Introduction
Combination medicine treatment typically only
results in short-term effects in the treatment of malignant
leukemia (1). The combination of
fludarabine, cytarabine, granulocyte colony-stimulating factor and
idarubicin is traditionally used for standard induction regimens
for the treatment of leukemia (2).
Unfortunately, the use of various chemotherapeutics for the
treatment malignant leukemia has failed to improve the overall
survival, where only 40% of patients with leukemia achieve 5-year
survival (3). The reason behind
these modest results is considered to be caused by the combined use
of multiple drugs, which eventually leads to intrinsic or acquired
multidrug resistance (MDR) (4). MDR
remains a formidable challenge for the successful treatment of
leukemia and is the major cause resulting in relapse of leukemia
(5). Therefore, MDR has become a
potential target for therapeutic intervention. A number of studies
reported that the most common cause of MDR is the abnormally high
expression of ATP-binding cassette (ABC) transporters in leukemia
cells (6,7). In fact, it was shown that the
overexpression of ABCB1, ABCC1 and ABCG2 was involved in the efflux
of various antitumor agents out of the cells (8). These proteins have the ability to
transport a plethora of chemotherapeutics with diverse structures
and functions outside of the cells. Antagonizing these transporters
has been identified to prevent drug efflux, thereby increasing the
accumulation of the drug inside the cells and provide a more
effective treatment for patients with leukemia (9). Therefore, ABC transporters have been
proposed as promising targets to circumvent drug resistance and
enhance the therapeutic efficacy of chemotherapeutics.
Recently, ABCG2 has received increasing attention
due to its mutable pharmacological binding sites (10). Evidence suggested that the
upregulation of the ABCG2 gene may be closely associated with the
high recurrence rate and adverse therapeutic response of
hematological malignancies (11).
For instance, the latest data obtained from 178 elderly patients
with acute myeloid leukemia (AML) revealed that a subset of samples
with the worst overall survival rate and the highest incidence of
drug-resistant disease had upregulated expression levels of ABCG2
(12). Furthermore, ABCG2 was
recognized as a documented marker for the side population (SP)
phenotype, which is highly rich in leukemia stem cells (LSCs) and
protects LSCs from antineoplastic agents (13). These results suggested that ABCG2 may
be a potential treatment target for MDR in leukemia.
As a potential therapeutic target, effectively
exploiting ABCG2 transporter inhibitors is the most commonly
adopted approach to overcome MDR. Unfortunately, to date, the
majority of ABCG2 inhibitors have failed in clinical trials due to
their unfavorable side effects, insufficient therapeutic effects or
unpredictable pharmacokinetic interactions (14). Thus, determining novel functions of
drugs already in clinical use is one of the most imperative
strategies for identifying safer and more efficient ABCG2
inhibitors.
KD025 (also known as SLx-2119) is a novel selective
inhibitor of Rho-associated protein kinase 2 (ROCK) 2, which is
200-fold more selective for ROCK-2 than ROCK-1 (15). ROCK was reported to serve an
important role in numerous intracellular processes and the aberrant
activation of the Rho-kinase pathway has been proven to contribute
to cardiovascular, renal and neurological disorders in
non-hematopoietic cells (16). In
addition, a study reported that KD025 could restrain adipogenesis
in 3T3-L1 cells by regulating key pro-adipogenic factors; the
results further implied that KD025 may be a potential obesity agent
(17). Data from Zanin-Zhorov et
al (18) indicated that KD025
may restore impaired immune homeostasis and serve a role in
autoimmunity therapy.
Upon screening for novel ABCG2 inhibitors in
leukemia cells, KD025 was discovered to markedly potentiate the
efficacy of conventional chemotherapeutic agents in
ABCG2-overexpressing leukemia cells and primary leukemia blast
cells derived from patients with leukemia. In addition, KD025
significantly inhibited the efflux of [3H]-mitoxantrone
and the accumulation of higher levels of
[3H]-mitoxantrone in HL60/ABCG2 cells. Thus, the present
study aimed to determine the effects of KD025 on the protein
expression levels and cellular location of ABCG2 in leukemia cells.
The findings of the current study may provide a novel perspective
for overcoming MDR in malignant leukemia.
Materials and methods
Chemicals and reagents
KD025 was purchased from Selleck Chemicals.
Mitoxantrone, topotecan, cisplatin and fumitremorgin C (FTC) were
purchased from Sigma-Aldrich (Merck KGaA). RPMI 1640, bovine serum
albumin (BSA), fetal bovine serum (FBS), penicillin/streptomycin
and 0.25% trypsin were purchased from HyClone (Cytiva). Primary
monoclonal antibody against ABCG2 (cat. no. MAB4145; clone BXP-34)
and AlexaFluor488-conjugated goat anti-mouse IgG secondary antibody
(cat. no. A-10684) were purchased from Thermo Fisher Scientific,
Inc. HRP-conjugated rabbit anti-sheep IgG secondary antibody (cat.
no. AP147P) was purchased from Sigma-Aldrich (Merck KGaA). Primary
antibody against GAPDH (cat. no. KC-5G4) was purchased from
Aksomics Inc. [3H]-mitoxantrone (4 Ci/mmol) was
purchased from Moravek Biochemicals Inc. DMSO, MTT, DAPI and
paraformaldehyde were purchased from Sigma-Aldrich (Merck KGaA).
Mitoxantrone, topotecan and FTC were used in place of KD025 as
positive controls to confirm the mechanism of drug resistance in
leukemia cell line models. Cisplatin (a non-substrate of ABCG2) was
used as a negative control.
Cell lines and culture
The human leukemia cell lines HL60 and K562 were
purchased from the Institute of Hematology & Blood Diseases
Hospital, Chinese Academy of Medical Sciences & Peking Union
Medical College. P388 cell lines were purchased from the Cell Bank,
Institute of Cell Biology, Chinese Academy of Sciences. HL60/ABCG2,
K562/ABCG2, and P388/ABCG2 cells (which overexpress ABCG2) were
established by the transduction of HL60, K562 and P388 cells,
respectively, with a Ha-breast cancer resistant protein (BCRP)
retrovirus that carried Myc-tagged human BCRP (ABCG2) cDNA in the
Ha retrovirus vector (19,20). HL60, HL60/ABCG2, K562 and K562/ABCG2
cell lines were cultured in RPMI 1640 containing 10% FBS, 100 U/ml
penicillin and 100 U/ml streptomycin at 37°C with 5%
CO2.
Cytotoxicity evaluation by MTT
assay
MTT (purity 99.59%) reagent was used to determine
the cell sensitivity to drugs with minor modifications as described
previously (21). The
IC50, which was defined as the drug concentration
resulting in 50% cell death, was calculated from survival curves
using the Bliss method. Cells were collected and seeded in 96-well
plates with appropriate density (5×103 cells/well in 160
µl medium). After plating for 24 h at 37°C, cells were
pre-incubated with 0.25, 0.5 and 1 µM KD025 for another 72 h at
37°C. Subsequently, the cells were treated with a range of
concentrations of chemotherapeutic agents by 2-fold dilution
(mitoxantrone range, 0.5–50 µM; topotecan range, 0.5–50 µM; and
cisplatin range, 0.5–50 µM) for another 68 h at 37°C, and 5 mg/ml
MTT (20 µl/well) was added to the cells and further incubated for 4
h (37°C). Subsequently, the medium was discarded, and 200 µl DMSO
was added to each well to dissolve the formazan product formed from
the metabolism of MTT. The absorbance was determined at a
wavelength of 540 nm with a background subtraction at 670 nm using
a Model 550 microplate reader (Bio-Rad Laboratories, Inc.)
