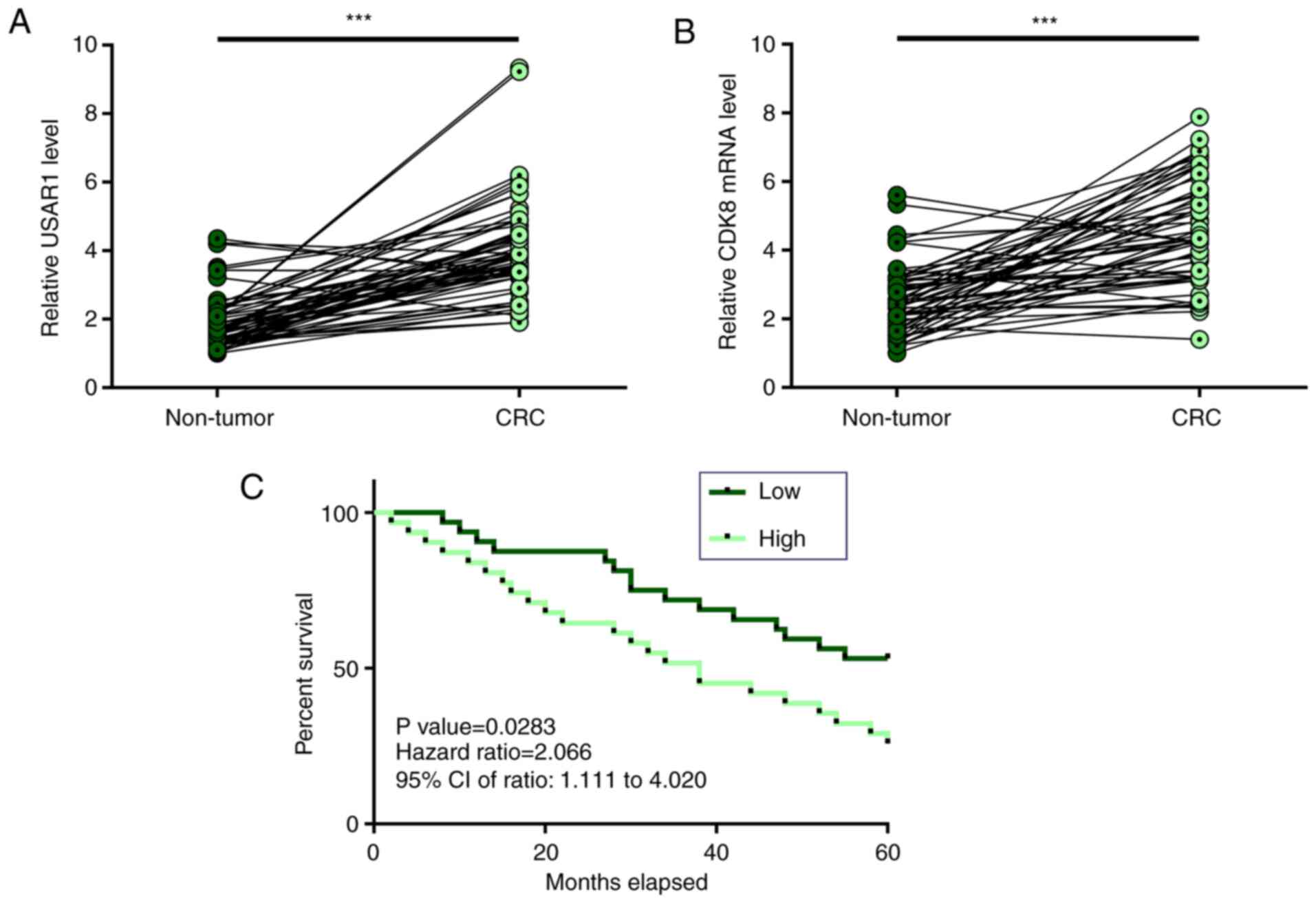
UASR1 and CDK8 were upregulated in CRC
and high expression levels of UASR1 in CRC predicted poor
survival
Expression of UASR1 and CDK8 in paired non-tumor and
CRC tissues from 62 patients with CRC were analyzed by performing
RT-qPCR. Compared with non-tumor tissues, the expression levels of
UASR1 (Fig. 1A) and CDK8 (Fig. 1B) were significantly higher in CRC
tissues (P<0.001). Survival curves were plotted for both high
and low UASR1 expression groups. Compared with patients in the low
expression group, patients in the high expression group exhibited
higher mortality rates (Fig. 1C). It
is worth noting that miR-107 expression was not significantly
correlated with patients' survival (P=0.488; HR=1.123; 95% CI:
0.598–2.117; Fig. S1A), while CDK8
expression was correlated with patients' survival (P=0.0167;
HR=2.376; 95% CI: 1.237–4.417; Fig.
