Introduction
Pancreatic neuroendocrine tumours (PNETs) are rare
neuroendocrine neoplasms that originate from diffuse neuroendocrine
cells (1). The incidence of PNETs
has been increasing rapidly in the last 50 years. The age-adjusted
incidence rate increased 6.4-fold from 1973 (1.09/100 000) to 2012
(6.98/100 000), partly as a result of increased detection using
endoscopic and imaging techniques (2,3). Surgery
remains the mainstay of therapy for patients diagnosed with both
functional and non-functional PNETs (4). Regarding biological behaviour, PNETs
have traditionally been considered to be less aggressive than
pancreatic adenocarcinomas; however, the pathological potential of
PNETs is increasingly being recognized as highly variable (5). Outcomes after surgical resection vary
widely, with recurrence rates ranging between 17 and 76% (6–8). The
prominent heterogeneity of PNETs creates an urgent need for
prognostic factors. Various studies have specifically investigated
factors that are associated with PNET progression (1,4,8). However, the pathophysiology involved in
the progression and prognosis of PNETs remains incompletely
characterized.
CD44 belongs to the adhesion molecule family
(9), which serves important roles in
cell proliferation, apoptotic resistance, motility, metastasis and
chemotherapy resistance (10–12).
Studies have reported that CD44 overexpression is associated with
metastasis and a poor prognosis in various types of cancer,
including gastric cancer, breast cancer and hepatocellular
carcinoma (13–20). Additionally, CD44 has been used as a
specific marker of cancer stem cells (CSCs) in a number of human
tumours (10,21,22).
Furthermore, CD44 serves an important role in invasion and
metastasis in a variety of human cancer types, including pancreatic
adenocarcinoma (23,24).
CD133, a member of the pentaspan transmembrane
glycoprotein family, is another marker of CSCs (25). CD133 was first described as a
hematopoietic stem cell marker and later reported as a marker of
CSCs in solid tumours (26).
Previous studies have focused on CD44 and CD133 co-expression; high
CD133 and CD44 expression is associated with invasion, metastasis,
recurrence and decreased survival time in colon cancer, gastric
cancer, oesophageal cancer, medullary thyroid carcinoma and
hepatoblastoma (14,19,27–32).
CSC subpopulations are critical in cancer
progression and serve as a promising therapeutic target (33). Numerous investigations have sought to
identify CSC populations based on their surface markers (33–35).
CSCs are also present in NETs (35),
where several CSC markers have been investigated, including
aldehyde dehydrogenase (ALDH), CD73 and CD24 (35–37). NET
cells with high ALDHA expression exhibit CSC-like properties
(35). High CD73 expression in PNET
tissues is strongly associated with invasion into adjacent organs
(37). CD24 expression is frequently
noted in primary and metastatic midgut NETs, but is rare in PNETs
(36). However, to the best of our
knowledge, studies on CD44 and CD133 expression in PNETs and their
prognostic value have not been performed. Therefore, the present
study aimed to analyse CD44 and CD133 expression in a cohort of
patients with PNETs, as well as the association between protein
expression and clinicopathological characteristics, while further
investigating the prognostic values of CD44 and/or CD133 in this
group.
Materials and methods
Patients and samples
Patients who underwent radical surgery for a PNET
between January 2,000 and December 2016 at the Department of
General Surgery, Guangdong Provincial People's Hospital (Guangzhou,
China) were included. Formalin-fixed paraffin-embedded primary
specimens were obtained from all patients, with protocols approved
by the Medical Ethics Review Committee of Guangdong General
Hospital, and written informed consent was provided by all
patients. The entire study was performed in accordance with the
Declaration of Helsinki.
The histological types and grades of all samples
were determined by experienced pathologists. The clinical stage of
patients with PNETs was evaluated based on the TNM classification
system (American Joint Committee on Cancer, TNM Staging System for
Pancreatic Neuroendocrine Tumours, 7th edition, 2010) (38). Histological grades of the tumours
were assessed according to the World Health Organization (WHO) 2010
classification (39). Routine
pathology staining was used for Ki-67 and to calculate percentage
as Ki-67 index, the detail is the same as percentage of CD44/CD133
in the immunohistochemistry method. Mitotic count and Ki-67 index
were assessed independently by two pathologists who evaluated ≥10
high-power fields for each section. The results of Ki-67 index and
mitotic count were further verified by a senior chief
pathologist.
The inclusion criteria for patients were as follows:
i) Initial treatment, including radical resection; ii) pathological
confirmation of PNET by postoperative histopathological diagnosis;
iii) no adjuvant therapy prior to surgery; iv) tumour lacking
involvement of the celiac axis or the superior mesenteric artery,
or without exhibiting distal metastasis; and v) no history of other
malignancies. In total, 5 patients were excluded based on these
criteria. Additionally, a single patient succumbed to a massive
abdominal haemorrhage during the perioperative period and was
excluded. Finally, a total of 71 eligible patients were
identified.
Information regarding clinicopathological
characteristics was collected for each patient. Follow-up
information on prognosis was collected through clinic visits in
outpatient departments, telephone calls and questionnaires.
Disease-free survival (DFS) was calculated from the date of
diagnosis to local recurrence or distal metastasis. Overall
survival (OS) was measured from the date of diagnosis to death due
to any cause, in addition to perioperative death caused by surgical
complications.
Immunohistochemistry
Slides (4-µm thick, two serial sections for each
sample) of formalin-fixed (37–40% for 24 h) at room temperature,
paraffin-embedded specimens with the highest tumour content were
used for immunohistochemical staining. Briefly, immunochemistry for
CD44 (rabbit monoclonal antibody; 1:100; cat. no. ab51037; Abcam)
and CD133 (rabbit polyclonal antibody; 1:200; cat. no. orb99113;
Biorbyt, Ltd.) and Ki-67 (rat polyclonal antibody; 1:2,000; MIB-1;
Gene Tech Co., Ltd.) was performed using commercially available
antibodies. Sections were heated at 60°C for 1 h and
de-paraffinized in xylene and rehydrated in a graded ethanol
series. Subsequently, antigen retrieval was performed using a
microwave at 110°C for 3 min. Endogenous peroxidase activity was
blocked using 3% hydrogen peroxide. Non-specific binding was
blocked using 3% bovine serum albumin (cat. no. G5001; Servicebio,
http://www.servicebio.cn/search-result?search=G5001)
in PBS at room temperature for 30 min. The aforementioned primary
antibodies were added overnight at 4°C. After sufficient PBS washes
at room temperature for 5 min (three times), sections were stained
at room temperature for 1 h with horseradish peroxidase-labelled
goat anti-rabbit antibodies (1:200; cat. no. K5007; Dako; Agilent
Technologies, Inc.). The sections were subsequently stained with
3,3′-diaminobenzidine. Slides were observed under a light
microscope (XSP-C204; CIC, magnification, ×100).
