Introduction
Primary central nervous system lymphoma (PCNSL)
belongs to the group of non-Hodgkin's lymphomas (NHLs) occurring
outside the lymph nodes, of which 90% are forms of diffuse large
B-cell lymphoma (DLBCL) (1). These
tumors account for 3–5% of all cases of primary brain tumors
globally and are characterized by single or multiple intracranial
lesions without extracranial dissemination (1). Over the past 25 years, the incidence of
PCNSL has quadrupled, especially in the human immunodeficiency
virus (HIV)-infected population (1).
Due to the lack of specific neuroimaging features,
the differential discriminative diagnosis of PCNSL as a condition
distinct from other brain tumors, such as glioma and metastatic
encephaloma, is quite difficult, and misdiagnoses are likely to
occur (2,3). An analysis of 46 PCNSL cases with a
pathological primary diagnosis in Daping Hospital (Chongqing,
China) between 2007 and 2016 showed that the agreement between MRI
data and pathological evidence was only 39.1% (unpublished data).
The sensitivity (88%) and specificity (86%) of positron emission
tomography-computed tomography (PET-CT) are reported to be higher
than those of MRI (4). However,
PET-CT examination is usually delayed due to the rapid development
of neurological symptoms stemming from multifocal and diffuse
invasive growth of PCNSL. Additionally, the role of surgery in
PCNSL is usually limited to stereogenic biopsy to allow a clear
pathological diagnosis (5). In
addition, surgical resection increases the risk of permanent
neurological impairment and prolongs the time from diagnosis to
chemotherapy administration (6).
HD-MTX is currently considered the basis for the application of
systemic chemotherapy in newly diagnosed PCNSL (7). This treatment regimen is significantly
different from that of other solid tumors, such as glioma for which
the temozolomide (TMZ) based concurrent radiotherapy has been well
established (8).
Therefore, on the basis of MRI and PET-CT
examinations, there is an urgent need to identify novel
high-sensitivity, high-specificity biomarkers that can be used to
quickly and simply evaluate patient diagnoses and differential
diagnoses, screen treatment regimens, evaluate efficacy and
prognosis, and monitor for early recurrence.
Neopterin (Npt), interleukin (IL)-6 or IL-10 and
microRNA are considered to play roles in the diagnosis of PCNSL,
with relatively high sensitivity and specificity (9–12).
However, in our retrospective study on CSF Npt, this biomarker
showed a false-positive ratio of 16%, and the accuracy of clinical
diagnosis was greatly reduced (13).
IL-6 is a potent pluripotent inflammatory factor produced by cells
that promotes the growth and differentiation of lymphocytes
(14); it is a powerful growth and
differentiation factor for lymphocytes that is secreted by various
cells, such as benign and malignant B lymphocyte, monocytes,
macrophages, fibroblasts and hepatocytes (15,16). In
a previous study, a primary B-cell lymphoma cell line was found to
produce IL-6 in an autocrine manner, promoting maturation of the
secreting B cells into plasma cells that produce antibodies. In
NHL, IL-10 plays a central role in the growth of B lymphocytes and
chemotactic factors, and can induce cells to release immunoglobulin
(17), which has been proven to be
associated with the occurrence and prognosis of lymphoid
malignancies (18,19). IL-10 is secreted by type 2 helper
cells, monocytes, macrophages and B lymphocytes in normal and
malignant lymphoid tissues, which promotes the growth and
differentiation of lymphocytes, and activates B cells to release
immunoglobulin (20–22). IL-10 is considered to be the
strongest immunosuppressive or anti-inflammatory cytokine, and
plays a variety of roles in the development and progression of
lymphoma (20,22,23).
Therefore, the purpose of the present study was to investigate the
potential of CSF IL-6 and IL-10 levels as novel biomarkers for
PCNSL diagnosis, differential diagnosis, efficacy evaluation,
treatment prognosis estimation and early recurrence monitoring.
Materials and methods
Patient recruitment
The present study was prospective in nature, and
patients with suspected PCNSL based on neuroimaging data obtained
between October 2016 and December 2018 were recruited as subjects
to undergo CSF IL-6 and IL-10 examinations. The sampling procedure
and the objective of the study were explained to the patients and
their guardians or family members, and written informed consent was
obtained. All baseline characteristics including gender, age,
Karnofsky performance scale (KPS) (24), results from Pandy's test (25), WHO grade (26), chemotherapy administration and
radiotherapy scheme was extracted from clinical records. The study
protocol was approved by The Ethics Committee of Daping
Hospital.
Patients were included if they met the following
criteria: i) Preoperative MRI or CT findings suggesting PCNSL, a
malignant brain tumor or cerebral inflammation; and ii)
HIV-seronegative status without a history of autoimmune diseases or
organ transplantation. The exclusion criteria were as follows: i)
Age <12 years; ii) history of trauma; iii) persistent high
intracranial pressure; iv) CT or ultrasonic findings indicating
diseases outside the CNS; v) other contraindications of lumbar
puncture; and vi) HIV-seropositive status. Patients were withdrawn
from the study if they met the following criteria: i) Pathological
examination indicating the absence of PCNSL, with follow-up
examinations being halted as a result; ii) withdrawal of consent by
the patients or their family members; iii) contraindications of
lumbar puncture; and iv) coma or death of the patient. Patients
histologically confirmed with intracranial metastatic tumor or
glioma comprised the control group. These patients were treated
according to NCCN guidelines (27).
CSF collection and IL-6 or IL-10
measurement
Within 2 weeks of the neuroimaging examinations and
1 week before the operation, the patients underwent a lumbar
puncture and venipuncture to obtain 2–5 ml of CSF and 2–5 ml of
blood, respectively. After the results of histological examinations
confirmed PCNSL (28), the patients
underwent the following protocol: The tumor was evaluated by MRI
scans before the operation and prior to and during 2–4 courses of
chemotherapy (rituximab [R; 375 mg/m2 day (d)1],
high-dose methotrexate (H-MTX, 4 g/m2, d1) and TMZ (150
mg/m2, d1-5). At 1 week prior to and after the MRI
examinations, CSF and blood were collected again using the
aforementioned techniques, and both IL-6 and IL-10 concentrations
were measured. For patients with severe encephaledema, an
intravenous infusion of 20% mannitol was performed for 2–3 days and
30 min prior to lumbar puncture. The flow rate of CSF was set at 1
drip/second, and the needle was removed after 2–5 ml of CSF was
obtained, after which the patients lay in the supine position
without a pillow for 2–4 h. The obtained CSF was equally divided
into two parts: One part was used for routine biochemical and cell
count analysis, and the other was centrifuged for 10 min at 2,000 ×
g at room temperature and supernatant was stored in −80°C before
determination of the IL-6 or IL-10 concentration.
CSF and blood IL-6 levels were measured by
electrochemiluminescence immunoassay using an Automatic Immune
Analyzer (Cobas e601, Roche Diagnostics GmbH) with an IL-6 test kit
(cat. no. 36459902, Roche Diagnostics GmbH), which detects IL-6
within a dynamic range of 1.50–5,000 pg/ml. IL-10 levels were
measured by using an chemiluminescence immunoassay analyzer with a
IL-10 test kit (cat. no. 0408, Roche Diagnostics GmbH), which
detects IL-10 within a dynamic range of 1.00–1,000 pg/ml. All the
analytical procedures were performed in accordance with the
manufacturer's instructions.
Therapeutic regimen determination
A total of 38 patients with PCNSL underwent the
following therapeutic and follow-up protocols. Multiple programs
devised by different departments at Daping Hospital ran parallel.
In total, 26 patients chose induction chemotherapy as the primary
treatment, among which 21 received whole-brain radiotherapy, 2 were
monitored without further therapy, 1 received stem cell
transplantation and 2 were administered TMZ. Another 9 patients
chose radiotherapy and 3 chose Gamma Knife radiosurgery as the
primary treatment. On the basis of the National Comprehensive
Cancer Network (NCCN) Clinical Practice Guidelines recommendations
in 2018 (27), whole-brain
radiotherapy with a dose of 23.4 Gy was performed for patients
exhibiting a complete response CR, an irradiation dose up to 45 Gy
was administered around the tumor mass for patients not achieving a
CR and 24–36 Gy whole-brain radiotherapy with 45-Gy irradiation
around the tumor mass was performed for patients who were
unsuitable for chemotherapy.