S1B).
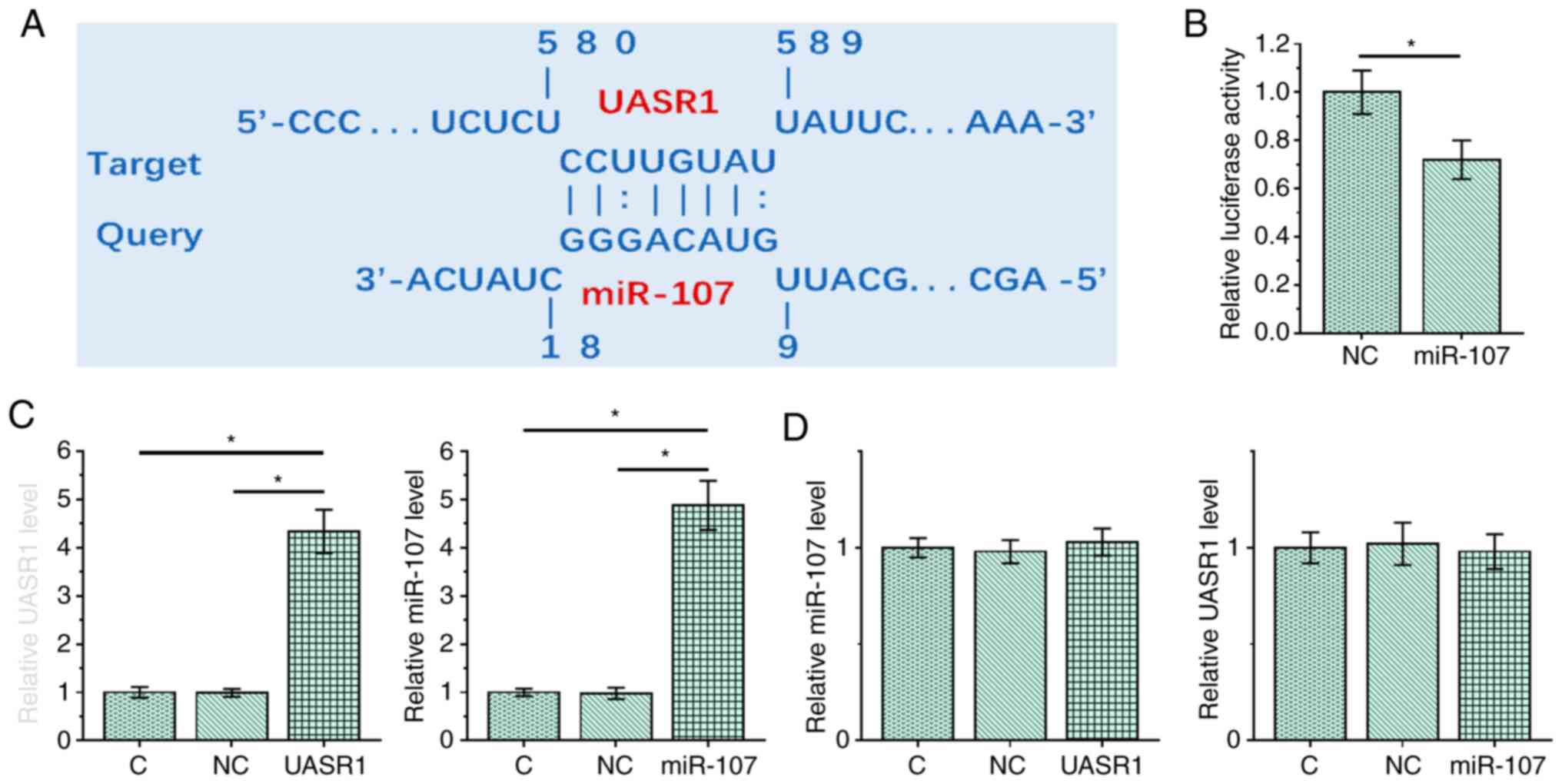
UASR1 and miR-107 interacted with each
other but did not regulate the expression of each other
The interaction between UASR1 and miR-107 was
predicted using IntaRNA2.0, which showed that UASR1 and miR-107
could form strong base pairing (Fig.