CD44 and CD133 immunostaining were blindly scored by
two independent pathologists using a semi-quantitative method that
included staining intensity (scored from 0 to 3) and the percentage
of positively stained tumour cells (scored from 0 to 100). Briefly,
staining intensities were scored as follows: 0, no staining; 1,
weak staining; 2, moderate staining; or 3, intense and strong
staining. The percentage of positively stained tumour cells was
determined by counting the number of positive staining cells and
the number of all tumour cells in ≥10 random-selected high-power
fields (HPFs), and calculated by the formula: Percentage (range,
0–100)=Number of stained cells/Total number of cells ×100. A total
score was calculated for each sample using the following formula:
Total score (range, 0–300)=Staining intensity scores (range,
0–3)xPercentage of positively stained cells (range, 0–100).
Statistical analysis
Statistical analysis was performed using SPSS
software v24.0 (IBM Corp.). The presentation of data adopt mean ±
SD. Frequency distributions and categorical variables were compared
using the χ2 test or ordinal regression, and continuous
variables were compared using one-way ANOVA, differences among
groups were compared using one-way ANOVA followed by LSD post hoc
test. The Kaplan-Meier survival method with the log-rank test was
used to assess survival time. P<0.05 (two-tailed) was considered
to indicate a statistically significant difference.
Results
Patient characteristics
The present study included a total of 71 patients,
of whom 42 were men (59.2%). The mean age was 45.2 years (range,
10–78 years). A total of 31 (43.7%) patients had functional PNETs,
while 40 (56.3%) patients had non-functional PNETs. All patients
underwent an intended curative resection. A total of 40 (56.3%) of
these patients underwent a pancreaticoduodenectomy or distal
pancreatectomy, 7 patients (9.9%) had a segmental pancreatectomy,
16 patients (22.5%) had an enucleation, 7 patients (9.9%) had a
local resection and 1 patient (1.4%) had a duodenum-preserving
resection of the pancreatic head,. Only 1 patient exhibited an R1
surgical margin, where the tumour was adjacent to the adrenal
gland. A total of 31 (43.6%) patients were categorized as G1 grade
and 30 (42.3%) patients were categorized as G2 grade and 10 (14.1%)
patients were categorized as G3 grade, according to the 2010 WHO
classification of tumours of the digestive system. A total of 15
patients (21.1%) experienced recurrence, with a median time to
recurrence of 2.5 years (range, 0.5–8.0 years; data not shown).
CD44 and CD133 expression in
PNETs
Both CD44 and CD133 expression were observed in PNET
tissues. CD44 and CD133 were primarily detected in the cytoplasm
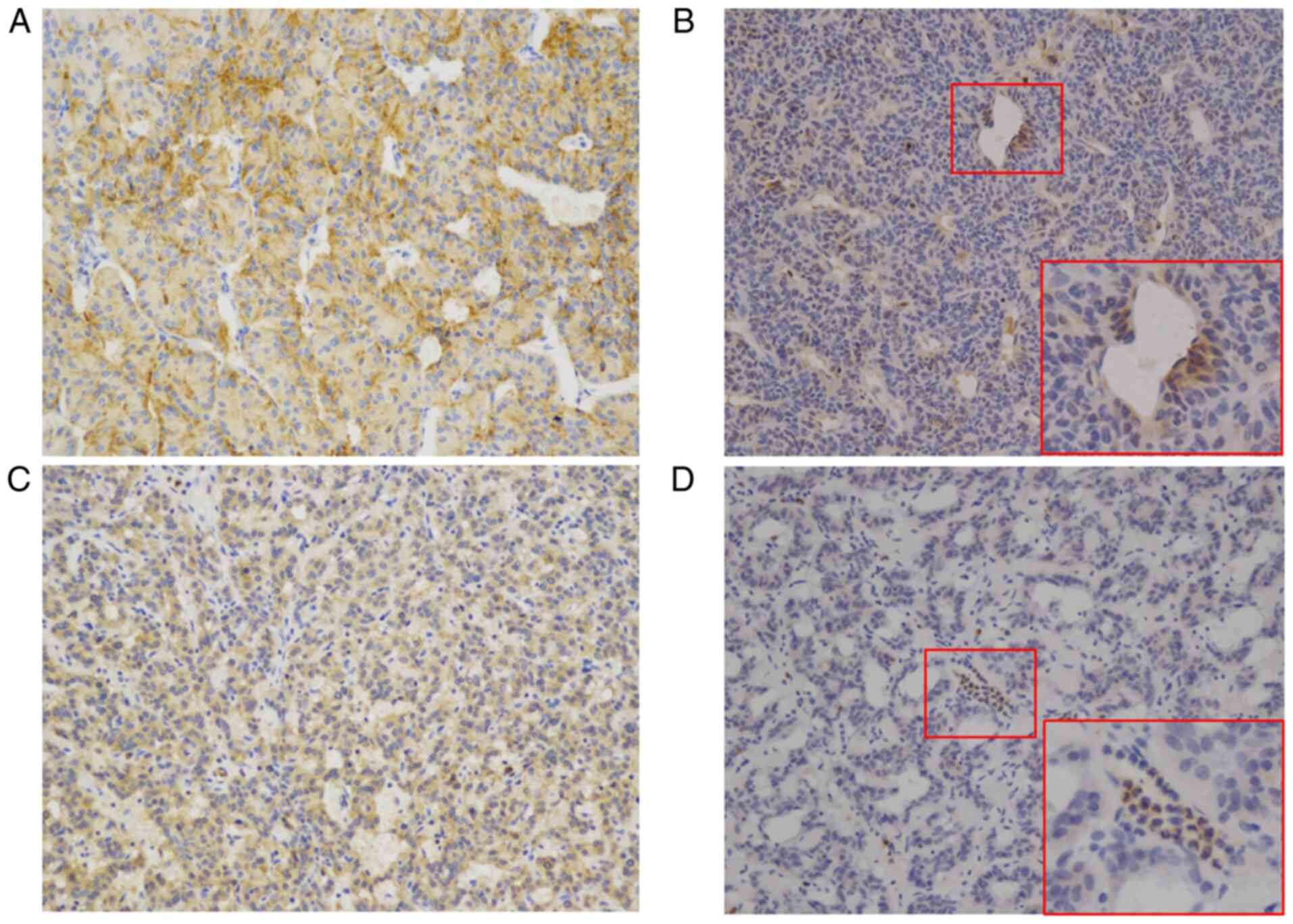
and cytomembrane of cells. CD44 exhibited two staining patterns:
Diffuse staining and scattered staining (Fig. 1A and B). The same staining patterns
were also noted for CD133 (Fig. 1C and
D).
