Introduction
Checkpoint immunotherapy has emerged as a promising
strategy for cancer treatments (1,2)
particularly for the treatment of melanoma (3) and lung cancer (4,5). Despite
the attempts made to utilize checkpoint immunotherapy for
pancreatic adenocarcinoma (PAAD) treatment, no clinical benefits
have yet been observed (6,7). It has been proposed that the specific
immunosuppressive tumor microenvironment of pancreatic cancer is
accountable for the limited clinical benefit of immunotherapy
(8,9). Immunosuppressive myeloid cells are one
of the numerous barriers in immunotherapy for pancreatic cancer
(10), as well as high stromal
density (11). Hence, researchers
established a combination of agents to facilitate checkpoint
immunotherapy in pancreatic cancer. It has been demonstrated that
the inhibition of interleukin 6 (IL-6) may enhance the efficacy of
programmed death-ligand 1 (PD-L1) in pancreatic cancer (12). Dickkopf-3 neutralization also leads
to an improvement in the immunotherapy effect in pancreatic cancer
(13). However, there is the
possibility that pancreatic cancer cells are able to utilize other
cell surface markers in order to avoid checkpoint immunotherapy and
thus facilitate disease progression.
The identification of cell surface markers for
pancreatic cancer provides therapeutic and diagnostic strategies.
Through the assistance of mass spectrometry, a cell surface
proteoglycan, glypican-1, was found to be specifically enriched in
pancreatic cancer cells and the associated exosomes (14), which provided a non-invasive
diagnostic and screening tool to detect early stages of pancreatic
cancer. Recently, it has been confirmed that plasminogen receptor
S100A10 is enriched on the pancreatic cancer cell surface and
contributes to cancer cell invasion (15). Consequently, it is important to
characterize novel pancreatic cancer-specific cell surface markers
that benefit immunotherapy by providing novel target sites and
improve patient survival through facilitating curative surgical
therapy via early diagnosis. To identify pancreatic cancer-specific
cell surface markers, the present study utilized mass spectrometry
and analyzed seven paired pancreatic adenocarcinoma tissues and
adjacent tissues. The analysis led to the discovery of a novel
panel of membrane proteins that predict the survival outcome of
patients with pancreatic cancer, as well as potential diagnostic
markers and therapeutic targets for pancreatic adenocarcinoma.
Materials and methods
PAAD samples
All patients involved in the present study provided
written informed consent, and all procedures were approved by the
Ethics Committee of Chinese PLA General Hospital (Beijing, China)
in accordance with the Declaration of Helsinki. All PAAD and
adjacent normal tissues (≥10 cm from the tumor tissue) were
obtained during surgeries between July and August 2017 at the
Department of Hepatobiliary and Pancreatic Surgical Oncology. PAAD
samples were confirmed by two blinded independent pathologists
(with 10 years of experience with pathologic diagnosis of the
pancreas) from the Department of Pathology at Chinese PLA General
Hospital. The diagnostic criteria were based on clinical practice
guidelines for pancreatic cancer issued by the National
Comprehensive Cancer Network (16).
PAAD and adjacent normal tissues were immediately dissected
following surgery and directly frozen at −80°C. Patient information
are listed in Table I.
 | Table I.Information of the patients with
pancreatic adenocarcinoma. |
Table I.
Information of the patients with
pancreatic adenocarcinoma.
| Sample no. | Sex | Age, years | Pathological
type |
|---|
| P1 | Male | 73 | Moderately
differentiated |
| P14 | Male | 45 | Moderately-poorly
differentiated |
| P22 | Male | 53 | Moderately-poorly
differentiated |
| P32 | Male | 75 | Moderately
differentiated |
| P36 | Male | 50 | Moderately-poorly
differentiated |
| P40 | Male | 50 | Poorly
differentiated |
| P45 | Female | 57 | Moderately
differentiated |
Mass spectrometry (MS) analysis
PAAD and adjacent normal tissues were homogenized in
a homogenizer (cat. no. JXSFTPRP-48; Shanghai Jingxin Medical
Instruments Co., Ltd.) at 120 Hz for 2 min, with 2, 2-mm steel
beads in cell lysis buffer (50 mM Tris-HCl, pH 7.5; 165 mM sodium
chloride; 10 mM EDTA; 10 µg/ml aprotinin; 10 µg/ml leupeptin, and
1% NP-40). Homogenized samples were lyophilized overnight at 4°C
with a Labconco Lyph-lock 1L (model 77400; Labconco Corporation).
Protein samples were digested in a 10 µg/ml sequencing
grade-modified trypsin (Promega Corporation) in 30% acetonitrile
(ACN), 50 mM ammonium bicarbonate, and 5 mM DTT overnight at 30°C.
Digested proteins were lyophilized overnight at 4°C and dissolved
in 0.1% formic acid. MS was performed as previously described
(17).
An Agilent 1100 LC/MSD Trap XCT (Agilent
Technologies, Inc.) was used for high performance liquid
chromatography and MS/MS. Each sample (25 µl) was used and
separated in columns (Zorbax 300SB-C18; 75 µm; 150 mm; Agilent
Technologies, Inc.). The Agilent 1100 capillary pump (Agilent
Technologies, Inc.) was operated under the following conditions:
Solvent A, 0.1% formic acid; solvent B, ACN in 0.1% formic acid;
column flow, 0.3 µl/min; primary flow, 300 µl/min; gradient, 0–5
min; 2% solvent B, 60 min 60% solvent B; stop time, 60 min. Protein
identification was performed using the Agilent Spectrum MILL MS
proteomics workbench (Rev BI.07.00.208; Agilent Technologies, Inc.)
and the Swiss-Prot protein sequence database search engine
(http://kr.expasy.org/sprot/) and the
MASCOT MS/MS Ions search engine (http://www.matrixscience.com/search_form_select.html).
The positive identification criteria of proteins were set as
follows: Protein score, >10.0 and peak intensity score, >8%
or peptide score.
