Introduction
Hepatocellular carcinoma is the second leading cause
of cancer-associated mortality worldwide, with a morbidity rate of
782,000 and a mortality rate of 746,000 in 2012 (1). Liver cancer has several types based on
the types of cells that become cancerous, including hepatic
angiosarcoma, cholangiocarcinoma and hepatocellular carcinoma
(2). hepatocellular carcinoma
accounts for >80% of liver cancer cases worldwide (3), and more than half of these occur in
China (4). In the past years, the
mortality rate of the majority of liver cancer types has decreased
with the development of targeted therapy (5,6).
However, the hepatocellular carcinoma mortality rates in China did
not decrease (7), thus the
identification of new molecular targets for hepatocellular
carcinoma treatment is required. Therefore, this was the aim of the
present study.
Rhophilin Rho GTPase binding protein 2 (RHPN2)
belongs to the rhophilin family of Ras-homologous
(Rho)-GTPase-binding proteins (8).
RHPN2 has been implicated in the organization of actin cytoskeleton
(8). RHPN2 is reported to drive
mesenchymal transformation by triggering RhoA activation in
malignant glioma (9). However, the
function of RHPN2 in hepatocellular carcinoma remains unknown.
Previous studies have demonstrated a significant
reduction of hepatocyte nuclear factor 1α (HNF1α) and therapeutic
effects in hepatocellular carcinoma tissues (10,11).
Therefore, the present study aimed to investigate the role of RHPN2
in hepatocellular carcinoma, whether there was a correlation
between RHPN2 and HNF1α, and whether RHPN2 could represent a novel
therapeutic target.
Materials and methods
Bioinformatics analysis
The cBio Cancer Genomics Portal (http://cbioportal.org) was used to explore the role of
RHPN2 in cancer genomic data. The portal is an open-access resource
for interactive exploration of multidimensional cancer genomics
data sets, and currently provides access to the data of >5,000
tumor samples from 20 cancer studies (12,13). The
Cancer Genome Atlas (14) PanCancer
Atlas Studies (10,953 patients/10,967 samples in 32 studies) were
selected and queried by RHPN2. The PanCancer Atlas figures were
obtained and 372 liver cases were selected and queried by RHPN2.
The survival of patients with liver cancer was then obtained. The
possible microRNAs (miRs) that may target RHPN2 were
bioinformatically predicted using TargetScan Human (http://www.targetscan.org/vert_72/) (15,16).
Tissue samples
A total of 31 hepatocellular carcinoma tissues and
the matched adjacent normal tissues were acquired from the Sichuan
Provincial Cancer Hospital (Sichuan, China). All tissues were
obtained from surgery. The histology of the 31 hepatocellular
carcinoma tissues was confirmed by the senior pathologist of the
Department of Pathology of the hospital. The inclusion criteria
included: ≥18 years of age, histologically confirmed diagnosis of
hepatocellular carcinoma and patients who underwent first surgery.
The exclusion criteria included: Secondary liver cancer,
hepatocellular carcinoma recurrence and patients who received
surgery before chemotherapy or sorafenib. The distance between the
carcinoma and adjacent normal tissues was 0.5–1 cm. The mean age of
the 31 patients (27 male and 4 female) with hepatocellular
carcinoma was 59±11.4 years (age range, 39–72 years). The present
study was approved by The Ethics Committee of Sichuan University
(Chengdu, China) and written informed consent was obtained from all
patients enrolled in the present study. For survival analysis,
patients were then divided into two groups (high and low RHPN2
expression groups) according to the RHPN2 mRNA expression levels in
the tumor tissues, and the median value (expression=2.28;
expression SEM=0.51) of the 31 hepatocellular carcinoma tissues was
chosen as the cut-off point. Patients were followed up for 60
months.
Immunohistochemical (IHC) analysis and
point-scoring system
A total of ten hepatocellular carcinoma tissues were
processed as per the standard protocol of IHC analysis (17). The tumor tissues were fixed in 10%
neutral buffered formalin at room temperature for at least 5 days.
After fixation, the tissues were dehydrated by immersion in
increasing concentrations of alcohol (75% alcohol for 2 h, 80% for
2 h, 85% for 2 h, 90% for 2 h and twice, 95% for 1 h and 100% for 1
h). Subsequently, the alcohol was cleared by incubation in xylene
prior to paraffin embedding. Paraffin is typically heated to 60°C
and then allowed to harden overnight. For IHC staining, tissue
samples were cut into 4-µm-thick sections, deparaffinized, hydrated
(at 70°C in xylene) and microwaved at full power for 20 min for
antigen retrieval (using sodium citrate pH 6.0). BSA (cat. no.
37520; Thermo Fisher Scientific, Inc.) was used as the blocking
reagent overnight in 4°C. The slides were incubated overnight at
4°C with anti-RHPN2 primary antibody (1:500; cat. no. PA5-62469;
Thermo Fisher Scientific). The slides were washed three times with
PBS and incubated with goat anti-rabbit polyclonal horseradish
peroxidase-conjugated secondary antibody (1:50; cat. no. 32260;
Thermo Fisher Scientific, Inc.) at room temperature for 2 h. The
slides were developed by diaminobenzidine staining as described
previously (18). IHC staining was
scored according to the following criteria: - (0–10% of the
nucleated cells were stained), + (11–40% stained), ++ (41–70%
stained) and +++ (71–100% stained). Tissues were analyzed using a
light microscope (Leica DM750; Leica Microsystems GmbH) and images
were captured at a magnification of ×200.