(22). The resistance fold-change
was calculated by dividing the IC50 values of substrates
in the presence or absence of the inhibitor by the IC50
of the parental cells without inhibitor treatment (23).
Patient samples
The present study was approved by the Ethics Review
Committee of Sun Yat-Sen University. Bone marrow blood (3 ml) was
obtained from nine patients diagnosed with AML or acute
lymphoblastic leukemia (ALL) according to FAB classification
(24). All patients provided written
informed consent. Leukemia blasts were isolated using
Ficoll-Hypaque density gradient centrifugation and cultured in
RPMI-1640 medium containing 20% FBS (25). Western blotting was performed to
determine ABCG2 expression levels in patient samples.
[3H]-mitoxantrone
accumulation and efflux assays
Following treatment for 12 h with or without 0.25,
0.5 or 1 µM KD025, 0.2 µM [3H]-mitoxantrone was added
into the and incubated for another 2 h at 37°C. Subsequently, the
cells were washed three times with ice-cold PBS and lysed in 10 mM
lysis buffer. The radioactivity of the cells was measured using a
Packard TRI-CARB® 1900CA liquid scintillation analyzer
from PerkinElmer, Inc.
Following the accumulation assay, the cells were
incubated in the presence or absence of 0.25, 0.5 or 1 µM KD025 and
2.5 µM FTC overnight. Subsequently, the cells were suspended in
PRMI 1640 medium containing 0.2 µM [3H]-mitoxantrone
with or without reversal agent at 37°C for 2 h. After washing three
times with ice-cold PBS, the cells were collected at various time
points (0, 60, 120 and 240 min). Each sample was placed in
scintillation fluid and the radioactivity was analyzed as described
previously (26).
Western blotting
Western blotting was performed to test the
expression levels of ABCG2 protein after treatment with 0.25, 0.5
or 1 µM KD025 for 48 h or with 2.5 µM KD025 for 0, 24, 36, 48 and
72 h (27). Following 12 h of
incubation with 0.25, 0.5 or 1 µM KD025, whole cells were harvested
and washed twice with ice-cold PBS. Cell extracts were collected
using a cell lysis buffer (PBS containing 1% Nonidet P-40, 0.5%
sodium deoxycholate, 0.1% SDS, 100 mg/ml PMSF, 10 mg/ml aprotinin
and 10 mg/ml leupeptin). Protein concentration was determined using
a BCA Protein assay (Thermo Fisher Scientific, Inc.). Equal amounts
of protein (60 µg/lane) were separated via 10% SDS-PAGE. The
separated proteins were subsequently transferred onto
nitrocellulose membranes and blocked with TBS-Tween-20 (TBST)
buffer (10 mmol/I Tris-HCl, 150 mmol/I NaCl and 0.1% Tween-20; pH
8.0) for 2 h at room temperature. The membranes were then incubated
overnight at 4°C with primary monoclonal antibodies against ABCG2
(1:200) or GAPDH (1:1,000). After washing three times with TBST,
the membranes were incubated with HRP-conjugated secondary antibody
(1:5,000) for 2 h at room temperature. The protein-antibody
complexes were then washed with TBST, and protein bands were
visualized using an enhanced chemiluminescence detection system
(Phototope TM-HRP Detection kit; Cell Signaling Technology, Inc.).
The protein bands were analyzed using Scion Image 4.0.3 software
(Scion Corporation). The protein expression levels were quantified
using gray value analysis software (Image Lab 3.0; Bio-Rad
Laboratories, Inc.) GAPDH was used as a loading control.
Immunofluorescence staining
Following overnight incubation in 24-well plates,
2.5 µM KD025 was added to the cells and incubated for 72 h. The
cells were then fixed in 4% paraformaldehyde for 15 min and
permeabilized using 0.1% Triton X-100 for 10 min both at room
temperature, before blocking with 6% BSA for 1 h at room
temperature. Subsequently, the cells were incubated overnight at
4°C with a monoclonal antibody against ABCG2 (1:500). Following
incubation, the cells were washed with ice-cold PBS and incubated
with AlexaFluor488-conjugated goat anti-mouse IgG secondary
antibody for 1 h (1:1,000). Cell nuclei were dyed with 1 µg/ml DAPI
for 72 h at 4°C (Sigma-Aldrich; Merck KGaA). Immunofluorescence
images were captured using an inverted confocal microscope in 6–8
random microscopic fields (magnification, ×400; model IX70; Olympus
Corporation) with IX-FLA fluorescence and a Charge-Coupled Device
camera.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
(SPSS, Inc.). Data are presented as the mean ± SD of 3–5
independent experimental repeats. Statistical differences between
the data were determined using one-way ANOVA and Student's t-test.
One-way ANOVA was used to assess significant differences between
the means of multiple groups, followed by Dunnett's post hoc test.
