Introduction
Mucinous tubular and spindle cell carcinoma (MTSCC)
of the kidney is a relatively rare tumor that was defined in the
2004 World Health Organization Classification (1). The tumor was previously believed to be
low grade with a favorable prognosis (2). The origin of MTSCC is controversial and
has been speculated to be either the loop of Henle or the
collecting duct (3). This tumor is
usually diagnosed by pathology, with tubular and spindled cords
surrounded by an abundant extracellular matrix (4). However, very few cases have been
classified so far as mucin-poor (5).
There is no uniform standard for the diagnosis, especially the
imaging diagnosis, or the treatment of MTSCC. Based on the findings
of the analysis of the histological and imaging features of MTSCC,
The present study provides hypotheses on the origin and growth mode
of MTSCC, aimed at the identification and development of more
accurate treatment methods and a precise evaluation of the
prognosis of patients.
Case report
Case 1
A 70-year-old man with intermittent gross hematuria,
intermittent renal colic, and radiating pain in the groin that
recurred two or three times per month over a year, presented to the
Third Affiliated Hospital of Soochow University (Changzhou, China)
in February 2016. A physical examination revealed left lower
abdominal pain and left renal percussion pain. Left ureteral
calculi with left hydronephrosis were identified by B-ultrasound,
and the patient reported discharging two small stones 2 days after
the initiation of antispasmodic and anti-inflammatory therapy.
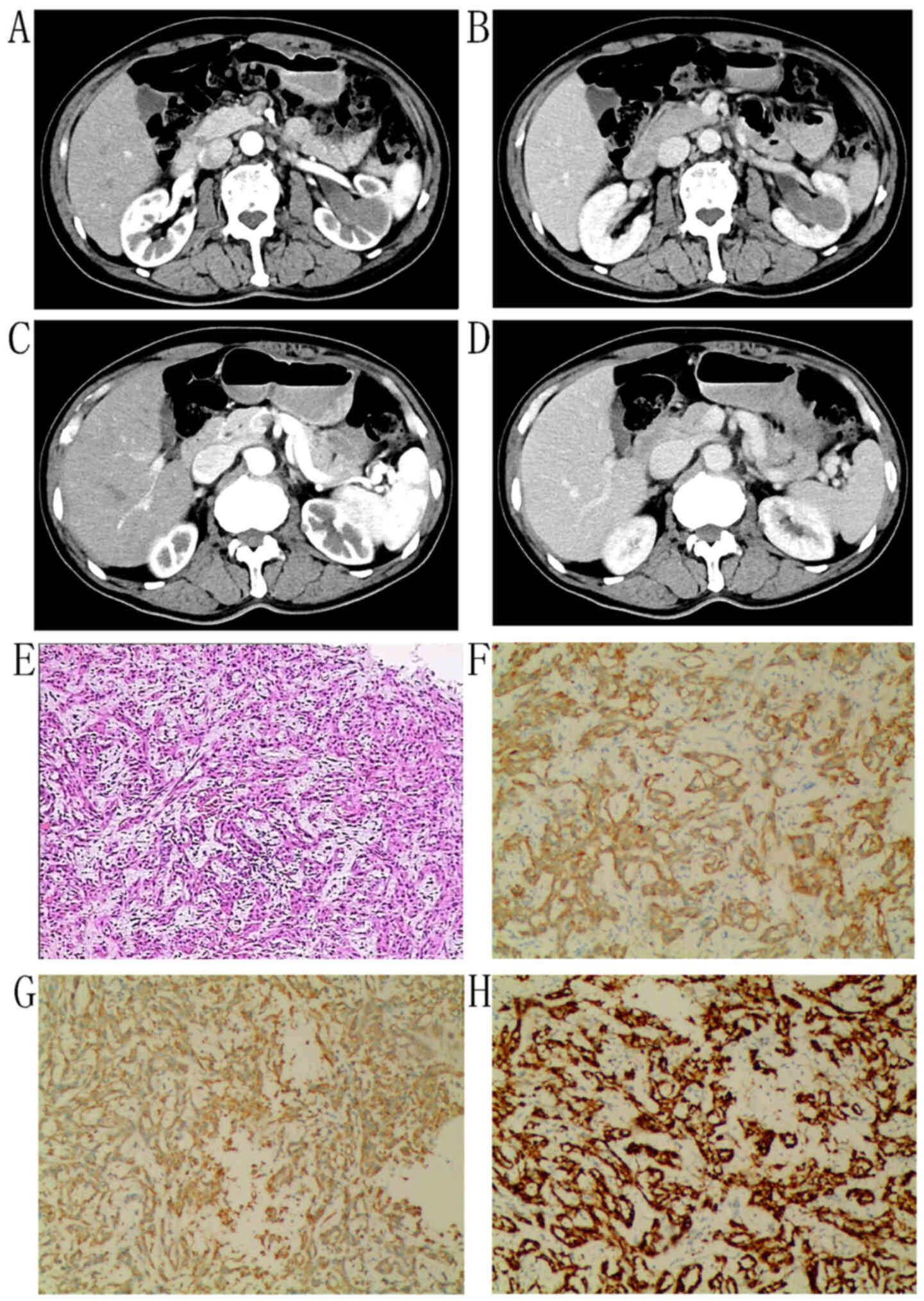
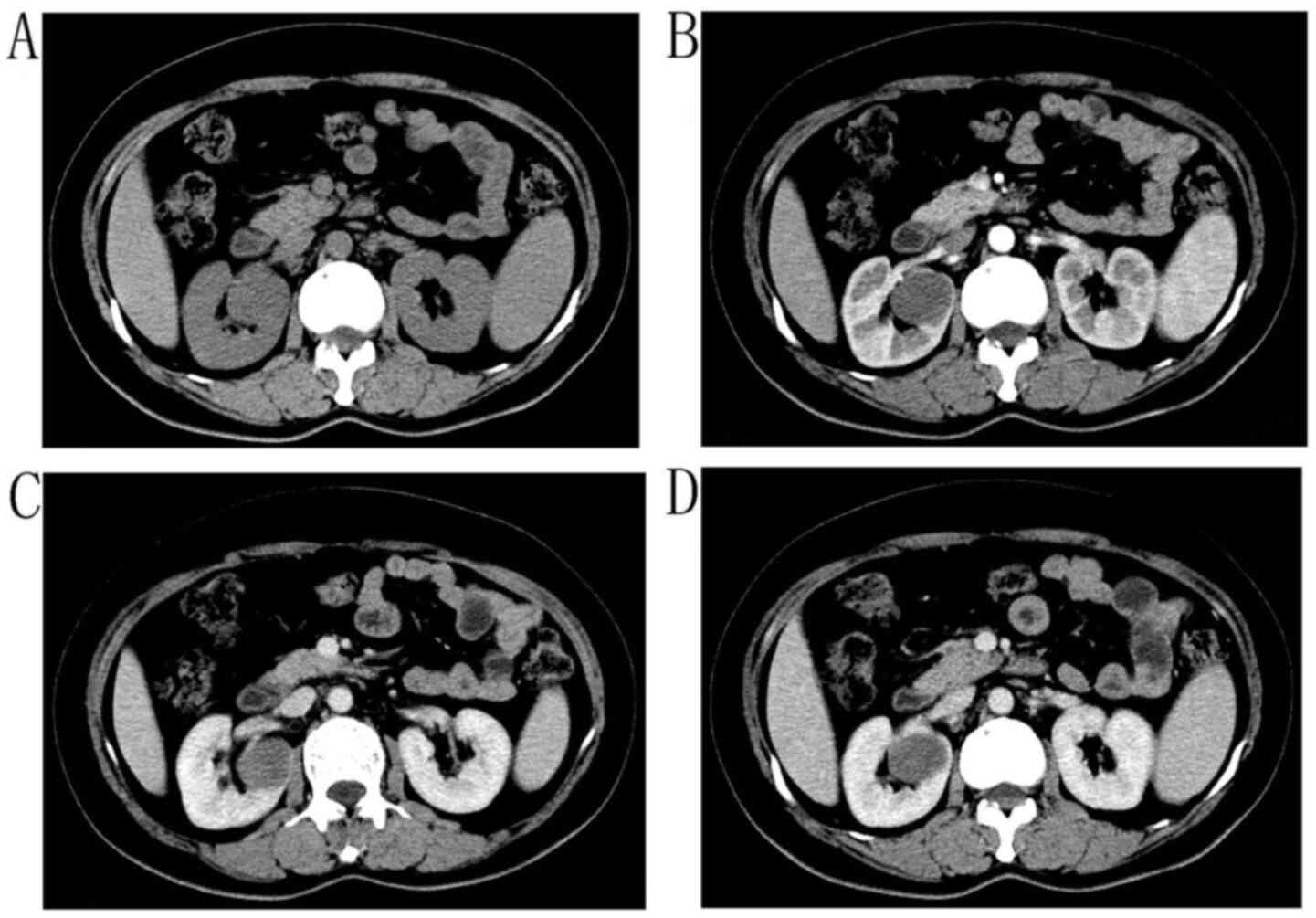
Total abdominal enhanced computed tomography (CT) scans showed a