2A). The interaction between UASR1 and miR-107 was investigated
by performing dual luciferase activity assay, which was performed
by co-transfecting CR4 cells with UASR1 luciferase vector + miR-107
mimic (miR-107 group) or UASR1 luciferase vector + NC miRNA (NC
group). Compared with the NC group, relative luciferase activity
was significantly lower in miR-107 group (Fig. 2B; P<0.05). To further investigate
the interaction between UASR1 and miR-107, CR4 cells were
transfected with the UASR1 expression vector or miR-107-mimic.
Overexpression of UASR1 and miR-107 was confirmed by RT-qPCR
(Fig. 2C; P<0.05). Compared with
the NC and C groups, overexpression of UASR1 and miR-107 did not
significantly affect the expression of each other (Fig. 2D).
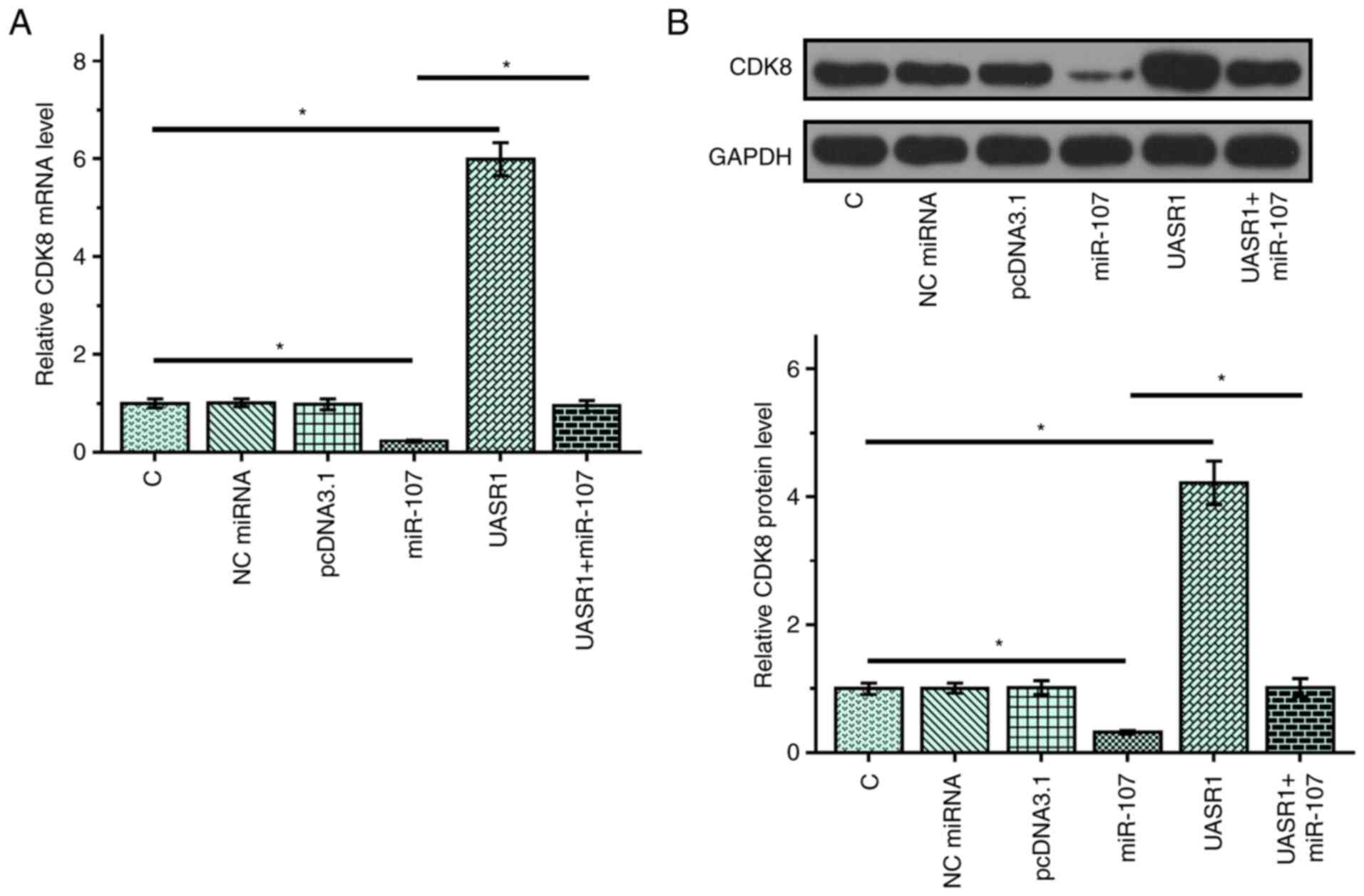
The expression of CDK8 was upregulated
in CR4 cells with overexpression of UASR1
To test the possibility of UASR1 as an internal
sponge of miR-107, the effects of overexpression of UASR1 and
miR-107 on the expression of CDK8, a miR-107 target, were evaluated