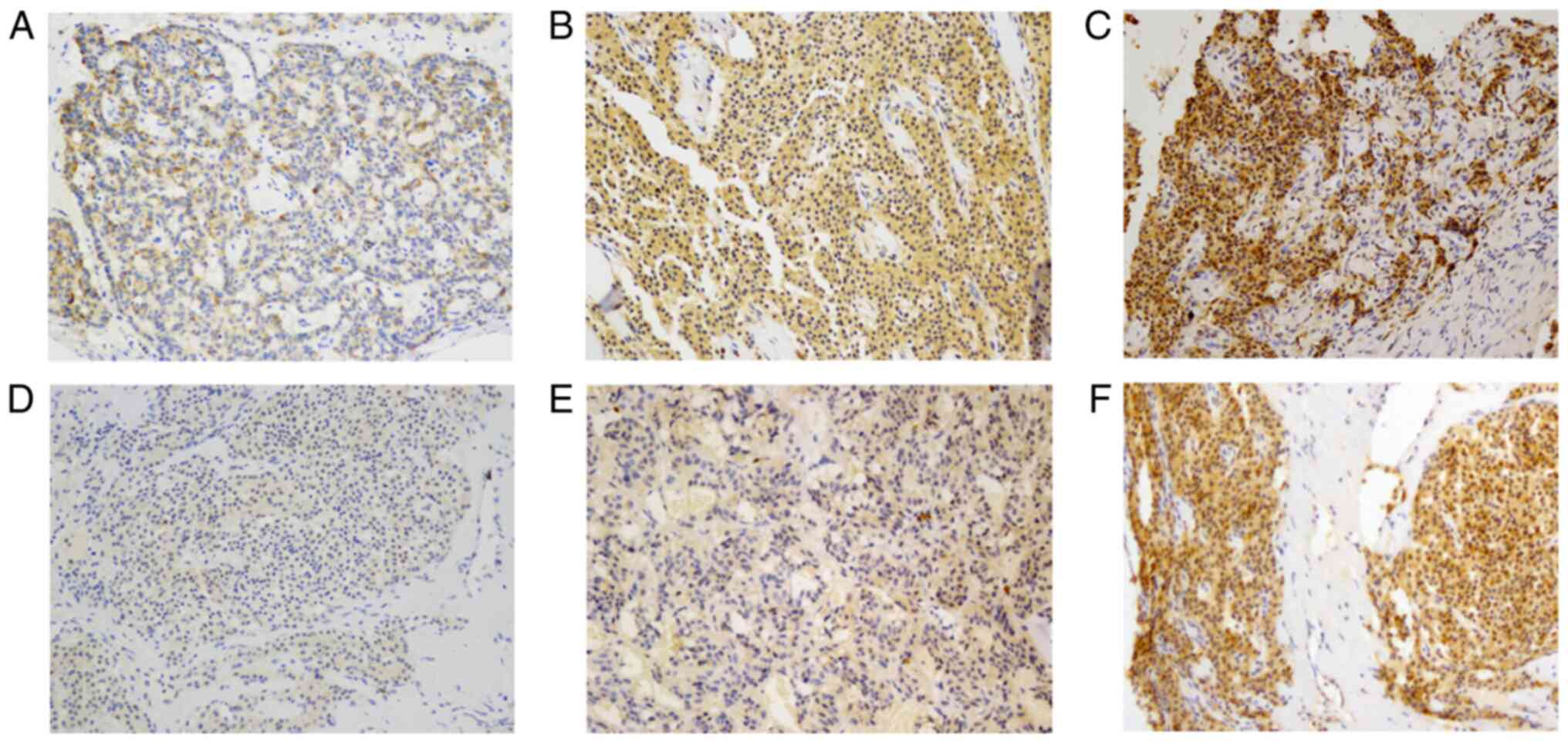
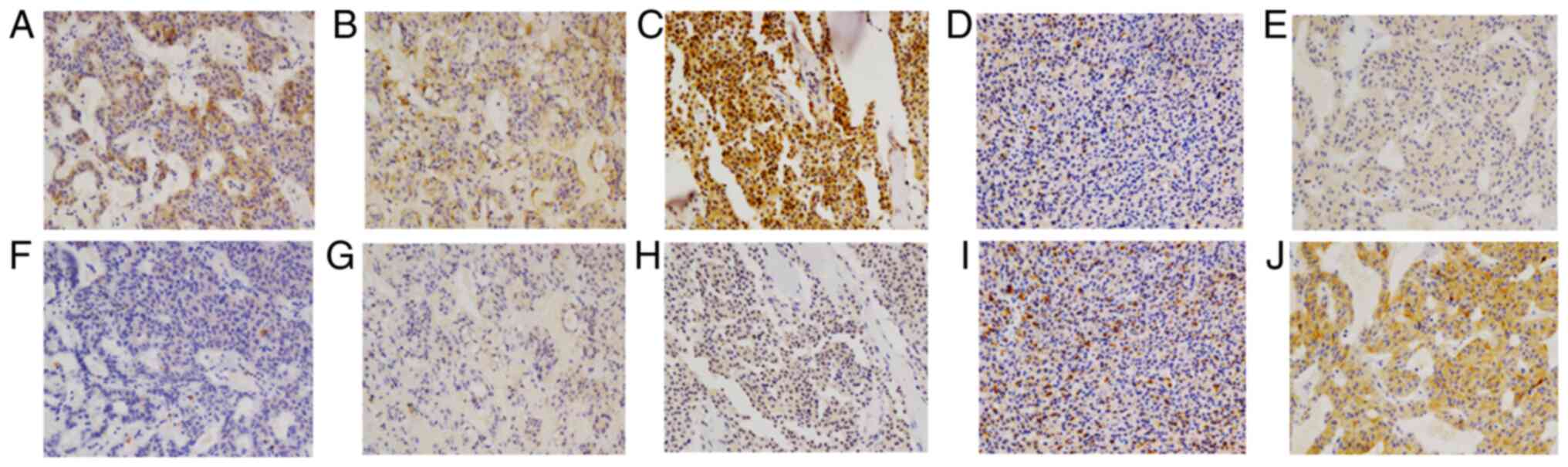
The expression levels of CD44 and CD133 were
evaluated in serial sections. The obtained staining scores ranged
from 0 (no staining) to 264 for CD44 staining and from 0 to 243 for
CD133 staining. For further analysis, CD44/133 expression was
divided into 4 levels: Level 0, no staining; level 1, score 1–100;
level 2, score 101–200; and level 3, score 201–300. Representative
images of staining levels are presented in Fig. 2. The number of cases in each level is
presented in Table I. Overall, CD44
staining was stronger than CD133 staining (Table I; Fig.