Parameters and evaluation criteria in
MRI imaging
A Siemens 3.0T superconducting MR scanner (Magnetom
verio, Siemens Healthineers) with a 32-channel head coil was
employed. The scan sequences and parameters were as follows:
T1-weighted imaging (T1WI): Repetition time (TR)/echo time (TE),
440/2.48 msec; T2WI: TR/TE, 4,900/96 msec; FLAIR sequence: TR/TE,
8,000/94 msec; diffusion-WI sequence: b value set at 0 and 1,000
sec/mm2; perfusion-WI sequence: TR/TE, 1,872/30 msec;
susceptibility-WI sequence: TR/TE, 27/20 msec; and MRS sequence:
TR/TE, 1,700/135 msec. Enhanced scans with Gd-DTPA were conducted
on all the patients. Post-processing was performed using the
software package NUMARIS/4 (version syngo MR 2004V) included in the
Siemens workstation.
Enhanced MRI and IL-6/IL-10 measurements were
performed once at each of the following time points: Before the
operation, after the operation, after every 2–4 courses of
induction chemotherapy, before radiotherapy and 1 month after
radiotherapy. During the follow-up period, these assessments were
repeated once every 3 months and a response status assigned. CR
indicates that no tumor mass was present on enhanced MRI after
therapy. Partial response (PR) indicates that the size of the tumor
mass (sum of the maximal diameters of the tumor mass) decreased by
at least 50%. Stable disease indicates that the tumor size
decreased by the extent below the standard of PR or increased by no
more than 25%. Progressive disease indicates that the tumor size
increased by more than 25%. Progression-free survival (PFS) is
defined as the period from the beginning of therapy to relapse or
disease progression, and overall survival (OS) is defined as the
period from the beginning of therapy to the last evaluation or
death from any cause.
Statistical analysis
The IL-6/IL-10 concentration was measured by
laboratory technicians from the Cancer Center laboratory at Daping
Hospital who were blinded to the patients' clinical
characteristics. The χ2 test was used to compare the
basic pathological features between control (non-PCNSL patients)
and PCNSL groups. Fisher's exact probability test was adopted when
necessary. The concentrations of biochemical indices, as well as
the IL-10/IL-6 levels in serum or CSF, were represented by median
and interquartile ranges, and the differences between groups were
analyzed by using the Kruskal-Wallis test followed by pairwise
comparisons with Bonferroni's correction for multiple tests. The
correlation between CSF IL-10/IL-6 levels and tumor size was
determined by Spearman's rank correlation coefficient. The receiver
operating characteristic (ROC) curve and area under curve (AUC) was
used to assess the diagnostic efficacy of CSF IL-6 or IL-10 levels
for PCNSL. The method of Youden index was used to determine optimal
cut-off value (29). Median PFS and
OS times, as well as their corresponding 95% confidence intervals,
were determined by Kaplan-Meier survival analysis. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed with SPSS 21.0 software (IBM
Corp.).
Results
Clinical characteristics of the
patients
A total of 91 patients with suspected PCNSL on the
basis of neuroimaging examinations or clinicians' impressions
underwent IL-6/IL-10 measurements and prospective analysis. In 3
patients, the definitive diagnosis was based on neuroimaging and
CSF IL-10 results without confirmation through biopsy. Histological
examinations confirmed DLBCL in 35 patients and other tumors in the
remaining 53 patients, including 22 patients with glioma (13 with
grade IV glioblastoma, 5 with anaplastic glioma and 4 with diffuse
astrocytoma), 3 with ependymoma, 13 with brain metastasis, 10 with
a medulloblastoma/primitive neuroectodermal tumor, 4 with germinoma
and 1 with tuberculoma. Among the patients with PCNSL, 20 exhibited
multiple lesions on MRI and 12 were followed up regularly. None of
the patients showed severe complications such as cerebral
hernia.
CSF IL-6 and IL-10 levels in PCNSL
were higher than those in other brain tumors
Baseline characteristics of the entire patient
population are shown in Table I.
There was no significant intergroup difference in sex distribution
(P=0.097) and the number of intracranial lesions (P=0.086).
However, the proportion of female patients, patients with >3
lesions or those showing negative results in Pandy's test was
higher in the PCNSL group than in the control group. Among the
continuous variables, age (P=0.001), KPS (P<0.001), serum IL-10
level (P=0.024), CSF IL-10 level (P<0.001), leukocyte count
(P=0.019), CSF glucose concentration (P<0.001) and the ratio of
IL-10 to IL-6 (P<0.001) exhibited significant differences
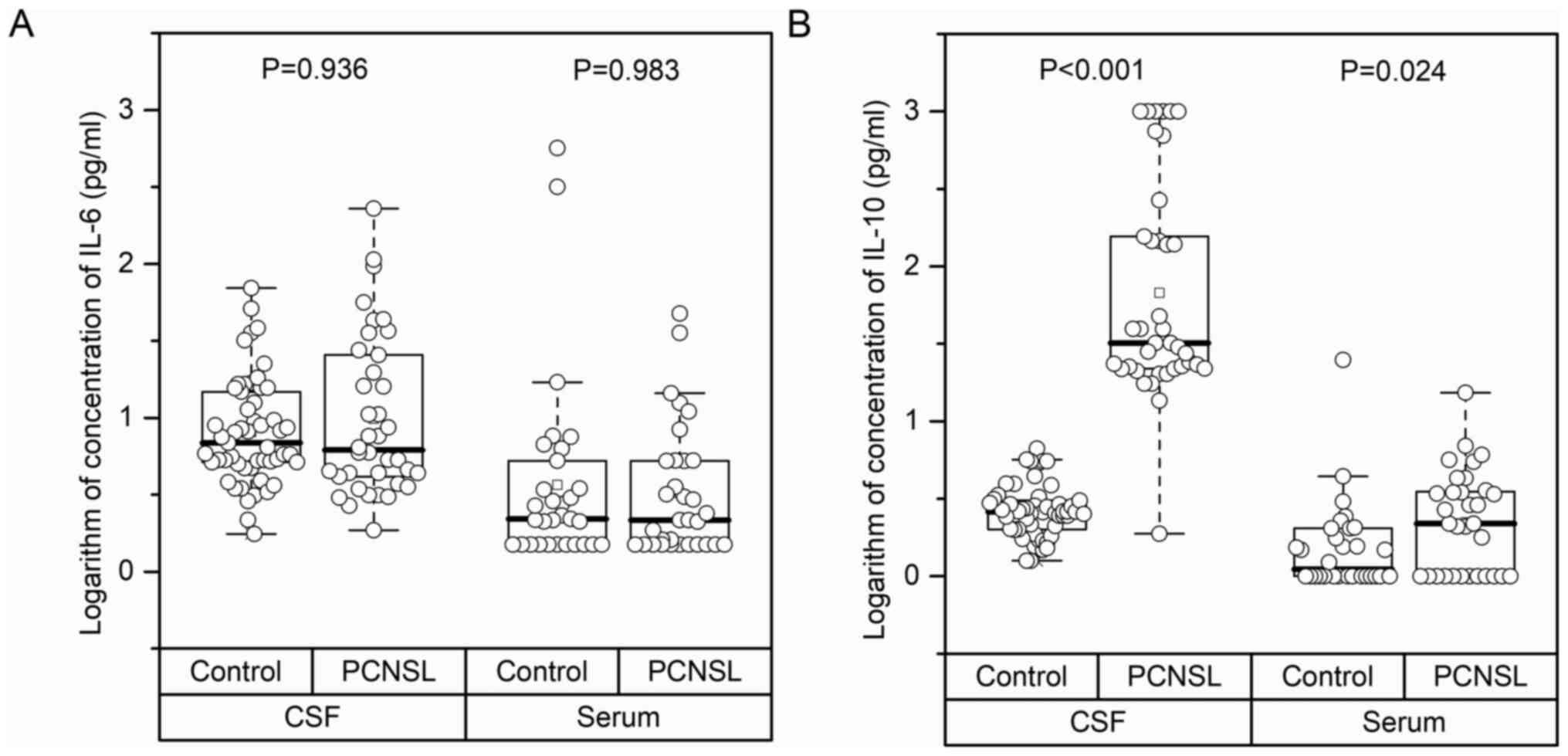
between the PCNSL and control groups (Table I). The boxplot in Fig. 1 shows the distribution of serum IL-6,
serum IL-10, CSF IL-6 and CSF IL-10 levels in the two groups. In
comparison with the control group, the serum and CSF IL-6 levels in
the PCNSL group showed no obvious difference; however, both serum
and CSF IL-10 levels increased significantly (P<0.001 and
P=0.024, respectively).
 | Table I.Comparison of baseline
characteristics between control and PCNSL groups. |
Table I.