Online analysis of candidate
genes
Candidate genes were analyzed using the GEPIA
database and the default settings (18). The Cancer Genome Atlas (TCGA)
database (https://portal.gdc.cancer.gov/) and The Broad
Institute Cancer Cell Line Encyclopedia (CCLE) database (https://portals.broadinstitute.org/ccle)
were screened for the potential genes with default settings; the
datasets were downloaded, and the histograms were drawn using
GraphPad Prism 5.0 (GraphPad Software, Inc.).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5.0. For the comparison of protein levels from MS data, all
datapoints were presented, and two-tailed Student's t-test was
performed to analyze the differences. For survival analysis,
log-rank test, also termed the Mantel-Cox test, was performed on
GEPIA. P<0.05 was considered to indicate a statistically
significant difference.
Results
Upregulated membrane proteins in
pancreatic adenocarcinoma
To identify novel pancreatic cancer specific
membrane proteins, total protein from seven paired PAAD and
adjacent normal tissues were subjected to mass spectrum analysis,
and membrane proteins were selected for further analysis. The
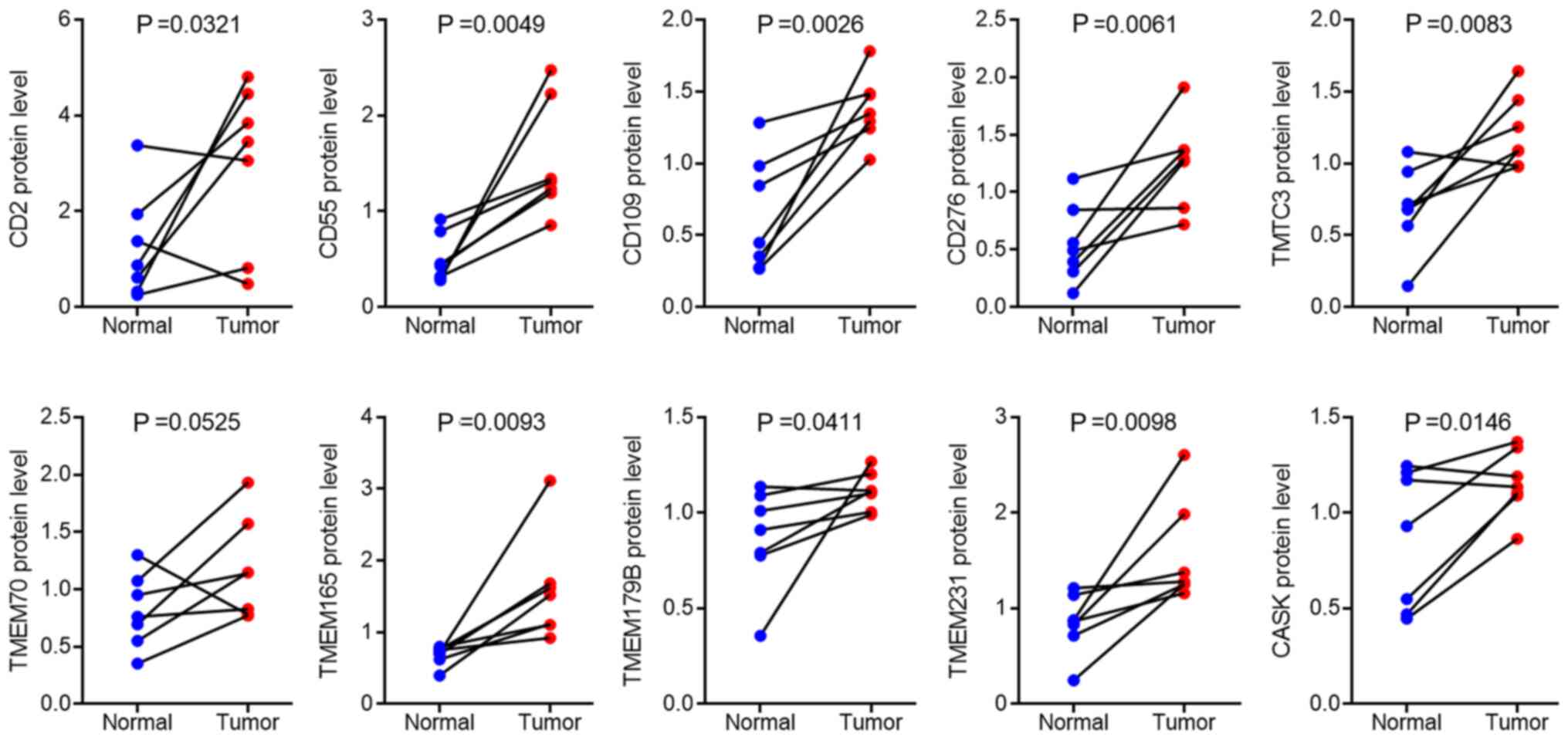
results demonstrated that the protein expression levels of CD2,
CD55, CD109, CD276, transmembrane and tetratricopeptide repeat
containing 3 (TMTC3), transmembrane protein (TMEM) 70, TMEM165,