Cell culture and reagents
HepG2 hepatocellular carcinoma and THLE-2 normal
liver cell lines were purchased from the Cell Bank of the Third
Military Medicine University. The HepG2 hepatocellular carcinoma
cell lines were authenticated by short tandem repeat profiling.
These cell lines and the hepatocellular carcinoma cells obtained
from patients were cultured in Dulbecco's Modified Eagle's Medium
containing 10% fetal bovine serum (both Gibco; Thermo Fisher
Scientific, Inc.). Cisplatin was purchased from Shanghai Yaji
Biological Technology Co., Ltd. The final concentration of
cisplatin used in experiments was 2.0 µg/ml. Cells were treated
with cisplatin for 24 h.
Detection of RHPN2 and hepatocyte
nuclear factor 1 (HNF1)α using reverse transcription-quantitative
(RT-q)PCR
Total RNA from the tumor or normal tissues or cells
was extracted using TRIzol® reagent (cat. no. 15596026;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The cDNA was synthesized using
SuperScript™ IV First-Strand Synthesis System (cat. no. 15596026;
Invitrogen; Thermo Fisher Scientific, Inc.). Reactions were
incubated at 42°C for 50 min, followed by heat inactivation for 5
min at 80°C. The gene expression levels were assessed via qPCR
using the 2−ΔΔCq method (19). The PCR amplification was performed
using SYBR™ Green PCR Master Mix (cat. no. 4334973; Thermo Fisher
Scientific, Inc.). GAPDH was used as an internal control. RT-qPCR
was performed using the following primers: RHPN2 Forward,
5′-AAGGGCTGTAATCCCCTTGC-3′ and reverse, 5′-CCGCACCTTTGAGTTTGTGG-3′;
HNF1α forward, 5′-AGCCGAGCCATGGTTTCTAA-3′ and reverse,
5′-GGCTCGTTAGGAGCTGAGGG-3; GAPDH forward,
5′-CTGACTTCAACAGCGACACC-3′ and reverse,
5′-TAGCCAAATTCGTTGTCATACC-3. Thermocycling conditions consisted of
50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C
for 15 sec and 60°C for 60 sec. RHPN2 mRNA expression levels in
THLE-2 cells were arbitrarily defined as 100%.
Small interfering (si) RNA-RHPN2 and
pcDNA3.1-RHPN2 transfection
siRNA-RHPN2 (si-RHPN2) and pcDNA3.1-RHPN2 were
designed and constructed by Shanghai Shengong Biotechnology Co.,
Ltd. Scrambled siRNA (si-NC) and pcDNA3.1 were used as controls.
Cells were seeded into 24-well plates at a density of
5×104 cells/well overnight. Transfection was performed
using Lipofectamine® 2000 (cat. no. 11668027; Thermo
Fisher Scientific, Inc.). siRHPN2 (0.6 µg) or pcDNA3.1-RHPN2 (1 µg)
were used separately. RHPN2 mRNA expression levels of the si-NC
transfection group and those of the pcDNA3.1 transfection group
were arbitrarily defined as 100%. MTT analysis was performed at 0,
24, 48 and 72 h following transfection. The cell apoptosis analysis
was performed 24 h following transfection.
Cell proliferation assay
Cell proliferation was analyzed using an MTT-based
colorimetric assay (20–24). Briefly, 5×105/well cells
were placed into 96-well plates. MTT reagent was then added into
the medium at a final concentration of 0.1 mg/ml. After formation
of insoluble formazan, 100 µl DMSO was added to each well to
solubilize formazan. The optical density was then measured using a
microplate reader equipped with a 570 nm filter.
Apoptosis analysis
Cells (5×105 cells/ml) were suspended in
Annexin V-fluorescein isothiocyanate-binding buffer (FITC; Abcam),
and incubated for 15 min at room temperature followed by addition
of propidium iodide (PI; Abcam) to each sample. All samples were
analyzed using a FACSCalibur flow cytometer (BD Biosciences) at 488
nm excitation (Argon-ion laser or solid-state laser). Emission was
detected at 530 (green for FITC) and 575–610 nm (orange for PI).
The early apoptosis rate (Annexin
V-FITC+/PI−) was calculated. The data were
analyzed using the BD FACSuite™ version 1.01 (BD Biosciences).
Network analyses
The network of RHPN2 was analyzed using Cytoscape
software v3.8.0 (25). The complete
analysis was performed by the Department of Bioinformatics of
Sichuan University.