by RT-qPCR (Fig. 3A) and western
blot analysis (Fig. 3B). Compared
with the C group, overexpression of miR-107 led to the
downregulated expression of CDK8 (P<0.05). Overexpression of
UASR1 served an opposite role and decreased the effects of miR-107
overexpression (P<0.05).
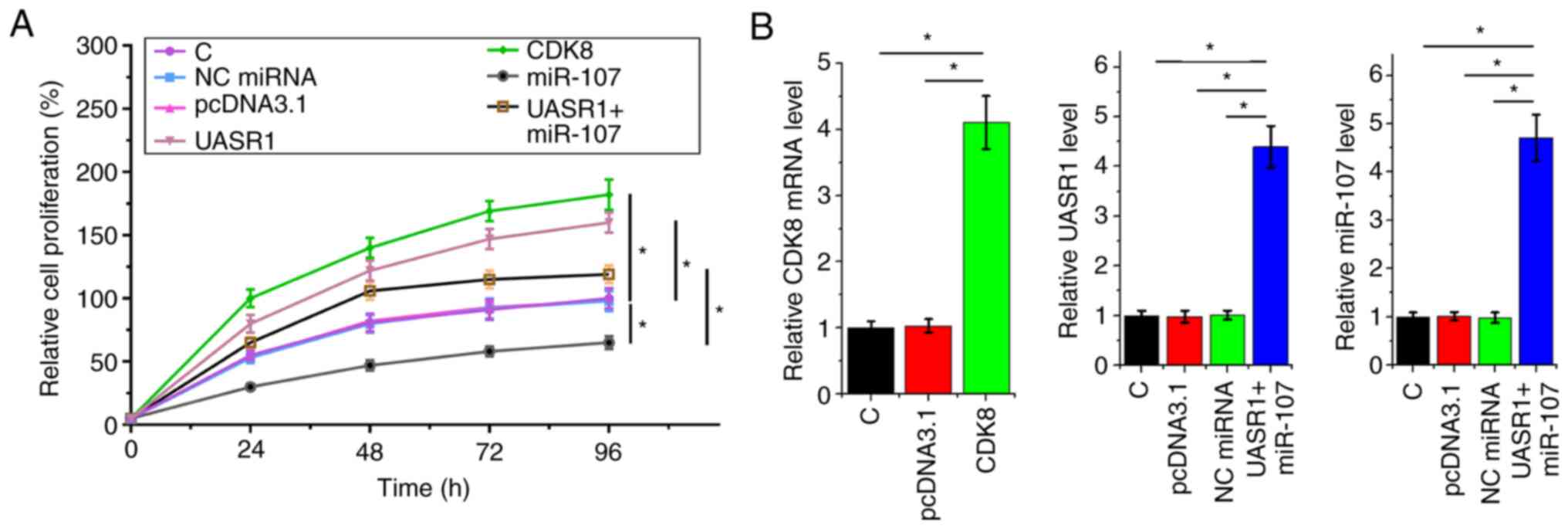
UASR1 promoted CR4 cell proliferation
through the miR-107/CDK8 axis
The effects of overexpression of UASR1, miR-107 and
CDK8 on the proliferation of CR4 cells were analyzed by performing
cell proliferation. Overexpression of UASR1 and CDK8 led to
increased proliferation rate of CR4 cells (P<0.05). By contrast,
overexpression of miR-107 led to decreased cell proliferation rate
(P<0.05). Furthermore, overexpression of UASR1 decreased the
inhibitory effects of miR-107 on cell proliferation (Fig. 4A; P<0.05). Overexpression of CDK8
in cells with CDK8 expression vector and the overexpression of
UASR1 and miR-107 in cells co-transfected with UASR1 expression
vector and miR-107 mimic were confirmed by RT-qPCR (Fig. 4B; P<0.05).