TMEM179B, TMEM231 and calcium/calmodulin-dependent serine protein
kinase (CASK) were the top 10 increased membrane proteins in
pancreatic cancer tissues compared with that in adjacent normal
tissues (Fig. 1). For further
evaluation on the potential of these 10 membrane proteins, the
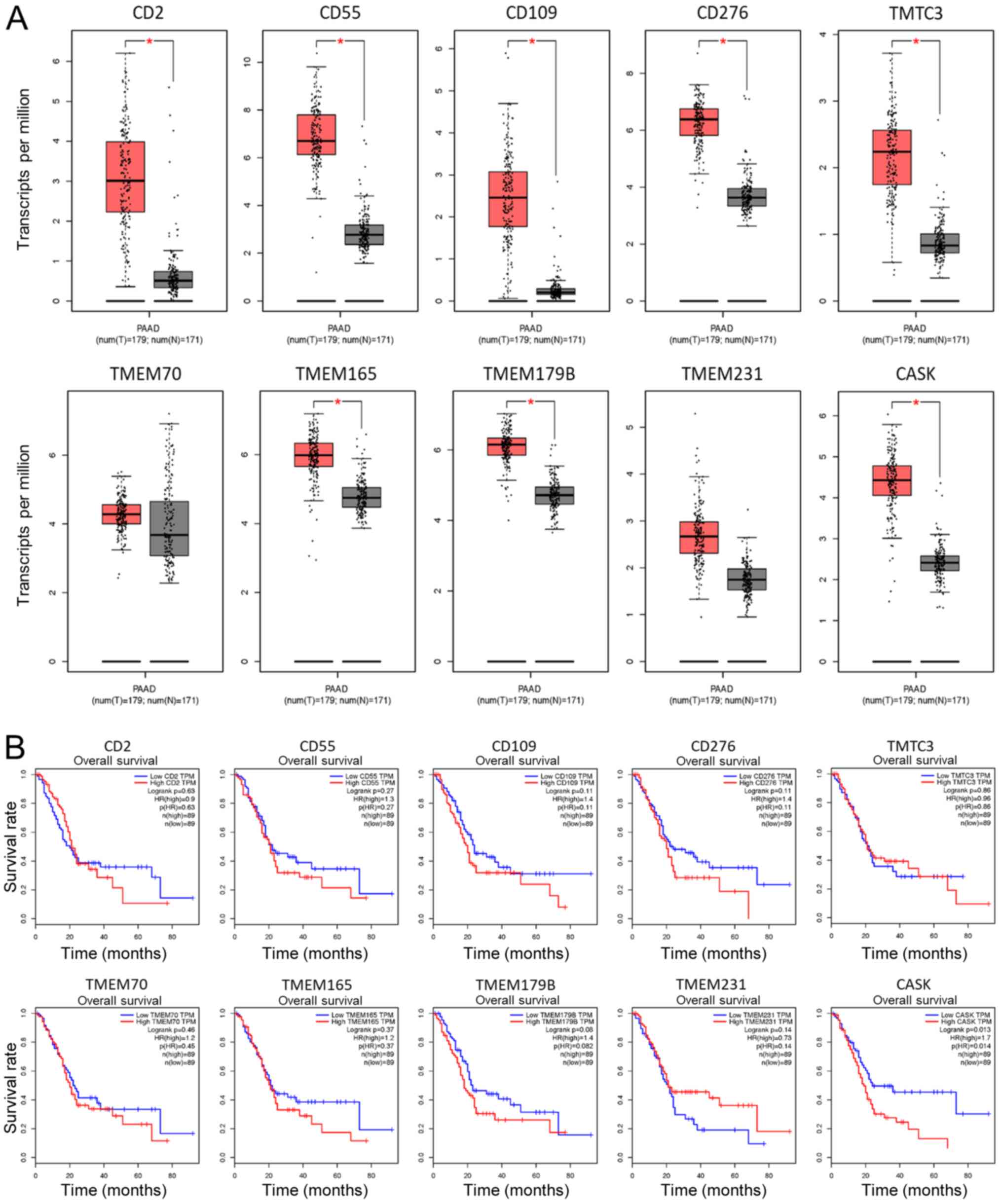
GEPIA online database was used, so that the mRNA expression level
of these membrane proteins in pancreatic cancer samples could be
investigated. Notably, the mRNA expression levels of all ten
membrane proteins were upregulated in PAAD samples compared with
those in the adjacent normal tissues, as illustrated in Fig. 2A, indicating the validity of the mass
spectrum data. Furthermore, overall survival time of patients with
PAAD, included in the GEPIA database was analyzed and it was found
that high protein expression levels of CASK protein in patients
with PAAD was significantly associated with poor overall survival
time (Fig. 2B) suggesting poor
outcome of patients with PAAD and high expression levels of
CASK.
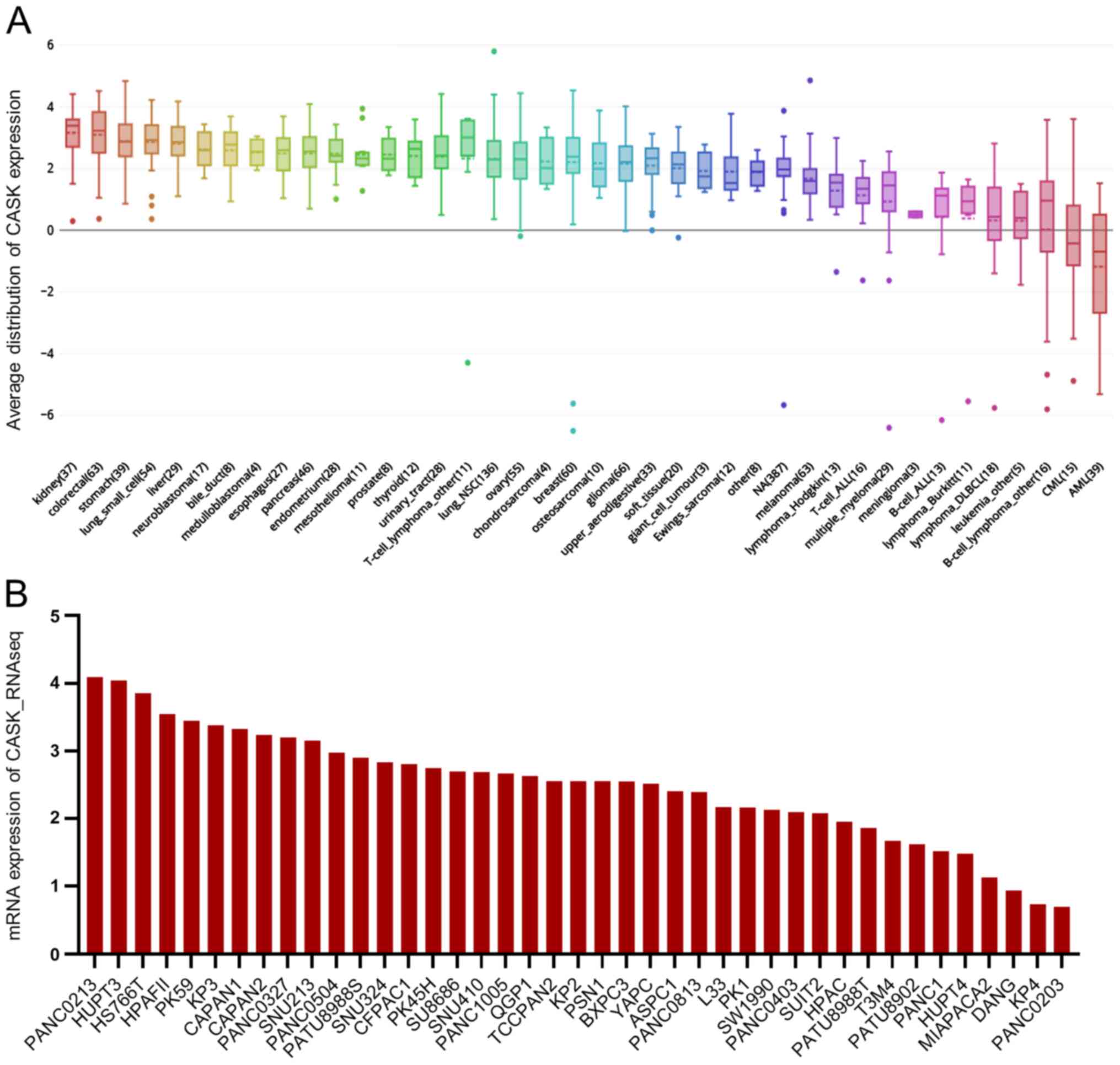
Next, the mRNA expression level of CASK was analyzed
in from the CCLE database and found that CASK was upregulated in
PAAD cell lines (ranking 10th) compared with that in other types of
cancer cells (Fig. 3A) and the
relative mRNA expression level of CASK was upregulated in most PAAD
cell lines (Fig. 3B).