4.4×2.3-cm isodense mass with clear boundaries in the left renal
pelvis (Fig. 1A and B)and a 1×1-cm
isodense mass on the surface of the left kidney (Fig. 1C and D). Urine cytology showed no
obvious tumor cells. Cystoscopy revealed a 1×0.8-cm gray-white mass
on the left wall of the bladder, which was excised. The bladder
tumor was diagnosed as MTSCC based on that Vimentin, CK8/18 and
P504S showed positive expression in tumoral cells. The preoperative
diagnosis was left renal pelvis carcinoma, left ureter carcinoma
and MTSCC of the bladder. Retroperitoneal laparoscopic radical
nephroureterectomy with bladder cuff resection and transurethral
resection of the bladder tumor was performed under general
anesthesia in March 2016. The dissection of the specimen revealed a
pelvic mass with a size of 3×3 cm, which was dark red. A 1×1-cm
grey nodule was available on the renal surface; the grey bladder
tumor had a total volume of 1.5×0.8×0.4 cm. The pathological
diagnosis was renal MTSCC with extensive necrosis and invasive
growth, suggesting a poor prognosis. Renal chromophobe cell
carcinoma (1×1-cm mass) on the renal surface and bladder MTSCC with
a tendency for renal tumor metastasis were also found. The
immunohistochemical results were consistent with the preoperative
pathological diagnosis. The patient was transferred to the
Department of Oncology, where he received chemotherapy consisting
of 1.2 g gemcitabine and 60 mg cisplatin through intravenous
injection every 3 months. The patient underwent chest and abdominal
enhanced CT, routine blood tests, liver and kidney function and
cystoscopy every 3 months. There were no signs of recurrence or
metastasis in any other organs and blood tests were normal after 36
months of follow-up.