Comparison of baseline
characteristics between control and PCNSL groups.
| Clinicopathological
characteristics | Control group | PCNSL group |
χ2/H | P-value |
|---|
| Sexa |
|
|
|
|
|
Female | 20 (37.7) | 21 (55.3) | 2.746 | 0.097 |
|
Male | 33 (62.3) | 17 (44.7) |
|
|
| Anatomical
sitea |
|
|
|
|
|
Cerebellum | 11 (20.8) | 3
(7.9) | 4.504 | 0.105 |
|
Mixture | 11 (20.8) | 14 (36.8) |
|
|
|
Superatentorial region | 31 (58.5) | 21 (55.3) |
|
|
| Number of
lesionsa |
|
|
|
|
| 1 | 40 (75.5) | 21 (55.3) | 4.912 | 0.086 |
| 2 | 4
(7.5) | 8
(21.1) |
|
|
| ≥3 | 9
(17.0) | 9
(23.7) |
|
|
| Radiographic
diagnosisa |
|
|
|
|
|
Non-PCNSL | 50 (94.3) | 21 (55.3) | 19.708 | <0.001 |
|
PCNSL | 3
(5.7) | 17 (44.7) |
|
|
| Pandy
testa |
|
|
|
|
| − | 34 (64.2) | 32 (84.2) | – | 0.071c |
| + | 18 (34.0) | 6
(15.8) |
|
|
| ++ | 1
(1.9) | 0
(0.0) |
|
|
| Age,
yearsb | 52 (43–57) | 55.5 (52–63) | 10.491 | 0.001 |
| KPSb | 80 (80–90) | 70 (60–90) | 14.621 | <0.001 |
| Total tumor volume,
cm3b | 3.444
(1.918–8.456) | 4.948
(2.985–12.353) | 1.129 | 0.288 |
| Serum LDH,
U/lb | 225.6
(165–508) | 208
(161–550) | 0.077 | 0.782 |
| Serum IL-6,
pg/mlb | 2.2
(1.50–5.26) | 2.16
(1.50–5.26) | 0.000 | 0.983 |
| Serum IL-10,
pg/mlb | 1.115
(1.00–2.04) | 2.18
(1.00–3.52) | 5.130 | 0.024 |
| CSF IL-6,
pg/mlb | 6.87
(5.14–14.78) | 6.18
(4.14–25.60) | 0.006 | 0.936 |
| CSF IL-10,
pg/mlb | 2.61
(2.01–3.08) | 32
(21.90–156.00) | 60.463 | <0.001 |
| CSF cell
countb | 0.005
(0.003–0.015) | 0.01
(0.003–0.021) | 0.678 | 0.410 |
| CSF Leukocyte
count, ×109/lb | 0.002
(0.001–0.005) | 0.0045
(0.002–0.012) | 5.541 | 0.019 |
| CSF LDH,
U/lb | 18.5
(10.9–23.5) | 14.9
(10.9–24.2) | 0.233 | 0.629 |
| CSF glucose,
mmol/lb | 3.46
(3.18–3.93) | 4.32
(3.93–4.95) | 23.217 | <0.001 |
| CSF total protein,
g/lb | 0.34
(0.20–0.57) | 0.38
(0.32–0.57) | 3.460 | 0.063 |
| Ratio of IL-10 to
IL-6 in serumb | 0.667
(0.246–0.815) | 0.667
(0.407–1.393) | 1.754 | 0.185 |
| Ratio of IL-10 to
IL-6 in CSFb | 0.341
(0.214–0.526) | 7.880
(5.573–11.672) | 56.867 | <0.001 |
Contribution of CSF IL-6 and IL-10 to
the diagnosis of PCNSL
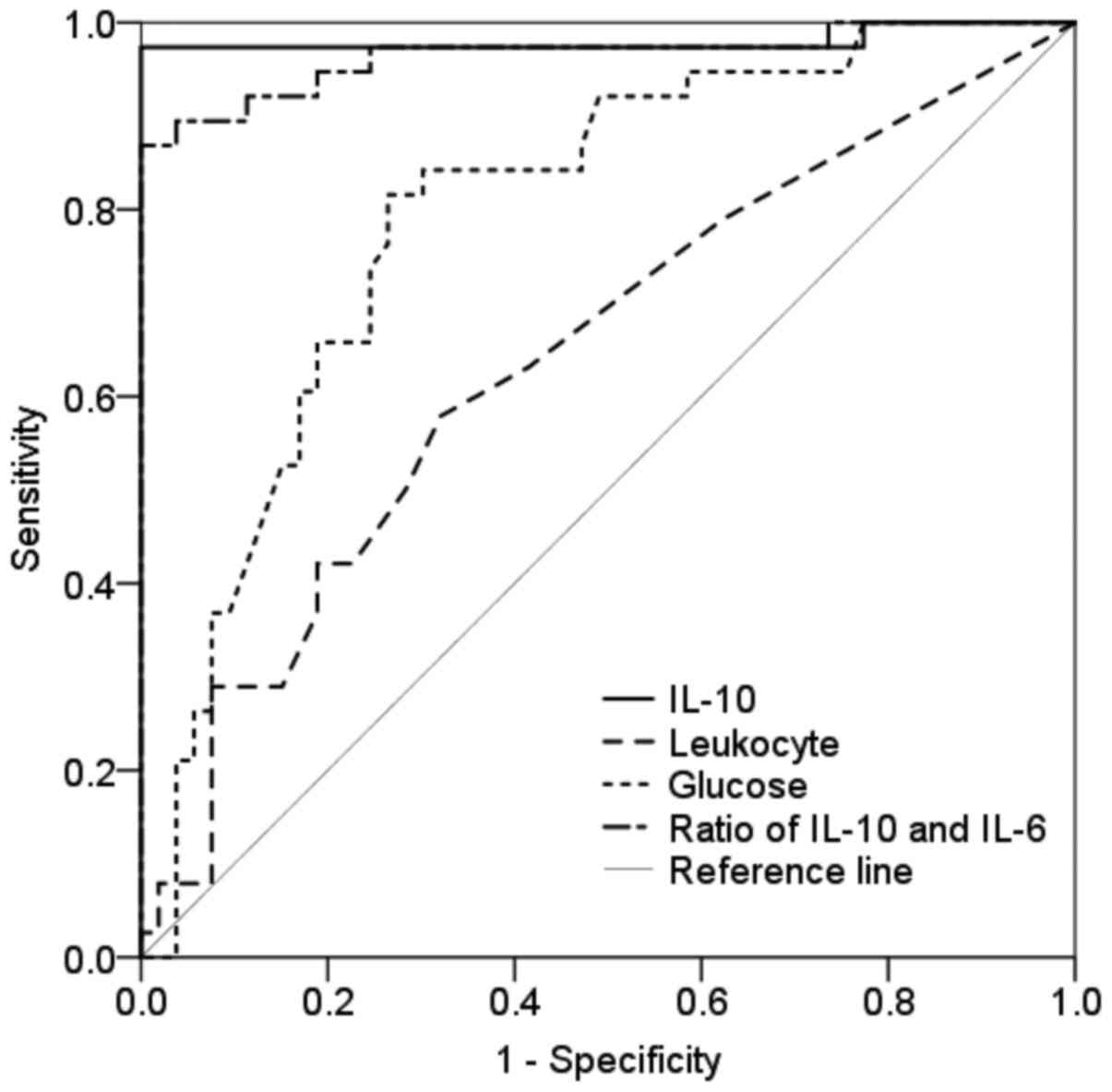
ROC curve analysis was performed for each parameter
that showed significant differences in Table I. As shown in Fig. 2 and Table
II, serum IL-10 level (P=0.030), CSF IL-10 level (P<0.001),
CSF leukocyte count (P=0.020), CSF glucose concentration
(P<0.001) and the ratio of IL-10 to IL-6 levels in the CSF
(P<0.001) showed the ability to discriminate patients with PCNSL
from those without PCNSL.
 | Table II.AUCs and corresponding 95%
confidential intervals of serum IL-10 and four other CSF
biochemical indicators for discriminating primary central nervous
system lymphoma from control cases. |
Table II.