Significant differences between two groups were evaluated using the
unpaired Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
KD025 markedly enhances the
cytotoxicity of antitumor drugs in leukemia cells overexpressing
ABCG2
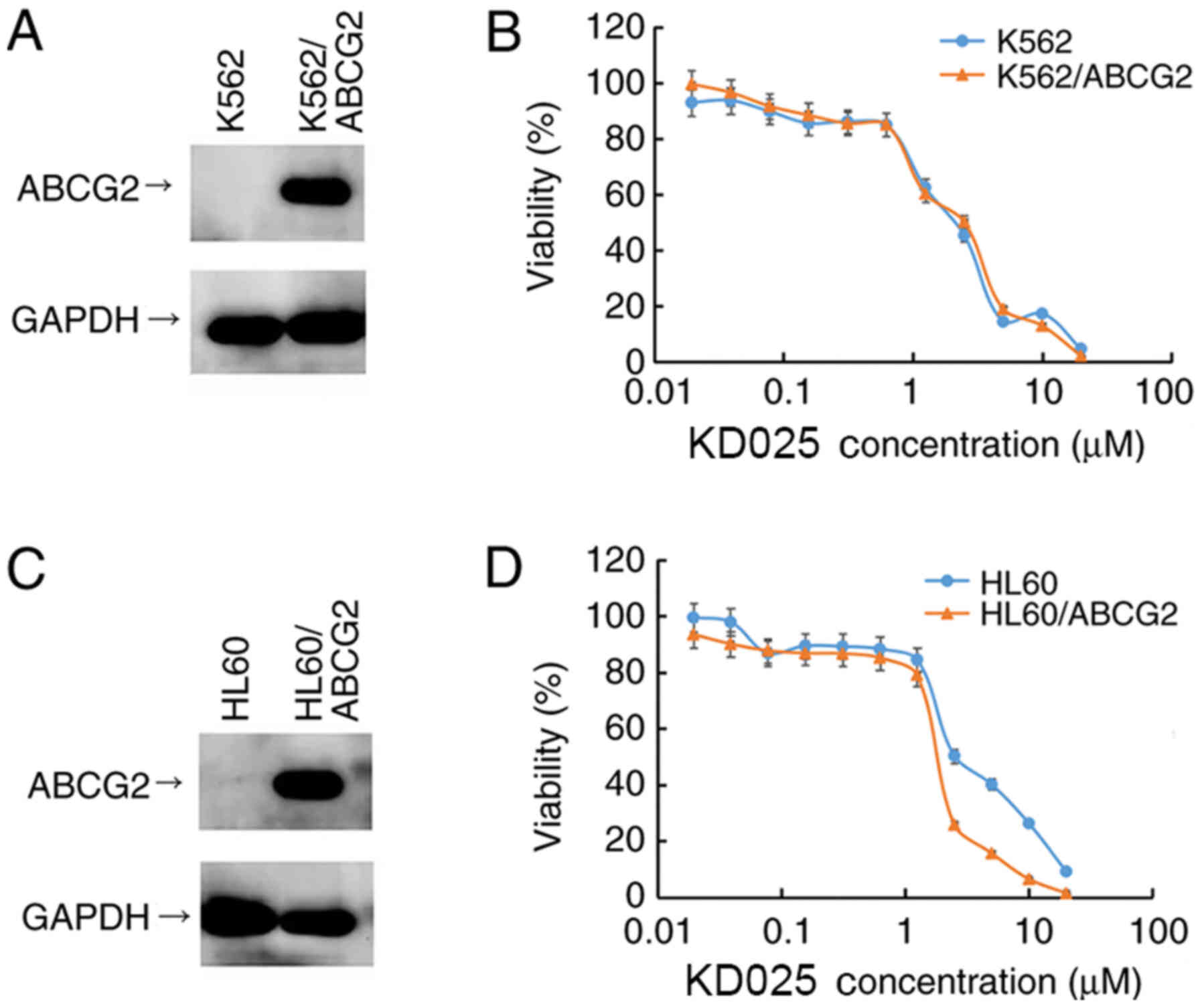
HL60/ABCG2 and K562/ABCG2 cell lines were
established by transfecting HL60 and K562 cells with a HaBCRP
retrovirus, which were subsequently selected for treatment with 4.0
µM mitoxantrone for 7 days. Prior to investigating the cytotoxicity
of KD025, the expression levels of the ABCG2 protein in the
transfected cell lines used in the study were confirmed using
western blotting analysis, respectively. The protein expression
levels of ABCG2 were overexpressed in K562/ABCG2 and HL60/ABCG2
cell lines compared with their parental cell lines HL60 and K562,
respectively (Fig. 1A and C).
MTT assays were subsequently performed to determine
the cytotoxicity of KD025 treatment in different leukemia cell
lines. As shown in Fig. 1B and D,
>70% of the ABCG2-overexpressing cell lines, HL60/ABCG2 and
K562/ABCG2, and their parental cell lines, HL60 and K562, survived
1 µM KD025 treatment, indicating that KD025 may be used as a
treatment up to a concentration of 1 µM. Therefore, all following
antineoplastic drug combination assays were performed with ≤1 µM
KD025. Based on these findings, the IC50 of various
drugs in leukemia-sensitive cells and in their resistant
counterparts with or without the accompanying treatment with
different concentrations of KD025 were determined (Tables I and II). The IC50 values of
mitoxantrone and topotecan in HL60/ABCG2 and K562/ABCG2 cell lines
were markedly higher than their respective values in HL60 and K562
cell lines (P<0.05). Following treatment with KD025, the
cytotoxicity of mitoxantrone and topotecan significantly increased
in both ABCG2-overexpressing HL60/ABCG2 and K562/ABCG2 cell lines
compared with cell lines without KD025 treatment, but not in their
parental HL60 or K562 cell lines. The IC50 of
mitoxantrone in both HL60/ABCG2 and K562/ABCG2 cells reduced from
17.217±1.058 to 0.891±0.042 µM and from 23.581±0.59 to 0.992±0.040
µM, respectively. Meanwhile, the IC50 of topotecan in
HL60/ABCG2 and K562/ABCG2 cells decreased from 18.726±1.054 to
0.975±0.039 µM and from 17.995±0.661 to 0.781±0.022 µM,
respectively. In addition, the effect of KD025 was similar to that
of 2.5 µM FTC, which was sensitive to ABCG2-overexpressing cells
and used as a positive control inhibitor of ABCG2. Conversely,
KD025 treatment did not alter the IC50 value of
cisplatin, which is not a substrate of ABCG2. These results
suggested that KD025 may significantly potentiate the cytotoxicity
of mitoxantrone and topotecan in ABCG2-overexpressing leukemia cell
lines in a concentration-dependent manner.
 | Table I.Effects of KD025 on reversing
ABCG2-mediated MDR in HL60 and HL60/ABCG2 cells. |
Table I.
Effects of KD025 on reversing
ABCG2-mediated MDR in HL60 and HL60/ABCG2 cells.
|
| IC50 ±
SD (µM) (Resistance fold) |
|---|
|
|
|
|---|
| Treatment | HL60 | HL60/ABCG2 |
|---|
| Mitoxantrone | 0.942±0.067
(1.00) | 17.217±1.058
(18.23) |
| + 0.25
µM KD025 | 0.980±0.083
(1.04) | 4.404±0.089
(4.29)b |
| + 0.5
µM KD025 | 0.928±0.074
(0.99) | 1.862±0.050
(1.98)b |
| + 1 µM
KD025 | 0.937±0.081
(0.99) | 0.891±0.042
(0.95)b |
| + 2.5
µM FTC | 0.506±0.039
(0.54)a | 0.825±0.031
(0.88)b |
| Cisplatin | 13.416±0.094
(1.00) | 20.173±0.920
(1.50) |
| + 1 µM
KD025 | 14.003±0.113
(1.04) | 19.251±0.908
(1.43) |
| Topotecan | 0.75±0.035
(1.00) | 18.726±1.054
(24.97) |
| + 0.25
µM KD025 | 0.648±0.030
(0.86) | 5.631±0.085
(7.51)b |
| + 0.5
µM KD025 | 0.601±0.025
(0.80) | 3.022±0.064
(4.03)b |
| + 1 µM
KD025 | 0.475±0.017
(0.63) | 0.975±0.039
(1.30)b |
| + 2.5
µM FTC | 0.248±0.018
(0.33)a | 0.860±0.024
(1.15)b |
| Cisplatin | 14.002±0.097
(1.00) | 18.566±1.079
(1.33) |
| + 1 µM
KD025 | 16.051±0.147
(1.15) | 19.805±1.093
(1.41) |
 | Table II.Effects of KD025 on reversing
ABCG2-mediated MDR in K562 and K562/ABCG2 cells. |
Table II.