Discussion
In the present study, the interactions between
UASR1, miR-107 and CDK8 were studied in CRC. It was demonstrated
that UASR1 was upregulated in CRC and that it may upregulate CDK8
by sponging miR-107, thereby promoting CRC cell proliferation.
The functionality of UASR1 has only been
investigated in breast cancer (13).
It was observed that UASR1 was upregulated in breast cancer and
that it interacted with the AKT/mTOR pathway to promote the
migration and proliferation of CRC cells. Another study reported
that UASR1 was upregulated in CRC and that it interacted with the
mTOR signaling pathway to promote cancer cell proliferation
(14). The present study confirmed
the upregulation of UASR1 in CRC. In addition, increased
proliferation of CRC cells was observed following overexpression of
UASR1. Therefore, UASR1 may serve an oncogenic role in CRC by
promoting cancer cell proliferation.
Distant metastasis is common in patients with CRCs
(18). Once distant metastasis has
occurred, the prognosis will be extremely poor. The present study
demonstrated that the high expression levels of UASR1 were closely
correlated with the poor survival of patients with CRC. Therefore,
measuring the expression levels of UASR1 prior to therapy may aid
in the determination of treatment approaches, thereby improving the
survival of patients with CRC. It is worth noting that high
expression levels of CDK8 were also correlated with the poor
survival of patients with CRC, while miR-107 expression had no
significant effects on patient survival. The mechanism that
correlates CDK8, but not miR-107, with patient survival remains to
be investigated.
miR-107 serves tumor suppressive roles in several
types of cancer (15,19). However, the role of miR-107 in CRC is
unknown. A recent study reported that miR-107 may target CDK8 to
suppress the migration and proliferation of breast cancer cells
(15). The present study showed that
miR-107 also serves a tumor suppressive role in regulating CRC cell
proliferation by targeting CDK8.
The present study showed that UASR1 and miR-107
interacted with each other in CRC cells. However, overexpression of
miR-107 did not affect the expression of UASR1. Therefore, UASR1 is
unlikely to be a target of miR-107. Notably, overexpression of
UASR1 decreased the inhibitory effects of overexpression of miR-107
on the expression of CDK8 and CRC cell proliferation. The results
of the present study suggested that UASR1 is likely to be an
internal sponge of miR-107.
It is known that miR-107 may also target CDK6
(20). However, in the CR4 CRC cell
line used in the present study, miR-107-overexpression failed to
significantly affect the expression of CDK6. Therefore, miR-107 may
have different functions in different cells lines and/or different
cancer types. It is worth noting that multiple CRC cell lines were
used at the beginning. However, multiple cell transfections were
included in the present study, and the CR4 cell line is the only
cell line that showed satisfactory transfection efficiency of all
the transfections.
The present study characterized a novel UASR1/miR-
107/CDK8 pathway in CRC. However, the present study is limited by
the small sample size. In addition, the in vivo interactions
among UASR1, miR-107 and CDK8 have not been investigated. Future
studies are required to include in vivo animal experiments
and enroll more patients to further confirm these conclusions.