Statistical analysis
Data were presented as the mean ± standard deviation
of three independent repeats. Kaplan-Meier analysis was used for
survival curves, and the log-rank test was used to compare the
difference between two groups. Paired two-tailed Student's t-tests
were used to analyze the mean values of paired groups (tumor and
non-tumor tissue from the same patient). Unpaired two-tailed
Student's t-tests were used to analyze the mean values of THLE and
HepG2 cells. One-way ANOVA followed by Tukey's post hoc test was
used to analyze the mean values ≥3 groups. Correlation analysis was
performed using two-tailed Pearson's correlation coefficient
analysis. All analyses were performed using SPSS software (version
16.0; SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Bioinformatics analysis of patients'
survival associated with RHPN2 expression
Initially, the role of RHPN2 was investigated using
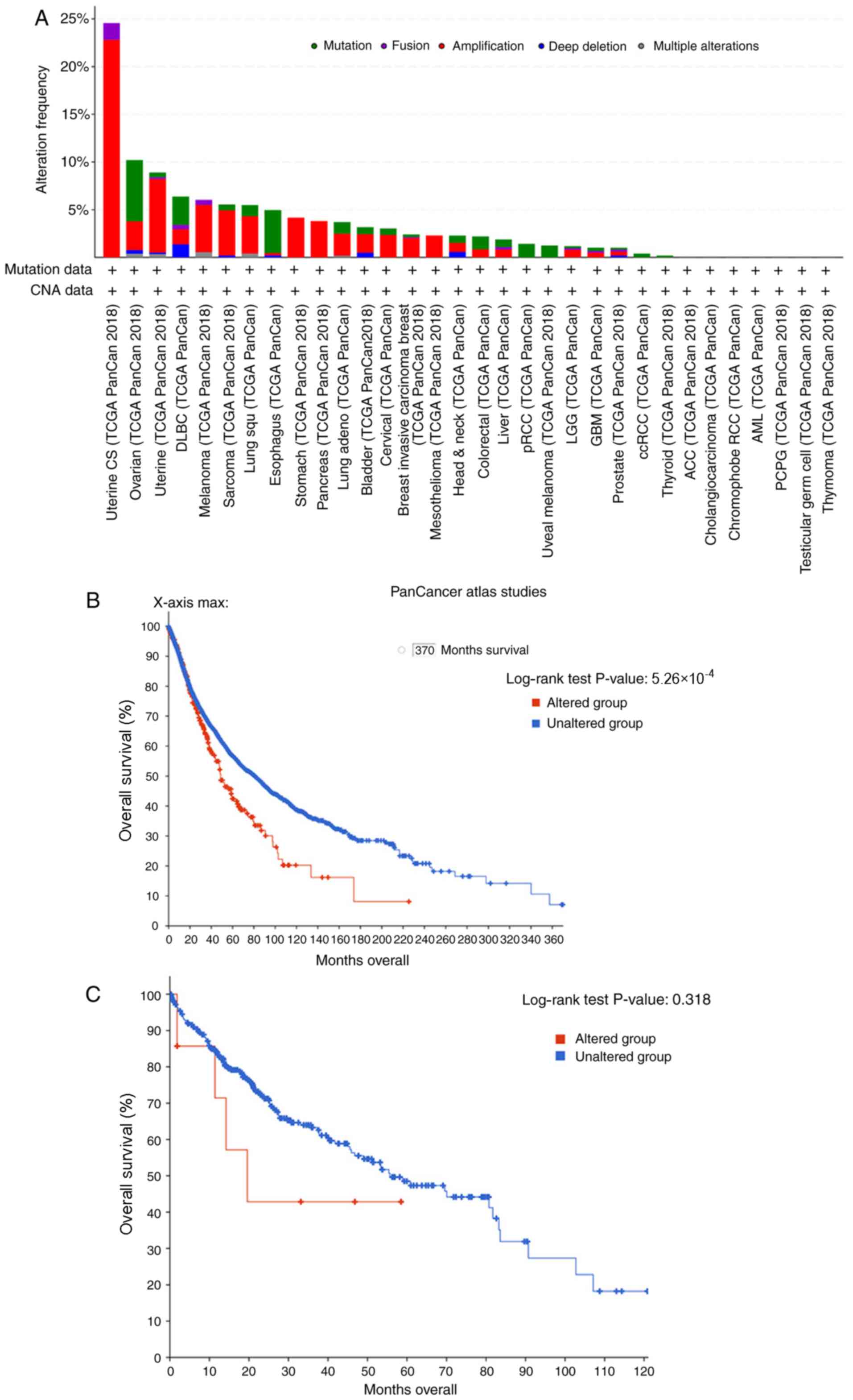
bioinformatics analysis and cBioPortal. The results revealed that
amplified RHPN2 is the most common mutation in various types of
cancer, including uterine, ovarian, stomach, esophageal, lung and
liver cancer (Fig. 1A). Next, the
overall survival of patients with numerous types of cancer
according to RHPN2 mutation were studied. The results demonstrated
that the altered group exhibited a lower survival rate compared
with that of the unaltered group (Fig.
1B; log-rank P=5.260e-4). Additionally, the overall survival
rate of patients with hepatocellular carcinoma was analyzed
according to RHPN2 alterations, and no significant differences were
observed between the altered and unaltered group; however, this may
have been due to inclusion of a limited number of patients
(Fig. 1C). Thus, 31 hepatocellular
carcinoma tissues and their matched adjacent tissue samples were
collected for patient's survival analysis.