Effects of KD025 on reversing
ABCG2-mediated MDR in K562 and K562/ABCG2 cells.
|
| IC50 ±
SD (µM) (Resistance fold) |
|---|
|
|
|
|---|
| Treatment | K562 | K562/ABCG2 |
|---|
| Mitoxantrone | 1.536±0.027
(1.00) | 23.581±0.59
(15.16) |
| + 0.25
µM KD025 | 1.604±0.031
(1.04) | 7.041±0.098
(4.58)b |
| + 0.5
µM KD025 | 0.946±0.015
(0.62) | 2.809±0.047
(1.83)b |
| + 1 µM
KD025 | 0.633±0.014
(0.41)a | 0.992±0.040
(0.65)b |
| + 2.5
µM FTC | 0.482±0.018
(0.31)a | 1.057±0.013
(0.69)b |
| Cisplatin | 16.517±1.815
(1.00) | 17.092±1.659
(1.04) |
| + 1 µM
KD025 | 14.672±1.539
(0.89) | 16.590±1.731
(1.00) |
| Topotecan | 0.953±0.017
(1.00) | 17.995±0.661
(18.88) |
| + 0.25
µM KD025 | 0.841±0.021
(0.88) | 6.118±0.069
(6.42)b |
| + 0.5
µM KD025 | 0.710±0.013
(0.75) | 2.35±0.037
(2.47)b |
| + 1 µM
KD025 | 0.551±0.013
(0.58)a | 0.781±0.022
(0.82)b |
| + 2.5
µM FTC | 0.630±0.014
(0.66)a | 0.740±0.014
(0.78)b |
| Cisplatin | 13.915±1.489
(1.00) | 17.620±1.857
(1.27) |
| + 1 µM
KD025 | 15.481±1.693
(1.11) | 16.729±1.319
(1.20) |
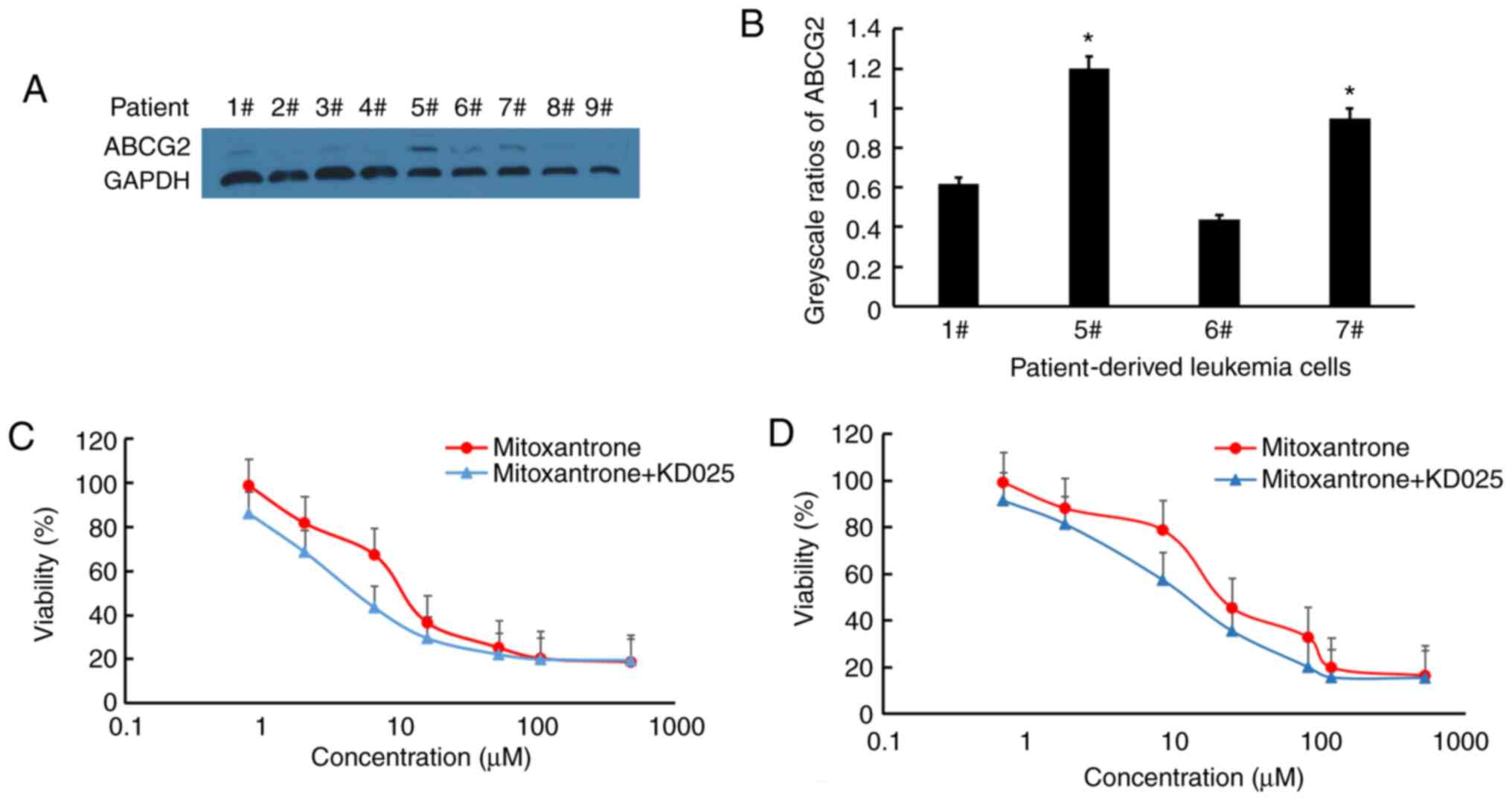
KD025 significantly increases the
cytotoxicity of mitoxantrone in patient-derived leukemic blast
cells
ABCG2 is widely expressed in patients with AML and
ALL (28). Thus, the expression
levels of ABCG2 and the cytotoxicity of mitoxantrone with or
without KD025 treatment in leukemia blast cells derived from
patients with leukemia were analyzed (Fig. 2). The results demonstrated that 4 of
9 patient samples displayed detectable ABCG2 expression (patient
nos. 1, 5, 6 and 7); the expression levels of ABCG2 in patient nos.
5 and 7 were significantly higher than the average of ABCG2
expression in the four samples, while the leukemic blast cells
derived from patient nos. 1 and 6 with low expression levels of
ABCG2 were excluded from subsequent analyses due to possible
experimental errors (P<0.05; Fig. 2A
and B). Treatment of leukemic blast cells with 2.5 µM KD025
effectively increased their sensitivity to the cytotoxicity of
mitoxantrone in leukemic blast cells derived from patient nos. 5
and 7 (Fig. 2C and D, respectively).
The results suggested that the combined use of KD025 and
mitoxantrone may result in effective clinical effects.