3), and a significant association was observed between CD44 and
CD133 expression (P<0.001; Table
I).
 | Table I.Association between CD44 and CD133
expression levels. |
Table I.
Association between CD44 and CD133
expression levels.
|
| CD44 levels |
|
|---|
|
|
|
|
|---|
| CD133 levels | 0 (n=10) | 1 (n=19) | 2 (n=32) | 3 (n=10) |
P-valuea |
|---|
| 0 (n=16) | 6 | 8 | 2 | 0 | <0.001 |
| 1 (n=27) | 4 | 8 | 12 | 3 |
|
| 2 (n=21) | 0 | 3 | 15 | 3 |
|
| 3 (n=7) | 0 | 0 | 3 | 4 |
|
CD44/CD133 expression and
clinicopathological parameters in PNETs
Patients were stratified according to the total
score of IHC staining, and the association between CD44/CD133
expression, and clinical characteristics of the enrolled patients
were compared. The associations between CD44 or CD133 expression
and the clinicopathological characteristics of patients with PNET
are presented in Tables II and
III, respectively. Increased CD44
expression was associated with poor tumour differentiation
(P=0.007), high Ki-67 index (P=0.001), added mitotic count
(P=0.003), high histological grade (P=0.001) and advanced stage
(P=0.025) (Table II). Increased
CD133 expression was also associated with high Ki-67 index
(P=0.014), age (P=0.028) and added mitotic count (P=0.012), but not
with tumour differentiation (P=0.118), tumour histologic grade
(P=0.126) and stage (P=0.203) (Table
III). No significant associations were observed between
CD44/133 expression and other clinical parameters, such as sex,
tumour location, tumour size, TNM stage and functionality (Tables II and III).
 | Table II.Association between CD44 expression
and clinicopathological characteristics in patients with pancreatic
neuroendocrine tumours (n=71). |
Table II.
Association between CD44 expression
and clinicopathological characteristics in patients with pancreatic
neuroendocrine tumours (n=71).
|
|
| CD44 expression
levels |
|
|---|
|
|
|
|
|
|---|
| Variables | Values | 0 (n=10) | 1 (n=19) | 2 (n=32) | 3 (n=10) |
P-valuea |
|---|
| Mean age ± SD,
years | 45.2±17.5 | 40.3±15.1 | 47.1±21.6 | 45.0±16.4 | 47.2±16.2 | 0.856 |
| Sex, n |
|
|
|
|
| 0.738 |
|
Female | 29 | 5 | 8 | 11 | 5 |
|
|
Male | 42 | 5 | 11 | 21 | 5 |
|
| Mean tumour size ±
SD, cm | 3.3±2.1 | 2.6±1.6 | 3.4±1.9 | 3.1±2.4 | 4.1±2.0 | 0.408 |
| Function, n |
|
|
|
|
| 0.395 |
|
Functional | 31 | 5 | 8 | 16 | 2 |
|
|
Non-functional | 40 | 5 | 11 | 16 | 8 |
|
| Location, n |
|
|
|
|
| 0.369 |
|
Head/uncinate | 34 | 7 | 9 | 12 | 6 |
|
| Body
and/or tail | 37 | 3 | 10 | 20 | 4 |
|
| Margin status,
n |
|
|
|
|
| 0.601 |
| R0 | 70 | 10 | 19 | 31 | 10 |
|
| R1 | 1 | 0 | 0 | 1 | 0 |
|
| Differentiation,
n |
|
|
|
|
| 0.007 |
|
Well/moderate | 64 | 10 | 18 | 30 | 6 |
|
|
Poor | 7 | 0 | 1 | 2 | 4 |
|
| Ki-67 index, n |
|
|
|
|
| 0.001 |
|
≤2% | 39 | 9 | 13 | 14 | 3 |
|
|
3-20% | 26 | 1 | 6 | 16 | 3 |
|
|
>20% | 6 | 0 | 0 | 2 | 4 |
|
| Mitotic
countb, n |
|
|
|
|
| 0.003 |
|
<2 | 38 | 10 | 14 | 13 | 1 |
|
|
2-20 | 25 | 0 | 4 | 15 | 6 |
|
|
>20 | 8 | 0 | 1 | 4 | 3 |
|
| Histological
gradec, n |
|
|
|
|
| 0.001 |
| G1 | 31 | 9 | 11 | 10 | 1 |
|
| G2 | 30 | 1 | 7 | 18 | 4 |
|
| G3 | 10 | 0 | 1 | 4 | 5 |
|
| TNM
staged, n |
|
|
|
|
| 0.025 |
| I | 49 | 7 | 13 | 26 | 3 |
|
| II | 22 | 3 | 6 | 6 | 7 |
|
 | Table III.Association between CD133 expression
and clinicopathological characteristics in patients with pancreatic
neuroendocrine tumours (n=71). |
Table III.
Association between CD133 expression
and clinicopathological characteristics in patients with pancreatic
neuroendocrine tumours (n=71).