RHPN2 expression levels in
hepatocellular carcinoma tissues and patient survival analysis
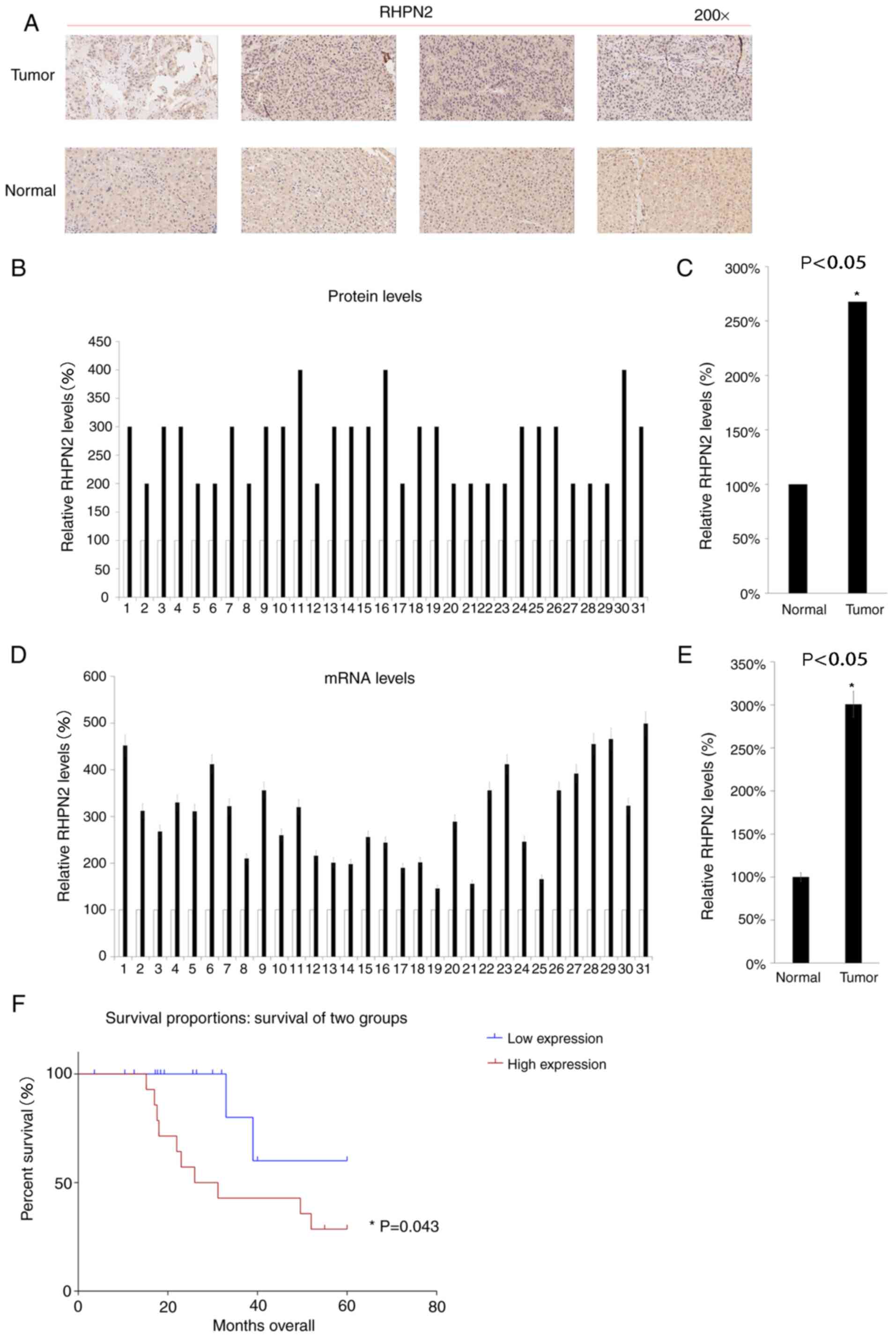
The protein levels of RHPN2 in 31hepatocellular
carcinoma tissues and their matching adjacent tissue samples were
analyzed using IHC. Hepatocellular carcinoma tissues consistently
exhibited higher protein expression levels of RHPN2 compared with
those of matched healthy tissues (Fig.
2A-C). Next, the RHPN2 mRNA expression levels were analyzed in
the 31 hepatocellular carcinoma tissues and matched samples. The
results demonstrated higher RHPN2 mRNA expression levels in the 31
hepatocellular carcinoma tissues compared with those of matching
healthy tissues (Fig. 2D and E). The
patients were then divided into two groups (RHPN2 high expression
group and RHPN2 low expression group) according to RHPN2 mRNA
expression levels in the tumor tissues, and the median value of the
31 hepatocellular carcinoma tissues was chosen as the cut-off
point. These patients were followed up for 60 months, and the RHPN2
low expression group demonstrated an improved prognosis compared
with that of RHPN2 high expression group (Fig. 2F).
