Introduction
Ovarian cancer is the sixth most frequently
diagnosed cancer among women worldwide (11.8 cases per 100,000
women in 2014), the second most common gynecologic malignancy in
female patients, and the most fatal tumor of the female
reproductive system (1,2). Among of ovarian tumors, ~90% are
epithelial ovarian serous cancers that occur primarily in
postmenopausal women (3,4). Despite the high response rates in
several patients receiving initial chemotherapy, the majority of
patients with advanced ovarian cancer ultimately develop recurrent
disease that is resistant to chemotherapy (5,6). The
identification of biomarkers has contributed to the management of
ovarian cancer by enabling the monitoring of patients' response to
treatment and recurrence, distinction of benign and malignant
pelvic masses, and detection of the disease at an earlier stage
(7,8). Thus, the identification of novel
biomarkers would benefit the prediction of patient prognosis and
the development of novel therapeutic strategies.
The tumor necrosis factor-α-induced protein 8-like
(TIPE/TNFAIP8) family is a recently identified family of proteins
that participate in the regulation of immunity and tumorigenesis
(9). The family comprises four
members: TIPE, TIPE1, TIPE2 and TIPE3 (9). TIPE2 has been demonstrated to be a
negative regulator of inflammation and cellular immunity (10,11),
whereas TIPE expression is downregulated in several malignances,
such as lung cancer, and is associated with a favorable patient
prognosis (12,13). TIPE1 is considered a cell death
inducer in both normal and cancer cells (12,13).
Downregulated TIPE1 expression has been observed in hepatocellular
carcinoma (14), lung cancer
(15) and gastric cancer (16). Ectopic TIPE1 expression promotes
apoptosis and inhibits cell invasion and migration in several types
of cancer (14–16); however, in cervical cancer, TIPE1
facilitates cell proliferation and suppresses the susceptibility of
cells to cisplatin chemotherapy (17). Furthermore, ectopic TIPE1 expression
significantly impairs osteosarcoma tumor growth in vivo by
inhibiting macrophage infiltration (18). However, the role of TIPE1 in ovarian
serous cancer remains unclear.
The present study aimed to determine the level of
expression and function of TIPE1 in ovarian cancer, using in
vitro and xenograft models. In addition, the underlying
molecular mechanism by which TIPE1 regulates ovarian cancer growth
was investigated via flow cytometric analysis, western blotting and
rescue experiments. The results of the present study expand the
understanding of TIPE1 and provide a potential prognostic predictor
and therapeutic target for ovarian cancer.
Materials and methods
Cell culture and reagents
Ovarian cancer cells (A2780, PA-1 and SKOV3) were
purchased from the American Type Culture Collection, while the
fallopian tube secretory epithelial cells (FTSECs) were purchased
from Shanghai Yu Bo Biotech Co., Ltd. Cells were cultured in DMEM
or RPMI-1640 medium supplemented with 10% fetal calf serum (all
from Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. The lentivirus-based TIPE1 expression system (based
on the LV6-EF1 α-Puro vector) and an empty vector control system
were purchased from Shanghai GenePharma Co., Ltd. and were used to
infect the ovarian cancer cells. Briefly, 1×105 A2780
and SKOV3 cells were seeded into each well of a 6-well plate and
incubated at 37°C with 5% CO2. At 24 h, 2×106
lentivirus (MOI=20) in 20 µl was added to infect the cells at 37°C
with 5% CO2, and 24 h later the culture medium was
removed. The stably infected cells were selected by treatment with
2 µg/ml puromycin (Selleck Chemicals) for 48 h at 37°C with 5%
CO2. The caspase-specific inhibitor z-VAD was purchased
from Selleck Chemicals and dissolved in DMSO for ovarian cancer
cell treatment (20 µM) for 24 h at 37°C with 5% CO2
after selecting the stably infected cells.
Tissue microarray
A microarray containing 90 human malignant ovarian
cancer tissues and 29 adjacent normal tissues (>5 cm away from
the tumor) were purchased from Shanghai Outdo Biotech Co., Ltd. IHC
staining was performed as previously described (19). Briefly, the tissue microarray was
subjected to dewaxing and hydration. Following antigen retrieval,
the slides were incubated with primary antibodies against TIPE1
(cat. no. 201986; 1:200 dilution; Abcam) overnight at 4°C.
Following incubation with the specific secondary antibodies (cat.
nos. SP9001 and SP9002; OriGene Technologies, Inc.) at 37°C for 1
h, the bound antibodies were visualized using 3,3′-diaminobenzidine
(Fuzhou Maixin Biotech Co., Ltd.). The nuclei were counterstained
with hematoxylin at room temperature for 10 min. The TIPE1 staining
in the tumor sections was scored by counting the number of positive
cells. TIPE1 staining was scored based on the percentage of
TIPE1-positive cells as follows: 0 points, <5%; 1 point, >5%
and <15%; 2 points, >15% and <25%; 3 points, >25% and
<35%; 4 points, >35%. The tumors with a score of 0 or 1 were
classified as TIPE1 low-expression tumors, while those with a score
of 2–4 were classified as TIPE1 high-expression tumors. The
analysis of the association between TIPE1 expression and patient
prognosis was performed as previously described (20).
Bioinformatics analyses
The expression raw data of TIPE1 in ovarian tumors
(n=98) and adjacent normal tissues (n=20) was downloaded from The
Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga).
Relative TIPE1 expression for each sample was calculated after
normalizing it to one normal sample. Wilcoxon signed-rank test was
used to analyze the data.
Cell viability assay
Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology) assay was performed to measure cell viability,
according to the manufacturer's instructions. Briefly, 1,000
cells/well were seeded into 96-well plates, and cell viability was
measured using CCK-8 at 0, 24, 48 and 72 h by measuring absorbance
at 450 nm using a microplate reader. A total of four independent
experiments were performed for each group.
Western blotting
Ovarian cancer cells and tumor tissues were
collected and lysed using RIPA lysis buffer (Beyotime Institute of
Biotechnology) supplemented with 1% protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA). Following centrifugation at 12,000 × g
for 15 min at 4°C, the supernatants were collected for protein
concentration determination via the Bradford assay (Beyotime
Institute of Biotechnology). A total of 20 µg of protein was loaded
for electrophoretic separation on 10% SDS/polyacrylamide gels and
transferred onto PVDF membranes (MilliporeSigma). Membranes were
blocked with 5% milk in TBS/Tween-20 (0.1%) buffer at room
temperature for 2 h, and subsequently probed with primary
antibodies against: TIPE1 (cat. no. 201986; 1:600 dilution; Abcam),
caspase-3 (cat. no. 9662; 1:400 dilution), caspase-8 (cat. no.