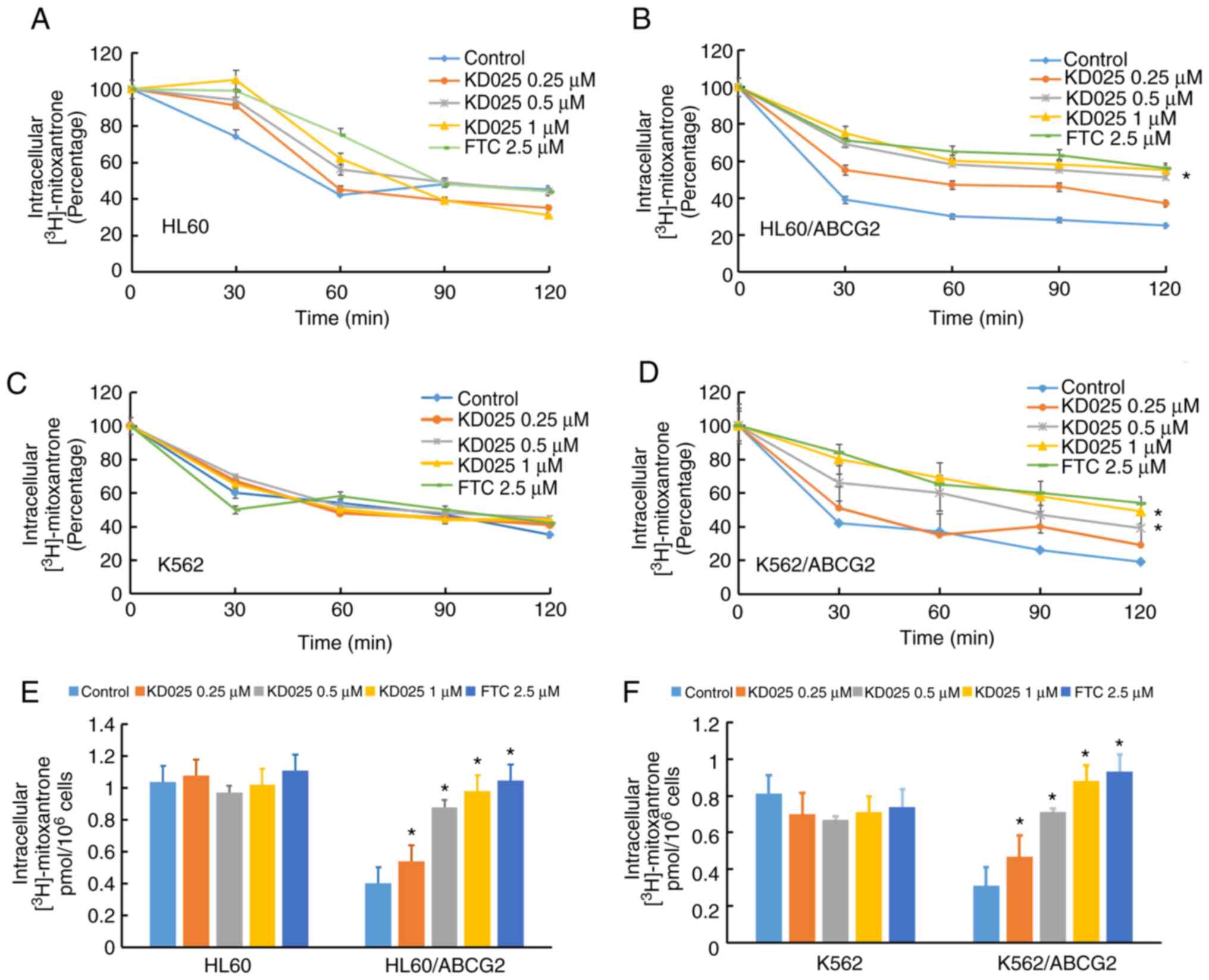
KD025 increases the accumulation of
[3H]-mitoxantrone and antagonizes its efflux in
ABCG2-overexpressing leukemia cell lines
Transporter inhibitors typically enhance anticancer
activity through preventing transporter-mediated efflux, which
leads to an increase in the accumulation of the intracellular drug
(29). To investigate the potential
mechanism of KD025 sensitizing ABCG2-overexpressing leukemia cells
to antineoplastic drugs, the intracellular levels of
[3H]-mitoxantrone were analyzed in the presence or
absence of KD025 treatment. KD025 treatment significantly increased
the intracellular accumulation levels of
[3H]-mitoxantrone in HL60/ABCG2 cells (Fig. 3B). Meanwhile, the accumulative effect
of [3H]-mitoxantrone following 1 µM KD025 treatment was
similar to that with 2.5 µM FTC. In addition, KD025 treatment
significantly reduced the efflux of [3H]-mitoxantrone in
HL60/ABCG2 cells compared with in cells without KD025 treatment
(Fig. 3E). Similarly, pretreatment
of KD025 also effectively improved the intracellular levels of
[3H]-mitoxantrone and decreased its efflux in K562/ABCG2
cells (Fig. 3B, D and F). However,
KD025 treatment did not significantly alter the efflux or
accumulation of [3H]-mitoxantrone in the parental HL60
or K562 cells (Fig. 3A and C). These
results indicated that KD025 treatment may increase the
intracellular accumulation of [3H]-mitoxantrone and
antagonize its efflux in ABCG2-overexpressing leukemia cell lines
in a concentration-dependent manner.
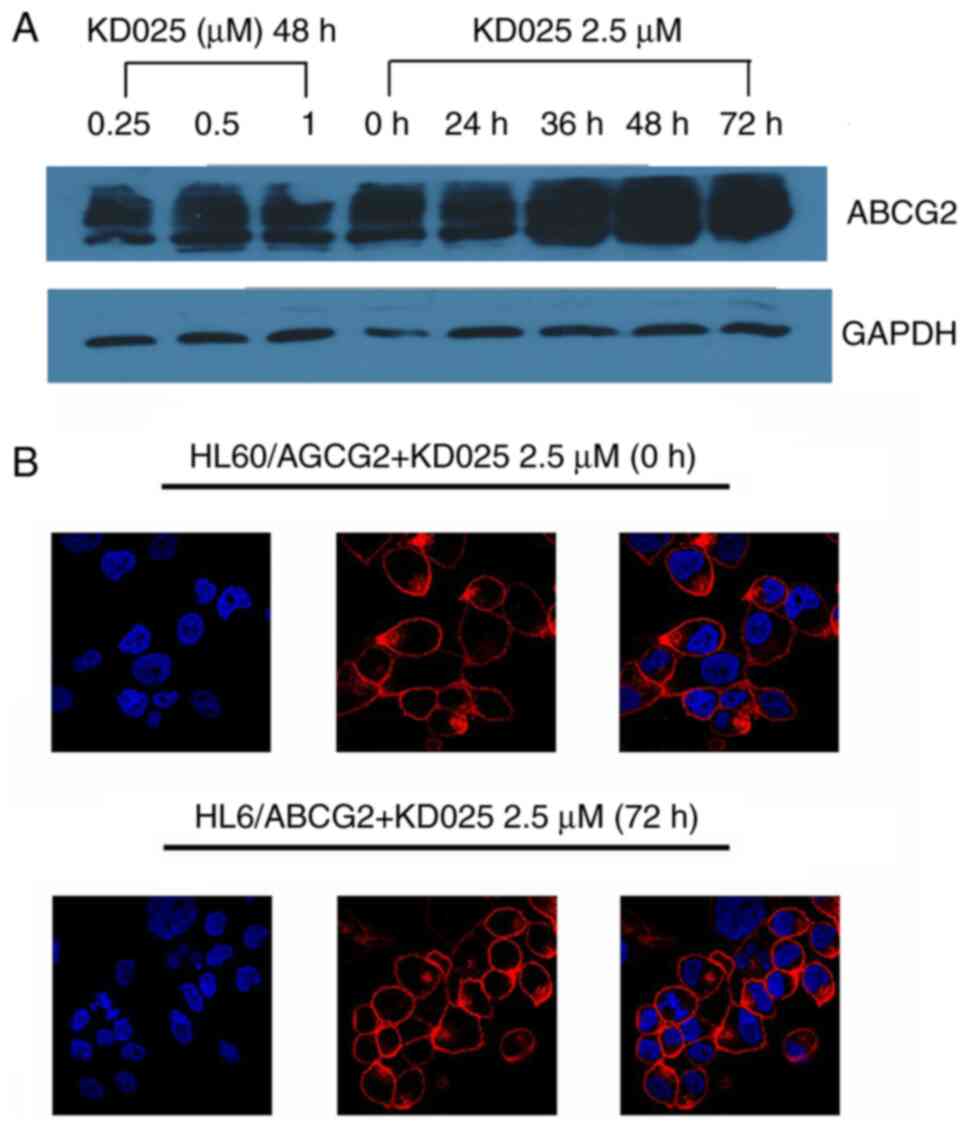
KD025 does not alter the expression
levels and subcellular locations of ABCG2
To determine the effect of KD025 treatment on the
expression levels and subcellular locations of ABCG2, western
blotting and immunofluorescence assays were performed on HL60/ABCG2
cells. ABCG2 protein expression levels were not altered following
72 h of treatment with 2.5 µM KD025 or with treatment of different
concentrations KD025 for 48 h in HL60/ABCG2 cell lines (Fig. 4A). In addition, there were no
significant changes identified in the cellular localizations of the
ABCG2 transporters following the treatment with 1 µM KD025 for up
to 72 h in HL60/ABCG2 cell lines (Fig.