AUCs and corresponding 95%
confidential intervals of serum IL-10 and four other CSF
biochemical indicators for discriminating primary central nervous
system lymphoma from control cases.
| Factors tested | AUC (95% CI) | P-value |
|---|
| Serum
IL-10a | 0.660
(0.521–0.800) | 0.030 |
| CSF IL-10 | 0.980
(0.940–1.000) | 0.000 |
| CSF Leukocyte
count | 0.644
(0.528–0.760) | 0.020 |
| CSF glucose | 0.797
(0.705–0.890) | 0.000 |
| Ratio of CSF IL-10
to IL-6 | 0.965
(0.924–1.000) | 0.000 |
Comparison of the diagnostic efficiencies of serum
and CSF IL-10 levels showed that the former was substantially
inferior to the latter. On the basis of the Youden index, the
optimal serum IL-10 cut-off value in all 91 patients was determined
to be 2.07 pg/ml, and the sensitivity, specificity, positive
predictive value (PPV) and negative predictive value (NPV) of this
cut-off value were 59.4, 83.3, 79.2, and 65.8%, respectively. By
contrast, the cut-off CSF IL-10 value was 10.13 pg/ml, and the
sensitivity, specificity, PPV and NPV of this cut-off value were
97.4, 100, 100 and 98.1%, respectively.
In the PCNSL group, 34 out of 35 cases (97.1%)
showed CSF IL-10 levels above the cut-off value. The CSF IL-10
level of 1 patient was below the cut-off value due to steroid
treatment, with the CSF IL-6 and IL-10 levels being 3.55 and 1.88
ng/ml, respectively. At 2 weeks post-withdrawal of steroid
treatment, the CSF IL-6 and IL-10 levels increased to 4.23 and 57.8
pg/ml, respectively, suggesting that steroid treatment can cause
false-negative outcomes. Another case diagnosed as left
occipitoparietal lymphadenoma showed a CSF IL-6 level of 69.69
pg/ml and a CSF IL-10 level of 1.56 pg/ml, but the histological
examination after craniotomy revealed a tuberculous granuloma. In
general, the diagnostic efficacy of IL-6 levels was substantially
lower than that of IL-10 levels.
Relevance of CSF IL-6 or IL-10 levels
to the number of tumor lesions and imaging and histological
manifestations
Among the 53 cases with a histological diagnosis of
non-PCNSL, 50 were primarily detected by MRI, resulting in a
specificity of 94.3%. By contrast, among the 38 PCNSL cases, 17
were primarily detected by MRI with a sensitivity of 44.7%. Among
these, 3 were confirmed by MRI scans and IL-10 assessment without
confirmation through biopsy, and the PET-CT assessments in these
cases revealed hypermetabolic foci. A total of 9 cases exhibited
characteristics of DLBCL on histological examination. Among the 12
PCNSL cases with regular follow-up assessments, 4 (33.3%) were
primarily and 5 (41.7%) were secondarily detected by MRI scan,
while the remaining 3 cases were diagnosed as other tumors. As
shown in Fig. 3, the low sensitivity
of MRI for the diagnosis of PCNSL was substantially compensated by
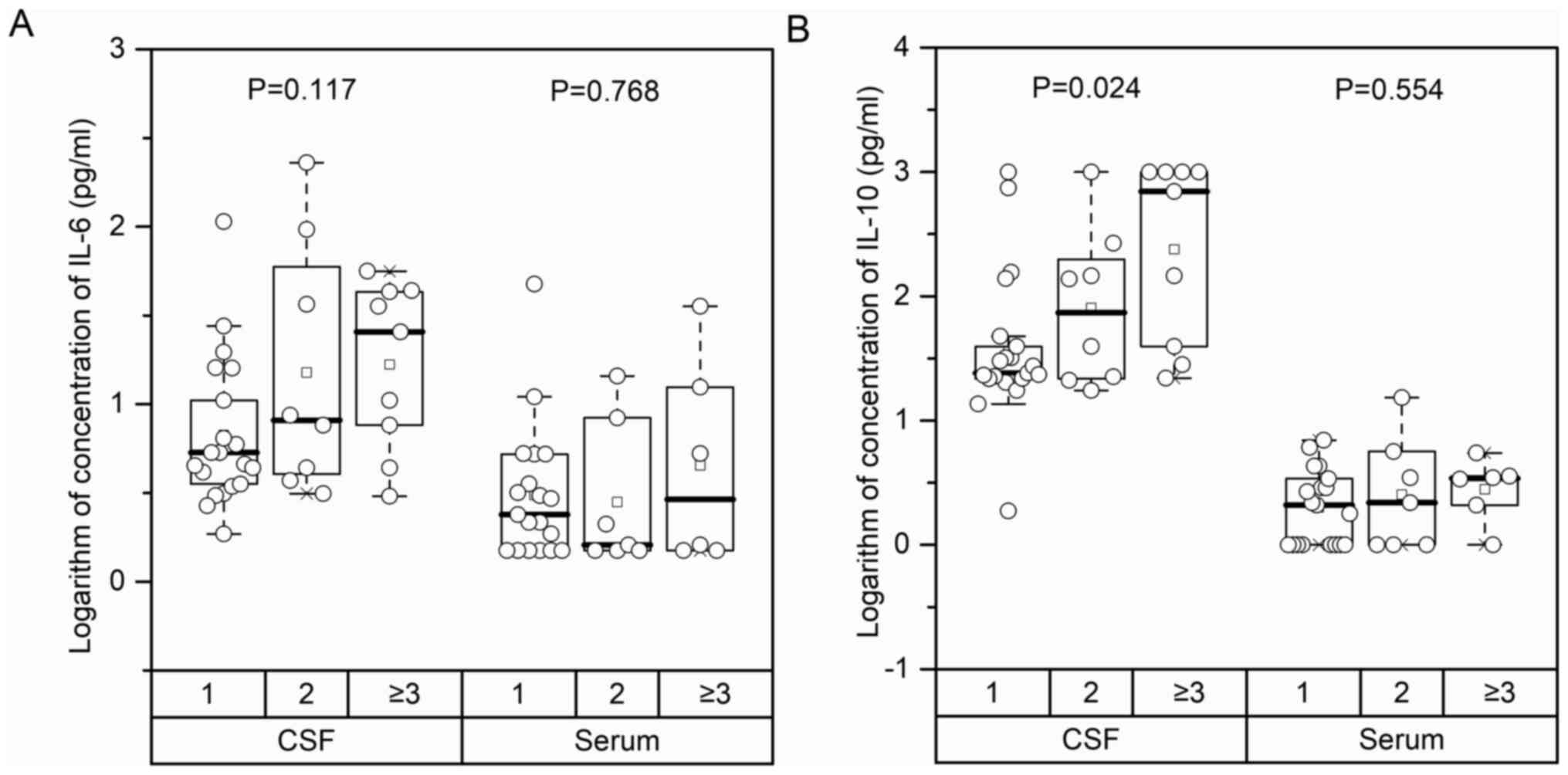
CSF IL-10 evaluations. In the 38 PCNSL cases, CSF IL-10
concentration rose significantly along with an increase in the
number of tumor lesions [Number of tumor lesion: 1 vs. 2 vs. ≥3,
median (IQR): 24.15 (21.71–39.50) vs. 88.75 (21.81–206.50) vs.