4927, 1:500 dilution), caspase-9 (cat. no. 9502; 1:400 dilution),
cleaved poly(ADP-ribose) polymerase (PARP; cat. no. 9548; 1:400
dilution), total PARP (cat. no. 5625; 1:1,000 dilution) and GAPDH
(cat. no. 5174; 1:2,000 dilution) (all from Cell Signaling
Technology, Inc.). Following the primary incubation, membranes were
incubated with horseradish peroxidase-conjugated anti-rabbit
immunoglobulin G (IgG)/anti-mouse IgG secondary antibody (cat. no.
ZDR-5306 and ZDR-5307; 1:10,000 dilution; OriGene Technologies,
Inc.) at room temperature for 2 h, and an ECL kit (MilliporeSigma)
was used to detect the expression of the target proteins.
Colony formation assay
For the colony formation assay, 2,000 ovarian cancer
cells were seeded into 6-well plates containing 2 ml DMEM
containing 10% FBS. Cells were subsequently cultured in a
humidified atmosphere of 5% CO2 at 37°C for 10–14 days.
The plated cells were then fixed with 4% paraformaldehyde at room
temperature (22–25°C) for 15 min, and stained with 0.2% crystal
violet (Beyotime Institute of Biotechnology) for 15 min at room
temperature. After washing with distilled water, the colonies in
each well were counted.
RNA isolation and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. The concentration of RNA was
determined by NanoDrop 2000 (Thermo Fisher Scientific, Inc.) and a
total of 1 µg RNA was used for RT using a Reverse Transcription kit
(cat. no. RR045A; Takara Bio, Inc.) according to the manufacturer's
instructions. For qPCR amplification, the reaction was performed in
a 20 µl reaction volume containing 10 µl SYBR-Green PCR Master mix
(cat. no. RR087A; Takara Bio, Inc.), using a real-time PCR system
(Roche Bioscience). The thermocycling conditions were as follows: 1
cycle at 95°C for 30 sec, followed by 42 cycles at 95°C for 5 sec
and 58°C for 30 sec. GAPDH was used as a loading control to
normalize TIPE1 expression. qPCR was subsequently performed, as
previously described (21). The
following primer sequences were used for qPCR: TIPE1 forward,
5′-CAGTGACCTGCTAGATGAG-3′, and reverse, 5′-CAAGGTGCTGAGTGAAGT-3′;
and GAPDH forward, 5′-GGTGAAGGTCGGAGTCAACG-3′, and reverse,
5′-CAAAGTTGTCATGGATGACC-3′. The results were analyzed as previously
described (22).
Flow cytometric analysis of apoptosis
and cell cycle
Ovarian cancer cells were seeded into a 6-well plate
at a density of 5×105 cells/well, and subsequently
incubated with 20 nM z-VAD for 24 h at 37°C. Cells were collected
by centrifugation at 1,500 × g at room temperature for 3 min,
washed with ice-cold phosphate buffered saline (PBS) three times,
and fixed with ice-cold 70% ethanol for 15 min at 4°C. For
apoptosis analysis, the apoptotic cells were stained with Annexin
V-fluorescein isothiocyanate and propidium iodide (PI; Nanjing
KeyGen Biotech Co., Ltd.), according to the manufacturer's
instructions. For cell cycle analysis, the cells were stained with
propidium iodide (cat. no. C1052; Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Apoptotic cells and the cell cycle were subsequently analyzed using
a FACSCanto II flow cytometer (BD Biosciences). The results were
analyzed by FlowJo software (version 10.0; FlowJo, LLC).
Animal study
All animal experiments were performed in compliance
with the provisions of the regulation for the Care and Use of
Laboratory Animals of Southern Medical University (Guangzhou,
China). Balb/c female nude mice (age, 5–6 weeks; weight, 15–16 g; 6
mice/group) were purchased from Beijing HFK Bioscience Co., Ltd.,
and maintained under specific pathogen-free conditions, with free
access to water and sterile food at 22–25°C and 12-h light/dark
cycle. To establish xenograft mouse models, the ovarian cancer
cells were collected and washed three times with sterile PBS. After
counting the cell number, cells were suspended in sterile PBS
(5×107 cells/ml), and 100 µl was subcutaneously
inoculated into the ventral of the mice. The tumor width and length
were measured from day 10 to day 25 post-injection using a vernier
caliper. The tumor volumes were calculated as follows: Tumor volume
= length × width2 × 0.52. No multiple tumors were
presented. At day 25 post-injection, the mice were anesthetized by
intraperitoneal injection with 60 µl 10% chloral hydrate (300
mg/kg; Beyotime Institute of Biotechnology) and sacrificed by
cervical dislocation. After confirming that the heartbeat of the
mice had stopped, the tumors were collected for further
analysis.
Immunohistochemical (IHC) staining and
terminal-deoxynucleoitidyl transferase mediated nick end labeling
(TUNEL) assay
IHC staining was performed, as previously described
(19). Briefly, ovarian tumor
tissues were fixed with 4% paraformaldehyde at room temperature for
48 h and embedded in paraffin. The 4-µm slides were sectioned and
subjected to dewaxing and hydration. Following antigen retrieval,
the slides were incubated with primary antibodies against TIPE1
(cat. no. 201986; 1:200 dilution; Abcam) and caspase-3 (cat. no.
9662; 1:100 dilution; Cell Signaling Technology, Inc.) overnight at
4°C. Following incubation with the specific secondary antibodies
(cat. nos. SP9001 and SP9002; OriGene Technologies, Inc.) at 37°C
for 1 h, the bound antibodies were visualized using
3,3′-diaminobenzidine (Fuzhou Maixin Biotech Co., Ltd.). The nuclei
were counterstained with hematoxylin at room temperature for 10
min. TUNEL assay was performed according to the manufacturer's
instructions (cat. no. QIA39; MilliporeSigma). Briefly, following
dewaxing and hydration, the slides were incubated with 2 mg/ml
Proteinase K (1:100 in 10 mM Tris, pH 8) at 37°C for 30 min. After
washing with TBS three times, the slides were incubated with 1X TdT
Equilibration Buffer at room temperature for 15 min and with 1X TdT
Labeling Reaction Mixture at 37°C for 1.5 h. After washing with TBS
three times, the slides were covered with mounting medium
containing a cell nuclear stain. TUNEL-positive cells were observed
in six randomly selected fields under a BX51 fluorescence
microscope The number of TUNEL-positive cells per frame were
counted by two experimenters.
5-Bromo-2-deoxyuridine staining (BrdU)
staining
A2780 cells (1×104) were seeded into each
well of a 6-well plate. At 24 h, the cells were treated with 10 µM
BrdU (Beyotime Institute of Biotechnology) at 37°C for 1 h, fixed
with 2% formaldehyde for 10 min at room temperature, washed with 1X
PBS, permeabilized in 0.2% Triton X-100 for 10 min at room
temperature, and washed with 1X PBS. The cells were incubated with
a phycoerythrin-conjugated-BrdU antibody (cat. no. 50230; 1:500
dilution; Cell Signaling Technology, Inc.) at 37°C for 2 h. The
cell nuclei were stained with DAPI (Beyotime Institute of
Biotechnology). The positive cells were observed in four randomly
selected fields under a BX51 fluorescence microscope.