4B). The present results suggested that the reversal effects of
KD025 were not accomplished by altering the expression levels nor
changing the intracellular localization of ABCG2 in HL60/ABCG2 cell
lines.
Discussion
Chemotherapy is the most effective therapeutic
method for patients with malignant leukemia, and the development of
standard induction therapy has resulted in complete hematological
remission (2). However, in recent
decades, the survival rate of leukemia has remained very low
(30), without any significant
improvements being made. The main cause is that most patients with
leukemia are either resistant to any initial treatment or acquire
resistance to chemotherapy (31).
Thus, MDR, which is described as the resistance to structurally and
functionally unrelated drugs, remains a challenge to the successful
treatment of patients with malignant leukemia undergoing
chemotherapy (32). MDR that occurs
at the molecular level has been reported to be more difficult to
overcome due to the reduced toxicity of intracellular antitumor
drugs (33). One mechanism of
cellular MDR is the overexpression of the ABC superfamily of
membrane transporters; aberrant activation of the drug efflux pumps
of the ABC protein effectively reduce intracellular concentration
of antineoplastic drugs (34). It
was reported that ABCB1 (also known as P-glycoprotein and MDR1),
ABCC1 (MRP1) and ABCG2 (BCRP) were closely associated with MDR in
numerous types of cancer cells, such as high-grade serous ovarian
carcinoma, refractory acute lymphoblastic leukemia and anaplastic
lymphoma kinase-rearranged lung cancer (35). These transporters have the ability to
efflux a large number of antitumor drugs from the cells, but each
transporter has its own unique substrates (4). Therefore, inhibiting the efflux
function of these proteins may provide more promising therapeutic
effects for patients with leukemia.
Notably, the BCRP gene encoding ABCG2 has
demonstrated enhanced clinical significance in malignant leukemia.
For example, a study on AML revealed that 33% of AML blasts had
upregulated BCRP expression levels and were inversely correlated
with disease prognosis and overall survival (36). In addition, ABCG2 has been widely
recognized as a promising therapeutic target for eradication of
LSCs, since ABCG2 is highly enriched in LSCs and serves a crucial
role in the differentiation, proliferation and self-renewal of LSCs
(37). Although intensive methods
and drugs have been designed to inhibit ABCG2, unfortunately, even
with the most advanced treatments to date, the reversal of
ABCG2-mediated MDR remains unsatisfactory and the relapse risk of
patients with leukemia remains high in the first two years
(38). Furthermore, as of yet,
appropriate inhibitors of ABCG2 are lacking, since these agents are
not very potent or are toxic, and their ability to inhibit ABCG2
has not been verified in patients (4). Thus, exploiting effective and safe
ABCG2 transporter inhibitors is currently the considered method to
improve the clinical efficacy of leukemia chemotherapy.
ROCK1 and ROCK2, and their isoform-mediated signals,
have been reported to be responsible for cell morphology, adhesion
and migration (39). Furthermore,
the ROCK pathway was also discovered to serve an important role in
physiological conditions and the abnormal activation of
myeloproliferative diseases (40). A
previous study demonstrated that ROCK inhibition effectively
reduced leukemic cell proliferation (41). Therefore, the deregulation of ROCK
signaling is emerging as a potential key target in leukemia. KD025
is a highly selective inhibitor of ROCK2 and has entered Phase I
clinical trials for psoriasis (15),
idiopathic pulmonary fibrosis and systemic sclerosis (42). A previous study revealed that KD025
treatment inhibited the adipocyte differentiation at the
intermediate stage in 3T3-L1 preadipocytes (43). Moreover, KD025 was suggested to
restore impaired immune homeostasis and serve a role in
autoimmunity therapy (44). The
present study indicated that KD025 treatment may be an effective
inhibitor for reversing MDR in leukemia cell lines. These results
were consistent with other previous studies on the ROCK signaling
pathway in leukemia (41,45). However, the reversal efficiency and
mechanism of KD025 treatment in ABCG2-mediated MDR remains
unclear.
Data from the present study revealed that KD025
treatment may interact with ABCG2 in leukemia cells. Cytotoxicity
assays demonstrated that KD025 treatment significantly altered the
sensitivity of the ABCG2 substrates mitoxantrone and topotecan in
ABCG2-overexpressing leukemia cells in a dose-dependent manner.