Downregulation of RHPN2 decreases
hepatocellular carcinoma cell proliferation and increases the
apoptotic rate
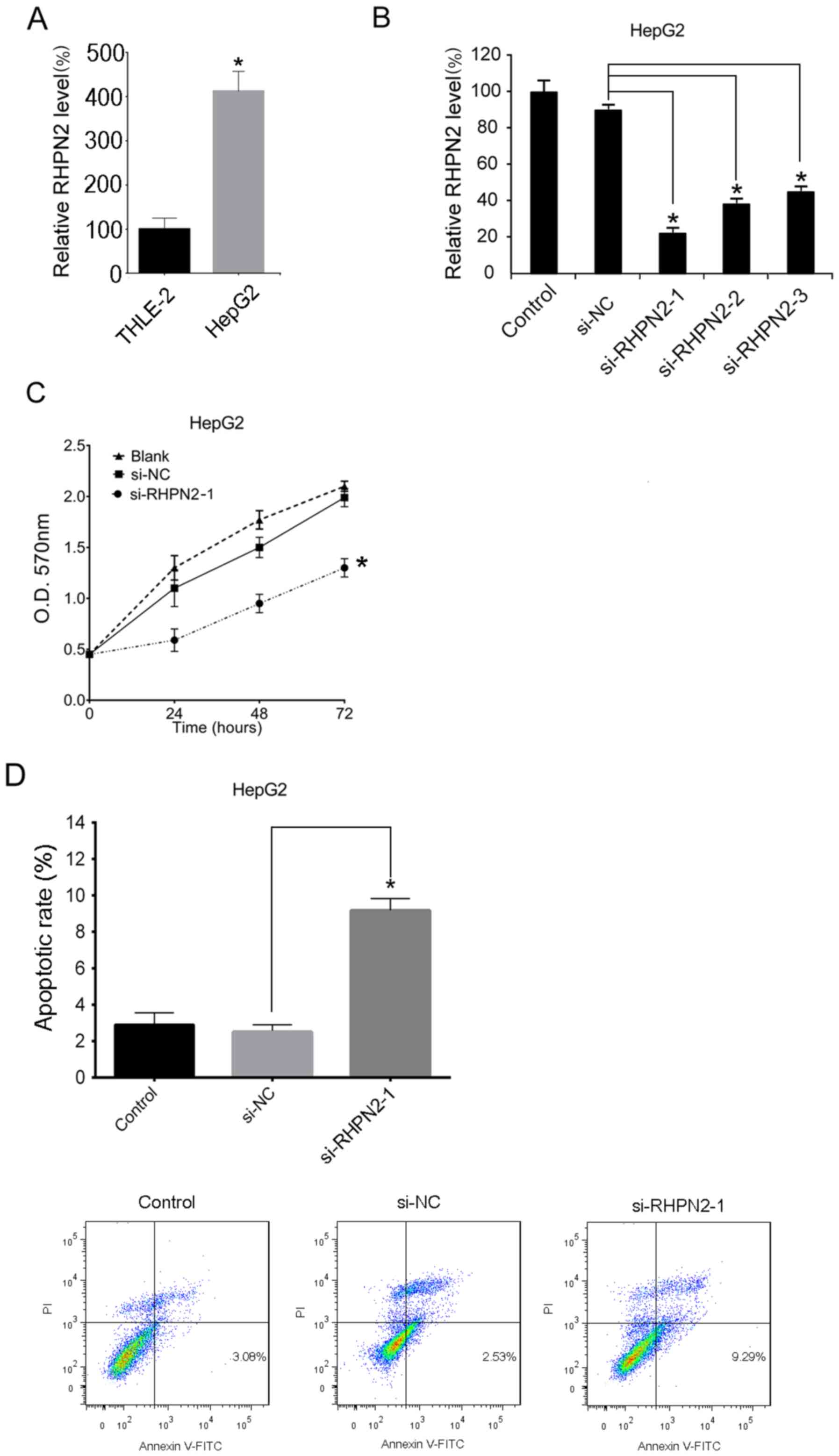
RHPN2 mRNA expression levels were determined in
THLE-2 and HepG2 cells. HepG2 cells displayed significantly higher
RHPN2 mRNA expression levels compared with those of THLE-2 cells
(Fig. 3A). The HepG2cells were then
transfected with si-RHPN2 or si-NC. RT-qPCR was used to analyze
RHPN2 mRNA levels after 24 h. The results demonstrated that the
levels of RHPN2 mRNA were significantly decreased following
si-RHPN2 transfection compared with si-NC (Fig. 3B). In addition, HepG2 cells
transfected with si-RHPN2 exhibited significantly decreased
cellular proliferation compared with the control group (si-NC;
Fig. 3C). Additionally, apoptosis
was analyzed using Annexin V/PI double-staining. The results
demonstrated that downregulation of RHPN2 significantly increased
the apoptotic rate of HepG2 cells compared with that of cells
transfected with si-NC (Fig.
3D).
Overexpression of RHPN2 promotes
hepatocellular carcinoma cell proliferation and reduces the
apoptotic rate