Statistical analysis. Statistical analysis was
performed using GraphPad Prism 5 (GraphPad Software, Inc.) and data
are presented as the mean ± standard deviation differences between
two groups were evaluated using Student's t-test, and differences
among multiple groups were evaluated using analysis of variance,
followed by Tukey's multiple comparison test. The paired t-test was
used for analyzing the IHC score in paired samples, and Wilcoxon
signed-rank test was performed for TCGA data analysis. Kaplan-Meier
followed by log-rank test was performed to analyze the rate of
disease-free survival and overall survival. P<0.05 was
considered to indicate a statistically significant difference.
Results
Downregulation of TIPE1 predicts the
progression of ovarian cancer
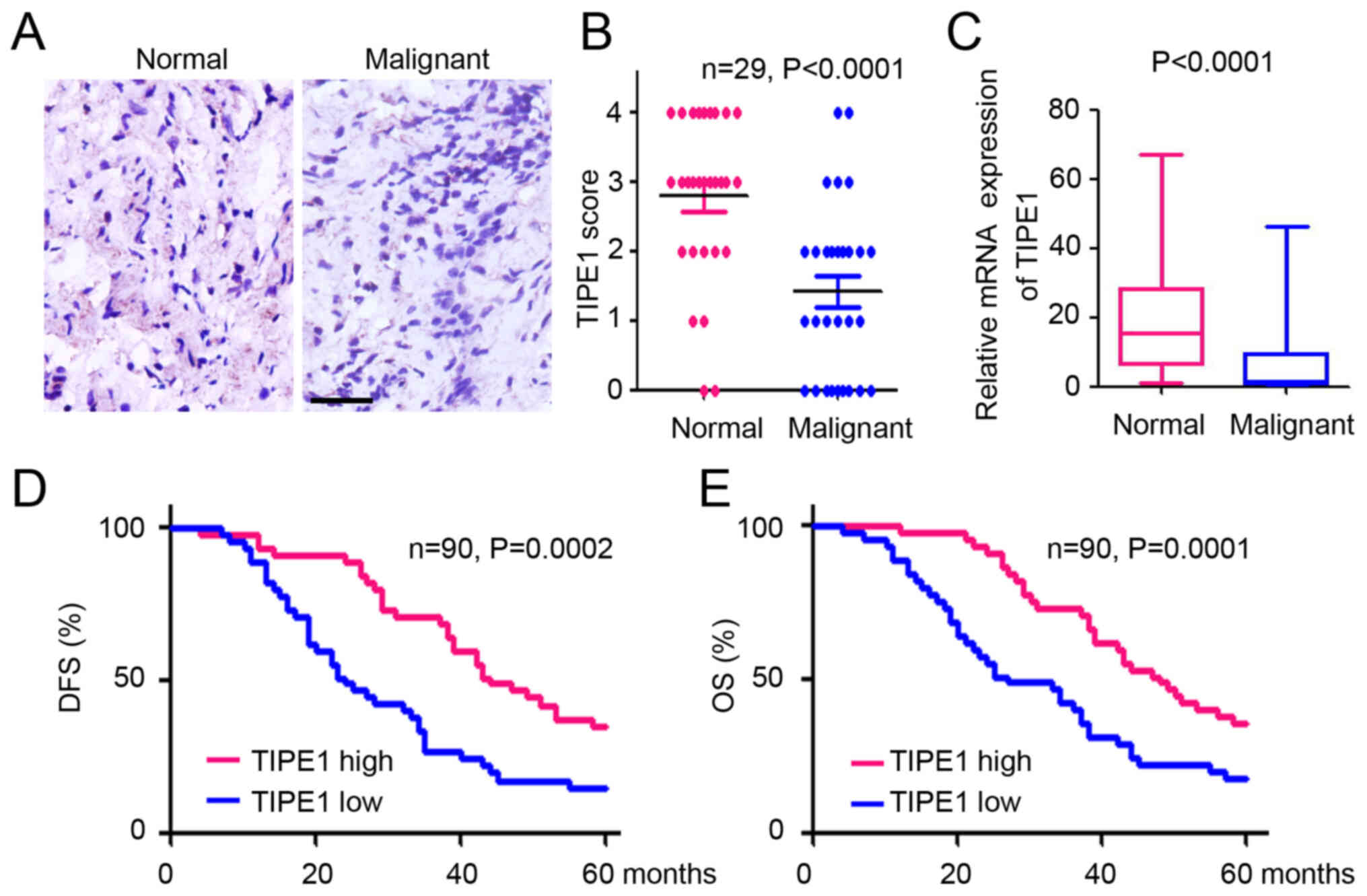
To investigate TIPE1 expression in ovarian cancer,
IHC staining was performed with a tissue microarray containing
normal and malignant ovarian tissues. TIPE1 expression was observed
in normal ovarian tissues, whereas few TIPE1-positive cells were
observed in malignant tissues (Fig.
1A). Further statistical analysis demonstrated a significant
decrease in TIPE1 expression in malignant ovarian tissues compared
with normal tissues, based on the IHC scores (Fig. 1B). A similar downregulation of TIPE1
was also observed in the ovarian serous tumors based on TCGA
database (Fig. 1C). To better
understand the potential role of TIPE1 in predicting the prognosis
of patients with ovarian cancer, patients were divided into high
and low TIPE1 groups, respectively, according to IHC scores of
TIPE1 expression. As presented in Fig.
1D, patients with a higher level of TIPE1 expression had a
higher rate of disease-free survival than patients with lower
levels of TIPE1 expression. Furthermore, lower TIPE1 expression
levels were associated with poorer overall survival in patients
with ovarian cancer (Fig. 1E).
Collectively, these results demonstrated that TIPE1 expression was
decreased in ovarian cancer tissues, and its expression levels were
associated with a favorable prognosis of patients with ovarian
cancer.