Taken together, the data showed that CASK was
increased in PAAD samples and cell lines and was associated with of
poor outcome in patients with PAAD.
Downregulated membrane proteins in
pancreatic adenocarcinoma
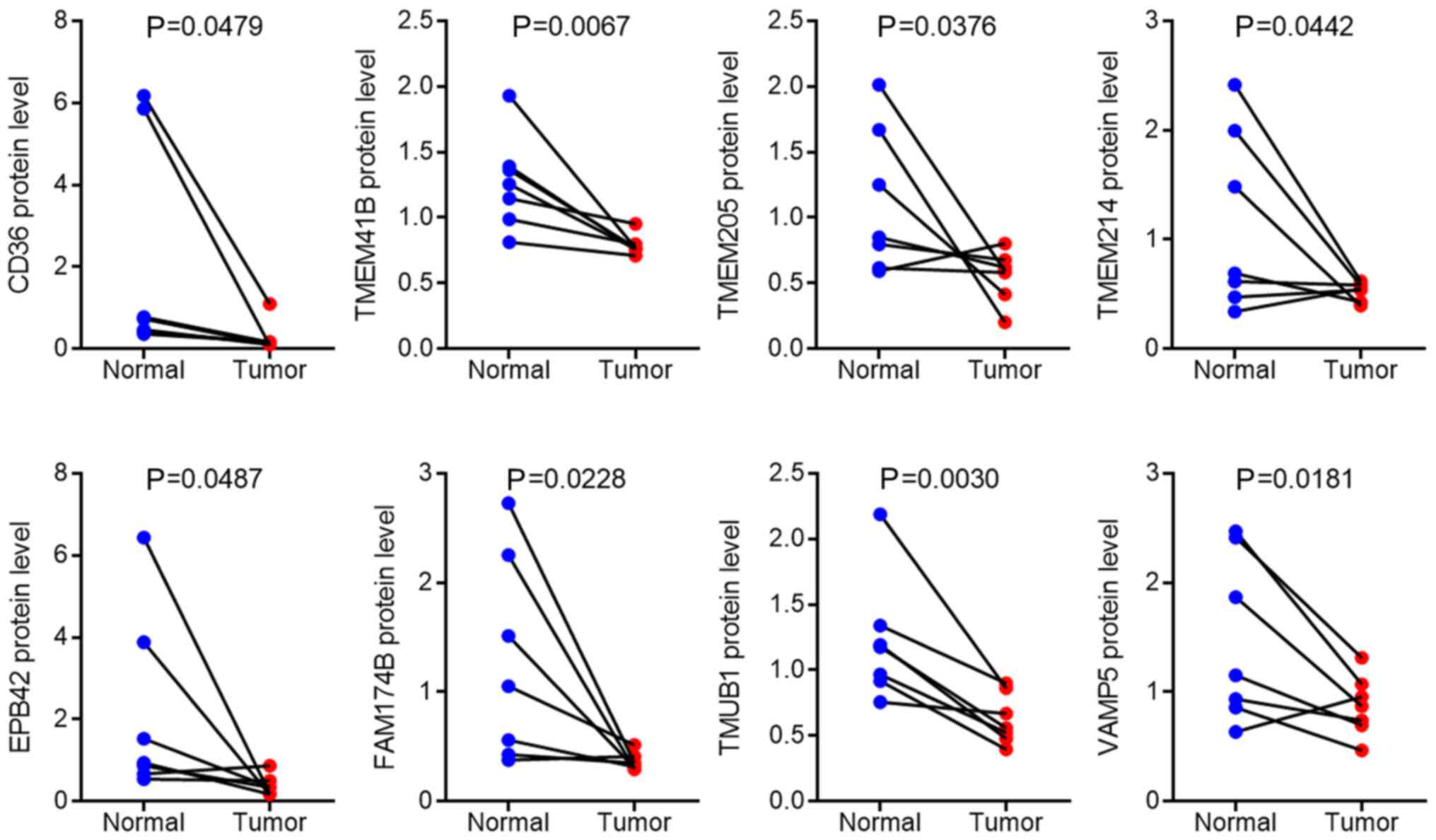
Subsequently, the downregulated membrane proteins
were analyzed in the mass spectrum data and eight proteins were
significantly decreased, CD36, TMEM41B, TMEM205, TMEM214,
erythrocyte membrane protein band 4.2 (EPB42), family with sequence
similarity 174 member B (FAM174B), transmembrane and ubiquitin-like
domain-containing 1 (TMUB1), and vesicle-associated membrane
protein 5 (VAMP5) in pancreatic cancer tissues compared with that
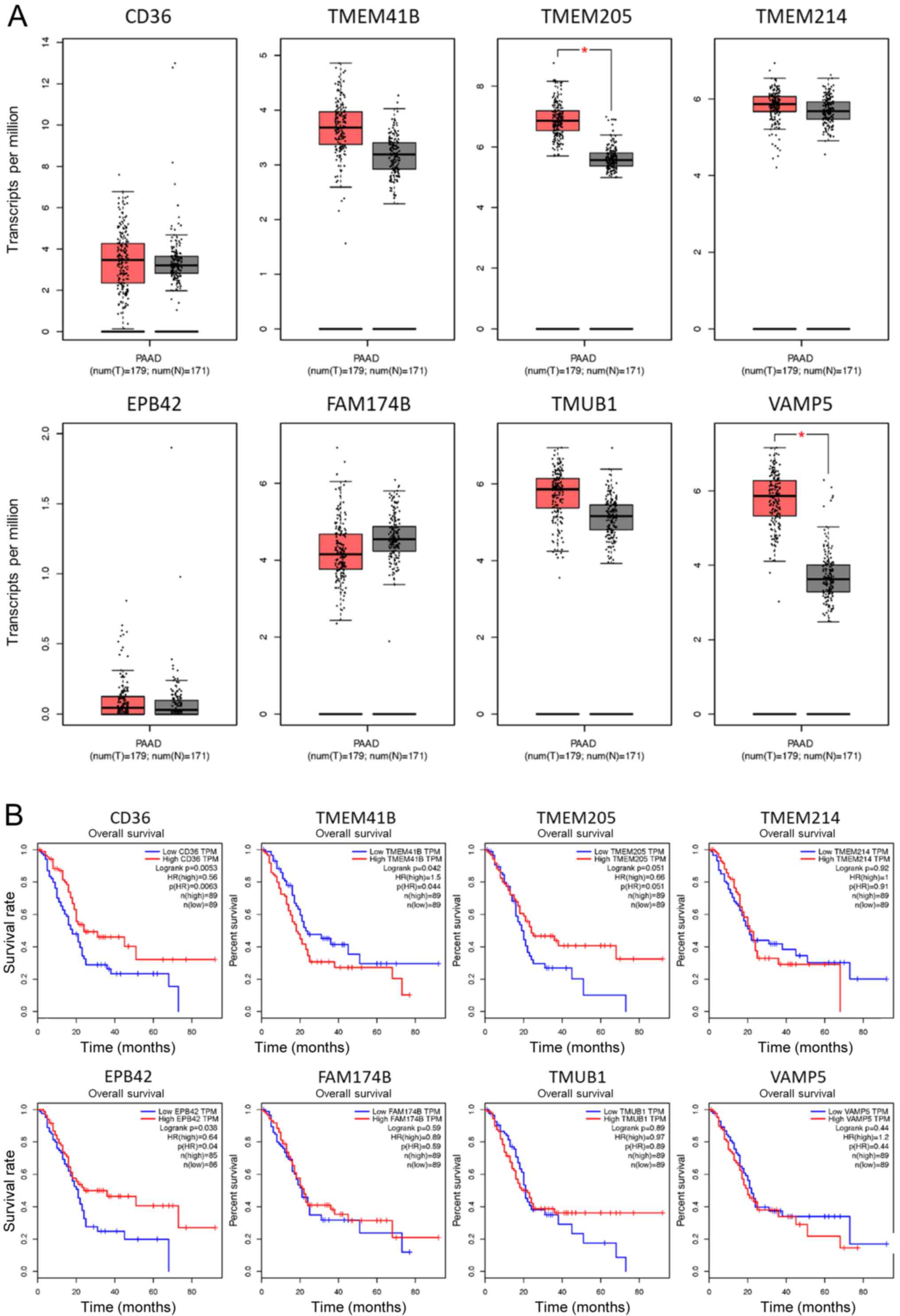