Case 2
A 61-year-old man, with left lower back pain that
had lasted a year, received treatment for chronic hepatitis B and
underwent B-ultrasound in March 2012. This showed a huge malignant
tumor (10.7×7.4 cm) in the lower pole of the left kidney and a
thrombus in the renal vein. Physical examination revealed left
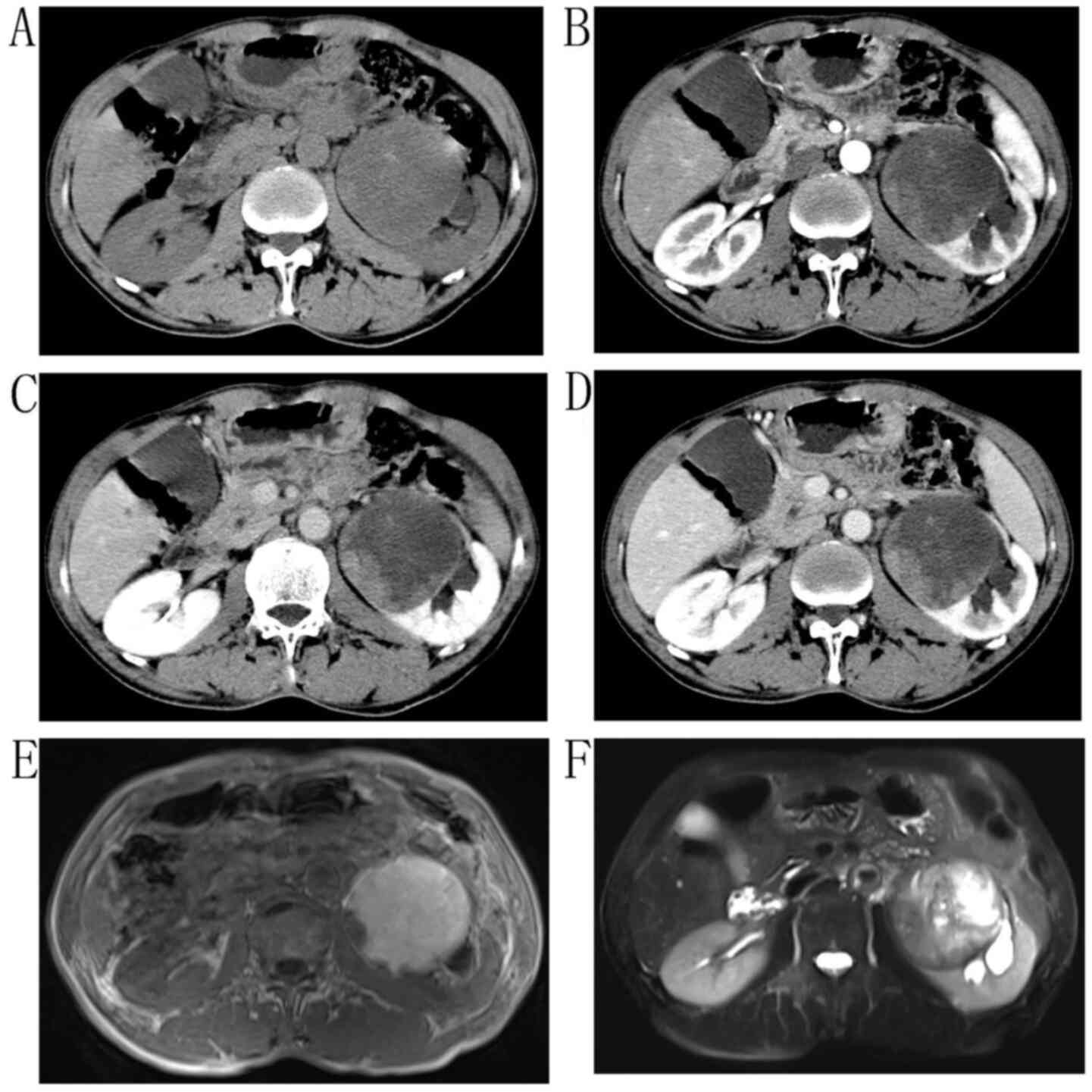
renal percussion pain. Enhanced CT revealed that the left kidney
was significantly enlarged; the capsule was not smooth, and a
cystic-solid soft mass of ~9.7×8.0 cm in size was present. The
internal density was uneven with small high-density shadows, and
the low-density area was considered to be the liquefaction area
(Fig. 2A-D). On magnetic resonance
imaging (MRI), a large 9.7×8×6-cm mass in the left kidney exhibited
a mixed signal of equal height in T2-weighted imaging (T2WI), and a
low-hybrid signal in T1WI (Fig. 2E and
F). The left renal vein was invasive. The patient underwent an
open left radical nephrectomy and renal pedicle lymph-node
dissection. After the tumor was incised, a 9.7×8×6-cm nodular mass,
which was grayish yellow and caseous, was found in the left renal
cortex. The tumor was diagnosed as MTSCC, and the renal pedicle
lymph nodes had no metastasis (0/3 lymph nodes). The patient
received chest and abdominal enhanced CT, routine blood tests,
liver and kidney function every 3 months during the follow-up
period of 80 months, and all these examinations showed no signs of
recurrence.