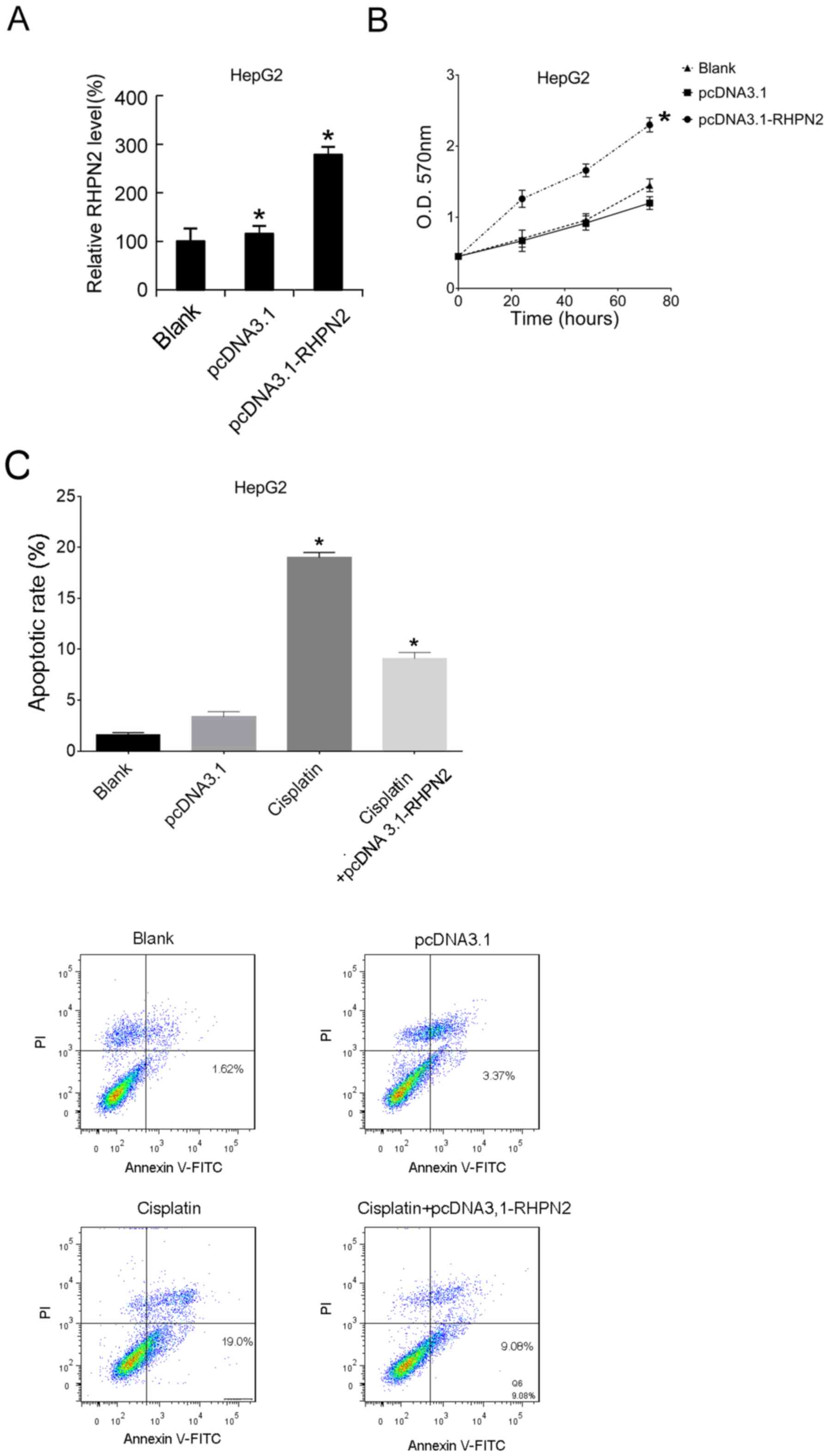
RHPN2-overexpression was induced by transfection
with an overexpression plasmid, pcDNA3.1-RHPN2, in HepG2 cells. The
RHPN2 mRNA expression levels in HepG2 cells were analyzed after 24
h using RT-qPCR. Transfection with pcDNA3.1-RHPN2 resulted in
upregulation of RHPN2 mRNA expression levels in HepG2 cells
compared with that of HepG2 cells transfected with pcDNA3.1
(Fig. 4A). MTT analysis demonstrated
that overexpression of RHPN2 significantly promoted hepatocellular
carcinoma cell proliferation compared with the blank and negative
controls (Fig. 4B). After 12 h of
pcDNA3.1-RHPN2 or pcDNA3.1 transfection, cisplatin was applied, and
then the apoptotic rates were assayed 12-h later. The results
demonstrated that, compared with the blank group, cisplatin
significantly increased the apoptotic rate, and that overexpression
of RHPN2 significantly reduced the cisplatin-induced increased
apoptotic rate compared with cisplatin-treated cells (Fig. 4C).
HNF1α is involved in the mechanism of
RHPN2
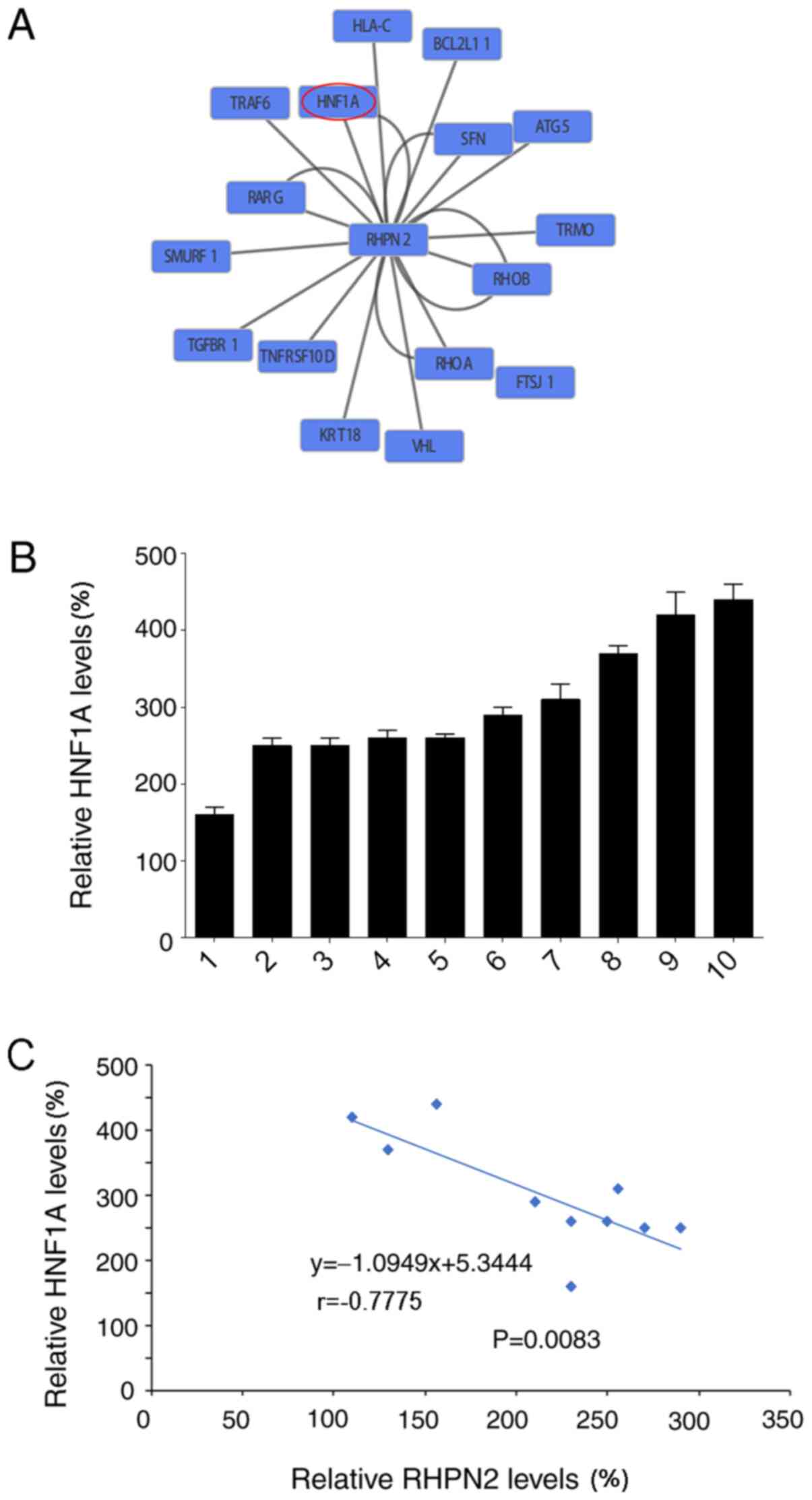
A network view of RHPN2 demonstrated the association
between HNF1α and RHPN2 (Fig. 5A).