in adjacent normal tissues, as illustrated in Fig. 4. Following validation of the mRNA
expression level of these genes using the GEPIA database, it was
found that the mRNA expression levels of these genes were not
significantly downregulated in PAAD samples compared with normal
tissues (Fig. 5A); however, it was
observed the mRNA levels of TMEM205 and VAMP5 were upregulated in
PAAD, which suggested a complicated posttranscriptional regulation
of these potential membrane markers in PAAD. Notably, low
expression level of CD36 or EPB42 was associated with poor overall
survival of patients with PAAD (Fig.
5B) suggesting that the reduction of CD36 or EPB42 was
associated with poor outcome in patients in PAAD.
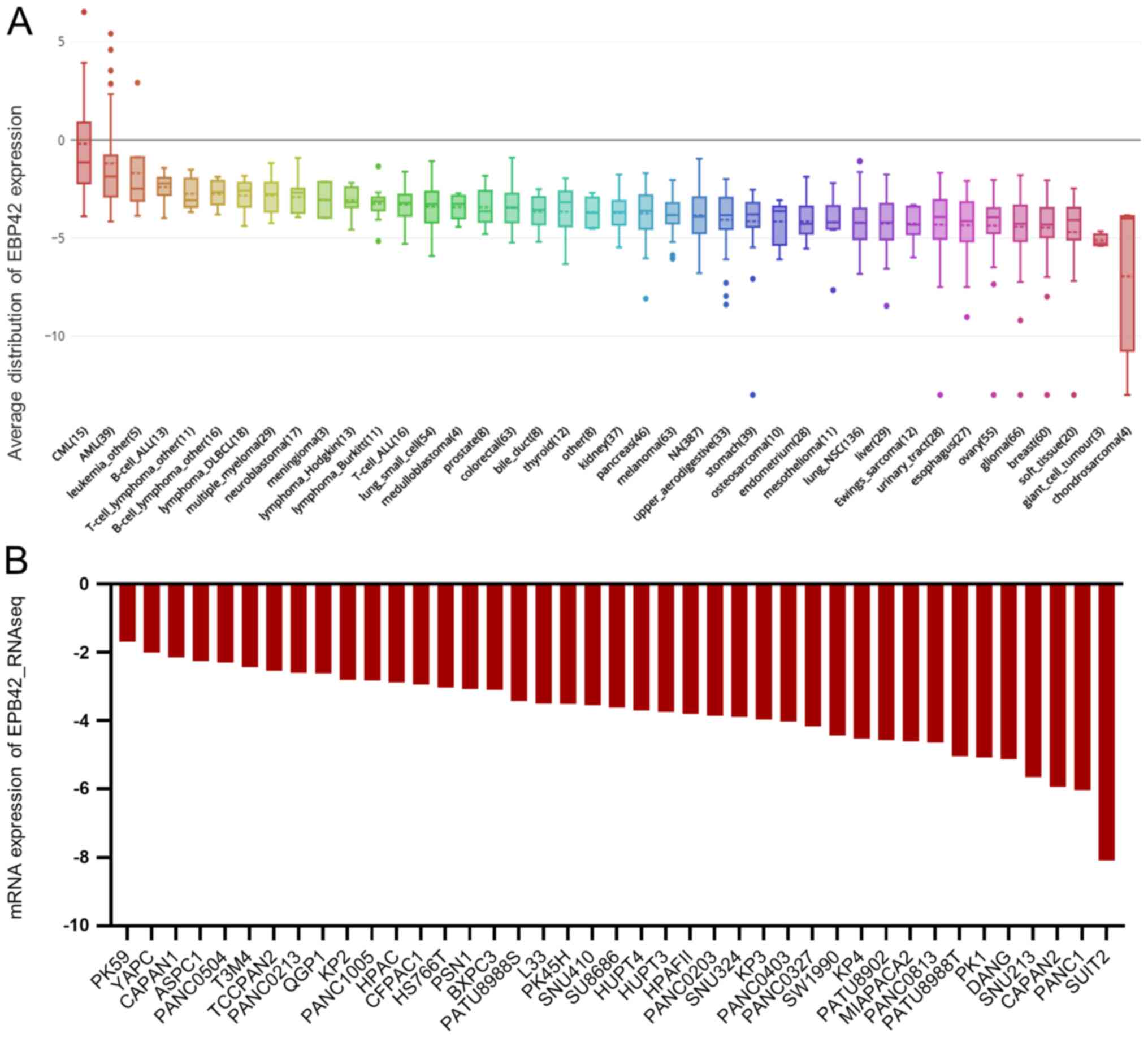
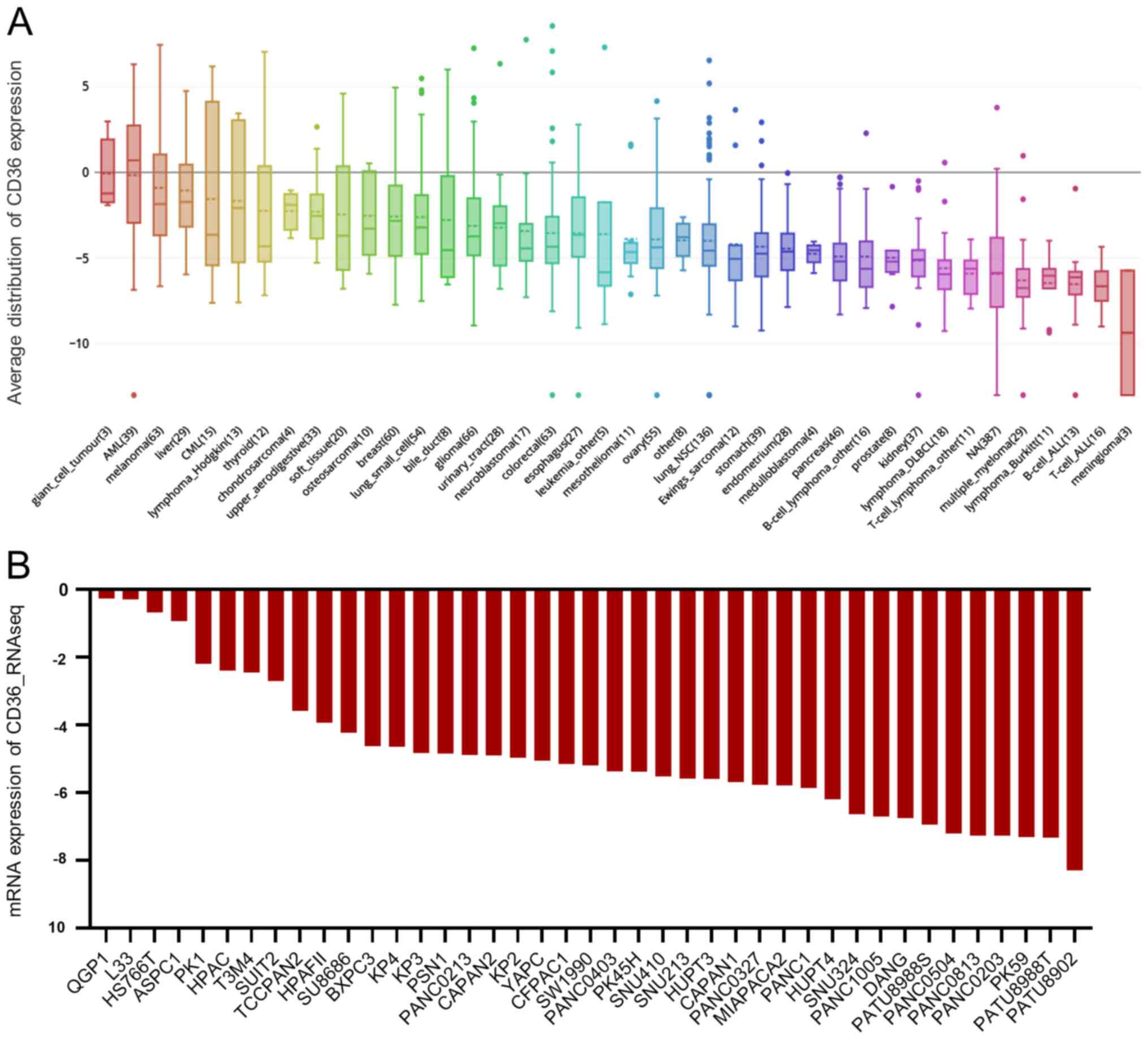
Furthermore, the mRNA expression level of CD36 and
EPB42 in the CCLE database was investigated and it was found that
both CD36 or EPB42 were decreased in PAAD cell lines compared with
that in other types of cancer cell lines (Figs. 6A and 7A, respectively). Furthermore, the mRNA
expression levels of CD36 and EPB42 were decreased in most PAAD
cell lines (Figs. 6B and 7B, respectively). Therefore, the results in
the present study suggested that the downregulation of CD36 and
EPB42 in PAAD samples predicted poorer survival outcomes in
patients with PAAD, and upregulation of CASK predicted poorer
survival outcomes in patients with PAAD.