Case 3
A 52-year-old woman without painless gross hematuria
or lower back pain, who had suffered from diabetes for 10 years,
was in hospital for a 4.3-cm mass in the left lower pole of the
kidney, which was found by B-ultrasound in July 2014. Physical
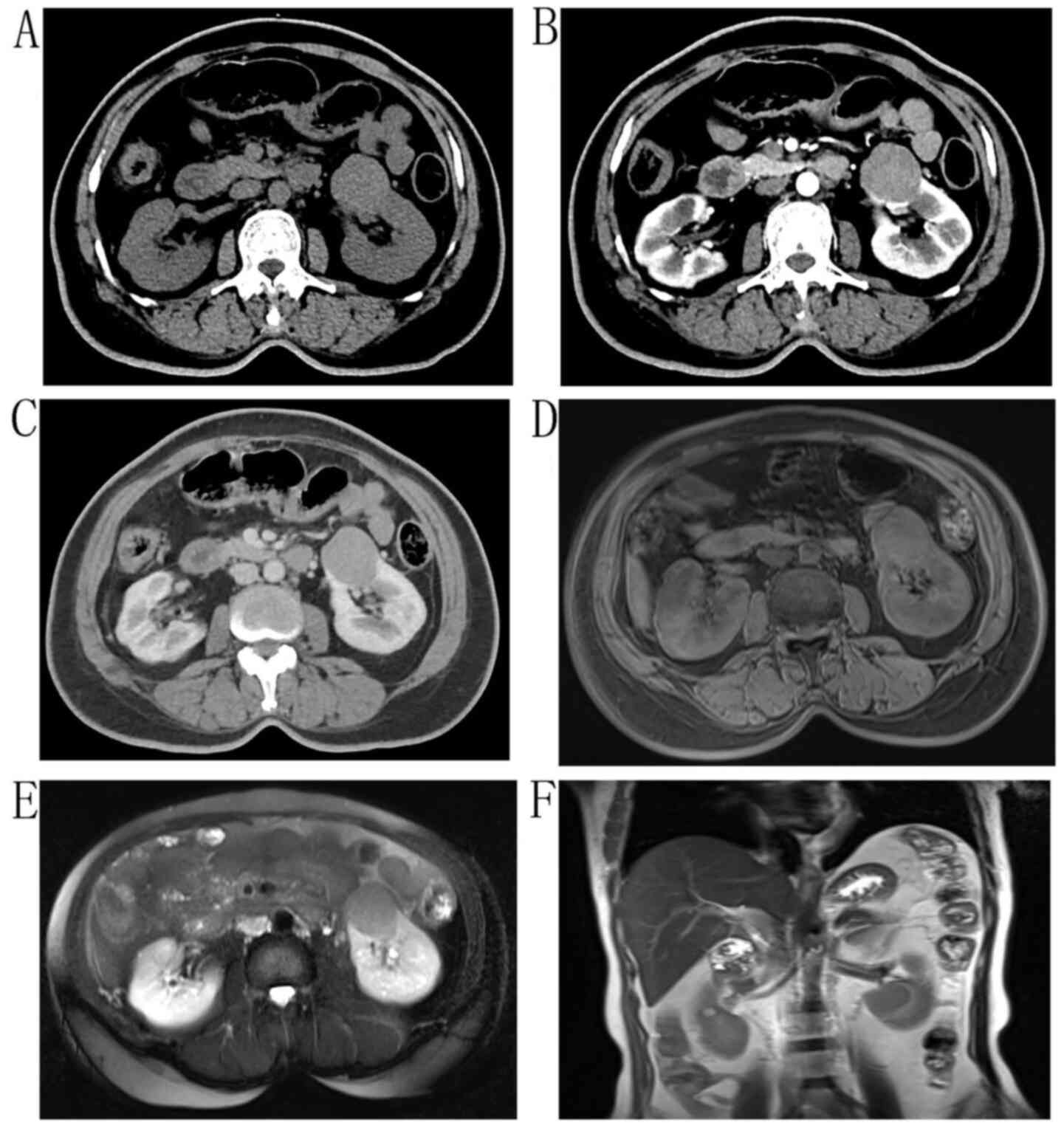
examination revealed slight left renal percussion pain. A circular
shadow with equal density and clear boundaries (Fig. 3A), which was weaker compared with
that of the cortex and greater compared with that of the medulla in
the arterial and venous phases, was found by enhanced CT (Fig. 3B and C). MRI revealed a circular
abnormal signal shadow of ~4 cm in diameter in the left renal
cortex, a moderate signal at T1, a slightly lower signal at T2,
slight enhancement in the enhanced scan and a low signal in
susceptibility weighted imaging (Fig.
3D-F). After a laparoscopic radical nephrectomy, the kidney was
cut open, and a 4.3×3-cm gray-white mass was observed in the renal
cortex. The mass were diagnosed as MTSCC. The patient, who
underwent chest and abdominal enhanced CT, routine blood tests,
liver and kidney function every 3 months, did not receive any other
treatment, and after a 56-month follow-up period, no recurrence was
found.