|
|
| CD133 expression
levels |
|
|---|
|
|
|
|
|
|---|
| Variables | Patients | 0 (n=16) | 1 (n=27) | 2 (n=21) | 3 (n=7) |
P-valuea |
|---|
| Mean age ± SD,
years | 45.2±17.5 | 43.1±19.3 | 40.6±17.2 | 48.4±17.1 | 58.0±11.9 | 0.028 |
| Sex, n |
|
|
|
|
| 0.674 |
|
Female | 29 | 8 | 13 | 5 | 3 |
|
|
Male | 42 | 8 | 14 | 16 | 4 |
|
| Mean tumour size ±
SD, cm | 3.3±2.1 | 3.0±1.7 | 2.6+±1.7 | 4.0±2.5 | 4.3±2.4 | 0.051 |
| Function, n |
|
|
|
|
| 0.061 |
|
Functional | 31 | 6 | 15 | 10 | 0 |
|
|
Non-functional | 40 | 10 | 12 | 11 | 7 |
|
| Location, n |
|
|
|
|
| 0.247 |
|
Head/uncinate | 34 | 11 | 10 | 10 | 3 |
|
| Body
and/or tail | 37 | 5 | 17 | 11 | 4 |
|
| Margin status,
n |
|
|
|
|
| 0.491 |
| R0 | 70 | 16 | 27 | 20 | 7 |
|
| R1 | 1 | 0 | 0 | 1 | 0 |
|
| Differentiation,
n |
|
|
|
|
| 0.118 |
|
Well/moderate | 64 | 16 | 23 | 20 | 5 |
|
|
Poor | 7 | 0 | 4 | 1 | 2 |
|
| Ki-67 index, |
|
|
|
|
| 0.014 |
|
≤2% | 39 | 11 | 19 | 9 | 0 |
|
|
3-20% | 26 | 5 | 7 | 9 | 5 |
|
|
>20% | 6 | 0 | 1 | 3 | 2 |
|
| Mitotic
countb, n |
|
|
|
|
| 0.012 |
|
<2 | 38 | 13 | 16 | 9 | 0 |
|
|
2-20 | 25 | 3 | 7 | 9 | 6 |
|
|
>20 | 8 | 0 | 4 | 3 | 1 |
|
| Histological
gradec, n |
|
|
|
|
| 0.126 |
| G1 | 31 | 10 | 13 | 8 | 0 |
|
| G2 | 30 | 6 | 10 | 9 | 5 |
|
| G3 | 10 | 0 | 4 | 4 | 2 |
|
| TNM
staged, n |
|
|
|
|
| 0.203 |
| I | 49 | 10 | 22 | 14 | 3 |
|
| II | 22 | 6 | 5 | 7 | 4 |
|
Survival analysis
The median follow-up time for this cohort was 57
months (range, 12–182 months). The single patient with an R1
surgical margin was excluded from the survival analysis to maintain
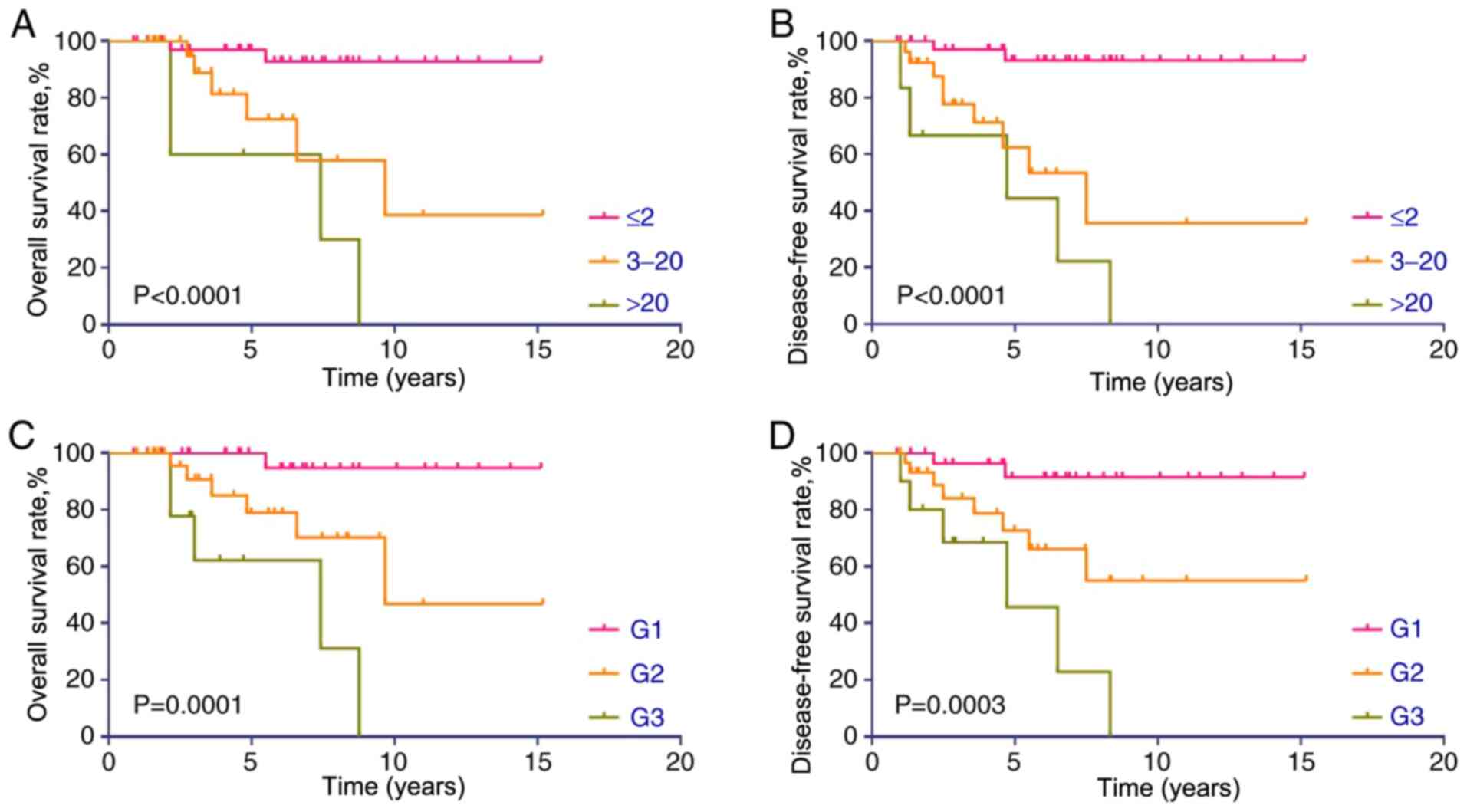
sample homogeneity. Kaplan-Meier survival curves for DFS and OS
stratified by Ki-67 index or histological grade are presented in
Fig. 4. Consistent with the
aforementioned immunohistochemistry observations, increased Ki-67
proliferative index and high histological grade were associated
with a poor prognosis in patients with PNET. Additionally,
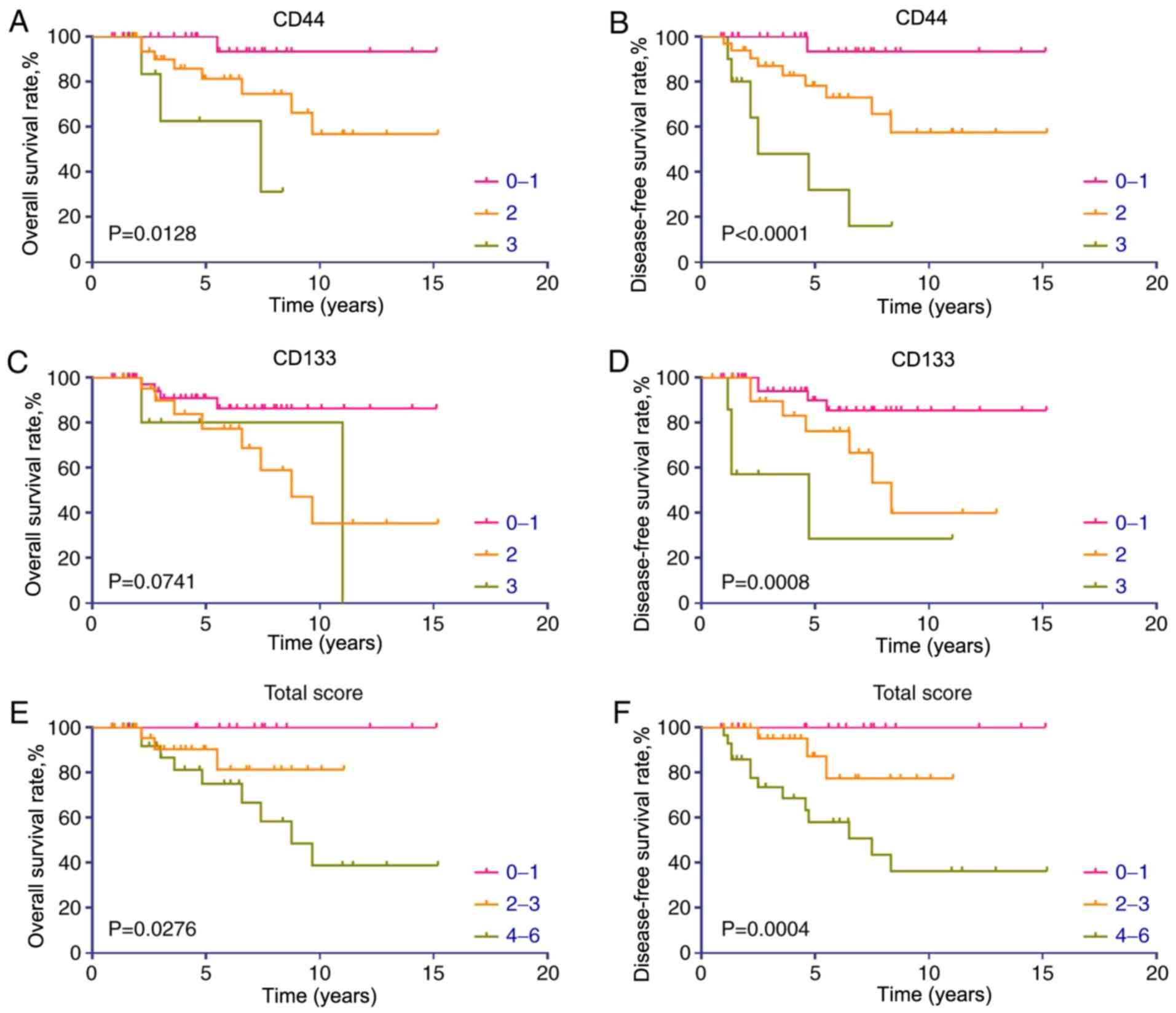
Kaplan-Meier survival curves revealed that patients with PNET with
low or no CD44 expression had significantly improved OS and DFS
rates (Fig. 5A and B). Increased
CD133 expression was associated with a poor OS rate (Fig. 5C); however, this association was not
significant (P=0.0741). However, CD133 expression was a significant
prognostic factor for DFS (P=0.0008; Fig. 5D).
To further evaluate the combined effect of CD44 and
CD133 co-expression on the prognosis in patients with PNET, the
CD44 expression levels were combined with the CD133 expression
levels for each sample, obtaining combined scores ranging from 0 to
6. Kaplan-Meier analysis revealed that patients with high combined
scores exhibited significantly decreased OS and DFS rates (Fig. 5E and F). Among the patients with a
combined score ≤1, none of the patients developed recurrence during
the follow-up period. Two G1 grade patients with a Ki-67 index ≤1%
experienced recurrence during the follow-up period, suggesting that
a total combined score ≤1 (indicating no CD44 and CD133 expression,
or that one of them is not expressed and the other is expressed at
a low level) may be a more effective predictor of a favourable
prognosis in patients with PNETs than low histological grade or low
Ki-67 index.
Discussion
Surgical resection remains the primary curative
modality in the management of PNETs (4). However, heterogeneous behaviour and
unpredictable pathology are a challenge to optimal treatment