697.00 (39.50–1,000.0), P=0.024]. However, there was no significant
correlation between CSF IL-6 (P=0.117), serum IL-6 (P=0.768), and
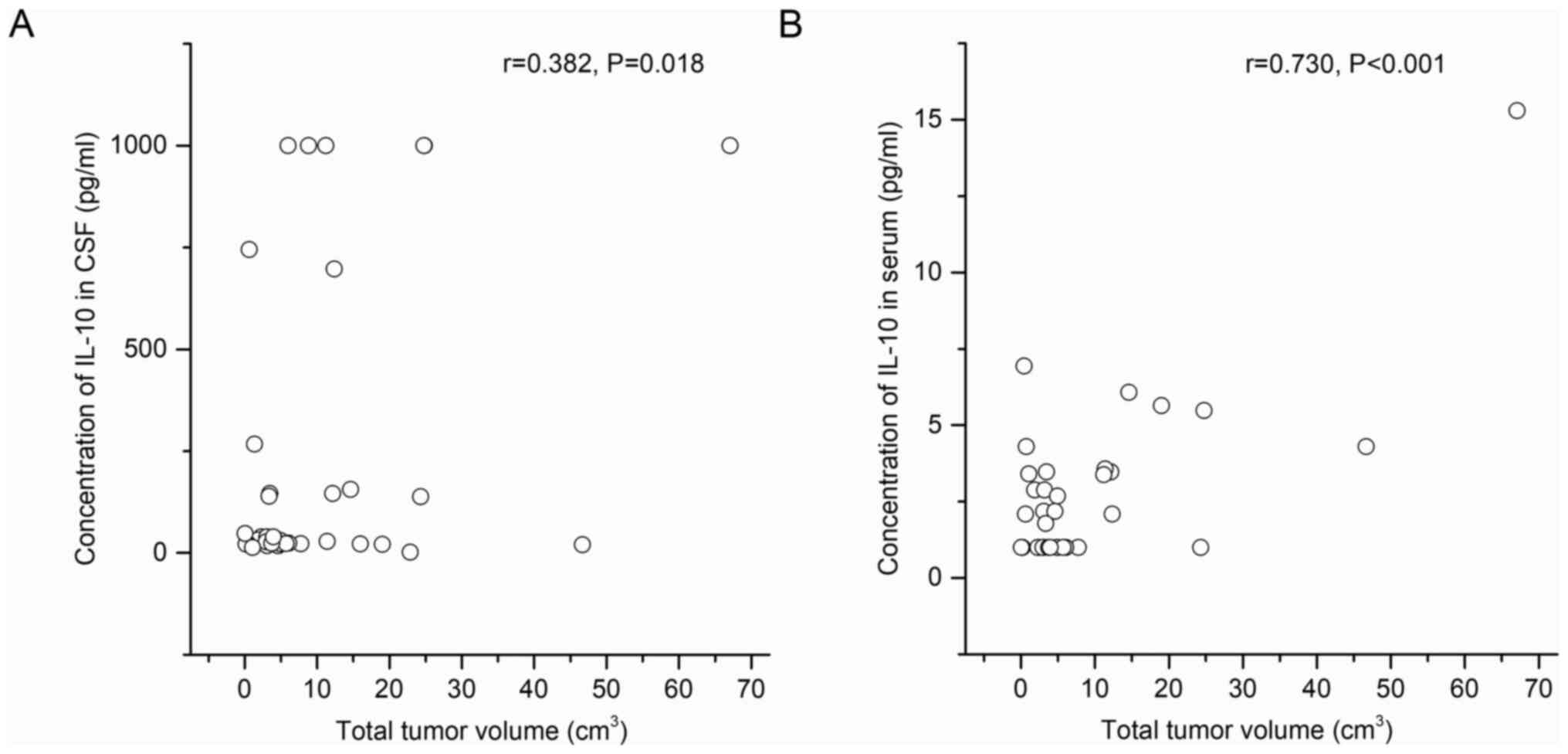
serum IL-10 levels (P=0.554) and tumor lesion number (Fig. 4). Nevertheless, tumor size correlated
positively with both serum and CSF IL-10 levels (r=0.730,
P<0.001; r=0.382, P=0.018; Fig.
5).
Role of CSF IL-10 levels in predicting
the curative effect and prognosis of PCNSL
Among the 38 patients with PCNSL, 12 were followed
up regularly, including 6 males and 6 females. The MRI scans
primarily showed 4 cases of PCNSL. Tumors were located in the
cerebellum, supratentorial region or at both sites in 2, 5 and 5
cases, respectively, while 1, 2 and 3 tumor lesions were present in
7, 3 and 2 cases, respectively. The follow-up periods ranged from 1
to 27 months, with a median period of 4 months, during which 4
cases showed disease progression and 3 patients died. The median
PFS time was 8 months (95% CI, 3.94–12.06), and the median OS time
was 17.5 months (95% CI, 11.55–23.45).
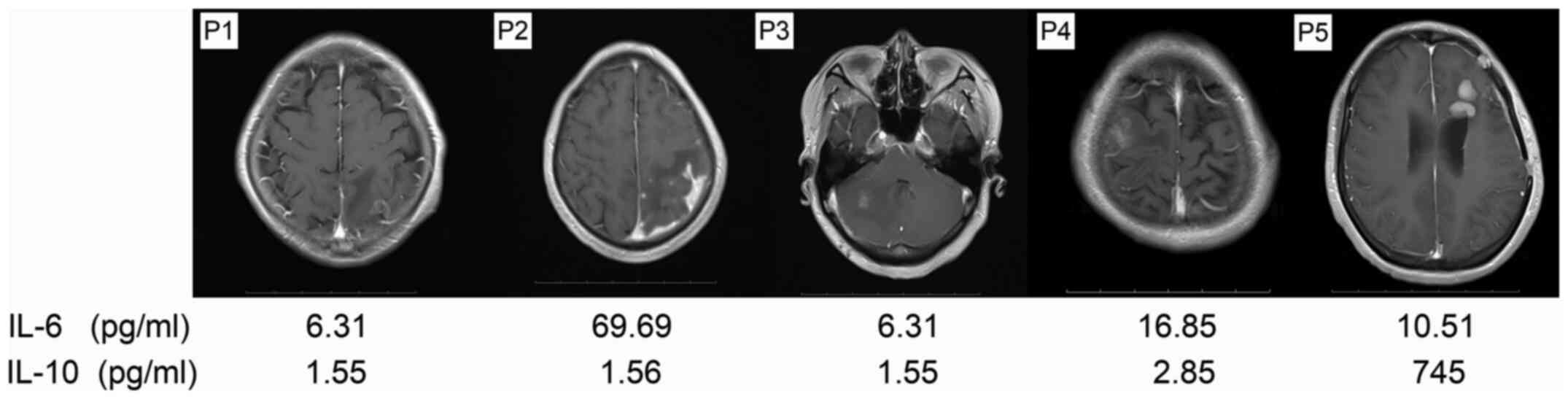
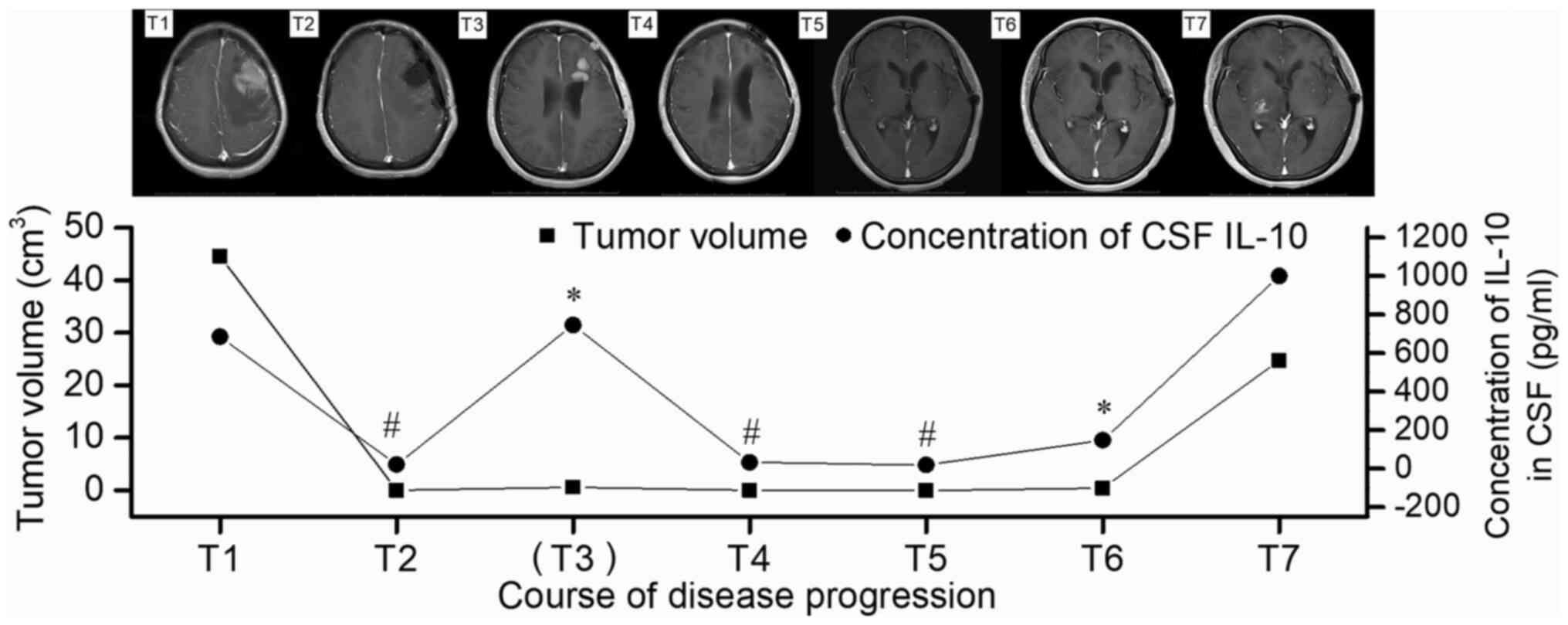
Case 2, which involved the most follow-up
examinations, was diagnosed as left frontal glioblastoma by MRI
before the operation. However, the CSF IL-10 level of the patient
was 683.4 pg/ml, indicating the presence of lymphadenoma. The case
was then confirmed to be intracranial primary diffuse large B-cell
lymphoma outside the germinal center by histological examination
after craniotomy (T1; Fig. 6). The
post-operative MRI indicated that the tumor had been CR, and this
was accompanied by a reduction in the CSF IL-10 level to 20.2 pg/ml
(T2; Fig. 6). The patient received
two courses of chemotherapy with R (375 mg/m2 d1), H-MTX
(4 g/m2, d1) and TMZ (150 mg/m2, d1-5), and
then underwent evaluation of CSF IL-10 levels, which had reached
745 pg/ml. The immediate enhanced MRI scan showed a lesion with a
size of 1.2×0.9 cm beside the left lateral ventricle that appeared
hypermetabolic on PET-CT, indicating tumor relapse (T3; Fig. 6). Therefore, the treatment was
changed to whole-brain radiotherapy at 23.4 Gy, with a local dose
of up to 45 Gy in a total number of 13 fractions. The CSF IL-10
level reduced to 32 pg/ml, corresponding to the CR observed on MRI
(T4; Fig. 6). After 4 courses of
chemotherapy with rituximab (R, 375 mg/m2 d1), high-dose
methotrexate (H-MTX, 4 g/m2, d1) and TMZ (150
mg/m2, d1-5), the CSF IL-10 level had reduced to 17.5
pg/ml, with MRI showing CR (T5; Fig.