Case 4
A 57-year-old man were underwent a B-ultrasound,
which revealed a 3.9×4-cm strong-echo mass in the upper middle
segment of the left kidney. A physical examination revealed no left
renal percussion pain. The shape of the mass was regular, with
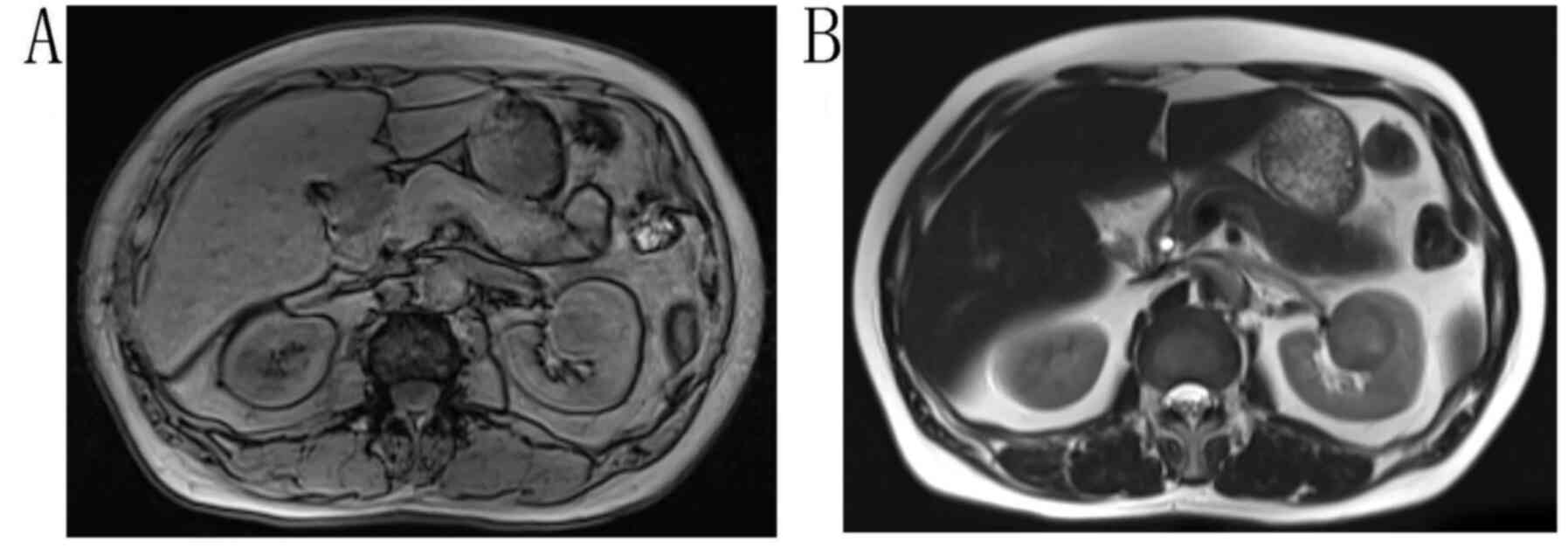
unclear boundaries. On MRI, a circular abnormal signal was observed
in the left kidney, with an equal signal in T1WI (Fig. 4A) and a slightly higher signal in
T2WI (Fig. 4B). At 3 days
post-hospitalization, the patient underwent a laparoscopic radical
resection of the left kidney, and a 3.7×4-cm gray-yellow mass was
found. The pathological result was left renal MTSCC.
Immunohistochemical results were as follows: CK19(+), CK(+),
CK8/18(+), Vimentin(+), CD10(+) and Ki-67 (+). The patient, who
received chest and abdominal enhanced CT, routine blood tests,
liver and kidney function every 3 months, did not receive any other
treatment and was without recurrence after 68 months of
follow-up.
Case 5
In March 2012, a 3.6×3.3-cm mass was found in the
right kidney of a 49-year-old woman who underwent B-ultrasound. The
mass was regular in shape, with an unclear boundary and uneven
internal echo. CT showed a 3.6×2.9-cm circular tumor in the right
kidney, which had a relatively uniform density. The tumor grew into
the renal pelvis and exhibited only slight enhancement (Fig. 5B). The patient underwent a
laparoscopic radical resection of the right kidney at 4-days
post-admission. After the dissection, a pale-yellow mass with clear
boundaries was located near the renal pelvis. Finally, it was
diagnosed as MTSCC. Without any other treatment after surgery, the
patient, who underwent who received chest and abdominal enhanced
CT, blood routine test, liver and kidney function every 3 months,
showed no signs of recurrence after 65 months of follow-up.
Discussion
As revealed by previous reports, MTSCC of the kidney
occurs predominantly in patients aged between 17 and 82 years, the
majority of who are female (female:male, 4:1) (6,7). This
result was not confirmed by the three male and two female patients
described in the five cases in the present study. Although we
consider that there is obvious disparity in the MTSCC sex ratio
presented, further statistics are required to elucidate the sex
ratio and its associations in such cases.
As reported by a previous study (8), no final conclusion exists as to whether
MTSCC originates from the loop of Henle or the collecting duct.
MacLennan et al (2) suggested
that the tumors, which had some overlapping features between MTSCC
and low-grade collecting duct carcinoma (CDC), originate from the
collecting duct epithelium. However, Parwani et al (9) put forth the contrary view that cuboidal
cells, elongated tubular glands and myxoid stroma, which are the
histological features in some MTSCC cases, were associated with the
loop of Henle. Epithelial neoplasms are usually polymorphic and
well circumscribed. The clinical manifestations and signs of MTSCC
are not significantly different from those in common renal tumors,
and the imaging findings of the polymorphic tumors are not
completely consistent. Thus, it is rather difficult to make a
precise preoperative diagnosis. Kenney et al (10) reviewed, analyzed and summarized the
findings of the CT images of 19 MTSCCs. It was established that the
mean tumor attenuation was 36 Hounsfield units in the pre-contrast
phase, 67 in the corticomedullary phase (CMP), 89 in the
nephrographic phase (NP) and 76 in the excretory phase (10). In the CT imaging of eight MTSCC cases
reported earlier by Wu et al (7), all tumors were slightly enhanced at
both the CMP and the NP, but less enhanced than the cortex and
medulla. In another study, the attenuation of MTSCC tumors in 17
cases was close to that in the cortex and medulla in the CT plain
scan, but less enhanced than the cortex and the medulla during all
phases of enhanced CT (11). Wu
et al (12) summarized the CT
features of 21 MTSCC cases and 18 CDC cases, and drew the following
conclusions: The attenuation value of CDC was greater than that of
MTSCC on the unenhanced CT; the enhancement of MTSCC and CDC was
less than that of the renal cortex and medulla; and the enhancement
of MTSCC was weaker than that of CDC in all phases of enhanced CT.