decision-making. The use of CD44 and CD133 as markers for CSCs,
which may promote tumourigenesis and regeneration, has been
actively investigated in various types of solid tumour, such as
gastric cancer, breast cancer and colon cancer (14,40,41).
Additionally, the presence of CSCs has been confirmed in NETs
(35). However, no evidence is
available on the expression levels of the CSC markers CD44 and
CD133 in PNETs and their effect on the prognosis in patients with
PNET.
In the present study, data from 71 patients with
PNET were obtained to examine the significance of CD44 and CD133 as
prognostic markers for survival. Immunohistochemical analysis
revealed that both CD44 and CD133 were expressed in most PNET
tissues and revealed a tendency toward co-expression. Overall, CD44
exhibited a higher positive rate and stronger staining intensity
compared with CD133. Survival analysis demonstrated that CD133
and/or CD44 upregulation may predict an unfavourable prognosis in
patients with PNETs.
CSC populations are primarily responsible for tumour
initiation, growth and metastasis (42). To date, studies on CSCs in NETs have
been rare. Gaur et al (35)
identified and characterized neuroendocrine CSCs from a midgut
carcinoid cell line (CNDT2.5) using ALDH as a surface marker,
revealing that CSCs are present in NETs. However, tumour biological
characteristics and stem cell markers may differ between midgut
NETs and PNETs. For example, CD24 is a CSC marker, and its
expression is frequently noted in primary and metastatic midgut
NETs, but is rarely observed in pancreatic and duodenal NETs
(36). In midgut NET cells, Gaur
et al (35) observed that
nearly all CNDT2.5 cells bind to CD44, whereas cells were not
labelled with CD133. In the present immunohistochemical assessment
of PNET tissues, CD44 and CD133 were co-expressed in PNETs. The
significant associations between CD44 and/or CD133 and the
prognosis in patients with PNETs suggest that these proteins are
important tumour promoters and potential CSC markers in PNETs.
Surgical resection remains the curative treatment
for patients with PNET. Most studies on prognostic factors for
outcomes after resection of PNET include patients with distal
metastases at resection, stage III patients with tumours involving
the celiac axis or the superior mesenteric artery, R1 resections or
patients with familial syndromes (43–46). In
the present study, a selective group of patients with stage I–II
PNETs with R0 resection was included, and risk factors for
prognosis were analysed. In the present cohort, recurrences were
noted in patients with Ki-67 index ≤1 and in G1 patients. However,
patients with a CD44/CD133 total combined score ≤1 exhibited no
risk of recurrence. By contrast, patients with an increased total
score exhibited a significantly increased risk of recurrence.
Therefore, the type of follow-up visit may be selected based on
different recurrence risks to be more cost-effective. To the best
of our knowledge, there is no evidence that evaluates the effects
of adjuvant therapy after radical surgical resection of PNETs. In
current clinical practice, the decision regarding adjuvant therapy
after surgery remains at the discretion of the attending physician.
The present findings may help to avoid unnecessary adjuvant
treatments in patients with a very low risk of recurrence and to
optimize patient selection to investigate the role of adjuvant
therapy based on recurrence risk in further clinical research.