TIPE1 inhibits ovarian cancer cell
proliferation in vitro
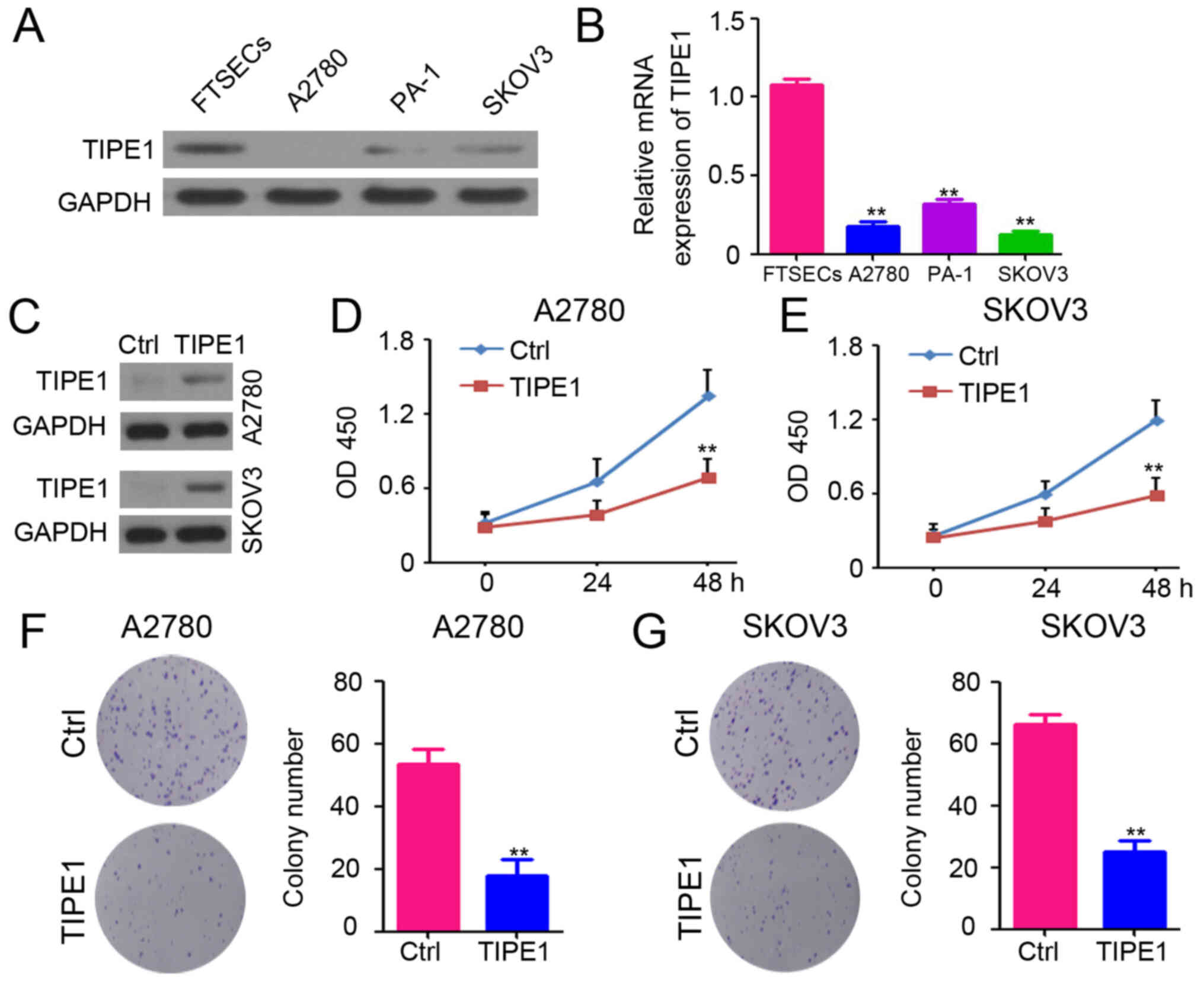
To select the best cells for determining the
functional role of TIPE1 in ovarian cancer, TIPE1 expression was
analyzed in normal human FTSECs and ovarian cancer cells (A2780,
PA-1 and SKOV3) via western blotting and RT-qPCR analyses. TIPE1
protein and mRNA expression levels were downregulated in ovarian
cancer cells (Fig. 2A and B).
Subsequently, A2780 and SKOV3 cells, two ovarian cancer cell lines
with low TIPE1 expression, were infected with a lentivirus based
TIPE1 expression system or a control system. Following selection
with puromycin, the stably infected cells were designated as
A2780-Ctrl, A2780-TIPE1, SKOV3-Ctrl and SKOV3-TIPE1, and expression
was confirmed via western blotting, which exhibited overexpression
of TIPE1 in A2780-TIPE1 and SKOV3-TIPE1 cells compared with the
controls (Fig. 2C). In order to
determine the cell viability of A2780 and SKOV3 cells, CCK-8 assay
was performed. As presented in Fig. 2D
and E, ectopic TIPE1 expression markedly inhibited the
proliferation of A2780 and SKOV3 cells. Colony formation assays
also confirmed the inhibitory role of TIPE1 on A2780 and SKOV3 cell
proliferation in vitro (Fig. 2F
and G). Taken together, these results provide evidence of the
inhibitory role of TIPE1 in ovarian cancer cell viability.
TIPE1 promotes caspase-dependent
apoptosis in ovarian cancer
To investigate the potential anticancer mechanisms
of TIPE1 control, dual staining using PI and Annexin V was
performed in ovarian cancer cells. The results demonstrated that
TIPE1 effectively promoted apoptosis of both A2780 and SKOV3 cells
(Fig. 3A and B; A2780-Ctrl, 3.2±1.0%
vs. A2780-TIPE1, 10.7±1.4%; SKOV3-Ctrl, 4.4±0.4% vs. SKOV3-TIPE1,
9.8±1.5%). In addition, flow cytometric analysis indicated that
TIPE1 had no significant effect on the distribution of ovarian
cancer cell cycle (Fig. S1A). BrdU
staining also indicated that there was no significant difference on
the proliferation between A2780-Ctrl and A2780-TIPE1 cells
(Fig. S1B). Western blot analysis
demonstrated that TIPE1 promoted the expression of caspase-8, −9,
−3, cleaved caspase-3, cleaved caspase-9 and cleaved PARP in both
A2780 and SKOV3 cells (Fig. 3C), and
had no significant effects on total PARP expression (Fig. 3C). Following inhibition of
caspase-dependent apoptosis using the specific inhibitor z-VAD,
TIPE1-mediated inhibition of proliferation (Fig. 3D) and induction of apoptosis
(Fig. 3E) in ovarian cancer cells
was attenuated. Collectively, these results suggest that TIPE1
promotes caspase-dependent apoptosis in ovarian cancer.