Yang et al (13) reported a
CT finding of MTSCC with a pattern of ‘lightly slow wash-in’ in
enhanced CT, which is very different from that of normal renal
carcinoma. The mass showed slight enhancement, but was less
enhanced than the normal renal parenchyma on the arterial and
venous phases; however, some areas of the mass showed slightly
patchy low attenuation (13). The
MRI scan of a MTSCC case reported by Marcela et al (14) showed a low signal on T1WI, but some
areas of the tumor had a high signal for necrosis and hemorrhage.
These areas revealed classic hypervascularization after the
injection of contrast medium. On T2WI, the tumor had an
intermediate to high signal. In the present study, on the CT scans
of cases 1, 2, 3 and 5, it was evident that the enhancement of the
tumor was weaker than that of the cortex and medulla in the
arterial and venous phases (Figs. 1A and
B, 2A-D, 3A-C and 5A-D). The MTSCC showed a mixed signal of
equal height on T2WI, a low-hybrid signal on T1WI. The left renal
vein was pressed forward (Fig. 2E and
F). In case 4, the tumor showed an equal signal on T1WI and a
slightly higher signal on T2WI (Fig. 4A
and B). It can be noted that the features of CT and MRI in the
present five cases were similar to those of the cases previously
reported, although some differences remained. Summarizing the CT
and MRI findings of other publications and the imaging features of
these five cases, it was found that the attenuation value of the
tumors in the CT plain scan were close to those of the renal cortex
and the medulla, and that the enhancement of the tumor was weaker
than that of the cortex and the medulla in both the arterial and
the venous phases. The tumors in cases 1 and 5 were endogenous, and
their attenuation value was significantly lower than that of the
cortex and the medulla on CT scan. Meanwhile, the other three
tumors were exogenous and their attenuation values were equal to or
higher than those of the cortex and the medulla. We speculate that
the reason for this phenomenon is the difference in the origin of
the tumor. Earlier reports showed attenuation values of CDC greater
than that of MTSCC on unenhanced CT. Therefore, we speculate that
the tumors had two origins: The tumors in cases 1 and 5 originated
from the loop of Henle, whereas the other three tumors originated
from the collecting tube. All these conclusions are, however,
speculative, and should be confirmed by more detailed pathology
studies. On MRI, the MTSCCs exhibited a low signal at T1WI but a
high signal at T2WI. Moreover, some areas of the tumor had a great
enhancement on CT and a high signal on MRI for necrosis and
hemorrhage. Although the imaging features of MTSCC have some
commonalities, the disease should be diagnosed by the pathological
results. As was evident in case 1, the pathological features of
MTSCC are long and narrow tubular epithelial cells arranged in the
sputum, filled with a white mucinous matrix and spindle cells
(Fig. 1E). As reported by Sarsik
et al (5), MTSCC is
subdivided into a classical and a mucin-poor type. In another
investigation, Ferlicot et al (15) summarized the immunohistochemical
results of 15 patients with MTSCC and found that almost all cases
had positive expression of epithelial membrane antigen, CK7, CK9,
α-methylacyl-CoA racemase, E-cadherin and cytokeratin AE1/AE3
(14). However, in case 1 in the
present study, mainly positive expression of Vimentin, CK8/18 and
P504s was present (Fig. 1F-H).
Sarsik et al (5) suggested
that it was difficult to distinguish MTSCC from papillary renal
cell carcinoma through immunohistochemical results. Therefore, we
speculated that these differences between the expression
established in previous cases and that in the present cases may be
due to the different origins of the tumors. Therefore, there may be
multiple origins possible for MTSCC.
MTSCC is a rare kidney tumor, and its main
treatments are radical nephrectomy or partial nephrectomy. For
patients with metastatic renal cell carcinoma, subtractive
nephrectomy plus cytokine therapy is the first choice (16). Retroperitoneal laparoscopic radical
nephroureterectomy with bladder cuff resection and transurethral
resection of the bladder tumor was therefore performed for case 1;
after the surgery the patient received intravesical instillation
therapy consisting of gemcitabine hydrochloride and cisplatin
chemotherapy every 3 months. In addition, it is still controversial
whether renal pedicle lymph-node dissection is needed for patients
for localized renal cell carcinoma and locally advanced renal cell
carcinoma. A study by Blom et al (17) showed that regional lymph-node
dissection did not significantly improve the incidence of
complications, overall survival time or disease progression time in
patients with localized renal cell carcinoma, and thus it was
concluded to be unnecessary. Renal cancer is not sensitive to
either radiotherapy or chemotherapy, and there is currently no
standard adjuvant treatment for radical nephrectomy. Simon et
al (18) reported a case in
which a patient with MTSCC and two other lesions in the thoracic
vertebral bodies received radiotherapy after tumor embolization and
radical nephrectomy with vertebral body resection. However, the
treatment effect was not ideal, and the patient died with
additional vertebral body lesions and liver lesions 3 weeks
postoperatively (18). The patients
in the present study in cases 2, 3, 4 and 5, who did not receive
additional radiotherapy or chemotherapy, had a good prognosis. For
patients with metastatic renal cell carcinoma, radical nephrectomy
and metastasis are feasible, and postoperative medical treatments
include molecular targeted therapy and chemotherapy. As reported
previously in 2010, sunitinib was effective for the treatment of a
patient with MTSCC (19). Another
study (20) administered Proleukin
to an MTSCC patient for 3 months, and they remained alive with no
recurrence at 9-years of follow-up (20). However, another study indicated that
pazopanib was ineffective for MTSCC (21). A further study (22) suggested that patients should receive
specific therapies depending on their condition. Molecular targeted
therapy with oral sorafenib was administered to an old woman, who
had a 7-cm solid mass in the left kidney with pulmonary metastases
and a shrunken right kidney, and she had no disease progression for
5 years Additionally, a 49-year-old male with a 19-cm left renal
mass and multiple other metastases, received pazopanib for 6 months
to reduce the renal tumor volume and underwent a left radical
nephrectomy (22). In the same
study, the patient diagnosed as MTSCC and metastasis of the bladder
received postoperative chemotherapy with gemcitabine in combination
with cisplatin, and had no recurrence within a 32-month follow-up
period.