Discussion
Pancreatic cancer exhibits a highly fatal
malignancy, which obstructs current immunotherapy (10). In the present study, 18 aberrantly
expressed cell surface proteins were identified in seven paired
PAAD and adjacent normal tissues. Further analysis using an online
database validated the mass spectrum results, even without
performing large-scale benchwork validations. Among the upregulated
membrane proteins, there was a significant increase in CASK in
patients with PAAD, revealed by the online database. CASK was
initially identified as an interactor of neurexins in neuronal
cells (19). It has been established
that CASK is a membrane-associated guanylate kinase, which can
enter the nucleus and acts as a coactivator to induce transcription
of cerebrocortical development-related genes, and whose mutation
resulted in microcephaly and hypoplasia of the brainstem and
cerebellum (20,21). Notably, a previous study showed that
CASK and its target gene, reelin, were co-upregulated in human
esophageal carcinoma (22).
Furthermore, it has been proven that CASK was increased in gastric
cancer cells and promoted their proliferation and invasion
(23). Therefore, it is important to
further investigate the role of CASK in pancreatic cancer
carcinogenesis.
The results in the present study also demonstrated
the increase of CD2 in PAAD samples, while a recent report
investigated the dose escalation of the anti-CD2 monoclonal
antibody in aggressive peripheral T-cell lymphomas (24). Although in the present study the
upregulation of CD2 was not associated with survival, testing this
antibody may be significant for the treatment of pancreatic cancer
since it provides novel insights into immunotherapy. Furthermore,
it was found that CD55 was increased in patients with pancreatic
cancer recruited into the study and from the online database. It
has been recently proposed that CD55 regulated anticancer drug
resistance in endometrioid tumors (25) and cervical cancer (26). All these data suggested an oncogenic
role of the membrane protein CD55. Notably, CD276 (B7-H3), which is
an important immune checkpoint member of the B7 and CD28 families
(26), was also increased in
patients with pancreatic cancer. It has been shown that CD276
protein expression was increased in pancreatic cancer tissues and
CD276 could block CD8+ T-cell infiltration to induce an
antitumor effect (27). The results
from the present study provided independent evidence that CD276 was
upregulated in PAAD and further strengthened the possibility to
develop immunotherapy and chemotherapy by targeting CD276.
Furthermore, CD109, TMTC3, TMEM165, and TMEM179B were also
increased in PAAD despite their roles in cancer being elusive.
Therefore, these targets may serve as novel diagnostic markers and
therapeutic targets when appropriate validations are provided.
In addition to the upregulated membrane proteins,
eight downregulated membrane proteins were identified in patients
with PAAD. TMEM205 was significantly downregulated in PAAD, and it
has been reported that TMEM205 was associated with cisplatin
resistance in PAAD (28,29). However, the genuine function of
TMEM205 requires further investigation. The mass spectrum data
revealed that VAMP5, the function of which in cancer is still
largely unknown, was downregulated in PAAD samples. It is important
to further dissect the biological function of TMEM205 in PAAD cell
lines. Notably, the results in the present study revealed the
decrease of CD36 and EPB42 in the seven patients with PAAD, while
low expression level of CD36 and EPB42 was associated with poor
outcome in patients with PAAD. CD36 is a critical fatty acid
receptor, which initiates the metastasis of human oral carcinomas
(30). Importantly, silencing of
CD36 also impaired metastasis in human melanoma- and breast
cancer-derived tumors (30). With
respect to the mRNA and protein expression levels of CD36 in PAAD,
revealing the role of CD36 in PAAD metastasis may provide a novel
strategy for PAAD treatment. The inconsistent expression pattern of
CD36 may result from the variety of patient samples. Further
in-depth analysis is required to characterize the role of CD36. On
the other hand, the role of EPB42, a crucial protein in hereditary
spherocytosis (31), in cancer is
less well-known. The mRNA expression levels of CD36 and EPB42 were
not statistically significantly decreased from the GEPIA database;
however, this could be due to post-transcriptional regulation. In
addition, a larger cohort of samples from different databases may
also be required to validate the results.
In summary, the results of the present study
identified multiple aberrantly expressed membrane proteins in
patients with PAAD, which may provide novel diagnostic markers and
drug targets for the immunotherapy of pancreatic cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81772798).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MM, CW, XG, SL, XX and ELH performed the literature
research. MM, XG, XX and ELH performed the clinical studies. MM,
CW, SL and XX performed the experiments. MM, CW, SL and ELH
performed statistical analysis. MM, CW, XX and ELH edited the
manuscript. SL and ELH were guarantors of the integrity of the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients involved in the study provided written
informed consent, and all procedures were approved by the Ethics
Committee of Chinese PLA General Hospital in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barkal AA, Brewer RE, Markovic M, Kowarsky
M, Barkal SA, Zaro BW, Krishnan V, Hatakeyama J, Dorigo O, Barkal
LJ and Weissman IL: CD24 signalling through macrophage Siglec-10 is
a target for cancer immunotherapy. Nature. 572:392–396. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao A, Hu XL, Saeed M, Chen BF, Li YP and
Yu HJ: Overview of recent advances in liposomal nanoparticle-based
cancer immunotherapy. Acta Pharmacol Sin. 40:1129–1137. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eroglu Z, Zaretsky JM, Hu-Lieskovan S, Kim
DW, Algazi A, Johnson DB, Liniker E, Kong B, Munhoz R, Rapisuwon S,
et al: High response rate to PD-1 blockade in desmoplastic
melanomas. Nature. 553:347–350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang XM, Xu YL, Huang MY, Zhang LL, Su
MX, Chen X and Lu JJ: Osimertinib (AZD9291) decreases programmed
death ligand-1 in EGFR-mutated non-small cell lung cancer cells.
Acta Pharmacol Sin. 38:1512–1520. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Royal RE, Levy C, Turner K, Mathur A,
Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I and
Rosenberg SA: Phase 2 trial of single agent Ipilimumab
(anti-CTLA-4) for locally advanced or metastatic pancreatic
adenocarcinoma. J Immunother. 33:828–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thind K, Padrnos LJ, Ramanathan RK and
Borad MJ: Immunotherapy in pancreatic cancer treatment: A new
frontier. Therap Adv Gastroenterol. 10:168–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bayne LJ, Beatty GL, Jhala N, Clark CE,
Rhim AD, Stanger BZ and Vonderheide RH: Tumor-derived
granulocyte-macrophage colony-stimulating factor regulates myeloid
inflammation and T cell immunity in pancreatic cancer. Cancer Cell.