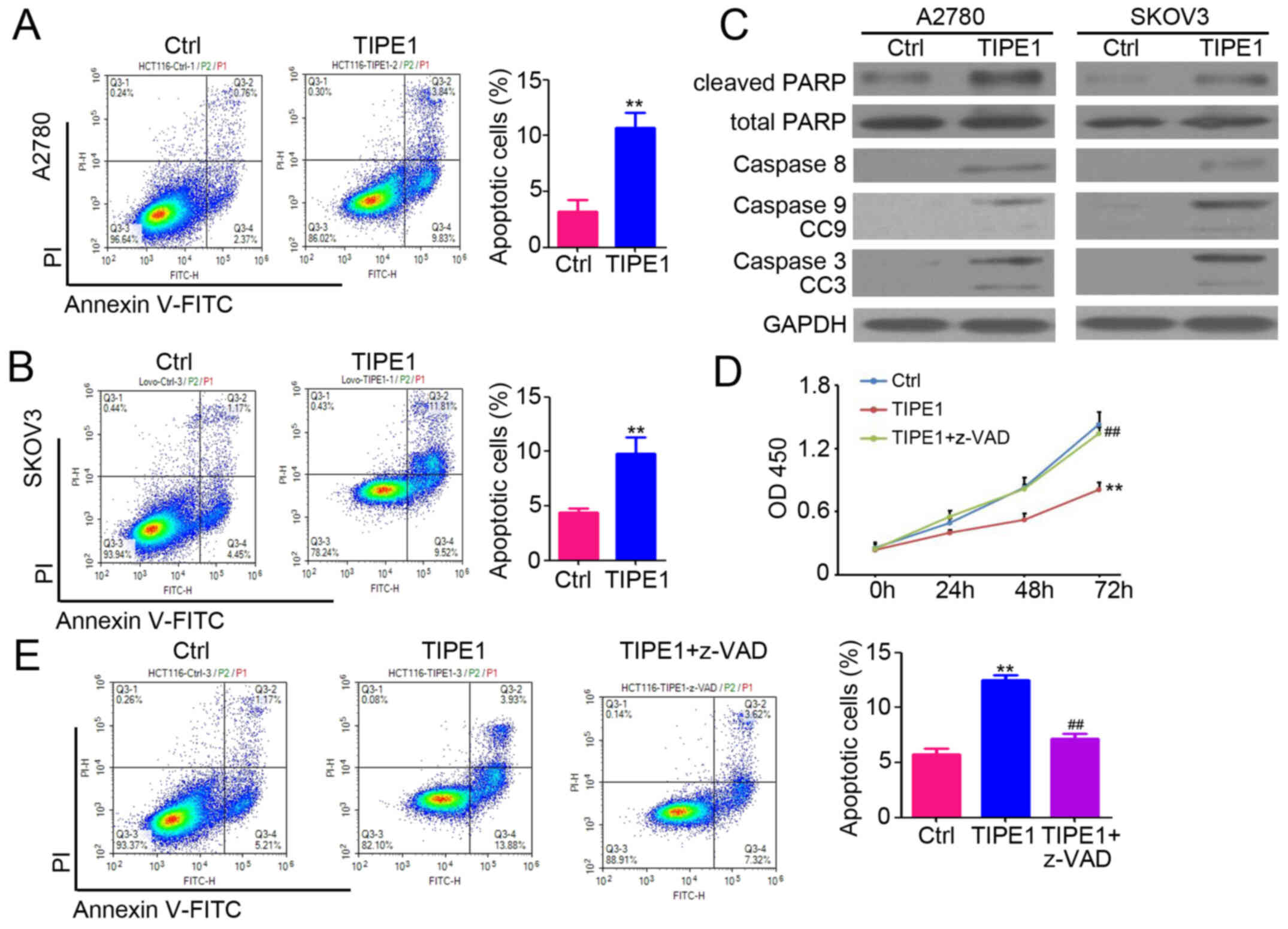 | Figure 3.TIPE1 promotes caspase-dependent
apoptosis in ovarian cancer. (A and B) Determination of apoptotic
cells by PI and Annexin V staining in A2780-Ctrl, A2780-TIPE1,
SKOV3-Ctrl and SKOV3-TIPE1 cells, followed by flow cytometry
analysis. The percentage of apoptotic cells was analyzed (n=3). (C)
Western blot analysis of caspase-8, caspase-9, CC9, caspase-3, CC3,
total PARP and cleaved PARP expression in A2780-Ctrl, A2780-TIPE1,
SKOV3-Ctrl and SKOV3-TIPE1 cells. GAPDH was used as a loading
control. (D) Cell Counting Kit-8 assay was performed to determine
the cell viability of A2780-Ctrl, A2780-TIPE1 and A2780-TIPE1 cells
treated with the z-VAD inhibitor. (n=5). (E) Determination of
apoptotic cells by PI and Annexin V staining in A2780-Ctrl,
A2780-TIPE1 and A2780-TIPE1 cells treated with the z-VAD inhibitor,
followed by flow cytometry analysis. The percentage of apoptotic
cells was analyzed (n=5). **P<0.01 vs. Ctrl;
##P<0.01 vs. TIPE1. TIPE1, tumor necrosis
factor-α-induced protein 8-like 1; PI, propidium iodide; CC,
cleaved caspase; PARP, cleaved poly(ADP-ribose) polymerase; Ctrl,
control; OD, optical density. |
TIPE1 impairs ovarian tumor growth in
vivo
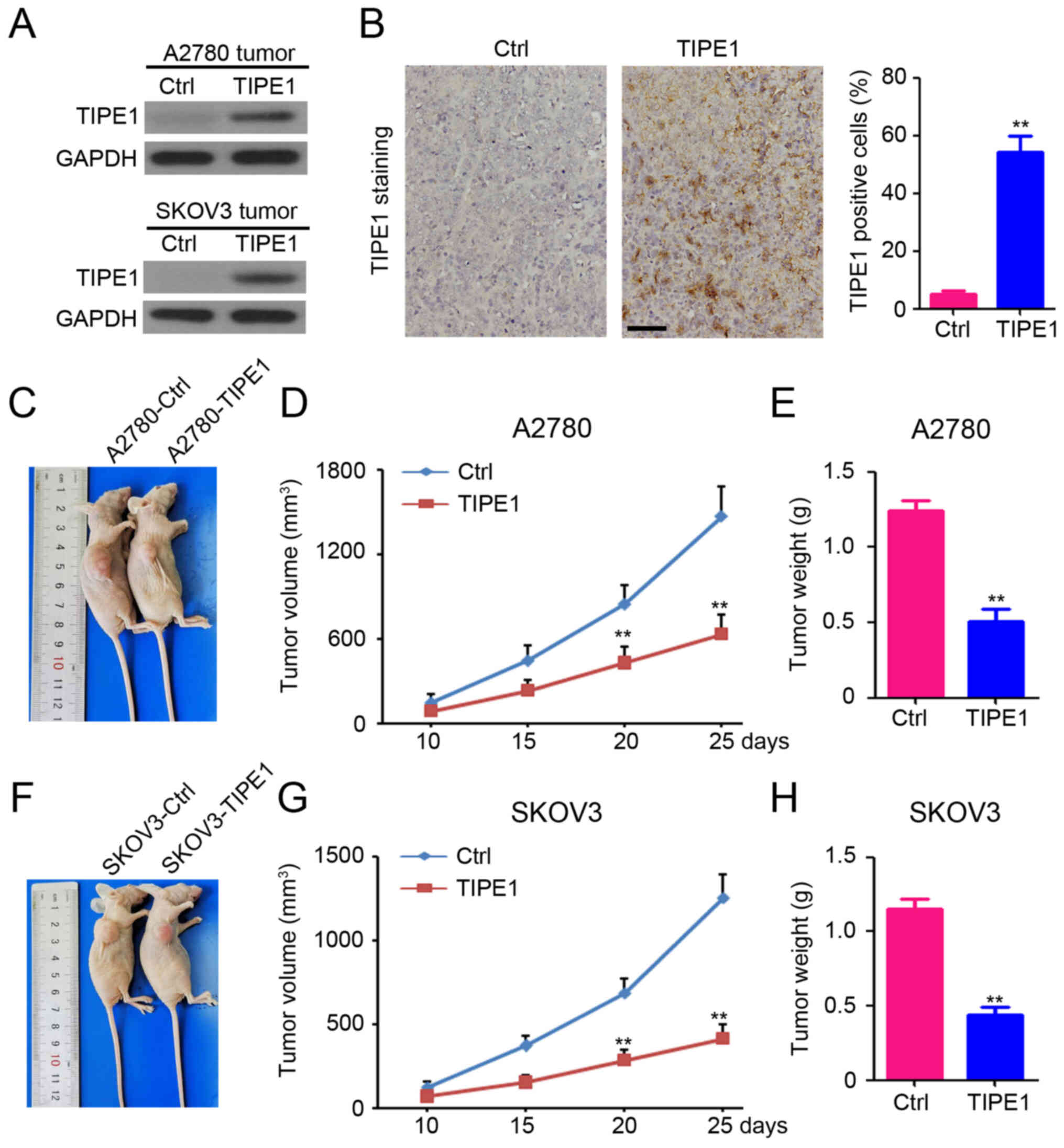
To further confirm the functional role of TIPE1 in
ovarian cancer, a xenograft mouse model was established by
subcutaneously injecting A2780 or SKOV3 cells into the right
ventricles of mice. Western blot analysis demonstrated the
upregulation of TIPE1 expression in both A2780-TIPE1 and
SKOV3-TIPE1 tumors compared with A2780-Ctrl and SKOV3-Ctrl tumors,
respectively (Fig. 4A). In addition,
a larger number of TIPE1-positive cells were observed in