As a rare low-grade and indolent renal tumor, MTSCC
is considered to have a good prognosis. All five cases included in
the present study had a good prognosis. However, increasingly more
cases of MTSCC with distant metastases are reported, some of which
have a poor prognosis. In an earlier study (21), mucin-poor MTSCC with multiple osseous
metastases without sarcomatoid differentiation was found two years
after bilateral nephrectomy. The patient received treatment with
pazopanib and focal radiotherapy to the lumbar and cervical
vertebrae metastases, but the effect was poor, and hepatic and
pulmonary metastases were recorded after 3 months of treatment. The
patient died a few months later. In another investigation, Sakatani
et al (23) reported that the
CT scan showed para-aortic lymph node metastasis and hepatic
metastasis 5 months after radical nephrectomy. The patient received
treatment with sunitinib but succumbed to a brain metastasis
(23). In a study by Shiro et
al (24), distant metastases
were revealed in the liver and bone by CT imaging after partial
nephrectomy, and the patient, who received molecular targeted
therapy and irradiation, succumbed due to tumor progression
(24). Patients with MTSCC and
sarcomatoid changes are considered to have a poor prognosis, with
previous evidence showing that three out of five patients developed
fatal distant metastasis (25,26). A
study by Ged et al (27)
included 25 cases of MTSCC, and found 3-year overall survival data
for 22 cases, while the remaining three cases succumbed to
metastasis within 3 years of being diagnosis (27). Although the review of certain cases
with distant metastasis would lead us to the conclusion that MTSCC
is a tumor with a high degree of malignancy and a poor prognosis,
other cases have revealed that MTSCC is indolent and has a good
prognosis (2,7,27).
In conclusion, most patients with MTSCC have a good
prognosis, but mucin-poor MTSCC with distant metastases or multiple
metastases has a bad prognosis. MTSCC may have unconfirmed
subtypes, therefore its origin is controversial. The CT features
associated with MTSCC include the weaker enhancement of the tumor
compared with that of the cortex and medulla in the arterial and
venous phases. Surgery is preferred for localized or progressive
MTSCC, and progressive cases should receive molecular targeted
therapies depending on the condition of the patient, as this may be
effective.
Acknowledgements
Not applicable.
Funding
The study was supported by National Science
Foundation of Jiangsu Province (grant no. 20150251).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
requests.
Authors' contributions
RX, TD, SY and XH analyzed and interpreted the
patient data regarding the renal cell carcinoma. PG, QZ and XW
performed the histological examination of the kidney, and were
major contributors in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committees of The First People's Hospital of Changzhou (Changzhou,
China), and all participants provided written informed consent to
participate.
Patient consent for publication
Written informed consent for publication was
obtained from all participants.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MTSCC
|
mucinous tubular and spindle cell
carcinoma
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
CDC
|
collecting duct carcinoma
|
|
CMP
|
corticomedullary phase
|
|
NP
|
nephrographic phase
|
References
|
1
|
Rzymkowska J, Dudek M, Ligaj M, Kalinowski
T and Demkow T: Mucinous tubular and spindle cell carcinoma. Cent
European J Urol. 65:164–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
MacLennan GT, Farrow GM and Bostwick DG:
Low-grade collecting duct carcinoma of the kidney: Report of 13
cases of low-grade mucinous tubulocystic renal carcinoma of
possible collecting duct origin. Urology. 50:679–684. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang G, Breyer BN, Weiss DA and MacLennan
GT: Mucinous tubular and spindle cell carcinoma of the kidney. J
Urol. 183:738–739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fine SW, Argani P, DeMarzo AM, Delahunt B,
Sebo TJ, Reuter VE and Epstein JI: Expanding the histologic
spectrum of mucinous tubular and spindle cell carcinoma of the
kidney. Am J Surg Pathol. 30:1554–1560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sarsik B, Simsir A, Karaarslan S and Sen
S: Mucinous tubular and spindle cell carcinoma of kidney and
problems in diagnosis. Turk Patoloji Derg. 27:116–126.
2011.PubMed/NCBI
|
|
6
|
Kato M, Soga N, Arima K and Sugimura Y: A
case of renal mucinous tubular and spindle cell carcinoma. Int J
Urol. 16:699–701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu XR, Chen YH, Sha JJ, Zhao L, Huang JW,
Bo JJ, Liu DM and Huang YR: Renal mucinous tubular and spindle cell
carcinoma: A report of 8 cases and review of the literature. Diagn
Pathol. 8:2062013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung SJ, Yoon HK, Chung JI, Ayala AG and
Ro JY: Mucinous tubular and spindle cell carcinoma of the kidney
with neuroendocrine differentiation: Report of two cases. Am J Clin
Pathol. 125:99–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parwani AV, Husain AN, Epstein JI,
Beckwith JB and Argani P: Low-grade myxoid renal epithelial
neoplasms with distal nephron differentiation. Hum Pathol.