HNF1α is a liver-enriched transcription factor that is considered
critical for the maintenance of hepatocyte function (10). Previous studies have demonstrated a
significant reduction of HNF1α and therapeutic effects in
hepatocellular carcinoma tissues (10,11).
Therefore, HNF1α mRNA expression levels were analyzed in tumor
tissues using RT-qPCR, and the relative HNF1α mRNA expression
levels were calculated (Fig. 5B).
Analysis of HNF1α mRNA expression levels demonstrated a negative
correlation between HNF1α and RHPN2 expression (Fig. 5C), suggesting that HNF1α may serve a
role in the mechanism of RHPN2 in hepatocellular carcinoma.
Additionally, bioinformatics analysis revealed RHPN2 as the target
gene of miR-141 and miR-200a (data not shown).
Discussion
The present study analyzed the function of RHPN2 in
hepatocellular carcinoma. IHC analysis revealed higher expression
levels of RHPN2 protein in hepatocellular carcinoma tissues
compared with those in adjacent normal tissues. RHPN2 promoted the
proliferation of hepatocellular carcinoma cells and suppressed
apoptosis. Gene network and correlation analyses revealed a
negative correlation between HNF1α and RHPN2 expression.
To the best of our knowledge, the present is the
study to investigate the oncogenic function of RHPN2 in
hepatocellular carcinoma as the oncogenic function of RHPN2 has
only been studied in malignant glioma (9). This previous study reported that RHPN2
drives mesenchymal transformation by triggering RhoA activation
(9). The results of the present
study demonstrated that RHPN2 promoted the proliferation of
hepatocellular carcinoma cells and suppressed apoptosis, providing
a possible explanation for the high expression levels of RHPN2 in
hepatocellular carcinoma tissues. More importantly, low expression
levels of RHPN2 in patients with human hepatocellular carcinoma
were associated with an improved prognosis rate. These survival
data highlighted on the importance of RHPN2 in hepatocellular
carcinoma.
Notably, bioinformatics analysis revealed RHPN2 as
the target gene of miR-141 and miR-200a by bioinformatics analysis
(data not shown). miR-141 and miR-200a belong to miR-200 family
(26) and both have been reported to
suppress the growth of various tumors, such as colon, gastric,
ovarian, lung and breast cancer (27–36).
Thus, it may be possible to treat hepatocellular carcinoma with
miR-141 and miR-200a targeting RHPN2.
The results of the present study demonstrated that
there was a negative correlation between HNF1α and RHPN2
expression, therefore it is possible that HNF1α-downregulation may
contribute to elevated RHPN2 levels in hepatocellular carcinoma.
However, the interaction between HNF1α and RHPN2 and the molecular
mechanism underlying this correlation remains unclear and requires
further investigation in future studies.
In conclusion, the results of the present study
suggested that overexpression of RHPN2 may promote hepatocellular
carcinoma and therefore inhibition of RHPN2 may delay the
progression of hepatocellular carcinoma.
Acknowledgements
The authors would like to thank Dr Fang (Department
of Brain Surgery, West China Hospital, Sichuan University, Chengdu,
China) for discussion of the study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BY collected the patient data. WB performed the
bioinformatics analysis. BY and XF performed PCR and transfection.
YH and WB performed the apoptosis analysis. BY and JZ contributed
to the study design and manuscript writing. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Sichuan University (Chengdu, China; approval no.
20160612) and written informed consent was provided by all patients
enrolled.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Srivatanakul P, Sriplung H and Deerasamee
S: Epidemiology of liver cancer: An overview. Asian Pac J Cancer
Prev. 5:118–125. 2004.PubMed/NCBI
|
|
3
|
Bosetti C, Turati F and La Vecchia C:
Hepatocellular carcinoma epidemiology. Best Pract Res Clin
Gastroenterol. 28:753–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuo TT, Zheng RS, Zhang SW, Zeng HM and
Chen WQ: Incidence and mortality of liver cancer in China in 2011.
Chin J Cancer. 34:508–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gish RG, Finn RS and Marrero JA: Extending
survival with the use of targeted therapy in the treatment of
hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 9 (Suppl
2):S1–S24. 2013.
|
|
6
|
Chen KW, Ou TM, Hsu CW, Horng CT, Lee CC,
Tsai YY, Tsai CC, Liou YS, Yang CC, Hsueh CW and Kuo WH: Current
systemic treatment of hepatocellular carcinoma: A review of the
literature. World J Hepatol. 7:1412–1420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsimberidou AM: Targeted therapy in
cancer. Cancer Chemother Pharmacol. 76:1113–1132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peck JW, Oberst M, Bouker KB, Bowden E and
Burbelo PD: The RhoA-binding protein, rhophilin-2, regulates actin
cytoskeleton organization. J Biol Chem. 277:43924–43932. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Danussi C, Akavia UD, Niola F, Jovic A,
Lasorella A, Pe'er D and Iavarone A: RHPN2 drives mesenchymal
transformation in malignant glioma by triggering RhoA activation.