However, KD025 treatment did not increase the cytotoxicity of
cisplatin, which is not a substrate of ABCG2. Furthermore, no
significant changes were identified in the antitumor effects in the
drug-sensitive parental leukemia HL60 and K562 cell lines. These
data suggested that the combination of KD025 treatment with
chemotherapeutic drugs may exert a preferable antineoplastic
effect. Moreover, the present study also investigated the
efficiency of KD025 in patient-derived leukemia blast cells. The
results indicated that KD025 treatment enhanced the cytotoxicity of
mitoxantrone in ABCG2-overexpressing samples. The significant
inhibitory effects of KD025 treatment on leukemia cells also
prompted the determination of its underlying mechanism. The drug
accumulation assay demonstrated that KD025 treatment effectively
improved the intracellular accumulation of mitoxantrone and
antagonized its efflux in ABCG2-overexpressing leukemia cells. It
has been previously proposed that ABCG2 inhibitors are divided into
two subtypes: Those only inhibiting ABCG2 activity (most ABCG2
inhibitors) and those that inhibit ABCG2 activity in addition to
reducing the expression levels of ABCG2 (for example afatinib)
(46). Thus, the present study
investigated the effects of KD025 treatment at different
concentrations on the protein expression levels and intracellular
locations of ABCG2 in leukemia cells through western blotting and
immunofluorescence assays, respectively. The results revealed that
KD025 treatment did not alter the expression levels or location of
ABCG2 in HL60/ABCG2 cell lines. Therefore, KD025 may exert its
reversal effect by directly inhibiting ABCG2 activity. Generally,
ABCG2 exerts its efflux functions depending on the ATPase activity,
of which the ATPase activity of ABC transporters is stimulated in
the presence of transport substrates (29,32).
Most reversal drugs block drug transport by acting as competitive
substrates that occupy the valid sites to prevent the drug
transport out of the cell (9,47). A
recent report suggested that KD025 may decrease the activity of ATP
in pulmonary microvascular endothelial cells (48). Thus, KD025 treatment may exert an
inhibitory effect on ABCG2 in leukemia cells through competitive
substrate transport blockade.
In conclusion, the results of the present study
suggested that KD025 treatment may significantly improve the
sensitivity of leukemia cells to chemotherapeutics by antagonizing
the efflux function of ABCG2. Therefore, KD025 treatment combined
with ABCG2 substrate antitumor drugs may be beneficial for the
treatment of MDR in patients with leukemia. While the anti-tumor
efficacy of the drug combination has been demonstrated, further
studies are still warranted to determine whether KD025 exerts
effects on LSCs or has any cell type or tissue specificity.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX and JP designed the research theme. WJ, XZ and RC
designed methods and experiments, performed the laboratory
experiments, analyzed the data, interpreted the results and wrote
the paper. WL, XY, WY and MZ co-designed the experiments and
discussed the analyses, interpretation and presentation of data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol for patient samples
studies was reviewed and approved by the Ethics Review Committee of
Sun Yat-Sen University. All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kayser S and Levis MJ: Advances in
targeted therapy for acute myeloid leukaemia. Br J Haematol.
180:484–500. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Kouchkovsky I and Abdul-Hay M: ‘Acute
myeloid leukemia: A comprehensive review and 2016 update’. Blood
Cancer J. 6:e4412016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sarkadi B, Homolya L, Szakács G and Váradi
A: Human multidrug resistance ABCB and ABCG transporters:
Participation in a chemoimmunity defense system. Physiol Rev.
86:1179–1236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robey RW, Pluchino KM, Hall MD, Fojo AT,
Bates SE and Gottesman MM: Revisiting the role of ABC transporters
in multidrug-resistant cancer. Nat Rev Cancer. 18:452–464. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaffer BC, Gillet JP, Patel C, Baer MR,
Bates SE and Gottesman MM: Drug resistance: Still a daunting
challenge to the successful treatment of AML. Drug Resist Updat.
15:62–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steinbach D, Sell W, Voigt A, Hermann J,
Zintl F and Sauerbrey A: BCRP gene expression is associated with a
poor response to remission induction therapy in childhood acute
myeloid leukemia. Leukemia. 16:1443–1447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yano K, Okabe C, Fujii K, Kato Y and
Ogihara T: Regulation of breast cancer resistance protein and
P-glycoprotein by ezrin, radixin and moesin in lung, intestinal and
renal cancer cell lines. J Pharm Pharmacol. 72:575–582. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR,
Liu DG, Ashby CJ Jr, Huang Y, Robey RW, Liang YJ, et al: Lapatinib
(Tykerb, GW572016) reverses multidrug resistance in cancer cells by
inhibiting the activity of ATP-binding cassette subfamily B member
1 and G member 2. Cancer Res. 68:7905–7914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strope JD, Peer CJ, Sissung TM, Hall OM,
Huang PA, Harris EM, Gustafson KR, Henrich CJ, Sigano DM, Pauly GT,
et al: Botryllamide G is an ABCG2 inhibitor that improves lapatinib
delivery in mouse brain. Cancer Biol Ther. 21:223–230. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yeheskely-Hayon D, Regev R, Eytan GD and
Dann EJ: The tyrosine kinase inhibitors imatinib and AG957 reverse
multidrug resistance in a chronic myelogenous leukemia cell line.
Leuk Res. 29:793–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilson CS, Davidson GS, Martin SB, Andries
E, Potter J, Harvey R, Ar K, Xu Y, Kopecky KJ, Ankerst DP, et al:
Gene expression profiling of adult acute myeloid leukemia
identifies novel biologic clusters for risk classification and
outcome prediction. Blood. 108:685–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding XW, Wu JH and Jiang CP: ABCG2: A
potential marker of stem cells and novel target in stem cell and
cancer therapy. Life Sci. 86:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mo W and Zhang JT: Human ABCG2: Structure,
function, and its role in multidrug resistance. Int J Biochem Mol
Biol. 3:1–27. 2012.PubMed/NCBI
|
|
15
|
Yiu ZZ and Warren RB: Novel oral therapies
for psoriasis and psoriatic arthritis. Am J Clin Dermatol.
17:191–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rozo C, Chinenov Y, Maharaj RK, Gupta S,
Leuenberger L, Kirou KA, Bykerk VP, Goodman SM, Salmon JE and
Pernis AB: Targeting the RhoA-ROCK pathway to reverse T-cell
dysfunction in SLE. Ann Rheum Dis. 76:740–747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Diep D, Hong K, Khun T, Zheng M, Ul-Haq A,
Jun HS, Kim YB and Chun KH: Anti-adipogenic effects of KD025
(SLx-2119), a ROCK2-specific inhibitor, in 3T3-L1 cells. Sci Rep.
8:24772018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zanin-Zhorov A, Weiss JM, Nyuydzefe MS,
Chen W, Scher JU, Mo R, Depoil D, Rao N, Liu B, Wei J, et al:
Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17
secretion in human T cells via STAT3-dependent mechanism. Proc Natl
Acad Sci USA. 111:16814–16819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sugimoto Y, Tsukahara S, Imai Y, Sugimoto
Y, Ueda K and Tsuruo T: Reversal of breast cancer resistance
protein-mediated drug resistance by estrogen antagonists and
agonists. Mol Cancer Ther. 2:105–112. 2003.PubMed/NCBI
|
|
20
|
Imai Y, Tsukahara S, Asada S and Sugimoto
Y: Phytoestrogens/flavonoids reverse breast cancer resistance
protein/ABCG2-mediated multidrug resistance. Cancer Res.