32:506–512. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kenney PA, Vikram R, Prasad SR, Tamboli P,
Matin SF and Wood CG: Mucinous tubular and spindle cell carcinoma
(MTSCC) of the kidney: A detailed study of radiological,
pathological and clinical outcomes. BJU Int. 116:85–92. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu Q, Zhu W, Wang Z and Wu J: Clinical
and CT imaging features of mucinous tubular and spindle cell
carcinoma. Chin Med J (Engl). 127:1278–1283. 2014.PubMed/NCBI
|
|
12
|
Wu J, Zhu Q, Zhu W, Chen W and Wang S:
Comparative study of CT appearances in mucinous tubular and spindle
cell carcinoma and collecting duct carcinoma of the kidney. Br J
Radiol. 88:201404342015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Zhang J, Huan Y and Xu J: CT of a
renal mucinous tubular and spindle cell carcinoma. Quant Imaging
Med Surg. 2:292–293. 2012.PubMed/NCBI
|
|
14
|
Lima MS, Barros-Silva GE, Pereira RA,
Ravinal RC, Tucci S Jr, Costa RS and Muglia VF: The imaging and
pathological features of a mucinous tubular and spindle cell
carcinoma of the kidney: A case report. World J Surg Oncol.
11:342013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferlicot S, Allory Y, Compérat E,
Mege-Lechevalier F, Dimet S, Sibony M, Couturier J and Vieillefond
A: Mucinous tubular and spindle cell carcinoma: A report of 15
cases and a review of the literature. Virchows Arch. 447:978–983.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Motzer RJ, Bacik J, Schwartz LH, Reuter V,
Russo P, Marion S and Mazumdar M: Prognostic factors for survival
in previously treated patients with metastatic renal cell
carcinoma. J Clin Oncol. 22:454–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blom JH, van Poppel H, Maréchal JM,
Jacqmin D, Schröder FH, de Prijck L and Sylvester R; EORTC
Genitourinary Tract Cancer Group, : Radical nephrectomy with and
without lymph-node dissection: Final results of European
Organization for Research and Treatment of Cancer (EORTC)
randomized phase 3 trial 30881. Eur Urol. 55:28–34. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simon RA, di Sant'agnese PA, Palapattu GS,
Singer EA, Candelario GD, Huang J and Yao JL: Mucinous tubular and
spindle cell carcinoma of the kidney with sarcomatoid
differentiation. Int J Clin Exp Pathol. 1:180–184. 2008.PubMed/NCBI
|
|
19
|
Larkin J, Fisher R, Pickering L, Thway K,
Livni N, Fisher C and Gore M: Metastatic mucinous tubular and
spindle cell carcinoma of the kidney responding to sunitinib. J
Clin Oncol. 28:e539–e540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao W, Huang B, Fei X, Huang X, Dai J,
Zhou W, Xu Z, Su H, Cheng K and Sun F: Clear cell changes in
mucinous tubular and spindle cell carcinoma: Cytoplasmic
pallor/clearing within tubules, vacuoles or hybrid conventional
clear cell carcinoma of kidney? Int J Clin Exp Pathol. 7:4350–4358.
2014.PubMed/NCBI
|
|
21
|
Sokolakis I, Kalogirou C, Frey L,
Oelschlager M, Krebs M, Riedmiller H, Kübler H and Vergho D:
Mucin-poor mucinous tubular and spindle cell carcinoma of the
kidney presented with multiple metastases two years after
nephrectomy: An atypical behaviour of a rare, indolent tumour. Case
Rep Urol. 2017:65975922017.PubMed/NCBI
|
|
22
|
Takeuchi H, Tokuyama N, Kuroda I and
Aoyagi T: Molecular targeted therapies of renal cell carcinoma
considering life stage of the patient: Two case reports. Exp Ther
Med. 15:3976–3980. 2018.PubMed/NCBI
|
|
23
|
Sakatani T, Okumura Y, Kuroda N,
Magaribuchi T, Nakano Y, Shirahase T, Watanabe J, Taki Y, Okigaki
M, Ikehara S and Adachi Y: Mucinous tubular and spindle cell
carcinoma with a high nuclear grade and micropapillary pattern: A
case report. Mol Clin Oncol. 7:976–890. 2017.PubMed/NCBI
|
|
24
|
Shiro U, Koyu S, Mieko U, Fumi N,
Chih-ping L, Abe E, Yamauchi T, Horiuchi S, Kamo M, Hattori K and
Nagashima Y: Mucin-poor and aggressive mucinous tubular and spindle
cell carcinoma of the kidney: Tow case reports. Mol Clin Oncol.
7:777–782. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dhillon J, Amin MB, Selbs E, Turi GK,
Paner GP and Reuter VE: Mucinous tubular and spindle cell carcinoma
of the kidney with sarcomatoid change. Am J Surg Pathol. 33:44–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bulimbasic S, Ljubanovic D, Sima R, Michal
M, Hes O, Kuroda N and Persec Z: Aggressive high-grade mucinous
tubular and spindle cell carcinoma. Hum Pathol. 40:906–907. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ged Y, Chen YB, Knezevic A, Donoghue MTA,
Carlo MI, Lee CH, Feldman DR, Patil S, Hakimi AA, Russo P, et al:
Mucinous tubular and spindle cell carcinoma of the kindey: Clinial
feature, genomic profiles, and treatment outcomes. Clin Genitourin
Cancer. 17:268–274. 2019. View Article : Google Scholar : PubMed/NCBI
|