A2780-TIPE1 tumors compared with A2780-Ctrl tumors (Fig. 4B). As presented in Fig. 4C-E, ectopic TIPE1 expression
effectively inhibited A2780 tumor growth, with a 56.9% reduction in
tumor volume (Fig. 4D; A2780-Ctrl
tumor volume, 1467.2±215.4 mm3 vs. A2780-TIPE1 tumor
volume, 632.9±132.8 mm3), and a 59.0% reduction in tumor
weight (Fig. 4E; A2780-Ctrl tumor
weight, 1.24±0.16 g vs. A2780-TIPE1 tumor weight, 0.51±0.20 g).
Furthermore, a significant antitumor effect of TIPE1 was also
observed in SKOV3 tumors, with a 67.1% reduction in tumor volume
(Fig. 4F and G; SKOV3-Ctrl tumor
volume, 1254.3±139.4 mm3 vs. SKOV3-TIPE1 tumor volume,
412.8±83.4 mm3), and a 61.8% reduction in tumor weight
(Fig. 4H; SKOV3-Ctrl tumor weight,
1.15±0.17 g vs. SKOV3-TIPE1 tumor weight, 0.44±0.13 g).
Collectively, these results suggest that TIPE1 impairs ovarian
tumor growth in vivo.
TIPE1 promotes ovarian tumor cell
apoptosis
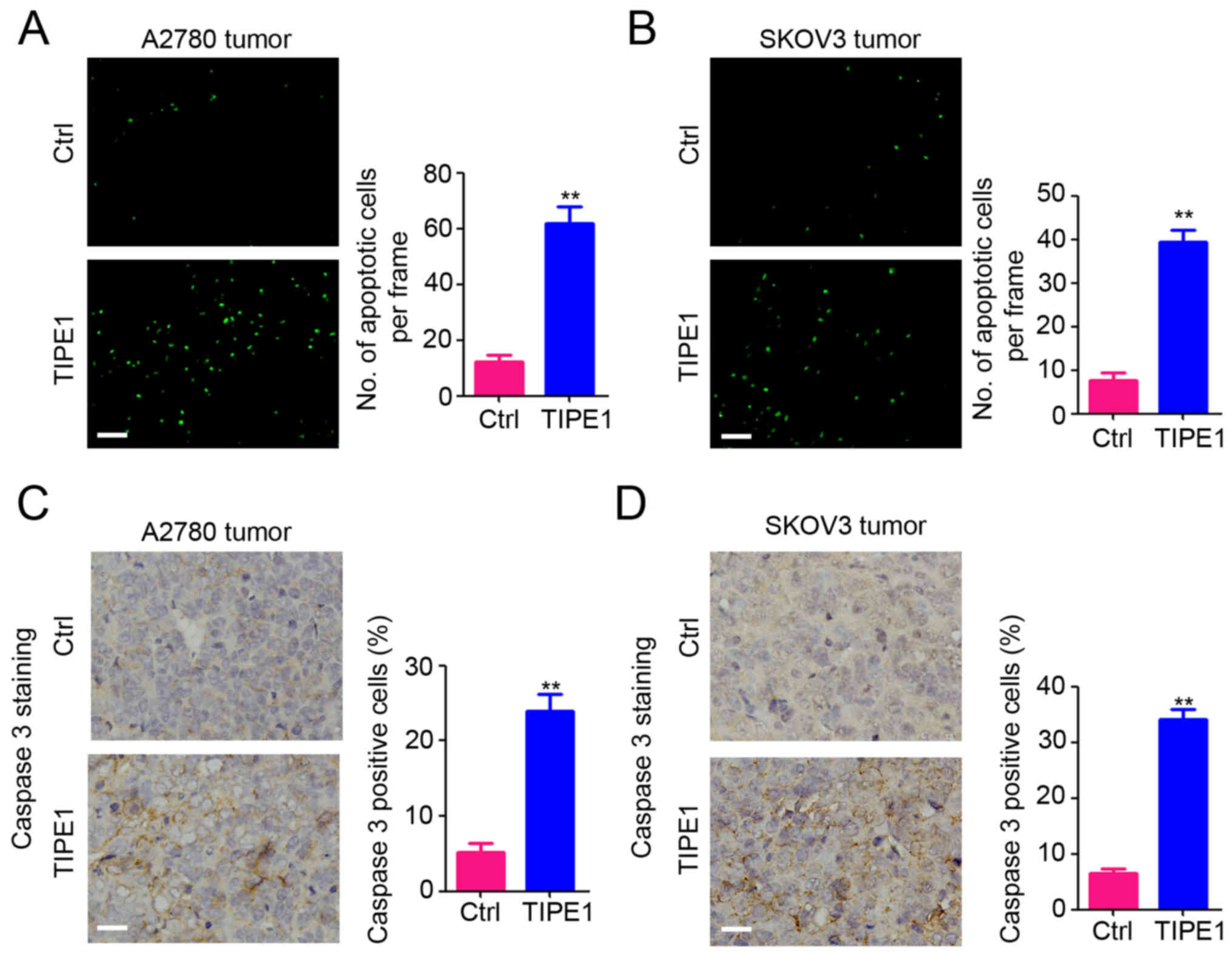
To determine the underlying molecular mechanism by
which TIPE1 inhibits ovarian tumor growth in vivo, TUNEL
assay was performed to detect apoptotic cells in A2780 and SKOV3
tumors. As presented in Fig. 5A, a
significantly greater number of apoptotic cells were observed in
A2780-TIPE1 tumors compared with A2780-Ctrl tumors. A similar
result was demonstrated for SKOV3 tumors (Fig. 5B). Furthermore, caspase-3 expression
was measured in A2780 and SKOV3 tumors by IHC staining, and the
results indicated that the percentage of caspase 3-positive cells
was significantly increased in both A2780-TIPE1 (Fig. 5C) and SKOV3-TIPE1 tumors (Fig. 5D). Collectively, these results
indicate that TIPE1 induces ovarian tumor cell apoptosis by
promoting caspase 3 expression, which was consistent with the in
vitro results.