64:4346–4352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo F, Luo M, Rong QX, Zhang H, Chen Z,
Wang F, Zhao HY and Fu LW: Niclosamide, an antihelmintic drug,
enhances efficacy of PD-1/PD-L1 immune checkpoint blockade in
Non-small cell lung cancer. J Immunother Cancer. 7:2452019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, Wang XK, Shi CJ, Zhang H, Hu YP,
Chen YF and Fu LW: Nilotinib enhances the efficacy of conventional
chemotherapeutic drugs in
CD34+CD38− stem cells and ABC
transporter overexpressing leukemia cells. Molecules. 19:3356–3375.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riss TL, Moravec RA, Niles AL, Duellman S,
Benink HA, Worzella TJ and Minor L: Cell viability assays. Assay
Guidance Manual [Internet] Bethesda (MD): Eli Lilly & Company
and the National Center for Advancing Translational Sciences; May
1–2004
|
|
24
|
Percival ME, Lai C, Estey E and Hourigan
CS: Bone marrow evaluation for diagnosis and monitoring of acute
myeloid leukemia. Blood Rev. 31:185–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang H, Patel A, Ma SL, Li XJ, Zhang YK,
Yang PQ, Kathawala RJ, Wang YJ, Anreddy N, Fu LW and Chen ZS: In
vitro, in vivo and ex vivo characterization of ibrutinib: A potent
inhibitor of the efflux function of the transporter MRP1. Br J
Pharmacol. 171:5845–5857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Kumar P, Anreddy N, Zhang YK, Wang
YJ, Chen Y, Talele TT, Gupta K, Trombetta LD and Chen ZS:
Quizartinib (AC220) reverses ABCG2-mediated multidrug resistance:
In vitro and in vivo studies. Oncotarget. 8:93785–93799. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XK, To KK, Huang LY, Xu JH, Yang K,
Wang F, Huang ZC, Ye S and Fu LW: Afatinib circumvents multidrug
resistance via dually inhibiting ATP binding cassette subfamily G
member 2 in vitro and in vivo. Oncotarget. 5:11971–11985. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ejendal KF and Hrycyna CA: Multidrug
resistance and cancer: The role of the human ABC transporter ABCG2.
Curr Protein Pept Sci. 3:503–511. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi Z, Tiwari AK, Shukla S, Robey RW, Kim
IW, Parmar S, Bates SE, Si QS, Goldblatt CS, Abraham I, et al:
Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine
kinase inhibitor AG1478. Biochem Pharmacol. 77:781–793. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Sun S, Zhang W and Shi Z:
Polymorphisms of ABCG2 and its impact on clinical relevance.
Biochem Biophys Res Commun. 503:408–413. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takeshita A: Efficacy and resistance of
gemtuzumab ozogamicin for acute myeloid leukemia. Int J Hematol.
97:703–716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tiwari AK, Sodani K, Dai CL, Ashby CJ and
Chen ZS: Revisiting the ABCs of multidrug resistance in cancer
chemotherapy. Curr Pharm Biotechnol. 12:570–594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi Z, Liang YJ, Chen ZS, Wang XW, Wang
XH, Ding Y, Chen LM, Yang XP and Fu LW: Reversal of
MDR1/P-glycoprotein-mediated multidrug resistance by vector-based
RNA interference in vitro and in vivo. Cancer Biol Ther. 5:39–47.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robey RW, To KK, Polgar O, Dohse M, Fetsch
P, Dean M and Bates SE: ABCG2: A perspective. Adv Drug Deliv Rev.
61:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Szakacs G, Varadi A, Ozvegy-Laczka C and
Sarkadi B: The role of ABC transporters in drug absorption,
distribution, metabolism, excretion and toxicity (ADME-Tox). Drug
Discov Today. 13:379–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen L, Manautou JE, Rasmussen TP and
Zhong XB: Development of precision medicine approaches based on
inter-individual variability of BCRP/ABCG2. Acta Pharm Sin B.
9:659–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lou H and Dean M: Targeted therapy for
cancer stem cells: The patched pathway and ABC transporters.
Oncogene. 26:1357–1360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alkharabsheh O and Frankel AE: Clinical
activity and tolerability of SL-401 (Tagraxofusp): Recombinant
diphtheria toxin and interleukin-3 in hematologic malignancies.
Biomedicines. 7:62019. View Article : Google Scholar
|
|
39
|
Borin TF, Arbab AS, Gelaleti GB, Ferreira
LC, Moschetta MG, Jardim-Perassi BV, Iskander AS, Varma NR, Shankar
A, Coimbra VB, et al: Melatonin decreases breast cancer metastasis
by modulating Rho-associated kinase protein-1 expression. J Pineal
Res. 60:3–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rocca S, Carra G, Poggio P, Morotti A and
Brancaccio M: Targeting few to help hundreds: JAK, MAPK and ROCK
pathways as druggable targets in atypical chronic myeloid leukemia.
Mol Cancer. 17:402018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takahashi N, Nobusue H, Shimizu T,
Sugihara E, Yamaguchi-Iwai S, Onishi N, Kunitomi H, Kuroda T and
Saya H: ROCK inhibition induces terminal adipocyte differentiation
and suppresses tumorigenesis in chemoresistant osteosarcoma cells.
Cancer Res. 79:3088–3099. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yoon JH, Nguyen TT, Duong VA, Chun KH and
Maeng HJ: Determination of KD025 (SLx-2119), a selective ROCK2
inhibitor, in rat plasma by high-performance liquid
chromatography-tandem mass spectrometry and its pharmacokinetic
application. Molecules. 25:13692020. View Article : Google Scholar
|
|
43
|
Diep D, Duong K, Choi H, Jun HS and Chun
KH: KD025 (SLx-2119) suppresses adipogenesis at intermediate stage
in human adipose-derived stem cells. Adipocyte. 8:114–124. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Flynn R, Paz K, Du J, Reichenbach DK,
Taylor PA, Panoskaltsis-Mortari A, Vulic A, Luznik L, MacDonald KK,
Hill GR, et al: Targeted Rho-associated kinase 2 inhibition
suppresses murine and human chronic GVHD through a Stat3-dependent
mechanism. Blood. 127:2144–2154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
D'Amato L, Dell'Aversana C, Conte M,
Ciotta A, Scisciola L, Carissimo A, Nebbioso A and Altucci L:
ARHGEF3 controls HDACi-induced differentiation via RhoA-dependent
pathways in acute myeloid leukemias. Epigenetics. 10:6–18. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang XK, He JH, Xu JH, Ye S, Wang F, Zhang
H, Huang ZC, To KK and Fu LW: Afatinib enhances the efficacy of
conventional chemotherapeutic agents by eradicating cancer
stem-like cells. Cancer Res. 74:4431–4445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nakamura Y, Oka M, Soda H, Shiozawa K,
Yoshikawa M, Itoh A, Ikegami Y, Tsurutani J, Nakatomi K, Kitazaki
T, et al: Gefitinib (‘Iressa’, ZD1839), an epidermal growth factor
receptor tyrosine kinase inhibitor, reverses breast cancer
resistance protein/ABCG2-mediated drug resistance. Cancer Res.
65:1541–1546. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee JY, Stevens RP, Kash M, Zhou C,
Koloteva A, Renema P, Paudel SS and Stevens T: KD025 shifts
pulmonary endothelial cell bioenergetics and decreases baseline
lung permeability. Am J Respir Cell Mol Biol. Jul 6–2020.(Epub
ahead of print). View Article : Google Scholar
|