In conclusion, UASR1 is upregulated in CRC and was a
predictor of poor survival. UASR1 may sponge miR-107 to upregulate
CDK8, thereby promoting cancer cell proliferation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
QZZ performed the clinical studies, experiments
work, data analysis and manuscript writing. ZSC performed the
literature research, experiment design and manuscript revision.
Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of The Second Hospital of
Shandong University approved the present study (approval no.
CRC201233255). All the patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Favoriti P, Carbone G, Greco M, Pirozzi F,
Pirozzi RE and Corcione F: Worldwide burden of colorectal cancer: A
review. Updates Surg. 68:7–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engstrand J, Nilsson H, Stromberg C, Jonas
E and Freedman J: Colorectal cancer liver metastases-a
population-based study on incidence, management and survival. BMC
Cancer. 18:782018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mahasneh A, Al-Shaheri F and Jamal E:
Molecular biomarkers for an early diagnosis, effective treatment
and prognosis of colorectal cancer: Current updates. Exp Mol
Pathol. 102:475–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Symonds EL, Pedersen S, Cole SR, Massolino
J, Byrne D, Guy J, Backhouse P, Fraser RJ, LaPointe L and Young GP:
Improving participation in colorectal cancer screening: A
randomised controlled trial of sequential offers of faecal then
blood based non-invasive tests. Asian Pac J Cancer Prev.
16:8455–8460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka S: Molecular pathogenesis and
targeted therapy of pancreatic cancer. Ann Surg Oncol. 23 (Suppl
2):S197–S205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li XL, Zhou J, Chen ZR and Chng WJ: P53
mutations in colorectal cancer-molecular pathogenesis and
pharmacological reactivation. World J Gastroenterol. 21:84–93.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heinemann V, Douillard JY, Ducreux M and
Peeters M: Targeted therapy in metastatic colorectal cancer-an
example of personalised medicine in action. Cancer Treat Rev.
39:592–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lalevee S and Feil R: Long noncoding RNAs
in human disease: Emerging mechanisms and therapeutic strategies.
Epigenomics. 7:877–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arun G, Diermeier SD and Spector DL:
Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol
Med. 24:257–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao Z, Wu P, Su M, Ling H, Khoshaba R,
Huang C, Gao H, Zhao Y, Chen J, Liao Q, et al: Long non-coding RNA
UASR1 promotes proliferation and migration of breast cancer cells
through the AKT/mTOR pathway. J Cancer. 10:2025–2034. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang W, Wang Z, Wang H, Li X and Wang HT:
Promoting effect of PAX5-activated lncRNA UASR1 on growth of
colorectal cancer by regulating the mTOR pathway. Eur Rev Med
Pharmacol Sci. 24:2986–2993. 2020.PubMed/NCBI
|
|
15
|
Li XY, Luo QF, Wei CK, Li DF, Li J and
Fang L: miRNA-107 inhibits proliferation and migration by targeting
CDK8 in breast cancer. Int J Clin Exp Med. 7:32–40. 2014.PubMed/NCBI
|
|
16
|
Weiser MR: AJCC 8th edition: Colorectal
cancer. Ann Surg Oncol. 25:1454–1455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benson AB III, Bekaii-Saab T, Chan E, Chen
YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton
MJ, et al: Metastatic colon cancer, version 3.2013: Featured
updates to the NCCN guidelines. J Natl Compr Canc Netw. 11:141–152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu C, Xie Z and Peng Q: miRNA-107 enhances
chemosensitivity to paclitaxel by targeting antiapoptotic factor
Bcl-w in non small cell lung cancer. Am J Cancer Res. 7:1863–1873.
2017.PubMed/NCBI
|
|
20
|
Chen L, Zhang R, Li P, Liu Y, Qin K, Fa
ZQ, Liu YJ, Ke YQ and Jiang XD: P53-induced microRNA-107 inhibits
proliferation of glioma cells and down-regulates the expression of
CDK6 and Notch-2. Neurosci Lett. 534:327–332. 2013. View Article : Google Scholar : PubMed/NCBI
|