Discussion
As an important regulator of homeostasis, the TIPE1
protein has been detected in a wide variety of normal tissues,
including neurons, hepatocytes, muscular tissues and epithelial
cells (23). Furthermore, several
studies have demonstrated the dysregulation of TIPE1 in various
types of cancer, including lung cancer (14–17,24). The
majority of these studies have reported the downregulation of TIPE1
expression in cancer tissues and cells (14–16).
However, enhanced TIPE1 expression has been reported in cervical
cancer tissues and cells (17). In
the present study, TIPE1 expression was demonstrated to be
downregulated in malignant tissues of patients with ovarian cancer
and ovarian cancer cells, which was consistent with the results of
previous studies on lung cancer (15), hepatocellular carcinoma (14) and gastric cancer (16). Based on the results demonstrating
altered TIPE1 expression in cancer tissues, the potential role of
TIPE1 in predicting patient prognosis was also assessed in the
present study. This has also been assessed in other types of cancer
in previous studies (14,15,17). The
results of the present study confirmed an inverse association
between TIPE1 expression and poor prognosis in patients with
ovarian cancer, which expands the role of TIPE1 in predicting
prognosis in various types of cancer.
TIPE1 plays crucial roles in diverse pathological
and physiological processes. In sepsis, TIPE1 functions as a
protective factor by inhibiting dendritic cell maturation, and
subsequently suppressing T-cell-mediated immunity (25). Antitumor effects of TIPE1 have also
been demonstrated in several types of cancer, including colorectal
and breast cancer (24,25). Recently, Ye et al (26) reported that ectopic TIPE1 expression
efficiently impairs stemness in colorectal cancer both in
vitro and in vivo. TIPE1 also inhibits breast cancer
cell proliferation, both in vivo and in vitro
(27), whereas it serves as an
oncogene in the pathogenesis of cervical cancer (17). Furthermore, TIPE1 overexpression
effectively inhibits osteosarcoma tumor growth in vivo,
which may contribute to the inhibition of macrophage infiltration
in osteosarcoma (18). In the
present study, the tumor suppressor role of TIPE1 in ovarian cancer
was confirmed by promoting apoptosis in vitro and in
vivo. Collectively, the results of the present study suggest
that TIPE1 may function as a potential therapeutic target for
ovarian cancer. Prospective studies will focus on investigating the
association and role of TIPE1 in chemotherapy-induced apoptosis in
ovarian cancer.
Promoting apoptosis is an efficient strategy for
inhibiting tumor progression and promoting chemotherapy sensitivity
in different types of cancer, including ovarian cancer (28,29).
Curcumin induces apoptosis in a dose- and time-dependent manner by
increasing the cytosolic Ca2+ concentration in ovarian
cancer cells (30). Luteolin
promotes apoptosis in cisplatin-resistant ovarian cancer cells and
enhances the cisplatin-induced reduction of tumor growth in mice
(31). The results of the present
study demonstrated the apoptosis-promoting role of TIPE1 in ovarian
cancer cells. In addition, ectopic TIPE1 expression was
demonstrated to impair ovarian cancer tumor growth in mice by
inducing apoptosis. Further investigations of the underlying
molecular mechanism indicated that TIPE1 increased the expression
of caspase-3, caspase-8 and caspase-9 in ovarian cancer cells.
Inhibition of caspase activity blocked the induction of apoptosis
by TIPE1 in ovarian cancer cells. β-catenin, Rac1 and p53
acetylation have been reported as the downstream targets of TIPE1
for the regulation of tumor growth (14,17,26).
Thus, further studies are required to identify the downstream
targets of TIPE1 for the regulation of ovarian cancer cell
proliferation.
In conclusion, the results of the present study
demonstrated the prognosis-predicting role and antitumor activity
of TIPE1 in ovarian cancer. However, further studies are required
to identify the direct targets of TIPE1 for the promotion of
caspase-dependent apoptosis. Furthermore, there is a long way to
demonstrate the therapeutic effect of TIPE1 in patients with
ovarian cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
National Natural Science Foundation of China (grant no. 81871214)
and the Merck Serono China Research Fund for Fertility Experts.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
SQ designed the experimental objective and made the
final decision for the manuscript. TL and LJ performed the main
experiments, and TL drafted the initial manuscript and performed
the literature review. YD helped with animal experiments. BW
performed the morphology experiments. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of Southern Medical University (20180416, Guangzhou,
China), and complied with the animal experiment guidelines of
Southern Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian cancer statistics, 2018. CA Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lheureux S, Braunstein M and Oza AM:
Epithelial ovarian cancer: Evolution of management in the era of
precision medicine. CA Cancer J Clin. 69:280–304. 2019.PubMed/NCBI
|
|
4
|
Partridge EE and Barnes MN: Epithelial
ovarian cancer: Prevention, diagnosis, and treatment. CA Cancer J
Clin. 49:297–320. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JY, Cho CH and Song HS: Targeted
therapy of ovarian cancer including immune check point inhibitor.
Korean J Intern Med (Korean Assoc Intern Med). 32:798–804.