6). However, after another two courses with the same regimen,
the patient experienced dizziness, which was associated with a
corresponding increase in the CSF IL-10 level to 146 pg/ml and the
presence of cloudy-like edema on the left frontal and basal ganglia
zone on MRI (T6; Fig. 6). Therefore,
a chemotherapy regimen based on rituximab and high-dose
methotrexate was recommended, but the patient rejected this
treatment Due to financial reasons and as no lesion was observed on
MRI. At 1 month post-discharge from the hospital, the condition of
the patient continued to deteriorate and symptoms of lethargy and a
cough appeared; therefore, the patient was readmitted to the
hospital. At this point, the CSF IL-10 level had increased to
>1,000 pg/ml, and MRI showed large cloud-like edema with an
irregular potentiated nodular focus in the left frontal and
bilateral basilar ganglia zones (T7; Fig. 6). The patient exhibited extremely
rapid disease progression and died with bedsores and a pulmonary
fungal infection.
In PCNSL patients, the variations in CSF IL-10
levels were in line with the tumor size on MRI imaging. High levels
of CSF IL-10 before therapy was associated closely with low KPS
scores and short PFS or OS times. In cases 1, 2, 3, 6 and 12, the
increase in CSF IL-10 levels appeared earlier than MRI features
after treatment, indicating that this biomarker held substantial
value in predicting tumor relapse. CSF IL-10 levels >138 pg/ml
before therapy occurred with markedly low KPS scores. A total of 6
cases with CSF IL-10 levels >1,000 pg/ml showed rapid disease
progression, signifying that CSF IL-10 levels >1,000 pg/ml could
be a crucial sign of a poor prognosis.
Discussion
Il-6 (14) and IL-10
(18,19) have been shown to be associated with
the occurrence and prognosis of lymphoid malignancies. In previous
studies, high serum IL-6 and IL-10 levels were considered to be
independent predictors of the prognosis of invasive and systemic
NHL (30,31). The cutoff value for serum IL-10 in 91
patients in the present study was 2.07 pg/ml, while the
sensitivity, specificity, and positive and negative predictive
values of serum IL-10 levels for the diagnosis of PCNSL were 59.4,
83.3, 79.2 and 65.8, respectively. The serum IL-6 level was lower
than the serum IL-10 level (P=0.030), and the serum IL-10 level was
lower than the CSF IL-10 level (P<0.001). In the present cohort,
1 patient presented with left occipital lymphoma by MRI
examination. The levels of CSF IL-6 and IL-10 in this patient were
69.69 and 1.56 pg/ml, respectively, and histological examination
showed tuberculous granuloma. Sasayama et al (10) found that when the cutoff value was
4.0 pg/ml, the sensitivity and specificity of the CSF IL-6 level
for diagnosing PCNSL were 77 and 63%, respectively. Serum and CSF
IL-6 levels have certain reference value in the diagnosis of PCNSL,
but have low specificity (32).
The CSF IL-10 level has a high sensitivity and
specificity in the diagnosis of PCNSL (10,11).
Among the 91 patients recruited in the present study, 38 had
confirmed PCNSL and 53 had other brain tumors. In the 38 patients
of the PCNSL group, the CSF IL-10 concentration increased
significantly with increasing number of lesions (1 vs. 2 vs. ≥3,
24.15 (21.71–39.50) vs. 88.75 (21.81–206.50) vs. 697.00
(39.50–1,000.0); P=0.024). There were significant positive
correlations between total tumor volume and serum or CSF IL-10
(r=0.730, P<0.001; r=0.382, P=0.018). Considering the
anti-inflammatory properties of IL-10, a continuously high level of
CSF IL-10 may trigger relatively strong immune activation, promote
cell proliferation and inhibit cell apoptosis (33,34). It
can be inferred that CSF IL-10 is produced by tumor cells, and that
the higher the level of CSF IL-10, the greater the tumor burden,
the lower the KPS score and the worse the prognosis.
According to the Youden index, the cutoff value for
serum IL-10 in the 91 patients of the present study was 2.07 pg/ml.
This cutoff value was sensitive (59.4%) and specific (83.3%), with
a PPV of 79.2% and a NPV of 65.8%. The cutoff value for CSF IL-10
was 10.13 pg/ml, and this cutoff value was sensitive (97.4%) and
specific (100%), with a PPV of 100% and an NPV of 98.1%. For the
differential diagnosis of PCNSL, CSF IL-10 has been recognized to
be significantly more effective than serum IL-10 in terms of
specificity and sensitivity, as the former can prevent a
false-positive diagnosis from interfering with the clinical
application of various biomarkers of PCNSL, including IL-10, which
has been previously reported as occurring (10,11,35–41).
Whitcup et al (36) first
found that the ratio of IL-10 to IL-6 in the CSF associated with
CNS lymphoma was increased, and Salmaggi et al (37) reported that the CSF IL-10
concentration in PCNSL was higher than that in other CNS tumors. In
the present study, only solid tumor patients suspected to have
PCNSL were included, and the specificity of CSF IL-10 in the
diagnosis of PCNSL was 100%, which is consistent with previous
reports (9,10). The most recent review article also
supports the high specificity of CSF IL-10 in the diagnosis of
PCNSL.
The influence of hormones on CSF IL-10 remains to be
elucidated. Among the 53 patients with brain tumors other than
PCNSL in the present study, 14 showed CSF IL-10 levels lower than
the minimal limit of the test (2 pg/ml). Conversely, only 1 PCNSL
patient had similarly low levels of CSF IL-10. The MRI scan of this
patient was indicative of PCNSL, and PET-CT examination highlighted
the hypermetabolic lesion. This patient was undergoing high-dose
steroid treatment for management of a constantly deteriorating
condition, and the CSF IL-6 and IL-10 levels after initiation of
this treatment were 3.55 and 1.88 pg/ml respectively. However, 3
weeks after withdrawal of steroid therapy, the CSF IL-6 level
increased to 4.23 pg/ml, while the CSF IL-10 level rose to 57.8
pg/ml. Steroids can suppress the survival of malignant cells, but
the tumor cells may recover soon after steroid treatment is halted.
Since patients receiving steroid treatment may show reduced CSF
IL-6 and IL-10 levels (36), it is
necessary to assess these levels again 2 to 3 weeks after
withdrawing steroid therapy. In addition, due to the fragility of
malignant lymphoma cells, these cells are not easily detected in
cytopathological examinations. The present study involved only one
case with detectable lymphoma cells in the CSF smear.