There are several limitations to the present study.
First, due to the low incidence of PNETs, the sample size of a PNET
cohort in a single centre is typically small, as in the current
study. Given that a large cohort is required to meet the
requirements of Cox regression analysis, it was not possible to
perform this analysis. Second, the aforementioned conclusions are
limited by the nature of single-centre data; an external validation
cohort is required to investigate whether the prognostic value of
CD44/CD133 is significant in other populations. Third, there was an
extended inclusion period in the present study. During this period,
follow-up strategies, surgical resection techniques and systemic
treatments have changed, which may have altered patient outcomes.
However, these limitations are difficult to overcome given the low
incidence and long course of PNETs. Further prospective and
multi-centre trials are warranted to discover prognostic factors
for PNETs and to reveal the underlying mechanisms involved in the
development of this type of tumour.
In conclusion, the present study revealed that
CD44/CD133 expression may be a useful biomarker to predict
prognosis after surgical resection of PNETs, and it may have a
pivotal role in the progression of PNETs. The current study was
limited by the nature of a retrospective design, and further
prospective studies and laboratory research are required to confirm
the present results and provide additional evidence for the role of
CD44/CD133 in PNETs.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Special Fund for Science and Technology Development of Guangdong
Province (grant no. 2016A030313769).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
BH conceived the present study. ZS and DL drafted
the initial manuscript. HW performed the immunohistochemical
examination of the sections. ZS was a major contributor to drafting
the final version of the manuscript. ZS, DL and BH analysed and
interpreted the patient data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Formalin-fixed, paraffin-embedded primary specimens
were obtained from all patients with protocols approved by the
Medical Ethics Review Committee of Guangdong Provincial People's
Hospital (Guangzhou, China; approval no. KY2020-169-01), and
written informed consent was provided by all patients. All the work
was performed in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PNETs
|
pancreatic neuroendocrine tumours
|
|
CSC
|
cancer stem cell
|
|
ALDH
|
aldehyde dehydrogenase
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
References
|
1
|
Vinik A, Casellini C, Perry RR, Feliberti
E and Vingan H: Diagnosis and management of pancreatic
neuroendocrine tumors (PNETS). De Groot LJ, Chrousos G and Dungan
K: Endotext, MDText.com, Inc. (South Dartmouth, MA). 2000.
|
|
2
|
Simard EP, Ward EM, Siegel R and Jemal A:
Cancers with increasing incidence trends in the United States: 1999
through 2008. CA Cancer J Clin. 62:118–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
United States. JAMA Oncol. 3:1335–1342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiruvella A and Kooby DA: Surgical
management of pancreatic neuroendocrine tumors. Surg Oncol Clin N
Am. 25:401–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kidd M, Modlin I and Oberg K: Towards a
new classification of gastroenteropancreatic neuroendocrine
neoplasms. Nat Rev Clin Oncol. 13:691–705. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haynes AB, Deshpande V, Ingkakul T, Vagefi
PA, Szymonifka J, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL and
Fernández-del Castillo C: Implications of incidentally discovered,
nonfunctioning pancreatic endocrine tumors: Short-term and
long-term patient outcomes. Ach Surg. 146:534–538. 2011.
|
|
7
|
Solorzano CC, Lee JE, Pisters PW, Vauthey
JN, Ayers GD, Jean ME, Gagel RF, Ajani JA, Wolff RA and Evans DB:
Nonfunctioning islet cell carcinoma of the pancreas: Survival
results in a contemporary series of 163 patients. Surgery.
130:1078–1085. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei IH, Harmon CM, Arcerito M, Cheng DF,
Minter RM and Simeone DM: Tumor-associated macrophages are a useful
biomarker to predict recurrence after surgical resection of
nonfunctional pancreatic neuroendocrine tumors. Ann Surg.
260:1088–1094. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bajorath J: Molecular organization,
structural features, and ligand binding characteristics of CD44, a
highly variable cell surface glycoprotein with multiple functions.
Proteins. 39:103–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thapa R and Wilson GD: The importance of
CD44 as a stem cell biomarker and therapeutic target in cancer.
Stem Cells Int. 2016:20872042016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naor D, Wallach-Dayan SB, Zahalka MA and
Sionov RV: Involvement of CD44, a molecule with a thousand faces,
in cancer dissemination. Semin Cancer Biol. 18:260–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ponta H, Sherman L and Herrlich PA: CD44:
from adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naor D, Nedvetzki S, Golan I, Melnik L and
Faitelson Y: CD44 in cancer. Crit Rev Clin Lab Sci. 39:527–579.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu L, Wu M, Sun L, Li W, Fu W, Zhang X and
Liu T: Clinicopathological and prognostic significance of cancer
stem cell markers CD44 and CD133 in patients with gastric cancer: A
comprehensive meta-analysis with 4729 patients involved. Medicine.
95:e51632016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang SJ and Bourguignon LY: Role of
hyaluronan-mediated CD44 signaling in head and neck squamous cell
carcinoma progression and chemoresistance. Am J Pathol.
178:956–963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang SJ, Ou-Yang F, Tu HP, Lin CH, Huang
SH, Kostoro J, Hou MF, Chai CY and Kwan AL: Decreased expression of
autophagy protein LC3 and stemness
(CD44+/CD24−/low) indicate poor prognosis in
triple-negative breast cancer. Hum Pathol. 48:48–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jian-Hui C, Er-Tao Z, Si-Le C, Hui W,
Kai-Ming W, Xin-Hua Z, Chaung-Qi C, Shi-Rong C and Yu-Long H: CD44,
Sonic Hedgehog, and Gli1 expression are prognostic biomarkers in
gastric cancer patients after radical resection. Gastroenterol Res
Pract. 2016:10130452016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Q, Zhou H, Liu Q, Cao Y, Wang G, Hu
A, Ruan L, Wang S, Bo Q, Chen W, et al: Prognostic value of the
expression of cancer stem cell-related markers CD133 and CD44 in
hepatocellular carcinoma: From patients to patient-derived tumor
xenograft models. Oncotarget. 7:47431–47443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Durko L, Wlodarski W, Stasikowska-Kanicka
O, Wagrowska-Danilewicz M, Danielewicz M, Hogendorf P, Strzelczyk J
and Malecka-Panas E: Expression and clinical significance of cancer
stem cell markers CD24, CD44, and CD133 in pancreatic ductal
adenocarcinoma and chronic pancreatitis. Dis Markers.