21:822–835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Özdemir BC, Pentcheva-Hoang T, Carstens
JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C,
Novitskiy SV, et al: Depletion of carcinoma-associated fibroblasts
and fibrosis induces immunosuppression and accelerates pancreas
cancer with reduced survival. Cancer Cell. 25:719–734. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panni RZ, Herndon JM, Zuo C, Hegde S, Hogg
GD, Knolhoff BL, Breden MA, Li X, Krisnawan VE, Khan SQ, et al:
Agonism of CD11b reprograms innate immunity to sensitize pancreatic
cancer to immunotherapies. Sci Transl Med. 11:eaau92402019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang H, Hegde S, Knolhoff BL, Zhu Y,
Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver
DT, et al: Targeting focal adhesion kinase renders pancreatic
cancers responsive to checkpoint immunotherapy. Nat Med.
22:851–860. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mace TA, Shakya R, Pitarresi JR, Swanson
B, McQuinn CW, Loftus S, Nordquist E, Cruz-Monserrate Z, Yu L,
Young G, et al: IL-6 and PD-L1 antibody blockade combination
therapy reduces tumour progression in murine models of pancreatic
cancer. Gut. 67:320–332. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou L, Husted H, Moore T, Lu M, Deng D,
Liu Y, Ramachandran V, Arumugam T, Niehrs C, Wang H, et al:
Suppression of stromal-derived Dickkopf-3 (DKK3) inhibits tumor
progression and prolongs survival in pancreatic ductal
adenocarcinoma. Sci Transl Med. 10:eaat34872018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 523:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bydoun M, Sterea A, Liptay H, Uzans A,
Huang WY, Rodrigues GJ, Weaver ICG, Gu H and Waisman DM: S100A10, a
novel biomarker in pancreatic ductal adenocarcinoma. Mol Oncol.
12:1895–1916. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tempero MA, Malafa MP, Chiorean EG, Czito
B, Scaife C, Narang AK, Fountzilas C, Wolpin BM, Al-Hawary M, Asbun
H, et al: Pancreatic adenocarcinoma, version 1.2019. J Natl Compr
Canc Netw. 17:202–210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuramitsu Y, Taba K, Ryozawa S, Yoshida K,
Zhang X, Tanaka T, Maehara SI, Maehara Y, Sakaida I and Nakamura K:
Identification of up- and down-regulated proteins in
gemcitabine-resistant pancreatic cancer cells using two-dimensional
gel electrophoresis and mass spectrometry. Anticancer Res.
30:3367–3372. 2010.PubMed/NCBI
|
|
18
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res.
45W:W98–W102. 2017. View Article : Google Scholar
|
|
19
|
Hata Y, Butz S and Südhof TC: Sudhof,
CASK: A novel dlg/PSD95 homolog with an N-terminal
calmodulin-dependent protein kinase domain identified by
interaction with neurexins. J Neurosci. 16:2488–2494. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsueh YP, Wang TF, Yang FC and Sheng M:
Nuclear translocation and transcription regulation by the
membrane-associated guanylate kinase CASK/LIN-2. Nature.
404:298–302. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Najm J, Horn D, Wimplinger I, Golden JA,
Chizhikov VV, Sudi J, Christian SL, Ullmann R, Kuechler A, Haas CA,
et al: Mutations of CASK cause an X-linked brain malformation
phenotype with microcephaly and hypoplasia of the brainstem and
cerebellum. Nat Genet. 40:1065–1067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Q, Lu J, Yang C, Wang X, Cheng L, Hu
G, Sun Y, Zhang X, Wu M and Liu Z: CASK and its target gene Reelin
were co-upregulated in human esophageal carcinoma. Cancer Lett.
179:71–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou X, Xu G, Yin C, Jin W and Zhang G:
Down-regulation of miR-203 induced by Helicobacter pylori infection
promotes the proliferation and invasion of gastric cancer by
targeting CASK. Oncotarget. 5:11631–11640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roswarski J, Roschewski M, Lucas A, Melani
C, Pittaluga S, Jaffe ES, Steinberg SM, Waldmann TA and Wilson WH:
Phase I dose escalation study of the anti-CD2 monoclonal antibody,
siplizumab, with DA-EPOCH-R in aggressive peripheral T-cell
lymphomas. Leuk Lymphoma. 59:1466–1469. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saygin C, Wiechert A, Rao VS, Alluri R,
Connor E, Thiagarajan PS, Hale JS, Li Y, Chumakova A, Jarrar A, et
al: CD55 regulates self-renewal and cisplatin resistance in
endometrioid tumors. J Exp Med. 214:2715–2732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leung TH, Tang HW, Siu MK, Chan DW, Chan
KK, Cheung AN and Ngan HY: Human papillomavirus E6 protein enriches
the CD55(+) population in cervical cancer cells, promoting
radioresistance and cancer aggressiveness. J Pathol. 244:151–163.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamato I, Sho M, Nomi T, Akahori T,
Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H and Nakajima Y:
Clinical importance of B7-H3 expression in human pancreatic cancer.
Br J Cancer. 101:1709–1716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen DW and Gottesman MM: RAB8 enhances
TMEM205- mediated cisplatin resistance. Pharm Res. 29:643–650.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen DW, Ma J, Okabe M, Zhang G, Xia D and
Gottesman MM: Elevated expression of TMEM205, a hypothetical
membrane protein, is associated with cisplatin resistance. J Cell
Physiol. 225:822–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pascual G, Avgustinova A, Mejetta S,
Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A,
Hueto JA, et al: Targeting metastasis-initiating cells through the
fatty acid receptor CD36. Nature. 541:41–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanzaki A, Hayette S, Morlé L, Inoue F,
Matsuyama R, Inoue T, Yawata A, Wada H, Vallier A, Alloisio N, et
al: Total absence of protein 4.2 and partial deficiency of band 3
in hereditary spherocytosis. Br J Haematol. 99:522–530. 1997.
View Article : Google Scholar : PubMed/NCBI
|