Although an optimal treatment strategy for PCNSL
based on high-dose methotrexate has not yet been determined,
whole-body radiotherapy and stem cell transplantation are currently
the most important treatment methods (7,42). In
the present study, 35 PCNSL patients were recruited from different
centers and received different treatments, making it difficult to
summarize PFS and OS data. However, 12 patients, including 2 who
received the most follow-up examinations, were regularly followed
up, with a 14-month interval from diagnosis to death. These results
indicate that the CSF IL-10 level might be indicative of the tumor
burden, and that the higher the IL-10 level, the more invasive the
tumor, the stronger the cellular immune response and the shorter
the survival time. In addition, the increased level of CSF IL-10 is
closely associated with poor survival and is an independent risk
factor for predicting the prognosis of PCNSL (20,36,43).
More importantly, in the present study, the increase in CSF IL-10
levels appeared earlier than the MRI abnormalities, suggesting the
CSF IL-10 may have value in monitoring the treatment of tumor
recurrence or clinical decision-making. In addition, increased CSF
IL-10 levels with increased tumor burden hinted that this cytokine
might be produced by tumor cells (44). However, high secretion of IL-10 by
cells was difficult to detect in the present study, as CSF IL-10
levels >1,000 pg/ml were associated with the rapidly
deteriorating condition of the patient, which means that the levels
of CSF IL-10, in addition to having an application in monitoring
the tumor state, may also be useful in predicting prognosis
(22). Biomarkers of these
characteristics make CSF IL-10 superior to other conventional tumor
biomarkers, as these conventional markers are essentially tumor
cell metabolites and therefore can be modified only on behalf of
the tumor itself. However, a previous study reported that IL-10 can
inhibit tumor angiogenesis, promote cytotoxic factor generation,
induce tumor elimination and improve survival rate (20).
IL-10 levels ≥138 and ≥1,000 pg/ml before and after
treatment, respectively, were important predictors of a poor
prognosis in the present study, which also indicates that CSF IL-10
expressed at high levels in the CSF accelerated the growth of tumor
cells resistant to both radiotherapy and chemotherapy. First, IL-10
may be produced by malignant B lymphocytes, which strongly
stimulates the proliferation and differentiation of B cells, and
promotes the occurrence and development of lymphoma (30). On the other hand, a high level of
IL-10 is associated with immunosuppressive effects, which can
suppress the antitumor immune response and interfere with immune
monitoring (21). An experimental
study also found that IL-10 could protect cells, promote cell
growth via autocrine or paracrine mechanisms to activate NHL,
promote proliferation, inhibit apoptosis and promote tumor cell
growth (45). A drug susceptibility
test showed that IL-10 is crucial in NHL resistance (33), which suggests that IL-10 not only is
very important during tumorigenesis, but also plays a promotive
role in the growth of tumor, as IL-10- or IL-10 receptor-specific
treatment may be an effective treatment strategy for PCNSL
(46). An IL-10 concentration
>1,000 pg/ml is considered a high-risk factor for PCNSL, and
targeting IL-10 will improve treatment intensity and patient PFS
and OS times, and reduce the CNS progression rate (47), which makes IL-10 worthy of further
in-depth study.
In regard to the limitations of the present study,
the small sample size was a primary limitation. Since PCNSL is a
rare disease of the brain, a single center will normally encounter
only a few cases, which may lead to data bias. The unitary disease
category was another limitation of the study. Due to its
prospective nature, this study recruited only patients with
suspected PCNSL shown by MRI. However, some reports have shown that
increased levels of CSF IL-10 are also found in idiopathic
inflammatory disease to a certain extent (41). Therefore, before drawing further
conclusions, studies with broader disease categories and larger
sample sizes should be conducted. The third limitation of the
present work was the incomplete basic clinical information of the
patients, which could be addressed by closer follow-up.
In summary, even with the numerous shortcomings, the
present study proved that the CSF IL-10 level was the most
significant biomarker for patients with suspected PCNSL by
neuroimaging and the presence of a tumor mass. Additionally, the
CSF IL-10 level served as an independent predictor for prognosis
that was closely associated with the poor survival of PCNSL
patients. These findings provide a reference for clinicians to
identify more effective therapeutic strategies. Further
prospective, multicenter studies would be helpful for clarifying
these issues in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MG, CC, and GW conceived and designed the
experiments. MG and YS performed the main experiments and analyzed
the data. HX, ZW and XD participated in the measurement and
analysis of IL-6 and IL-10 in the blood and CSF. MG and YS wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by The Medical Ethics
Committee at the Daping Hospital, Army Medical University (Third
Military Medical University).
Patient consent for publication
Written informed consent for the publication of
images and all other clinical data is obtained from the enrolled
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Su M, Huang D, Sun L, Dong Z, Wu L and Yu
S: A diagnostic challenge of primary Central nervous system
lymphoma: From the eyes to the brain. Int J Neurosci. 1–7.
2020.(Epub ahead of print). doi: 10.1080/00207454.2020.1773822.
View Article : Google Scholar
|
|
2
|
Bataille B, Delwail V, Menet E,
Vandermarcq P, Ingrand P, Wager M, Guy G and Lapierre F: Primary
intracerebral malignant lymphoma: Report of 248 cases. J Neurosurg.
92:261–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartmann M, Heiland S, Harting I, Tronnier
VM, Sommer C, Ludwig R and Sartor K: Distinguishing of primary
cerebral lymphoma from high-grade glioma with perfusion-weighted
magnetic resonance imaging. Neurosci Lett. 338:119–122. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bertaux M, Houillier C, Edeline V, Habert
MO, Mokhtari K, Giron A, Bergeret S, Hoang-Xuan K, Cassoux N,
Touitou V, et al: Use of FDG-PET/CT for systemic assessment of
suspected primary central nervous system lymphoma: A LOC study. J
Neurooncol. 148:343–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Josephson SA, Papanastassiou AM, Berger
MS, Barbaro NM, McDermott MW, Hilton JF, Miller BL and Geschwind
MD: The diagnostic utility of brain biopsy procedures in patients
with rapidly deteriorating neurological conditions or dementia. J
Neurosurg. 106:72–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khatab S, Spliet W and Woerdeman PA:
Frameless image-guided stereotactic brain biopsies: Emphasis on
diagnostic yield. Acta Neurochir (Wien). 156:1441–1450. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferreri AJM, Cwynarski K, Pulczynski E,
Fox CP, Schorb E, La Rosee P, Binder M, Fabbri A, Torri V,
Minacapelli E, et al: Whole-brain radiotherapy or autologous
stem-cell transplantation as consolidation strategies after
high-dose methotrexate-based chemoimmunotherapy in patients with
primary CNS lymphoma: Results of the second randomisation of the
International Extranodal Lymphoma Study Group-32 phase 2 trial.
Lancet Haematol. 4:e510–e523. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gilbert MR, Wang M, Aldape KD, Stupp R,
Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT,
et al: Dose-dense temozolomide for newly diagnosed glioblastoma: A
randomized phase III clinical trial. J Clin Oncol. 31:4085–4091.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Viaccoz A, Ducray F, Tholance Y, Barcelos
GK, Thomas-Maisonneuve L, Ghesquieres H, Meyronet D, Quadrio I,
Cartalat-Carel S, Louis-Tisserand G, et al: CSF neopterin level as
a diagnostic marker in primary central nervous system lymphoma.
Neuro Oncol. 17:1497–1503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sasayama T, Nakamizo S, Nishihara M,
Kawamura A, Tanaka H, Mizukawa K, Miyake S, Taniguchi M, Hosoda K
and Kohmura E: Cerebrospinal fluid interleukin-10 is a potentially
useful biomarker in immunocompetent primary central nervous system
lymphoma (PCNSL). Neuro Oncol. 14:368–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sasagawa Y, Akai T, Tachibana O and Iizuka
H: Diagnostic value of interleukin-10 in cerebrospinal fluid for
diffuse large B-cell lymphoma of the central nervous system. J
Neurooncol. 121:177–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baraniskin A, Kuhnhenn J, Schlegel U,
Maghnouj A, Zollner H, Schmiegel W, Hahn S and Schroers R:
Identification of microRNAs in the cerebrospinal fluid as biomarker
for the diagnosis of glioma. Neuro Oncol. 14:29–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geng M, Xiao H, Liu J, Song Y, Fu P, Cheng
X, Zhang J and Wang G: The diagnostic role and dynamic changes in
cerebrospinal fluid neopterin during treatment of patients with
primary central nervous system lymphoma. Cancer Med. 7:3889–3898.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoekzema R, Murray PI, van Haren MA, Helle
M and Kijlstra A: Analysis of interleukin-6 in endotoxin-induced
uveitis. Invest Ophthalmol Vis Sci. 32:88–95. 1991.PubMed/NCBI
|
|
15
|
Salles G and Coiffier B: Inherited
cytokine response and risk of lymphoma. Lancet Oncol. 7:3–4. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kurzrock R: The role of cytokines in
cancer-related fatigue. Cancer. 92 (Suppl 6):S1684–S1688. 2001.