2017.
|
|
6
|
Kroeger PT Jr and Drapkin R: Pathogenesis
and heterogeneity of ovarian cancer. Curr Opin Obstet Gynecol.
29:26–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong X, Men X, Zhang W and Lei P: Advances
in tumor markers of ovarian cancer for early diagnosis. Indian J
Cancer. 51 (Suppl 3):e72–e76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang WL, Lu Z and Bast RC Jr: The role of
biomarkers in the management of epithelial ovarian cancer. Expert
Rev Mol Diagn. 17:577–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bordoloi D, Banik K, Shabnam B, Padmavathi
G, Monisha J, Arfuso F, Dharmarajan A, Mao X, Lim LHK, Wang L, et
al: TIPE family of proteins and its implications in different
chronic diseases. Int J Mol Sci. 19:192018. View Article : Google Scholar
|
|
10
|
Jiang J, Pang X, Liu H, Yang X, Zhang Y,
Xiang X, Li J, Li T and Zhao P: Reduced TIPE2 expression is
inversely associated with proinflammatory cytokines and positively
correlated with bone mineral density in patients with osteoporosis.
Life Sci. 216:227–232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Q, Ma L, Liu T, Ge C, Zhou Q, Wei C
and Shi W: TIPE2 suppresses Pseudomonas aeruginosa keratitis by
inhibiting NF-kappaB signaling and the infiltration of inflammatory
cells. J Infect Dis. 220:1008–1018. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Ni XY, Chen RL, Li J and Gao FG:
TIPE attenuates the apoptotic effect of radiation and cisplatin and
promotes tumor growth via JNK and p38 activation in Raw264.7 and
EL4 cells. Oncol Rep. 39:2688–2694. 2018.PubMed/NCBI
|
|
13
|
Padmavathi G, Banik K, Monisha J, Bordoloi
D, Shabnam B, Arfuso F, Sethi G, Fan L and Kunnumakkara AB: Novel
tumor necrosis factor-α induced protein eight (TNFAIP8/TIPE)
family: Functions and downstream targets involved in cancer
progression. Cancer Lett. 432:260–271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Liang X, Gao L, Ma H, Liu X, Pan
Y, Yan W, Shan H, Wang Z, Chen YH, et al: TIPE1 induces apoptosis
by negatively regulating Rac1 activation in hepatocellular
carcinoma cells. Oncogene. 34:2566–2574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu X, Ma Y, Cheng J, Li X, Zheng H, Jiang
L and Zhou R: TIPE1 function as a prognosis predictor and negative
regulator of lung cancer. Oncotarget. 8:78496–78506. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu W, Chen Y, Xie H, Guo Y, Ren D, Li Y,
Jing X, Li D, Wang X, Zhao M, et al: TIPE1 suppresses invasion and
migration through down-regulating Wnt/β-catenin pathway in gastric
cancer. J Cell Mol Med. 22:1103–1117. 2018.PubMed/NCBI
|
|
17
|
Zhao P, Pang X, Jiang J, Wang L, Zhu X,
Yin Y, Zhai Q, Xiang X, Feng F and Xu W: TIPE1 promotes cervical
cancer progression by repression of p53 acetylation and is
associated with poor cervical cancer outcome. Carcinogenesis.
40:592–599. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen P, Zhou J, Li J, Zhang Q and Zuo Q:
TIPE1 suppresses osteosarcoma tumor growth by regulating macrophage
infiltration. Clin Transl Oncol. 21:334–341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai L, Cui X, Zhang X, Cheng L, Liu Y,
Yang Y, Fan P, Wang Q, Lin Y, Zhang J, et al: SARI inhibits
angiogenesis and tumour growth of human colon cancer through
directly targeting ceruloplasmin. Nat Commun. 7:119962016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ujiie D, Okayama H, Saito K, Ashizawa M,
Min AK, Endo E, Kase K, Yamada L, Kikuchi T, Hanayama H, et al:
KRT17 as a prognostic biomarker for stage II colorectal cancer.
Carcinogenesis. 41:591–599. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bao L, You B, Shi S, Shan Y, Zhang Q, Yue
H, Zhang J, Zhang W, Shi Y, Liu Y, et al: Metastasis-associated
miR-23a from nasopharyngeal carcinoma-derived exosomes mediates
angiogenesis by repressing a novel target gene TSGA10. Oncogene.
37:2873–2889. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui J, Zhang G, Hao C, Wang Y, Lou Y,
Zhang W, Wang J and Liu S: The expression of TIPE1 in murine
tissues and human cell lines. Mol Immunol. 48:1548–1555. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Liu Y, Hu C, Ni X and Huang X:
Tumor necrosis factor α-induced protein 8-like 1 promotes apoptosis
by regulating B-cell leukemia/lymphoma-2 family proteins in
RAW264.7 cells. Oncol Lett. 12:3506–3512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luan YY, Zhang L, Zhu FJ, Dong N, Lu JY
and Yao YM: Effect of TIPE1 on immune function of dendritic cells
and its signaling pathway in septic mice. J Infect Dis.
220:699–709. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye T, Yang B, Wang C, Su C, Luo J, Yang X,
Yu H, Yuan Z, Meng Z and Xia J: TIPE1 impairs stemness maintenance
in colorectal cancer through directly targeting β-catenin.
Carcinogenesis. 41:25–35. 2020.PubMed/NCBI
|
|
27
|
Hu W, Feng CM, Liu LY, Li N, Tian F, Du
JX, Zhao Y, Xiang XX, Liu K and Zhao PQ: TIPE1 inhibits breast
cancer proliferation by downregulating ERK phosphorylation and
predicts a favorable prognosis. Front Oncol. 9:4002019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tong Y, Zhang G, Li Y, Xu J, Yuan J, Zhang
B, Hu T and Song G: Corilagin inhibits breast cancer growth via
reactive oxygen species-dependent apoptosis and autophagy. J Cell
Mol Med. 22:3795–3807. 2018. View Article : Google Scholar
|
|
29
|
Yan XY, Zhong XR, Yu SH, Zhang LC, Liu YN,
Zhang Y, Sun LK and Su J: p62 aggregates mediated caspase 8
activation is responsible for progression of ovarian cancer. J Cell
Mol Med. 23:4030–4042. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seo JA, Kim B, Dhanasekaran DN, Tsang BK
and Song YS: Curcumin induces apoptosis by inhibiting
sarco/endoplasmic reticulum Ca2+ ATPase activity in
ovarian cancer cells. Cancer Lett. 371:30–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Luo Y, Qiao T, Wu Z and Huang Z:
Luteolin sensitizes the antitumor effect of cisplatin in
drug-resistant ovarian cancer via induction of apoptosis and
inhibition of cell migration and invasion. J Ovarian Res.
11:932018. View Article : Google Scholar : PubMed/NCBI
|