Cancer Res. 73:5140–5150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian H, Deng X, Huang ZW, Wei J, Ding CH,
Feng RX, Zeng X, Chen YX, Ding J, Qiu L, et al: An HNF1α-regulated
feedback circuit modulates hepatic fibrogenesis via the crosstalk
between hepatocytes and hepatic stellate cells. Cell Res.
25:930–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng X, Lin Y, Yin C, Zhang X, Ning BF,
Zhang Q, Zhang JP, Qiu L, Qin XR, Chen YX and Xie WF: Recombinant
adenovirus carrying the hepatocyte nuclear factor-1alpha gene
inhibits hepatocellular carcinoma xenograft growth in mice.
Hepatology. 54:2036–2047. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Lichtenberg T, Hoadley KA, Poisson
LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee
AV, et al: An integrated TCGA pan-cancer clinical data resource to
drive high-quality survival outcome analytics. Cell.
173:400–416.e11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garcia DM, Baek D, Shin C, Bell GW,
Grimson A and Bartel DP: Weak seed-pairing stability and high
target-site abundance decrease the proficiency of lsy-6 and other
microRNAs. Nat Struct Mol Biol. 18:1139–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agarwal V, Bell GW, Nam J-W and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar
|
|
17
|
Hofman F: Immunohistochemistry. Current
protocols in immunology. 49:21.24. 21–21.24. 23. 2002.
|
|
18
|
Quail DF, Bowman RL, Akkari L, Quick ML,
Schuhmacher AJ, Huse JT, Holland EC, Sutton JC and Joyce JA: The
tumor microenvironment underlies acquired resistance to CSF-1R
inhibition in gliomas. Science. 352:aad30182016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roehm NW, Rodgers GH, Hatfield SM and
Glasebrook AL: An improved colorimetric assay for cell
proliferation and viability utilizing the tetrazolium salt XTT. J
Immunol Methods. 142:257–265. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gerlier D and Thomasset N: Use of MTT
colorimetric assay to measure cell activation. J Immunol Methods.
94:57–63. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berridge MV, Tan AS, McCoy KD and Wang R:
The biochemical and cellular basis of cell proliferation assays
that use tetrazolium salts. Biochemica. 4:14–19. 1996.
|
|
24
|
Weichert H, Blechschmidt I, Schröder S and
Ambrosius H: The MTT-assay as a rapid test for cell proliferation
and cell killing: Application to human peripheral blood lymphocytes
(PBL). Allerg Immunol (Leipz). 37:139–144. 1991.PubMed/NCBI
|
|
25
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Korpal M and Kang Y: The emerging role of
miR-200 family of microRNAs in epithelial-mesenchymal transition
and cancer metastasis. RNA Biol. 5:115–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng H, Zhang L, Cogdell DE, Zheng H,
Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR and Zhang W:
Circulating plasma MiR-141 is a novel biomarker for metastatic
colon cancer and predicts poor prognosis. PLoS One. 6:e177452011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du Y, Xu Y, Ding L, Yao H, Yu H, Zhou T
and Si J: Down-regulation of miR-141 in gastric cancer and its
involvement in cell growth. J Gastroenterol. 44:556–561. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Jaarsveld MT, Helleman J, Boersma AW,
van Kuijk PF, van Ijcken WF, Despierre E, Vergote I, Mathijssen RH,
Berns EM, Verweij J, et al: miR-141 regulates KEAP1 and modulates
cisplatin sensitivity in ovarian cancer cells. Oncogene.
32:4284–4293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tejero R, Navarro A, Campayo M, Viñolas N,
Marrades RM, Cordeiro A, Ruíz-Martínez M, Santasusagna S, Molins L,
Ramirez J and Monzó M: miR-141 and miR-200c as markers of overall
survival in early stage non-small cell lung cancer adenocarcinoma.
PLoS One. 9:e1018992014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Neves R, Scheel C, Weinhold S, Honisch E,
Iwaniuk KM, Trompeter HI, Niederacher D, Wernet P, Santourlidis S
and Uhrberg M: Role of DNA methylation in miR-200c/141 cluster
silencing in invasive breast cancer cells. BMC Res Notes.
3:2192010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li A, Omura N, Hong SM, Vincent A, Walter
K, Griffith M, Borges M and Goggins M: Pancreatic cancers
epigenetically silence SIP1 and hypomethylate and overexpress
miR-200a/200b in association with elevated circulating miR-200a and
miR-200b levels. Cancer Res. 70:5226–5237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xia H, Ng SS, Jiang S, Cheung WK, Sze J,
Bian XW, Kung HF and Lin MC: miR-200a-mediated downregulation of
ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma
cell growth, migration and invasion. Biochem Biophys Res Commun.
391:535–541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mateescu B, Batista L, Cardon M, Gruosso
T, de Feraudy Y, Mariani O, Nicolas A, Meyniel JP, Cottu P,
Sastre-Garau X and Mechta-Grigoriou F: miR-141 and miR-200a act on
ovarian tumorigenesis by controlling oxidative stress response. Nat
Med. 17:1627–1635. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu Y, Lu J, Li X, Zhu H, Fan X, Zhu S,
Wang Y, Guo Q, Wang L, Huang Y, et al: MiR-200a inhibits
epithelial-mesenchymal transition of pancreatic cancer stem cell.
BMC Cancer. 14:852014. View Article : Google Scholar : PubMed/NCBI
|