View Article : Google Scholar
|
|
17
|
Rousset F, Garcia E, Defrance T, Peronne
C, Vezzio N, Hsu DH, Kastelein R, Moore KW and Banchereau J:
Interleukin 10 is a potent growth and differentiation factor for
activated human B lymphocytes. Proc Natl Acad Sci USA.
89:1890–1893. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fluckiger AC, Durand I and Banchereau J:
Interleukin 10 induces apoptotic cell death of B-chronic
lymphocytic leukemia cells. J Exp Med. 179:91–99. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stewart JP, Behm FG, Arrand JR and Rooney
CM: Differential expression of viral and human interleukin-10
(IL-10) by primary B cell tumors and B cell lines. Virology.
200:724–732. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mocellin S, Marincola FM and Young HA:
Interleukin-10 and the immune response against cancer: A
counterpoint. J Leukoc Biol. 78:1043–1051. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moore KW, de Waal MR, Coffman RL and
O'Garra A: Interleukin-10 and the interleukin-10 receptor. Annu Rev
Immunol. 19:683–765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mosser DM and Zhang X: Interleukin-10: New
perspectives on an old cytokine. Immunol Rev. 226:205–218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Garra A, Barrat FJ, Castro AG, Vicari A
and Hawrylowicz C: Strategies for use of IL-10 or its antagonists
in human disease. Immunol Rev. 223:114–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frappaz D, Bonneville-Levard A, Ricard D,
Carrie S, Schiffler C, Xuan KH and Weller M: Assessment of
Karnofsky (KPS) and WHO (WHO-PS) performance scores in brain tumour
patients: The role of clinician bias. Support Care Cancer. Aug
13–2020.(Epub ahead of print). doi: 10.1007/s00520-020-05663-y.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teusch W: Relationships between Pandy's
test and syphilis reactions in the spinal fluid. Med Monatsschr.
4:290–291. 1950.(In Undetermined Language). PubMed/NCBI
|
|
26
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nabors LB, Portnow J, Ammirati M, Baehring
J, Brem H, Butowski N, Fenstermaker RA, Forsyth P, Hattangadi-Gluth
J, Holdhoff M, et al: NCCN guidelines insights: Central nervous
system cancers, version 1.2017. J Natl Compr Canc Netw.
15:1331–1345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sugita Y, Muta H, Ohshima K, Morioka M,
Tsukamoto Y, Takahashi H and Kakita A: Primary central nervous
system lymphomas and related diseases: Pathological characteristics
and discussion of the differential diagnosis. Neuropathology.
36:313–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fluss R, Faraggi D and Reiser B:
Estimation of the Youden index and its associated cutoff point.
Biometrical J. 47:458–472. 2005. View Article : Google Scholar
|
|
30
|
El Far M, Fouda M, Yahya R and El Baz H:
Serum IL-10 and IL-6 levels at diagnosis as independent predictors
of outcome in non-Hodgkin's lymphoma. J Physiol Biochem.
60:253–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duletić AN, Stifter S, Dvornik S, Skunca Z
and Jonjić N: Correlation of serum IL-6, IL-8 and IL-10 levels with
clinicopathological features and prognosis in patients with diffuse
large B-cell lymphoma. Int J Lab Hematol. 30:230–239. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song Y, Zhang W, Zhang L, Wu W, Zhang Y,
Han X, Yang C, Zhang L and Zhou D: Cerebrospinal Fluid IL-10 and
IL-10/IL-6 as accurate diagnostic biomarkers for primary central
nervous system large B-cell lymphoma. Sci Rep. 6:386712016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alas S, Emmanouilides C and Bonavida B:
Inhibition of interleukin 10 by rituximab results in
down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin's
lymphoma to apoptosis. Clin Cancer Res. 7:709–723. 2001.PubMed/NCBI
|
|
34
|
Vega MI, Huerta-Yepaz S, Garban H,
Jazirehi A, Emmanouilides C and Bonavida B: Rituximab inhibits p38
MAPK activity in 2F7 BNHL and decreases IL-10 transcription:
Pivotal role of p38 MAPK in drug resistance. Oncogene.
23:3530–3540. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Westrhenen A, Smidt LCA, Seute T,
Nierkens S, Stork ACJ, Minnema MC and Snijders TJ: Diagnostic
markers for CNS lymphoma in blood and cerebrospinal fluid: A
systematic review. Brit J Haematol. 182:384–403. 2018. View Article : Google Scholar
|
|
36
|
Whitcup SM, Stark-Vancs V, Wittes RE,
Solomon D, Podgor MJ, Nussenblatt RB and Chan CC: Association of
interleukin 10 in the vitreous and cerebrospinal fluid and primary
central nervous system lymphoma. Arch Ophthalmol. 115:1157–1160.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salmaggi A, Eoli M, Corsini E, Gelati M,
Frigerio S, Silvani A and Boiardi A: Cerebrospinal fluid
interleukin-10 levels in primary central nervous system lymphoma: A
possible marker of response to treatment? Ann Neurol. 47:137–138.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rubenstein JL, Wong VS, Kadoch C, Gao HX,
Barajas R, Chen L, Josephson SA, Scott B, Douglas V, Maiti M, et
al: CXCL13 plus interleukin 10 is highly specific for the diagnosis
of CNS lymphoma. Blood. 121:4740–4748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mabray MC, Barajas RF, Villanueva-Meyer
JE, Zhang CA, Valles FE, Rubenstein JL and Cha S: The combined
performance of ADC, CSF CXC chemokine ligand 13, and CSF
interleukin 10 in the diagnosis of central nervous system lymphoma.
AJNR Am J Neuroradiol. 37:74–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nguyen-Them L, Costopoulos M, Tanguy ML,
Houillier C, Choquet S, Benanni H, Elias-Shamieh R, Armand M,
Faivre G, Glaisner S, et al: The CSF IL-10 concentration is an
effective diagnostic marker in immunocompetent primary CNS lymphoma
and a potential prognostic biomarker in treatment-responsive
patients. Eur J Cancer. 61:69–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ikeguchi R, Shimizu Y, Shimizu S and
Kitagawa K: CSF and clinical data are useful in differentiating CNS
inflammatory demyelinating disease from CNS lymphoma. Mult Scler.
24:1212–1223. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thiel E, Korfel A, Martus P, Kanz L,
Griesinger F, Rauch M, Roth A, Hertenstein B, von Toll T,
Hundsberger T, et al: High-dose methotrexate with or without whole
brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase
3, randomised, non-inferiority trial. Lancet Oncol. 11:1036–1047.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sasayama T, Tanaka K, Mizowaki T,
Nagashima H, Nakamizo S, Tanaka H, Nishihara M, Mizukawa K, Hirose
T, Itoh T and Kohmura E: Tumor-associated macrophages associate
with cerebrospinal fluid interleukin-10 and survival in primary
central nervous system lymphoma (PCNSL). Brain Pathol. 26:479–487.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dukers DF, Jaspars LH, Vos W, Oudejans JJ,
Hayes D, Cillessen S, Middeldorp JM and Meijer CJ: Quantitative
immunohistochemical analysis of cytokine profiles in Epstein-Barr
virus-positive and -negative cases of Hodgkin's disease. J Pathol.
190:143–149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Voorzanger N, Touitou R, Garcia E,
Delecluse HJ, Rousset F, Joab I, Favrot MC and Blay JY: Interleukin
(IL)-10 and IL-6 are produced in vivo by non-Hodgkin's lymphoma
cells and act as cooperative growth factors. Cancer Res.
56:5499–5505. 1996.PubMed/NCBI
|
|
46
|
Wu X, Hsu DK, Wang KH, Huang Y, Mendoza L,
Zhou Y and Hwang ST: IL-10 is overexpressed in human cutaneous
T-cell lymphoma and is required for maximal tumor growth in a mouse
model. Leuk Lymphoma. 60:1244–1252. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leppa S, Jorgensen J, Tierens A, Meriranta
L, Ostlie I, de Nully BP, Fagerli UM, Larsen TS, Mannisto S,
Munksgaard L, et al: Patients with high-risk DLBCL benefit from
dose-dense immunochemotherapy combined with early systemic CNS
prophylaxis. Blood Adv. 4:1906–1915. 2020. View Article : Google Scholar : PubMed/NCBI
|