2017:32768062017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iseki Y, Shibutani M, Maeda K, Nagahara H,
Ikeya T and Hirakawa K: Significance of E-cadherin and CD44
expression in patients with unresectable metastatic colorectal
cancer. Oncol Lett. 14:1025–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chanmee T, Ontong P, Kimata K and Itano N:
Key roles of hyaluronan and its CD44 receptor in the stemness and
survival of cancer stem cells. Front Oncol. 5:1802015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bourguignon LYW, Earle C and Shiina M:
Activation of matrix hyaluronan-mediated CD44 signaling, epigenetic
regulation and chemoresistance in head and neck cancer stem cells.
Int J Mol Sci. 18:18492017. View Article : Google Scholar
|
|
23
|
Ohara Y, Oda T, Sugano M, Hashimoto S,
Enomoto T, Yamada K, Akashi Y, Miyamoto R, Kobayashi A, Fukunaga K,
et al: Histological and prognostic importance of
CD44(+)/CD24(+)/EpCAM(+) expression in clinical pancreatic cancer.
Cancer Sci. 104:1127–1134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li XP, Zhang XW, Zheng LZ and Guo WJ:
Expression of CD44 in pancreatic cancer and its significance. Int J
Clin Exp Pathol. 8:6724–6731. 2015.PubMed/NCBI
|
|
25
|
Grosse-Gehling P, Fargeas CA, Dittfeld C,
Garbe Y, Alison MR and Corbeil D Kunz-Schughart LA: CD133 as a
biomarker for putative cancer stem cells in solid tumours:
Limitations, problems and challenges. J Pathol. 229:355–378. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bauer N, Fonseca AV, Florek M, Freund D,
Jaszai J, Bornhauser M, Fergeas CA and Corbeil D: New insights into
the cell biology of hematopoietic progenitors by studying
prominin-1 (CD133). Cells Tissues Organs. 188:127–138. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bellizzi A, Sebastian S, Ceglia P,
Centonze M, Divella R, Manzillo EF, Azzariti A, Silvestris N,
Montemurro S, Caliandro C, et al: Co-expression of CD133(+)/CD44(+)
in human colon cancer and liver metastasis. J Cell Physiol.
228:408–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mokrowiecka A, Veits L, Falkeis C, Musial
J, Kordek R, Lochowski M, Kozak J, Wierzchniewska-Lawska A, Vieth M
and Malecka-Panas E: Expression profiles of cancer stem cell
markers: CD133, CD44, Musashi-1 and EpCAM in the cardiac
mucosa-Barrett's esophagus-early esophageal adenocarcinoma-advanced
esophageal adenocarcinoma sequence. Pathol Res Pract. 213:205–209.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Wu Y, Gao W, Li F, Bo Y, Zhu M, Fu
R, Liu Q, Wen S and Wang B: Identification and characterization of
CD133+CD44+ cancer stem cells from human laryngeal squamous cell
carcinoma cell lines. J Cancer. 8:497–506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han SA, Jang JH, Won KY, Lim SJ and Song
JY: Prognostic value of putative cancer stem cell markers (CD24,
CD44, CD133, and ALDH1) in human papillary thyroid carcinoma.
Pathol Res Prac. 213:956–963. 2017. View Article : Google Scholar
|
|
31
|
Zhou JY, Chen M, Ma L, Wang X, Chen YG and
Liu SL: Role of CD44(high)/CD133(high) HCT-116 cells in the
tumorigenesis of colon cancer. Oncotarget. 7:7657–7666. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bahnassy AA, Fawzy M, El-Wakil M, Zekri
AR, Abdel-Sayed A and Sheta M: Aberrant expression of cancer stem
cell markers (CD44, CD90, and CD133) contributes to disease
progression and reduced survival in hepatoblastoma patients: 4-year
survival data. Transl Res. 165:396–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Putzer BM, Solanki M and Herchenroder O:
Advances in cancer stem cell targeting: How to strike the evil at
its root. Adv Drug Deliv Rev. 120:89–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leon G, MacDonagh L, Finn SP, Cuffe S and
Barr MP: Cancer stem cells in drug resistant lung cancer: Targeting
cell surface markers and signaling pathways. Pharmacol Ther.
158:71–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gaur P, Sceusi EL, Samuel S, Xia L, Fan F,
Zhou Y, Lu J Tozzi F, Lopez-Berestein G, Vivas-Mejia P, et al:
Identification of cancer stem cells in human gastrointestinal
carcinoid and neuroendocrine tumors. Gastroenterology.
141:1728–1737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Salaria S, Means A, Revetta F, Idrees K,
Liu E and Shi C: Expression of CD24, a stem cell marker, in
pancreatic and small intestinal neuroendocrine tumors. Am J Clin
Pathol. 144:642–648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Katsuta E, Tanaka S, Mogushi K, Shimada S,
Akiyama Y, Aihara A, Matsumura S, Mitsunori Y, Ban D, Ochiai T, et
al: CD73 as a therapeutic target for pancreatic neuroendocrine
tumor stem cells. Int J Oncol. 48:657–669. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
American Joint Committee on Cancer, TNM
Staging System for Pancreatic Neuroendocrine Tumours, . (7th).
2010.
|
|
39
|
World Health Organization Classification
of Tumours Pathology and Genetics of Pancreas Tumours, . 2010.
|
|
40
|
Hirata A, Hatano Y, Niwa M, Hara A and
Tomita H: Heterogeneity of Colon Cancer Stem Cells. Adv Exp Med
Biol. 1139:115–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Das PK, Rakib MA, Khanam JA, Pillai S and
Islam F: Novel therapeutics against breast cancer stem cells by
targeting surface markers and signaling pathways. Curr Stem Cell
Res Ther. 14:669–682, 2×x. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zagar TM, White RR, Willett CG, Tyler DS,
Papavassiliou P, Papalezova KT, Guy CD, Broadwater G, Clough RW and
Czito BG: Resected pancreatic neuroendocrine tumors: Patterns of
failure and disease-related outcomes with or without radiotherapy.
Int J Radiat Oncol Biol Phys. 83:1126–1131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mehta S, de Reuver PR, Gill P, Andrici J,
D'Urso L, Mittal A, Pavlakis N, Clarke S, Samra JS and Gill AJ:
Somatostatin receptor SSTR-2a expression is a stronger predictor
for survival than Ki-67 in pancreatic neuroendocrine tumors.
Medicine. 94:e12812015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boninsegna L, Panzuto F, Partelli S,
Capelli P, Fave GD, Bettini R, Pederzoli P, Scarpa A and Falconi M:
Malignant pancreatic neuroendocrine tumour: Lymph node ratio and
Ki67 are predictors of recurrence after curative resections. Eur J
Cancer. 48:1608–1615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oh TG, Chung MJ, Park JY, Bang SM, Park
SW, Chung JB and Song SY: Prognostic factors and characteristics of
pancreatic neuroendocrine tumors: Single center experience. Yonsei
Med J. 53:944–951. 2012. View Article : Google Scholar : PubMed/NCBI
|